Abstract
Background
This position statement provides clinical recommendations for the assessment of pain, level of sedation, iatrogenic withdrawal syndrome and delirium in critically ill infants and children. Admission to a neonatal or paediatric intensive care unit (NICU, PICU) exposes a child to a series of painful and stressful events. Accurate assessment of the presence of pain and non-pain-related distress (adequacy of sedation, iatrogenic withdrawal syndrome and delirium) is essential to good clinical management and to monitoring the effectiveness of interventions to relieve or prevent pain and distress in the individual patient.
Methods
A multidisciplinary group of experts was recruited from the members of the European Society of Paediatric and Neonatal Intensive Care (ESPNIC). The group formulated clinical questions regarding assessment of pain and non-pain-related distress in critically ill and nonverbal children, and searched the PubMed/Medline, CINAHL and Embase databases for studies describing the psychometric properties of assessment instruments. Furthermore, level of evidence of selected studies was assigned and recommendations were formulated, and grade or recommendations were added on the basis of the level of evidence.
Results
An ESPNIC position statement was drafted which provides clinical recommendations on assessment of pain (n = 5), distress and/or level of sedation (n = 4), iatrogenic withdrawal syndrome (n = 3) and delirium (n = 3). These recommendations were based on the available evidence and consensus amongst the experts and other members of ESPNIC.
Conclusions
This multidisciplinary ESPNIC position statement guides professionals in the assessment and reassessment of the effectiveness of treatment interventions for pain, distress, inadequate sedation, withdrawal syndrome and delirium.
Electronic supplementary material
The online version of this article (doi:10.1007/s00134-016-4344-1) contains supplementary material, which is available to authorized users.
Keywords: Assessment, Pain, Sedation, Distress, Withdrawal syndrome, Delirium
Introduction
This position statement provides clinical recommendations for the assessment of pain, level of sedation, iatrogenic withdrawal syndrome (IWS) and delirium in critically ill infants and children. Admission to a neonatal or paediatric intensive care unit (NICU, PICU) exposes a child to a series of painful and stressful events. The effects of these events are commonly resolved by the administration of analgesics (e.g. morphine, fentanyl) and/or sedatives (e.g. benzodiazepines, α2-selective adrenergic agonists) [1]. However, sedation with benzodiazepines in neonates is advised against in view of the unfavourable patient outcomes [2]. A recent survey showed wide variety in both the dosages and choices of drugs administered to neonates [3]. While adequate analgesia and sedation help reduce the stress response and improve the clinical and psychological outcomes [4], inadequate analgesia and sedation will lead to pain, pain-induced agitation or undersedation and possibly to accidental extubation or removal of vascular access devices. Overuse of analgesic and sedative agents, on the other hand, can lead to oversedation, prolonged ICU stay, longer ventilation times, drug tolerance and dependence. Furthermore, IWS and delirium could be identified as side effects of prolonged analgesia and sedation [5, 6]. Both are considered as concepts of non-pain-related distress in critically ill children. The current clinical guidelines on analgesic and sedative drugs use in adult and paediatric ICU populations [7, 8] are based on evidence of highly variable level.
