Abstract
We propose an integrated and adaptable approach to improve patient care and clinical outcomes through analgesia and light sedation, initiated early during an episode of critical illness and as a priority of care. This strategy, which may be regarded as an evolution of the Pain, Agitation and Delirium guidelines, is conveyed in the mnemonic eCASH—early Comfort using Analgesia, minimal Sedatives and maximal Humane care. eCASH aims to establish optimal patient comfort with minimal sedation as the default presumption for intensive care unit (ICU) patients in the absence of recognised medical requirements for deeper sedation. Effective pain relief is the first priority for implementation of eCASH: we advocate flexible multimodal analgesia designed to minimise use of opioids. Sedation is secondary to pain relief and where possible should be based on agents that can be titrated to a prespecified target level that is subject to regular review and adjustment; routine use of benzodiazepines should be minimised. From the outset, the objective of sedation strategy is to eliminate the use of sedatives at the earliest medically justifiable opportunity. Effective analgesia and minimal sedation contribute to the larger aims of eCASH by facilitating promotion of sleep, early mobilization strategies and improved communication of patients with staff and relatives, all of which may be expected to assist rehabilitation and avoid isolation, confusion and possible long-term psychological complications of an ICU stay. eCASH represents a new paradigm for patient-centred care in the ICU. Some organizational challenges to the implementation of eCASH are identified.
Keywords: ICU, Pain, Analgesia, Sedation, eCASH
Introduction
Various data have indicated a consistent and strong association between early deep sedation and poor long-term outcomes, including mortality, cognitive decline and psychological complications [1–6]. A number of interventions have been suggested to address this [7–11], and a shift towards light sedation was strongly advocated in the Pain, Agitation and Delirium (PAD) guidelines [12] and the recently published German Delirium, Analgesia, and Sedation guidelines [13], but experience from recent controlled trials suggests that deep sedation continues to be commonplace in the intensive care unit (ICU) [14, 15].
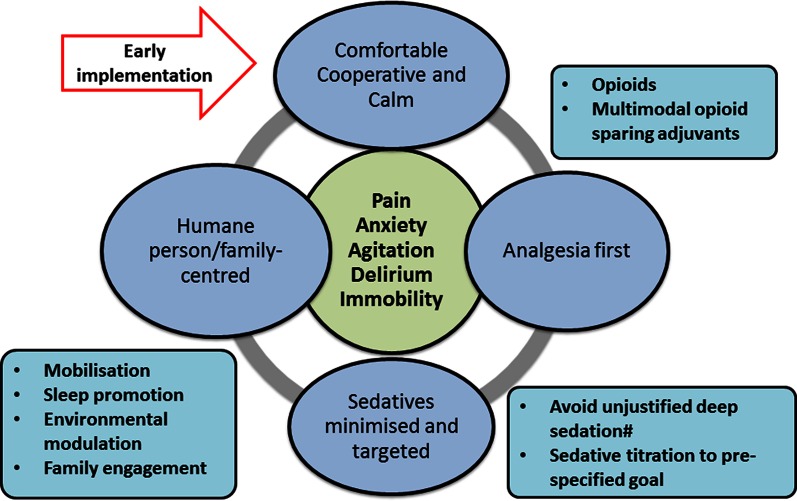
We propose an integrated and adaptable approach to achieve light sedation, initiated early during an episode of critical illness and as a priority of care: ideally, patients should be comfortable, calm and able to cooperatively engage with caregivers and family at all times. This process is designed to deliver effective analgesia, titrated goal-directed minimal sedation and a patient-centred focus. To that end, this strategy may be conveyed in the mnemonic eCASH—early Comfort using Analgesia, minimal Sedatives and maximal Humane care. As illustrated in Fig. 1, eCASH has several distinct features:
An emphasis on early implementation. The time component of eCASH is central to the concept.
Generalizability: this is a sequential process-of-care intervention that can be delivered to all patients.
Promotion and facilitation of patient-centred care.
Fig. 1.
The eCASH concept: early implementation to manage and prevent pain, anxiety, agitation, delirium and immobility and facilitate patient-centred care. (#Moderate or deep sedation remains relevant for some situations, including the management of severe respiratory failure with ventilator–patient dyssynchrony, prevention of awareness in patients receiving neuromuscular blocking agents, status epilepticus, surgical conditions necessitating strict immobilization and some cases of severe brain injury with intracranial hypertension)
This commentary assumes prior knowledge of the pharmacology of sedative and analgesic agents and familiarity with sedation, pain and delirium assessment scales and does not examine these matters in detail.
Heavy sedation: outdated but still used
Profound sedation used to be widespread in ICU patients, not least because the mechanical ventilation of the era was considered to be otherwise intolerable [16]. Significant improvements in ICU-related organ-support technology, such as advances in ventilator design, dialysis and extracorporeal circuits, have diminished that rationale for deep sedation and the PAD guidelines have identified the possibilities for improving clinical outcomes by achieving and maintaining light sedation [12].
However, it is left to clinicians at the bedside to decide when to implement these measures. This often results in exactly the outcome they were meant to avoid: unnecessary deep sedation. This fallacy may be especially marked during the first few days in the ICU, when depth of sedation is considered—wrongly—not to be a critical consideration. In fact, observations from multiple studies suggest a consistent link between early deep sedation and significant harm [1–4], which may be attributed to a range of deleterious effects (Table 1).
Table 1.