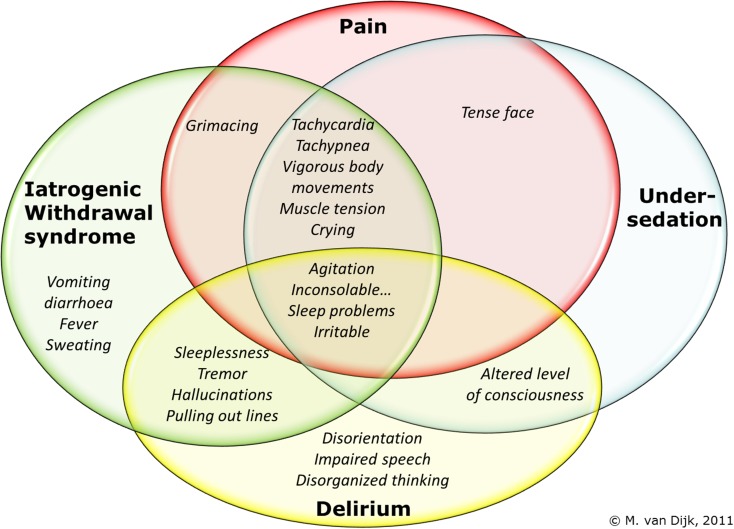
Accurate and regular measurement of pain and non-pain-related distress is essential, not only to establish their presence [9] but also to monitor the effectiveness of interventions. The effectiveness of pharmacological interventions should be monitored because this may be affected by the specific pharmacokinetics and pharmacodynamics in the individual critically ill child [10]. The gold standard of assessing patient comfort is self-reporting. Self-report is impossible, however, in preverbal and nonverbal children who are often sedated or when a tracheal tube is in place. In these cases, healthcare professionals must resort to observing the child’s physiological and behavioural responses. Still, healthcare professionals’ observations and assessments of pain and non-pain-related distress will depend on their ideas and beliefs on discomfort, pain, best drugs and treatment, and on their knowledge. On the other hand, as we know from adults [11], it may be difficult to discriminate between pain, distress, IWS and delirium in critically ill children, because the behavioural cues will overlap in part (Fig. 1). Therefore, standardized assessment tools have been proposed and validated so as to limit avoidable variability in assessment [12]. In practice, a patient’s individual analgesia and sedation requirements will be assessed by different nurses, with varying degrees of expertise, which may lead to inconsistent dosing of sedatives and analgesics [13]. Use of a standard tool may counteract this effect and promote continuity of care [14].
Fig. 1.
Overlap of behavioural cues in pain, sedation, withdrawal syndrome and delirium
This position paper specifically provides clinical recommendations for NICU and PICU healthcare clinicians on the assessment of pain, sedation, IWS and delirium in their patients.
Methods
A multidisciplinary group of expert clinicians and researchers in the fields of pain, sedation, withdrawal syndrome and delirium were recruited from the membership of the European Society of Paediatric and Neonatal Intensive Care (ESPNIC) to develop the position statement. The process of formulating the clinical recommendation comprised the following steps. First, questions were formulated regarding the clinical practice of assessment of pain-related and non-pain-related distress (e.g. adequacy of sedation, IWS and delirium) in critically ill and nonverbal children. Second, an extensive search of the literature on assessment tools was performed to find evidence for recommendations. For this reason, the PubMed/Medline, CINAHL and Embase databases were searched using the following MeSH and all fields search terms: (pain measurement, distress, sedation, iatrogenic withdrawal syndrome, delirium) AND (paediatric critical care OR neonatal intensive care) (see supplementary material for search strategy). The search scope was limited to studies in the English or French language published between August 2005 and August 2015, so as to provided the most up to date relevant research, which included paediatric or neonatal critical care nonverbal inpatients, with the age limits set from birth to 18 years. Neonates were included, as they can be admitted to PICUs in some European settings. In the past few decades more than 40 neonatal pain assessment tools have been developed and validated. From two recent systematic reviews we derived the most recent evidence of the psychometric properties of neonatal pain (e.g. acute, prolonged pain) assessment instruments [15, 16]. On the basis on this, we described the psychometric properties of the most commonly used neonatal instruments. Additional search terms, such as pain questionnaires, pain scales, pain tools, pain instruments and search of authors known in the field served to verify completeness of the search results. Cross-referencing of key articles and recently published systematic reviews describing psychometric properties of assessment instruments [17, 18] served as a final check. Prior to full-text retrieval, studies describing the psychometric properties of instruments to assess physiological and behavioural cues of pain-related and/or non-pain-related distress were selected on the basis of the title and abstract. Studies that did not report on psychometric properties of the tools and those that only reported on neonatal abstinence syndrome were excluded.
In the third phase, each of the articles selected was subjected to an independent grade of evidence review by at least two of the authors and differences in grading were resolved through discussion. The level of the evidence was assigned a grade using the definitions provided in a supplementary table and based on reference test, specific research design and methodology [19]. Subsequently, recommendations for assessment of pain-related and non-pain-related distress in children were formulated and discussed by the group during a meeting. The recommendations were assigned according to the level of evidence. Lastly, to achieve consensus the draft position statement was reviewed by independent members of the ESPNIC Nursing Science section (Pain & Sedation study group) and the Pharmacology section; they graded the importance of the statements related to the topic area. This process did not lead to any fundamental changes. The final version was endorsed by the Executive Board of ESPNIC.
This position statement puts a focus on the assessment of (1) pain-related distress and (2) non-pain-related distress (level of sedation, withdrawal and delirium) in the NICU/PICU as a first essential step in the management of pain and distress in these vulnerable populations.
Results
Evidence from a total of 32 full-text articles describing the psychometric properties of assessment tools for pain-related and non-pain-related distress in children was used to underpin the recommendations in this position statement (see supplementary material).
Assessment of pain-related distress
Pain assessment in hospitalised infants and children is notoriously difficult because of the different emotional and cognitive development stages of this patient group. Moreover, they are often ventilated and sedated, which complicates assessment of behaviours, and interpreting pain-related behaviours is often subjective, relying on the clinicians’ interpretation.
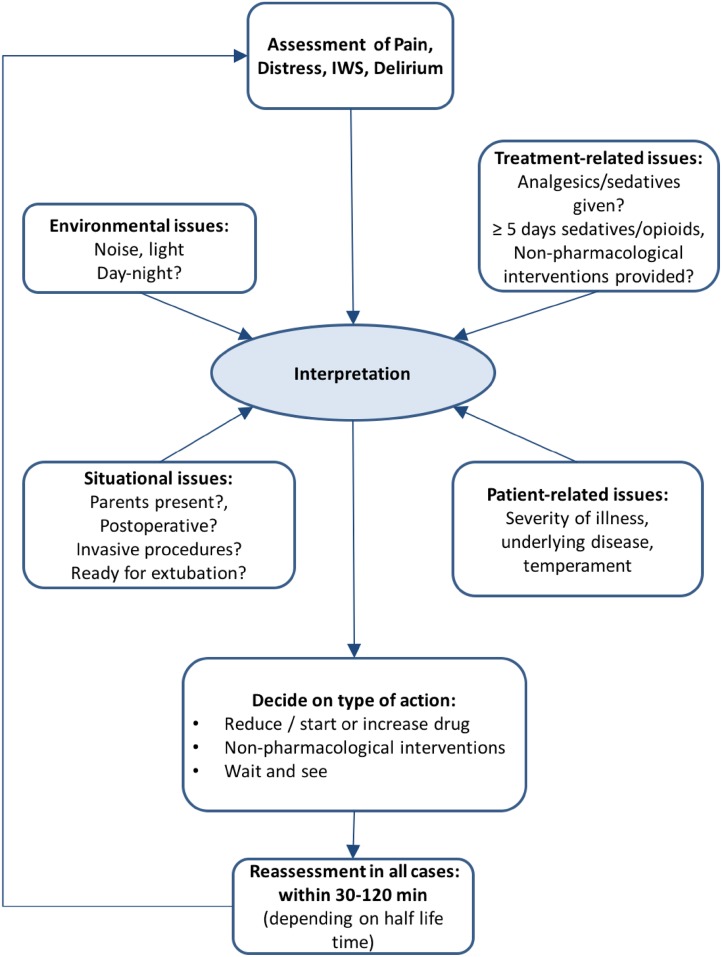
For intensive care settings, we can distinguish two relevant types of pain: (1) acute pain, including procedural and postoperative pain (e.g. pain caused by heelstick, suctioning, venepuncture, thoracic drainage) and postoperative pain; and (2) prolonged pain (see Table 1 for definitions). For clinical reasons it is important to explore the underlying pathogenesis and the context of pain (Fig. 2). However, different types of pain, e.g. neurogenic pain, visceral pain and somatic pain, can not be distinguished with the use of observational assessment tools.