Problems potentially associated with deep sedation
| Loss of human contact |
| Respiratory depression |
| Inactivity-induced diaphragm dysfunction |
| Myocardial depression and haemodynamic instability |
| Microvascular alterations |
| Altered gut function—ileus |
| Airway (micro)aspiration |
| Increased risk of pneumonia |
| Increased risk of thrombophlebitis |
| Risk of decubitus ulcers |
| Delirium |
| Risk of ICU-acquired weakness |
| Peripheral muscle weakness |
| Immunosuppression |
| Prolonged mechanical ventilation/weaning |
| Prolonged ICU and hospital stay |
| Permanent cognitive deficits |
| Chronic psychological illnesses |
| Costs |
Moderate or deep sedation remains relevant for some situations, including the management of severe respiratory failure with ventilator–patient dyssynchrony, prevention of awareness in patients receiving neuromuscular blocking agents, status epilepticus, surgical conditions necessitating strict immobilization and some cases of severe brain injury with intracranial hypertension. For the vast majority of ICU patients, however, achieving and maintaining a light level of sedation should be sufficient and may avoid the potential harm caused by early oversedation. In this context, it should be noted that, given the evolution of sophisticated ventilator technologies [17], the response to patient–ventilator asynchrony should firstly be efforts to identify a more tolerable ventilator setting and only subsequently an increased use of analgesics and/or sedatives. The ventilator should always be adapted to the patient, not the patient to the ventilator.
eCASH: a mnemonic for a modern approach
Early comfort with minimal sedation should be provided as a clinical priority on a par with early resuscitation, early sepsis management and an early lung-protective ventilation strategy. eCASH, with its emphasis on comfort, analgesia, minimal sedation and patient-centred care, encompasses this new outlook. Implicit in eCASH is the understanding that if analgesia and light sedation do not achieve a calm and cooperative patient then the causes of failure should be identified and corrected before any resort to deep sedation is considered.
Pain management: the starting point for eCASH
Pain management with effective analgesia is the first priority in the implementation of the eCASH premise, and there is still ample scope for improvement in this area.
A systematic approach to anticipating individual patient requirements for analgesia is likely to improve pain management (Table 2). Matters for consideration include pre-existing chronic pain syndromes (and associated medications) at admission and acute pain related to the presenting illness. During subsequent ICU care, analgesia is required for ongoing illness-related pain and discomfort related to routine treatments, such as suction and positioning. Intermittent increases in analgesia may also be required to address procedure-associated pain. (This requires appreciation of the pharmacokinetics of analgesics, especially the time-to-peak effect. Application of a nociceptive stimulation before the peak effect of the drug has been reached will give the impression that the analgesic is ineffective.) Regular evaluation and reassessment of analgesia requirements is therefore essential throughout the ICU stay.
Table 2.
A suggested systematic approach to assessing likely analgesia requirements to manage pain in the ICU
| Pain category | Examples | Potential therapeutic approaches | Candidate drugs |
|---|---|---|---|
| Pre-existing chronic pain or analgesia requirements | Chronic neuropathic pain syndromes | Continue chronic pain medications (e.g. gabapentin, amitriptyline) | Gabapentin, amitriptyline |
| Opioid addiction | Continue or introduce long-acting agents | Methadone | |
| Consider opioid-sparing agents | Paracetamol (acetaminophen) | ||
| Acute illness-related pain | Musculoskeletal trauma | Intermittent or continuous opioid drugs, preferably PCA | Paracetamol (acetaminophen) |
| Surgery | Opioid-sparing agents | Ketamine | |
| Visceral and inflammation-related pain | Adjunct analgesics | Dexmedetomidine | |
| Analgo-sedative agents | |||
| Continuous ICU treatment-related pain/discomfort | Endotracheal tube tolerance | Intermittent or continuous opioid | Ketamine |
| Mechanical ventilation | Opioid-sparing agents | Dexmedetomidine | |
| Pressure care | Analgo-sedative agents | ||
| Physiotherapy | |||
| Joint stiffness | |||
| Intermittent procedural pain | Drain insertion | Boluses of opioid | |
| Chest physiotherapy | Local anaesthesia | ||
| Tracheostomy |
NB Non-steroidal anti-inflammatory agents should be used with extreme caution, if at all. Analgesic agents should only be used to achieve pain relief and not as sedatives. For procedural analgesia, deep sedation may be required
PCA patient-controlled analgesia
In conscious, oriented patients a numeric rating scale (NRS) may be used to quantify pain [18]. This is not viable if deep sedation is used or if communication is impeded by brain dysfunction; for such patients, validated pain assessment scales have been developed, such as the Behavioral Pain Scale (BPS) and the Critical-Care Pain Observation Tool (CPOT) [19–21].
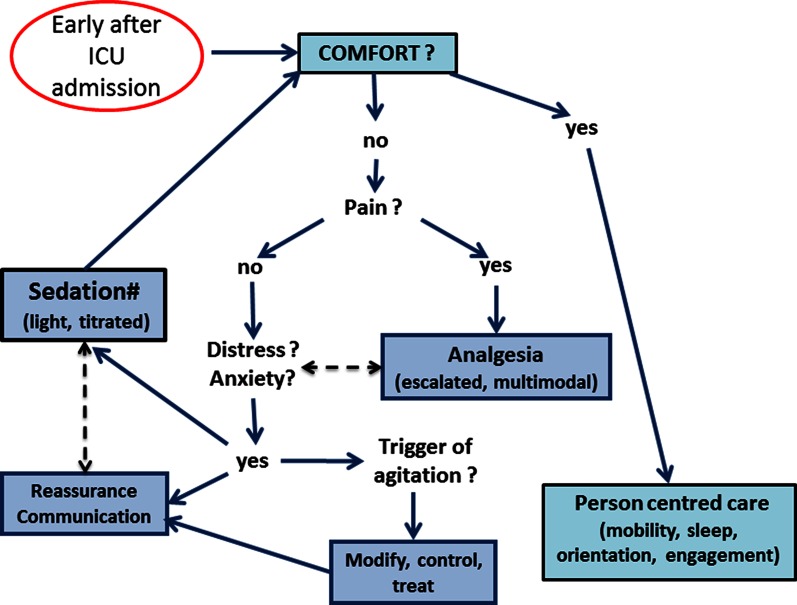
Opioids remain central to analgesia and the needs of many patients may be satisfied with low-dose opioids. Patient-controlled analgesia (PCA) should be preferred for patients who are able to use it. eCASH, however, emphasises the need to reduce total opioid exposure in order to avoid adverse effects such as respiratory depression, feeding intolerance, constipation and ileus, withdrawal, tolerance, hyperalgesia, physical dependence and depression of the immune system. The use of an escalated multimodal strategy is recommended for that purpose (Fig. 2). For example, the use of paracetamol can reduce the overall opioid load and associated adverse effects [22] and is feasible even in patients with hepatic impairment or injury. The introduction of adjunctive analgesics, such as pentins, lidocaine, alpha-2-agonists and low-dose ketamine, should be considered early if opioid needs are increasing. In the context of invasive mechanical ventilation, both ketamine and the alpha-2-agonists have been suggested to provide an opioid-sparing effect with a moderate sedative effect in patients who need both analgesia and sedation [23, 24].