Table 1.
Definitions of pain, distress, withdrawal syndrome and delirium
|
Pain
“An unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage. Note: The inability to communicate verbally does not negate the possibility that an individual is experiencing pain and is in need of appropriate pain-relieving treatment” (IASP 2014) Acute pain “Acute pain should be viewed as the initiation phase of an extensive, persistent nociceptive and behavioural cascade triggered by tissue injury. This cascade has the potential to span orders of magnitude of space and time, but generally subsides within weeks” [89] Postoperative pain Acute pain experienced post-surgery Prolonged pain The terms prolonged and recurrent are used interchangeably in the literature. Prolonged or persistent pain is primarily caused by disease e.g. peritonitis. Prolonged pain differs from chronic pain in that there is a clear stimulus caused by disease (e.g. peritonitis) or therapy (e.g. mechanical ventilation, insertion of tubes or drains), with a clear definable beginning and an expected endpoint. But less than 3 months and full recovery of tissue damage can be expected, which is not the case in chronic pain [23] |
|
Non-pain-related distress
Distress is an organism’s response to aversive internal and external stimuli and may include discomfort, anxiety and fear [25] Optimal sedation A state in which the patient is somnolent, responsive to the environment but untroubled by it, and with no excessive movements [13] |
|
Iatrogenic withdrawal syndrome
A clinical syndrome that manifests after stopping or reversing a drug after prolonged exposure to that drug [3, 4] Tolerance A decrease in a drug’s effect or the need to increase the dose to achieve the same effect [3, 4] Physiological dependence The requirement for continued administration of a sedative or analgesic to prevent signs of withdrawal syndrome |
|
Delirium
A neurocognitive disorder due to a somatic illness or its treatment [61] DSM-5 criteria: A. Disturbance in attention (i.e. reduced ability to direct, focus, sustain and shift attention) and awareness (reduced orientation to the environment) B. The disturbance develops over a short period of time (usually hours to a few days), represents an acute change from baseline attention and awareness, and tends to fluctuate in severity during the course of a day C. An additional disturbance in cognition (e.g. memory deficit, disorientation, language, visuospatial ability or perception) D. The disturbances in Criteria A and C are not better explained by a pre-existing, established or evolving neurocognitive disorder and do not occur in the context of a severely reduced level of arousal such as coma E. There is evidence from the history, physical examination or laboratory findings that the disturbance is a direct physiological consequence of another medical condition, substance intoxication or withdrawal (i.e. due to a drug of abuse or to a medication), or exposure to a toxin, or is due to multiple etiologies |
IASP international association for the study of pain
Fig. 2.
Interpretation of pain and non-pain-related distress in critically ill children, based on van Dijk et al. 2012 [16]
Children and neonates in the intensive care setting undergo numerous procedures which potentially cause pain, e.g. intravenous cannulation, chest drain insertion, intubation or discomfort, e.g. from invasive monitoring lines. Nurses and physicians should be aware, however, that daily care (e.g. turning) can be painful as well, and that what is considered painful in older children and adults [20] should also be considered painful for children and neonates. Neonates are particularly at risk of pain exposure with a reported mean of 10.0–22.9 procedures per day [21, 22]. Prolonged pain is poorly understood, but is characterised by a lack of clear stimulus, a variable duration and slow recovery [23]. Furthermore it is present after several days of hospitalisation and when no obvious cause for pain is present [24].
Recommendation
Identify potential sources of pain and take appropriate actions (grade of recommendation = D).
The use of pain assessment instruments has been widely recommended as a means to provide consistency between clinicians, to provide an indication that pain/discomfort is present and to assess the effect of pharmacological or non-pharmacological interventions.