Fig. 2.
The eCASH implementation map commences upon ICU admission and focuses on coordinated effective analgesia and pain management and titrated minimal and light sedation. Dashed double-headed arrows identify factors and/or interventions that need to be considered concurrently. (#Moderate and deep sedation remains relevant for specific clinical situations, as noted in Fig. 1)
The emphasis on low ketamine dosage is important: the primary aim of ketamine use in this context should be to provide analgesia, and this is best achieved at low doses. Ketamine has sedative effects at higher doses but there is also a dose-dependent increase in side effects, notably psychosis, hallucination and delirium, that make it unsuitable for general use as a sedative agent.
Non-pharmacological measures, including music therapy and relaxation techniques, may be opioid-sparing and analgesia-enhancing [25]. These interventions are safe according to the usual meaning of that term but they are not a reliable low-cost option, nor are they necessarily easy to provide.
Sedation in eCASH
Light sedation emphasises maintaining the patient in a state in which they are calm, comfortable and cooperative (the 3C rule). Ideally, the patient can be awake in order to maintain eye contact, interact with caregivers and family members and participate in physical and/or occupational therapy but permitted to drift off to sleep when uninterrupted. This state, broadly equivalent to a Richmond Agitation Sedation Scale (RASS) score of –1/0 [26], has been linked with favourable effects on a range of clinical outcomes [12].
Agitation in the critically ill patient can result from many factors, including pain, delirium, anxiety, drug withdrawal syndromes and discomfort resulting from poor ventilator synchrony, bowel dysfunction or pressure areas. Emphasis should be placed on diagnosing the cause of agitation rather than simply on treating the symptoms, which can result in unnecessary re-sedation. Suboptimal treatment of pain should also be considered as a major driver of agitation and treated diligently, with the emphasis on effective pain relief and not on masking signs of agitation. Attention must also be given to concomitant drivers of brain dysfunction, such as decreased cerebral perfusion, severe hypoxaemia, sepsis, high temperature, electrolyte imbalance, sleep disturbances and deliriogenic medications. Short-term sedation may be required while these precipitants are corrected. The presence or absence of delirium should be objectively tested by a validated tool, such as the Confusion Assessment Method for the ICU (CAM-ICU) [27].
Light or no sedation may be key to patient benefit
Successful provision of light sedation requires a combination approach with attention being paid to the choice of sedative agent(s) as well as targeted and titrated sedative intensity. These considerations have influenced a general retreat from benzodiazepines as first-line sedatives in the ICU and a move towards short-acting, easy-to-titrate agents such as propofol and dexmedetomidine.
A pilot study of early goal-directed sedation illustrated successful implementation of targeted early light sedation using dexmedetom-idine as the primary sedative [2]. Similarly, in a study comparing sedation with dexmedetomidine and midazolam for light sedation, sedation with dexmedetomidine was associated with lower rates of delirium, and shorter time on mechanical ventilation [28]. Comparative studies of propofol and dexmedetomidine are equivocal [29]; either agent may therefore be used when targeted light sedation is required.
A ‘no-sedation’ strategy has been reported by Strøm et al. [11] to increase ventilator-free days, albeit with limitations such as increased risk of agitation. This approach uses morphine and haloperidol and there has to be some question regarding whether this is truly a ‘no-sedative’ regimen or rather an opioid-based sedation strategy. There is in addition a widespread (though untested) belief that successful implementation of this approach requires quite a high staff:patient ratio (typically 1:1). Nevertheless, these concepts and approaches are consistent with the eCASH philosophy, some specific aspects of which are now examined in more detail.
Detailed examination of the pharmacology of first-line sedatives is beyond the scope of this discussion, but it is noteworthy that investigation of electroencephalography (EEG) patterns in patients administered different drugs gives substance to the view that different mechanisms of action may give rise to qualitatively different and distinct forms of sedation [30, 31].
Time to phase out benzodiazepines?
It is premature to signal the elimination of benzodiazepines from the sedative repertoire but, as noted above, their place as first-line sedatives is greatly diminished in the eCASH paradigm. Non-benzodiazepine sedatives deliver better effects on some ‘hard’ outcomes, such as ICU length of stay and duration of mechanical ventilation (but not short-term mortality or delirium), than benzodiazepines, particularly in mechanically ventilated patients [28, 32–35]. Benzodiazepines should be reserved for specific indications, such as amnesia for procedural sedation, convulsions, some instances of alcohol withdrawal, intractable agitation, palliation or severe brain pathologies. Whether or not intermittent, titrated application of predominantly anxiolytic gamma-aminobutyric acid (GABA)-agonists is beneficial is a matter of current research. When used, benzodiazepines should be titrated, preferably by intermittent rather than continuous infusion, which appears to be a risk factor for delirium [36].
Goodbye daily sedation stops: hello frequent titration protocols
The use of bedside sedation scales may move the ICU team away from deep and prolonged sedation (often based on use of benzodiazepines) towards continuous targeted light sedation with drugs that are relatively short-acting and easier to titrate. This will likely diminish the role and need for daily sedation stops as originally conceived and implemented, and is in accordance with the 3C principle already outlined.
Where sedation is required for optimizing patient care, sedative medications should be titrated to the lowest amount required to achieve the target sedation level deemed necessary. The medical team should constantly evaluate the need for ongoing sedation and down-titrate sedative medications, with the explicit goal of complete withdrawal at the earliest clinically warranted opportunity. Sedative and analgesic drugs may accumulate in critically ill patients despite careful titration and use of short-acting agents. A stage may be reached when sedation stops may be needed only in cases where drug accumulation is suspected. There is now reasonable evidence attesting to the positive impact of protocol use on standards of care in analgesia, sedation and delirium [37–41], although not all the data are conclusive and affirmative [42, 43]. It should be noted that the evidence for protocolized therapy derives substantially from studies of benzodiazepines [40] and that no firm conclusions on the impact of protocolization may be drawn for other agents. As Minhas et al. [40] have noted, explanations of the benefit seen with protocolization are likely to involve more than simply a reduction in total sedative use.
Validated sedation scales at the bedside: subjective but valuable
Checking for excess sedation and analgesia should be part of a routine bedside evaluation by nurses, like adequate feeding, having the bed elevated or applying thromboembolism prophylaxis [44].
The use of clinically validated scales to monitor sedation level is often recommended to facilitate targeted, goal-directed sedation and improve communication among care providers. Sedation scales rely on an interaction between patient and caregiver and it may be argued that they lead to confirmation bias, i.e., to confirming a level of sedation already presumed to have been achieved. Nevertheless, they yield a quantitative estimate of sedation depth that can be questioned and scrutinised and that may be particularly useful in patients with deep sedative states.
The development of objective and reliable neurophysiologic measures of sedation level, while proceeding at an experimental and proof-of-concept level, is still far from being a practical option at the bedside. A major problem with currently available systems, which were mostly designed for depth of anaesthesia monitoring, is the confounding effect of facial electromyography on EEG-based algorithms [45, 46]. The use of neurophysiologic monitors should, however, be considered in patients requiring deeper sedation to avoid a descent into profound sedation.
Patient-centred care, a core component of eCASH
The concept of eCASH is pivotal to the delivery of patient-centred care for ICU patients (Table 3). A programme that addresses all these elements should be implemented on ICU admission and continued throughout the ICU stay. Close attention should be given to identifying and mitigating factors that obstruct the early introduction of eCASH.
Table 3.
Components of patient-centred care
| Frequent and appropriate communication |
| Explanations of the care components |
| Time and space orientation |
| Noise reduction |
| Avoidance of unnecessary restraints |
| Sleep promotion at night |
| Physical activity/early mobilization |
| Mental stimulation |
| Occupational therapy including cognitive training |
| Family engagement |
Interdisciplinary collaboration is essential to the implementation and delivery of the eCASH premise. The team culture should focus on avoiding potentially harmful interventions and promoting best practice at all times. The role of bedside nurses and caregivers is pivotal within this framework, and adequate staff education programmes specific to sedation–analgesia management should be a core activity and competency [47, 48]. Although nurse:patient ratios differ between hospitals and may be regarded as suboptimal in many cases, inappropriately deep sedation is not a defensible response to staff shortages. In this context, it should be noted that intervention consistent with the eCASH philosophy was associated with less use of physical restraints in the pilot phase of a controlled trial in mechanically ventilated, critically ill patients [49]. These data provide some assurance that a general presumption towards light sedation need not be accompanied by greater need for physical restraints where nursing ratios are less than ideal.
Some matters for consideration are discussed in the next part of this commentary; additional local factors will apply in different ICUs.
Promotion of sleep
Many patients’ experience of sleep in an ICU is unsatisfactory and potentially disadvantageous, and poor sleep has been identified in some studies as a prelude to and risk factor for delirium [50, 51]. Restoration of more normal sleep may become a therapeutic goal in the ICU, though whether that is a benefit in its own right or a surrogate for other changes is difficult to determine at present [52].
There is evidence that, beyond discomfort, sleep deprivation/alteration is associated with immunodepressive effects that may be clinically relevant [53, 54]. These data provide an incentive to prevent/correct sleep disturbance in ICU patients, albeit that formal studies of the effects of correction are not available. These reflections also focus attention on differential effects of sedatives on immune function [54, 55].
Improving patient sleep can be justified as a quality-of-care issue, with non-pharmacological methods representing a natural starting point. The deployment of ‘sleep bundles’—which include maintenance of regular sleep–wake rhythms, reduction in night-time light, noise and care episodes and use of earplugs and music—may be helpful [25, 56, 57]. Too little is known about the effect of analgesics and various sedative agents on sleep patterns in the ICU [58]. The value of adjunct therapies, such as melatonin or tryptophan, is unclear [59, 60].
Early mobilization strategies
An important corollary of the eCASH strategy is that the patient is capable of involvement in programmes for early mental stimulation and physical activity. The benefits of early mobilization for adult ICU patients have been documented [61–64]; this may help to avoid agitation, delirium and ICU-acquired weakness and hence contributes to the eCASH paradigm [63, 64]. The risks of early mobilization appear to be smaller than might have been anticipated and the barriers to implementation, although impossible to ignore, are surmountable [65, 66]. It is therefore a matter of concern that outmoded approaches to sedation still appear to be a significant impediment to early mobilization [67, 68].
Communication with staff/relatives
Sedation strategy can significantly influence patients’ communication with staff/relatives. Patients’ experiences of interaction with ICU staff are often brief and fragmented and the effects of those contacts can be incomprehensible, confusing or alarming to the patient [69]. Clearly, the capacity of a patient to communicate and understand is linked to alertness: to that extent, the adoption of eCASH-style light sedation may be expected to benefit the patient. Conversely, enhancement of the patient’s capacity to interact will benefit the patient only if staff are responsive to what the patient communicates and able to respond in a way that is coherent and comprehensible to the patient. Various training and technical measures to enhance the quality of communication have been proposed [70]. Good communication—an inherent theme of eCASH—may be expected to decrease the need for physical restraints.
Reorientation can be promoted through simple measures such as return of hearing aids and spectacles and other measures that allow patients to re-establish meaningful contacts with their surroundings and with other people. Review of the visiting hours policy is an integral part of this aspect of care and rehabilitation. Attention to the needs of relatives is also relevant both for its effects on patients and as a quality improvement goal in its own right [71].
eCASH is about patients, their carers and families
Full realization of the goals of eCASH requires changes to the process and culture of care. Successful implementation of change requires a close interdisciplinary and interprofessional collaboration and consideration of the different interests and priorities of the participating healthcare specialists [72].
The focus must be on multimodal interventions, some of which will very likely fall outside the scope of what might usually be described as ‘medical’ [73]. Promoting factors include implementation planning, training/support and effective documentation, and perhaps debriefing, whereas excessive staff turnover, poor staff morale and lack of interdisciplinary respect are obstacles to implementation [72]. The development of robust systems to track and feed back the quality of sedation and analgesia provides potential methods for driving quality improvement in a manner similar to the successful programmes that have decreased infections in ICUs [74].