There is limited literature on pain assessment in the PICU; the available studies concern the validation of instruments such as the COMFORT scale [25, 26], the COMFORT-B scale1 [27–33], the FLACC scale [32, 34, 35] and the Multidimensional Assessment Pain Scale (MAPS) [36, 37]. The COMFORT-B scale has also been validated for patients with burns [38]. In contrast, more than 40 pain assessment instruments for neonates have been developed in the last few decades, but not all meet the minimum psychometric requirements for application in clinical practice [16]. The well-established, validated COMFORT-B scale and the FLACC scale (for infants and children) [39] and the promising PIPP-R (for neonates) [40, 41] are recommended (see Tables 2, 3 and supplementary material for their psychometric properties).
Table 2.
Panel of behavioural instruments specific to paediatric critical care
| COMFORT behavior scale | |
| Categories | Score |
| Alertness | 1–5 |
| Calmness/agitation | 1–5 |
| Respiratory response or cryinga | 1–5 |
| Physical movement | 1–5 |
| Muscle tone | 1–5 |
| Facial tension | 1–5 |
| Total score 6–30 | |
| Withdrawal Assessment Tool version 1 (WAT-1) | |
| Information from patient record | |
| Loose/watery stools | No = 0, yes = 1 |
| Vomiting/retching/gagging | No = 0, yes = 1 |
| Temperature > 37.8 °C | No = 0, yes = 1 |
| 2 min pre-stimulus observation | |
| State | SBS ≤ 0 = 0, SBS ≥ 1 = 1 |
| Tremor | No = 0, moderate/severe = 1 |
| Any sweating | No = 0, yes = 1 |
| Uncoordinated/repetitive movement | No = 0, moderate/severe = 1 |
| Yawning of sneezing | No = 0, yes = 1 |
| 1 min stimulus observation | |
| Startle to touch | No = 0, moderate/severe = 1 |
| Muscle tone | Normal = 0, increased = 1 |
| Post-stimulus recovery | |
| Time to gain calm state (SBS ≤ 0) | 0–2 |
| Total score 0–12 | |
| Sophia Observation withdrawal Symptoms-scale (SOS) | |
| Items | Score |
| Autonomic dysfunction | |
| Tachycardia | No = 0, yes = 1 (for all items) |
| Tachypnoea | |
| Fever (≥38.5 °C) | |
| Sweating | |
| CNS irritability | |
| Agitation | |
| Anxiety | |
| Tremors | |
| Increased muscle tension | |
| Inconsolable crying | |
| Grimacing | |
| Sleeplessness | |
| Motor disturbance | |
| Hallucinations | |
| Gastrointestinal dysfunction | |
| Vomiting | |
| Diarrhoea | |
| Total score 0–15 | |
SBS State Behavioural Scale
aCrying only in spontaneous breathing patients
Table 3.
Pain: summary of recommended assessment tools for neonates and critically ill children
| Neonates | Infants and children | ||||||
|---|---|---|---|---|---|---|---|
| PIPP [90–92] | PIPP-revised [40, 41] | N-PASS [93, 94] | COMFORTneo [95] | COMFORT behaviour scale [26, 29, 31, 33] | FLACC [35, 96] | Multidimensional Assessment of Pain Scale (MAPS) [36, 97] | |
| Age range | 28–40 weeks | 28–40 weeks | 23–40 weeks | 24–42 weeks | 0–3 years | 0–7 years | 0–31 months |
| Type of pain | Procedural and postoperative pain | Procedural pain | Procedural and prolonged pain | Prolonged pain | Postoperative pain | Postoperative pain | Postoperative pain |
| Variables assessed | Heart ratea
Oxygen saturationa Brow bulgea Eye squeezea Nasolabial furrowa Behavioural state |
Heart ratea
Oxygen saturationa Brow bulgeb Eye squeezeb Nasolabial furrowb Behavioural state |
Heart rate Respiratory rate Blood pressure Oxygen saturation crying, irritability Facial expressions Behavioural state Extremities/tone |
Alertness Calmness/agitation Respiratory response or crying Body movement Muscle tone Facial tension |
Alertness Calmness/agitation Respiratory response or crying Physical movement Muscle tone Facial tension |
Facial expression, movement of limbs Cry, consolability |
Vital signs HR and/ or BP Breathing pattern Facial expression Body movements State of arousal |
| Score range (cut-off point) |
0–21 0–6 no to mild pain 7–12 moderate pain >12 severe pain |
0–21 0–6 no to mild pain 7–12 moderate pain >12 severe pain |
Pain: 0–10 >3 |
6–30 6–13 no to mild discomfort 14–21 moderate discomfort >22 severe discomfort |
6–30 >17 pain |
0–10 1–3 mild discomfort 4–6 moderate discomfort 6–10 severe discomfort/pain |
|
| Adjustment for gestational age | Yes | Yesc | Yes | No | NA | NA | NA |
| Reliability data | + | – | + | + | + | + | + |
| Forms of validity established | Construct and concurrent | Construct and concurrent | Construct and convergent | Concurrent | Construct and concurrent | Construct and concurrent | |
| Clinical utility | + | – | + | + | + | ||
| Grade | A | A | B | B | A | B | A |