Transferring responsibility and ownership of sedation management to bedside staff requires appropriate training and education programmes within ICUs. Clear strategies to manage the causes of agitation are needed to ensure ‘buy-in’ from nursing teams, with access to support and advice for difficult cases. A supportive and ‘no-blame’ culture in relation to the identification and review of sedation-related adverse events is a key element in the implementation of nurse-led management.
Given the variety of circumstances encountered in ICUs, it is not feasible to offer detailed prescriptions for how to implement change. By extension, it is not possible to make claims for how much money (if any) might be saved by the full and successful implementation of such a formula. That, however, is to miss the point: eCASH is a process for optimising clinical benefit to patients, both during their time in the ICU and potentially beyond it; that goal is worthwhile in its own right.
Developing eCASH
The guiding principles of eCASH are clear and components of the eCASH concept are supported by current guidelines; details of this philosophy of care can be expected to evolve in response to clinical trial data. Among trials currently in progress that may be expected to influence the final form of eCASH are: SPICE III (NCT01728558); MENDS II (NCT01739933); NONSEDA (NCT0196768); AWARE (NCT01617265); DESIRE (NCT01760967); and VITALITY (NCT02143661), which examines environmental influences on ICU outcomes. A longer-term goal would be to evaluate the clinical effectiveness of a prespecified eCASH bundle in a randomised clinical trial.
Acknowledgments
Orion Pharma (Espoo, Finland) initiated and supported the round-table meeting from which this work emerged, but had no influence on the development of the views expressed. Writing and editorial assistance was provided by Hughes associates, Oxford, UK.
Compliance with ethical standards
Conflicts of interest
Authors received assistance from Orion Pharma, Espoo, Finland, to attend the meeting from which this work was developed. PS, CS and GCF declare personal consultancy agreements with Orion Pharma. GC and JM declare receipt of honoraria for engagements; GC further declares receipt of research grants from Orion Pharma to the Catholic University of Rome. DL declares unpaid editorship of a newsletter supported by Orion Pharma. All other authors declare no consultancy or similar agreements with Orion Pharma. The authors have no other relevant conflicts of interest to declare.
Footnotes
Take-home message:
Excessive deep sedation, even if restricted to initiation of ICU treatment, is associated with worse outcome for ICU patients: everything should be done to avoid it. We propose a new approach with the acronym eCASH—early Comfort using Analgesia, minimal Sedatives and maximal Humane care—predicated on the early achievement of pain relief and the maintenance of comfort with minimal sedation being used to facilitate natural sleep, early mobilization and engagement with caregivers and relatives.
References
- 1.Shehabi Y, Bellomo R, Reade MC, Bailey M, Bass F, Howe B, McArthur C, Seppelt IM, Webb S, Weisbrodt L. Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. Am J Respir Crit Care Med. 2012;186:724–731. doi: 10.1164/rccm.201203-0522OC. [DOI] [PubMed] [Google Scholar]
- 2.Shehabi Y, Chan L, Kadiman S, Alias A, Ismail WN, Tan MA, Khoo TM, Ali SB, Saman MA, Shaltut A, Tan CC, Yong CY, Bailey M. Sedation depth and long-term mortality in mechanically ventilated critically ill adults: a prospective longitudinal multicentre cohort study. Intensive Care Med. 2013;39:910–918. doi: 10.1007/s00134-013-2830-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanaka LM, Azevedo LC, Park M, Schettino G, Nassar AP, Réa-Neto A, Tannous L, de Souza-Dantas VC, Torelly A, Lisboa T, et al. Early sedation and clinical outcomes of mechanically ventilated patients: a prospective multicenter cohort study. Crit Care. 2014;18:R156. doi: 10.1186/cc13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balzer F, Weiß B, Kumpf O, Treskatsch S, Spies C, Wernecke KD, Krannich A, Kastrup M. Early deep sedation is associated with decreased in-hospital and two-year follow-up survival. Crit Care. 2015;19:197. doi: 10.1186/s13054-015-0929-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brummel NE, Jackson JC, Pandharipande PP, Thompson JL, Shintani AK, Dittus RS, Gill TM, Bernard GR, Ely EW, Girard TD. Delirium in the ICU and subsequent long-term disability among survivors of mechanical ventilation. Crit Care Med. 2014;42:369–377. doi: 10.1097/CCM.0b013e3182a645bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson JC, Pandharipande PP, Girard TD, Brummel NE, Thompson JL, Hughes CG, Pun BT, Vasilevskis EE, Morandi A, Shintani AK, et al. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med. 2014;2:369–379. doi: 10.1016/S2213-2600(14)70051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 8.Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, Taichman DB, Dunn JG, Pohlman AS, Kinniry PA, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (awakening and breathing controlled trial): a randomised controlled trial. Lancet. 2008;371:126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 9.Hughes CG, Girard TD, Pandharipande PP. Daily sedation interruption versus targeted light sedation strategies in ICU patients. Crit Care Med. 2013;41(9 Suppl 1):S39–S45. doi: 10.1097/CCM.0b013e3182a168c5. [DOI] [PubMed] [Google Scholar]
- 10.Pandharipande P, Banerjee A, McGrane S, Ely EW. Liberation and animation for ventilated ICU patients: the ABCDE bundle for the back-end of critical care. Crit Care. 2010;14:157. doi: 10.1186/cc8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strøm T, Martinussen T, Toft P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: a randomised trial. Lancet. 2010;375:475–480. doi: 10.1016/S0140-6736(09)62072-9. [DOI] [PubMed] [Google Scholar]