See supplemental material for detailed data regarding psychometric properties
aChanges expressed in per cent (in PIPP used to look at heart rate increases only but the revised version also takes heart rate declines into account)
bChanges expressed in seconds
cOnly if the score on the other items >0
Recommendation
Use an age-appropriate tool to assess acute and prolonged pain i.e. the PIPP(-revised) in neonates and the COMFORT behaviour scale, FLACC or MAPS in critically ill children (grade of recommendation = A).
The vital signs heart rate and mean arterial pressure have been moderately correlated with behaviour items [28, 42]. In children, these vital signs are probably less reliable indicators of pain than behavioural indicators. In heavily sedated or muscle-relaxed children, however, increases in heart rate and mean arterial pressure may indicate that the body is under some stress—in the absence of behavioural signs pain must be one of the considerations in this scenario, the more so as there is no other method to assess these children.
It must be remembered that in the case of pain or discomfort in the nonverbal child, reflected by a high score, the practitioner should acknowledge possibly contributing environmental factors (temperature, noise) or other factors such as the need for a change of position, infant teething or the need for nappy care. It is assumed that the nurse will check and modify these environmental factors first before making a treatment plan and reassess once an intervention has taken place.
Studies have shown that parents themselves wish to be more involved in the process of assessing pain in their child and urge for more consistent pain assessment and management practices by staff. Parents’ knowledge of their own child and how they may display pain or distress may enhance a clinician’s assessment and management practices. Further research is needed with regard to pain assessment involving families.
Recommendation
Parent and family assessment of pain should be considered in pain assessment (grade of recommendation = D).
There are no clear-cut recommendations in the literature on the frequency of pain assessment; this position statement merely provides the clinician with a consensus on the frequency. Furthermore, the frequency of assessment will depend on the goal of therapeutic treatment (e.g. weaning of ventilation, transfer to paediatric ward).
Recommendation
Pain assessment should take place routinely, depending on therapeutic goals, but at greater frequency (1–2 h) if the patient is receiving any analgesic infusion (grade of recommendation = D).
Audits of pain assessment should take place regularly (e.g. every 12 months) to evaluate the quality of patient care and patient outcomes [43].
Recommendation
Pain assessment audits should take place regularly (grade of recommendation = C).
Non-pain-related distress
Sedation assessment
Patients admitted to an intensive care unit are likely to develop physical and psychological distress. Non-pain-related distress in ventilated children is treated with sedatives. Optimal sedation has been described as a state in which the patient is somnolent, responsive to the environment but untroubled by it and without excessive movements [13] (Table 1). In practice this means that a child is conscious, breathes in synergy with the ventilator and is tolerant or compliant to other therapeutic procedures. Still it can be challenging to reach this level of sedation. A recent systematic review revealed that across all studies of paediatric patients (n = 25), patients were optimally sedated in 58 %, undersedated in 10 % and oversedated in 32 % of the observations [44]. Optimal level of sedation varies for each patient and careful consideration should be given to the underlying diagnosis and severity of illness [1, 13]. Oversedation may lead to longer duration of mechanical ventilation and increased healthcare costs. On the other hand, undersedation can lead to increased distress, self- or accidental extubation, accidental displacements of catheters, tubes and vascular access. In clinical practice it can be challenging to reach the optimal level of sedation in infants and children. The majority of children in the PICU are below 4 years of age and, in view of their development, not yet able to understand or make sense of their situation, and they will more easily become anxious and scared. For this reason they often need greater amounts of sedatives to ensure lines and tubes remain in situ.