- 12.Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 13.DAS-Taskforce 2015. Baron R, Binder A, Biniek R, Braune S, Buerkle H, et al. Evidence and consensus based guideline for the management of delirium, analgesia, and sedation in intensive care medicine. Revision 2015 (DAS-Guideline 2015)—short version. Ger Med Sci. 2015;13:Doc19. doi: 10.3205/000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The TEAM Study Investigators Early mobilization and recovery in mechanically ventilated patients in the ICU: a bi-national, multi-centre, prospective cohort study. Crit Care. 2015;19:81. doi: 10.1186/s13054-015-0765-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page VJ, Ely EW, Gates S, Zhao XB, Alce T, Shintani A, Jackson J, Perkins GD, McAuley DF. Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2013;1:515–523. doi: 10.1016/S2213-2600(13)70166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heffner JE. A wake-up call in the intensive care unit. N Engl J Med. 2000;342:1520–1522. doi: 10.1056/NEJM200005183422011. [DOI] [PubMed] [Google Scholar]
- 17.Kacmarek RM. The mechanical ventilator: past, present, and future. Respir Care. 2011;56:1170–1180. doi: 10.4187/respcare.01420. [DOI] [PubMed] [Google Scholar]
- 18.Chanques G, Viel E, Constantin JM, Jung B, de Lattre S, Carr J, Cissé M, Lefrant JY, Jaber S. The measurement of pain in intensive care unit: comparison of 5 self-report intensity scales. Pain. 2010;151:711–721. doi: 10.1016/j.pain.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 19.Young J, Siffleet J, Nikoletti S, Shaw T. Use of a behavioural pain scale to assess pain in ventilated, unconscious and/or sedated patients. Intensive Crit Care Nurs. 2006;22:32–39. doi: 10.1016/j.iccn.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Rijkenberg S, Stilma W, Endeman H, Bosman RJ, Oudemans-van Straaten HM. Pain measurement in mechanically ventilated critically ill patients: Behavioral Pain Scale versus Critical-Care Pain Observation Tool. J Crit Care. 2015;30:167–172. doi: 10.1016/j.jcrc.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Echegaray-Benites C, Kapoustina O, Gélinas C. Validation of the use of the Critical-Care Pain Observation Tool (CPOT) with brain surgery patients in the neurosurgical intensive care unit. Intensive Crit Care Nurs. 2014;30:257–265. doi: 10.1016/j.iccn.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Dahl JB, Nielsen RV, Wetterslev J, Nikolajsen L, Hamunen K, Kontinen VK, Hansen MS, Kjer JJ, Mathiesen O, Scandinavian Postoperative Pain Alliance (ScaPAlli) Post-operative analgesic effects of paracetamol, NSAIDs, glucocorticoids, gabapentinoids and their combinations: a topical review. Acta Anaesthesiol Scand. 2014;58:1165–1181. doi: 10.1111/aas.12382. [DOI] [PubMed] [Google Scholar]
- 23.McGuinness SK, Wasiak J, Cleland H, Symons J, Hogan L, Hucker T, Mahar PD. A systematic review of ketamine as an analgesic agent in adult burn injuries. Pain Med. 2011;12:1551–1558. doi: 10.1111/j.1526-4637.2011.01220.x. [DOI] [PubMed] [Google Scholar]
- 24.Patel A, Davidson M, Tran MC, Quraishi H, Schoenberg C, Sant M, Lin A, Sun X. Dexmedetomidine infusion for analgesia and prevention of emergence agitation in children with obstructive sleep apnea syndrome undergoing tonsillectomy and adenoidectomy. Anesth Analg. 2010;111:1004–1010. doi: 10.1213/ANE.0b013e3181ee82fa. [DOI] [PubMed] [Google Scholar]
- 25.Beaulieu-Boirė G, Bourque S, Chagnon F, Chouinard L, Gallo-Payet N, Lesur O. Music and biological stress dampening in mechanically-ventilated patients at the intensive care unit ward—a prospective interventional randomized crossover trial. J Crit Care. 2013;28:442–450. doi: 10.1016/j.jcrc.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, Gordon S, Francis J, Speroff T, Gautam S, Margolin R, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289:2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 27.Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, Speroff T, Gautam S, Bernard GR, Inouye SK. Evaluation of delirium in critically ill patients: validation of the confusion assessment method for the intensive care unit (CAM-ICU) Crit Care Med. 2001;29:1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, Whitten P, Margolis BD, Byrne DW, Ely EW, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 29.Jakob SM, Ruokonen E, Grounds RM, Sarapohja T, Garratt C, Pocock SJ, Bratty JR, Takala J. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. JAMA. 2012;307:1151–1160. doi: 10.1001/jama.2012.304. [DOI] [PubMed] [Google Scholar]
- 30.Akeju O, Pavone KJ, Westover MB, Vazquez R, Prerau MJ, Harrell PG, Hartnack KE, Rhee J, Sampson AL, Habeeb K, et al. A comparison of propofol- and dexmedetomidine-induced electroencephalogram dynamics using spectral and coherence analysis. Anesthesiology. 2014;121:978–989. doi: 10.1097/ALN.0000000000000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med. 2010;363:2638–2650. doi: 10.1056/NEJMra0808281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fraser GL, Devlin JW, Worby CP, Alhazzani W, Barr J, Dasta JF, Kress JP, Davidson JE, Spencer FA. Benzodiazepine versus nonbenzodiazepine-based sedation for mechanically ventilated, critically ill adults: a systematic review and meta-analysis of randomized trials. Crit Care Med. 2013;41(9 Suppl 1):S30–S38. doi: 10.1097/CCM.0b013e3182a16898. [DOI] [PubMed] [Google Scholar]
- 33.Klompas M, Li L, Szumita P, Kleinman K, Murphy MV, CDC Prevention Epicenters Program Associations between different sedatives and ventilator-associated events, length-of-stay, and mortality in mechanically ventilated patients. Chest. 2015 doi: 10.1378/chest.15-1389. [DOI] [PubMed] [Google Scholar]
- 34.Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, Shintani AK, Thompson JL, Jackson JC, Deppen SA, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298:2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 35.Carson SS, Kress JP, Rodgers JE, Vinayak A, Campbell-Bright S, Levitt J, Bourdet S, Ivanova A, Henderson AG, Pohlman A, et al. A randomized trial of intermittent lorazepam versus propofol with daily interruption in mechanically ventilated patients. Crit Care Med. 2006;34:1326–1332. doi: 10.1097/01.CCM.0000215513.63207.7F. [DOI] [PubMed] [Google Scholar]
- 36.Zaal IJ, Devlin JW, Hazelbag M, Klein Klouwenberg PM, van der Kooi AW, Ong DS, Cremer OL, Groenwold RH, Slooter AJ. Benzodiazepine-associated delirium in critically ill adults. Intensive Care Med. 2015;41:2130–2137. doi: 10.1007/s00134-015-4063-z. [DOI] [PubMed] [Google Scholar]
- 37.Skrobik Y, Ahern S, Leblanc M, Marquis F, Awissi DK, Kavanagh BP. Protocolized intensive care unit management of analgesia, sedation, and delirium improves analgesia and subsyndromal delirium rates. Anesth Analg. 2010;111:451–463. doi: 10.1213/ANE.0b013e3181d7e1b8. [DOI] [PubMed] [Google Scholar]
- 38.Dale CR, Kannas DA, Fan VS, Daniel SL, Deem S, Yanez ND, 3rd, Hough CL, Dellit TH, Treggiari MM. Improved analgesia, sedation, and delirium protocol associated with decreased duration of delirium and mechanical ventilation. Ann Am Thorac Soc. 2014;11:367–374. doi: 10.1513/AnnalsATS.201306-210OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ackrivo J, Horbowicz KJ, Mordino J, El Kherba M, Ellingwood J, Sloan K, Murphy J (2015) Successful implementation of an automated sedation vacation process in intensive care units. Am J Med Qual (pii: 1062860615593340, Epub ahead of print) [DOI] [PubMed]
- 40.Minhas MA, Velasquez AG, Kaul A, Salinas PD, Celi LA. Effect of protocolized sedation on clinical outcomes in mechanically ventilated intensive care unit patients: a systematic review and meta-analysis of randomized controlled trials. Mayo Clin Proc. 2015;90:613–623. doi: 10.1016/j.mayocp.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rose L, Fitzgerald E, Cook D, Kim S, Steinberg M, Devlin JW, Ashley BJ, Dodek P, Smith O, Poretta K, et al. Clinician perspectives on protocols designed to minimize sedation. J Crit Care. 2015;30:348–352. doi: 10.1016/j.jcrc.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 42.Quintard H, Tran-Marsalla L, Esquirole C, Ichai C. Economic and clinical impact of a controlled sedation procedure in an intensive care unit. Ann Fr Anesth Reanim. 2012;31:778–782. doi: 10.1016/j.annfar.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 43.Aitken LM, Bucknall T, Kent B, Mitchell M, Burmeister E, Keogh S. Sedation protocols to reduce duration of mechanical ventilation in the ICU: a Cochrane systematic review. J Adv Nurs. 2016;72:261–272. doi: 10.1111/jan.12843. [DOI] [PubMed] [Google Scholar]
- 44.Vincent JL. Give your patient a fast hug (at least) once a day. Crit Care Med. 2005;33:1225–1229. doi: 10.1097/01.CCM.0000165962.16682.46. [DOI] [PubMed] [Google Scholar]
- 45.Walsh TS, Ramsay P, Lapinlampi TP, Sarkela MO, Viertio-Oja HE, Merilainen PT. An assessment of the validity of spectral entropy as a measure of sedation state in mechanically ventilated critically ill patients. Intensive Care Med. 2008;34:308–315. doi: 10.1007/s00134-007-0858-x. [DOI] [PubMed] [Google Scholar]
- 46.Vivien B, Di Maria S, Ouattara A, Langeron O, Coriat P, Riou B. Overestimation of bispectral index in sedated intensive care unit patients revealed by administration of muscle relaxant. Anesthesiology. 2003;99:9–17. doi: 10.1097/00000542-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 47.Radtke FM, Heymann A, Franck M, Maechler F, Drews T, Luetz A, Nachtigall I, Wernecke KD, Spies CD. How to implement monitoring tools for sedation, pain and delirium in the intensive care unit: an experimental cohort study. Intensive Care Med. 2012;38:1974–1981. doi: 10.1007/s00134-012-2658-1. [DOI] [PubMed] [Google Scholar]
- 48.Warlan H, Howland L. Posttraumatic stress syndrome associated with stays in the intensive care unit: importance of nurses’ involvement. Crit Care Nurs. 2015;35:44–52. doi: 10.4037/ccn2015758. [DOI] [PubMed] [Google Scholar]
- 49.Shehabi Y, Bellomo R, Reade MC, Bailey M, Bass F, Howe B, et al. Early goal-directed sedation versus standard sedation in mechanically ventilated critically ill patients: a pilot study. Crit Care Med. 2013;41:1983–1991. doi: 10.1097/CCM.0b013e31828a437d. [DOI] [PubMed] [Google Scholar]
- 50.Zhang WY, Wu WL, Gu JJ, Sun Y, Ye XF, Qiu WJ, Su CQ, Zhang SQ, Ye WQ. Risk factors for postoperative delirium in patients after coronary artery bypass grafting: a prospective cohort study. J Crit Care. 2015;30:606–612. doi: 10.1016/j.jcrc.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 51.Kamdar BB, Niessen T, Colantuoni E, King LM, Neufeld KJ, Bienvenu OJ, Rowden AM, Collop NA, Needham DM. Delirium transitions in the medical ICU: exploring the role of sleep quality and other factors. Crit Care Med. 2015;43:135–141. doi: 10.1097/CCM.0000000000000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weinhouse GL. Delirium and sleep disturbances in the intensive care unit: can we do better? Curr Opin Anaesthesiol. 2014;27:403–408. doi: 10.1097/ACO.0000000000000093. [DOI] [PubMed] [Google Scholar]
- 53.Papaioannou V, Mebazaa A, Plaud B, Legrand M. ‘Chronomics’ in ICU: circadian aspects of immune response and therapeutic perspectives in the critically ill. Intensive Care Med Exp. 2014;2:18. doi: 10.1186/2197-425X-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanders RD, Hussell T, Maze M. Sedation & immunomodulation. Anesthesiol Clin. 2011;29:687–706. doi: 10.1016/j.anclin.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 55.Sanders RD, Godlee A, Fujimori T, Goulding J, Xin G, Salek-Ardakani S, Snelgrove RJ, Ma D, Maze M, Hussell T. Benzodiazepine augmented γ-amino-butyric acid signaling increases mortality from pneumonia in mice. Crit Care Med. 2013;41:1627–1636. doi: 10.1097/CCM.0b013e31827c0c8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patel J, Baldwin J, Bunting P, Laha S. The effect of a multicomponent multidisciplinary bundle of interventions on sleep and delirium in medical and surgical intensive care patients. Anaesthesia. 2014;69:540–549. doi: 10.1111/anae.12638. [DOI] [PubMed] [Google Scholar]
- 57.Kamdar BB, King LM, Collop NA, Sakamuri S, Colantuoni E, Neufeld KJ. The effect of a quality improvement intervention on perceived sleep quality and cognition in a medical ICU. Crit Care Med. 2013;41:800–809. doi: 10.1097/CCM.0b013e3182746442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oto J, Yamamoto K, Koike S, Onodera M, Imanaka H, Nishimura M. Sleep quality of mechanically ventilated patients sedated with dexmedetomidine. Intensive Care Med. 2012;38:1982–1989. doi: 10.1007/s00134-012-2685-y. [DOI] [PubMed] [Google Scholar]
- 59.Robinson TN, Dunn CL, Adams JC, Hawkins CL, Tran ZV, Raeburn CD, Moss M. Tryptophan supplementation and postoperative delirium—a randomized controlled trial. J Am Geriatr Soc. 2014;62:1764–1771. doi: 10.1111/jgs.12972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mistraletti G, Umbrello M, Sabbatini G, Miori S, Taverna M, Cerri B, Mantovani ES, Formenti P, Spanu P, D’Agostino A, et al. Melatonin reduces the need for sedation in ICU patients. A randomized controlled trial. Minerva Anestesiol. 2015;81:1298–1310. [PubMed] [Google Scholar]
- 61.Balas MC, Vasilevskis EE, Olsen KM, Schmid KK, Shostrom V, Cohen MZ, Peitz G, Gannon DE, Sisson J, Sullivan J, et al. Effectiveness and safety of the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility bundle. Crit Care Med. 2014;42:1024–1036. doi: 10.1097/CCM.0000000000000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, Spears L, Miller M, Franczyk M, Deprizio D, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patel BK, Pohlman AS, Hall JB, Kress JP. Impact of early mobilization on glycemic control and ICU-acquired weakness in critically ill patients who are mechanically ventilated. Chest. 2014;146:583–589. doi: 10.1378/chest.13-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jackson JC, Santoro MJ, Ely TM. Improving patient care through the prism of psychology: application of Maslow’s hierarchy to sedation, delirium, and early mobility in the intensive care unit. J Crit Care. 2014;29:438–444. doi: 10.1016/j.jcrc.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Engel HJ, Needham DM, Morris PE, Gropper MA. ICU early mobilization: from recommendation to implementation at three medical centers. Crit Care Med. 2013;41(9 Suppl 1):S69–S80. doi: 10.1097/CCM.0b013e3182a240d5. [DOI] [PubMed] [Google Scholar]
- 66.Klein K, Mulkey M, Bena JF, Albert NM. Clinical and psychological effects of early mobilization in patients treated in a neurologic ICU: a comparative study. Crit Care Med. 2015;43:865–873. doi: 10.1097/CCM.0000000000000787. [DOI] [PubMed] [Google Scholar]
- 67.Nydahl P, Ruhl AP, Bartoszek G, Dubb R, Filipovic S, Flohr HJ, Kaltwasser A, Mende H, Rothaug O, Schuchhardt D, et al. Early mobilization of mechanically ventilated patients: a 1-day point-prevalence study in Germany. Crit Care Med. 2014;42:1178–1186. doi: 10.1097/CCM.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 68.Berney SC, Harrold M, Webb SA, Seppelt I, Patman S, Thomas PJ, Denehy L. Intensive care unit mobility practices in Australia and New Zealand: a point prevalence study. Crit Care Resusc. 2013;15:260–265. [PubMed] [Google Scholar]
- 69.Meriläinen M, Kyngäs H, Ala-Kokko T. Patients’ interactions in an intensive care unit and their memories of intensive care: a mixed method study. Intensive Crit Care Nurs. 2013;29:78–87. doi: 10.1016/j.iccn.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 70.Happ MB, Garrett KL, Tate JA, DiVirgilio D, Houze MP, Demirci JR, George E, Sereika SM. Effect of a multi-level intervention on nurse–patient communication in the intensive care unit: results of the SPEACS trial. Heart Lung. 2014;43:89–98. doi: 10.1016/j.hrtlng.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schwarzkopf D, Behrend S, Skupin H, Westermann I, Riedemann NC, Pfeifer R, Günther A, Witte OW, Reinhart K, Hartog CS. Family satisfaction in the intensive care unit: a quantitative and qualitative analysis. Intensive Care Med. 2013;39:1071–1079. doi: 10.1007/s00134-013-2862-7. [DOI] [PubMed] [Google Scholar]
- 72.Carrothers KM, Barr J, Spurlock B, Ridgely MS, Damberg CL, Ely EW. Contextual issues influencing implementation and outcomes associated with an integrated approach to managing pain, agitation, and delirium in adult ICUs. Crit Care Med. 2013;41(9 Suppl 1):S128–S135. doi: 10.1097/CCM.0b013e3182a2c2b1. [DOI] [PubMed] [Google Scholar]
- 73.Trogrlić Z, van der Jagt M, Bakker J, Balas MC, Ely EW, van der Voort PH, Ista E. A systematic review of implementation strategies for assessment, prevention, and management of ICU delirium and their effect on clinical outcomes. Crit Care. 2015;19:157. doi: 10.1186/s13054-015-0886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Walsh TS, Kydonaki K, Lee RJ, Everingham K, Antonelli J, Harkness RT, Cole S, Quasim T, Ruddy J, McDougall M, et al. Development of process control methodology for tracking the quality and safety of pain, agitation, and sedation management in critical care units. Crit Care Med. 2016;44:564–574. doi: 10.1097/CCM.0000000000001463. [DOI] [PubMed] [Google Scholar]