The sedation goal may vary considerably from patient to patient and depends on severity of illness, type of disease and treatment as well as environmental factors, such as noise. When a child shows signs of agitation and fighting against the ventilator, the child should be sedated after confirmation that the ventilator settings are well adjusted to the child’s respiratory needs.
Recommendation
Search for potential causes of non-pain-related distress/discomfort to take appropriate actions (grade of recommendation = D).
Although clinical judgement of trained ICU professionals is important, the use of a sedation assessment tool is needed to determine the efficacy of sedatives and related interventions, to facilitate inter-institutional comparisons and to facilitate targeted sedation. Several behavioural sedation scoring scales (e.g. COMFORT scale [25, 45], COMFORT behaviour scale [14, 42], State Behaviour Scale [46]) have been described and validated for children (Tables 2, 4, supplementary material). Also, these tools are the most commonly used instruments in daily practice [47]. No single instrument has been shown to be superior for use in this population, and it is advisable to select a scale that has been validated for this patient population. The frequency of assessment reported in the included studies (n = 25) varied considerably i.e. from once daily to hourly [44]. Although the frequency of assessment will depend on whether symptoms have been controlled or not and on the goal of therapeutic treatment (e.g. weaning of ventilation), we recommend regular assessment at least once per shift and accurate documentation of the sedation score.
Table 4.
Sedation: summary of recommended assessment tools for critically ill children
| COMFORT scale [25, 45, 98] | COMFORT behaviour scale [14, 31, 32, 42, 99] | State Behavioural Scale (SBS) [46] | |
|---|---|---|---|
| Age range | 0–16 years | 0–16 years | 6 weeks–6 years |
| Variables assessed | Distress Heart rate Mean arterial pressure Alertness Calmness Respiratory response Movement Muscle tone Facial expression |
Distress Alertness Calmness/agitation Respiratory response or crying Physical movement Muscle tone Facial tension |
Respiratory drive Coughing Best response to stimuli Attentiveness to care provider Tolerance to care Consolability Movement after consoled |
| Score range (cut-off point) |
8–40 <17 oversedation 17–26 optimal sedation >26 undersedation |
6–30 <11 oversedation 11–22 adequate sedation >22 undersedation |
6-point scale; state behaviour on a scale of −3 to +2 0 = awake and calm |
| Reliability data | + | + | + |
| Forms of validity established | Face, construct and concurrent | Face, construct and concurrent, responsiveness | Face, construct |
| Clinical utility | Feasibility and utility established at bedside | Feasibility and utility established at bedside | |
| Grade | A | A | B |
See supplementary material for detailed data regarding psychometric properties
Recommendation
Use standardized sedation assessment tools with proven validity, reliability and clinical utility; the COMFORT behaviour scale (grade of recommendation = A).
Together with the vital signs, the level of sedation must be assessed and documented every 4–8 h or as indicated by the sedation score or the child’s clinical condition (grade of recommendation = D).
Iatrogenic withdrawal syndrome assessment in infants and children
Prolonged administration of opioids and/or benzodiazepines in infants and children may induce drug tolerance and physiological dependency. Abrupt discontinuation or (too rapid) weaning of these drugs in physically dependent infants and children may result in IWS (Table 1) [6, 48].
Tolerance and withdrawal symptoms may occur after 5 or more days of continuous infusion of opioids or benzodiazepines in infants as well as children. The onset of withdrawal can occur after 1 up to 48 h after tapering off or discontinuation [6, 48]. An estimated 10–34 % of all PICU patients are at risk of IWS [49, 50]. Fentanyl and morphine are the most frequently used analgesic drugs in the NICU and PICU that underlie opiate IWS, with prevalence rates of 9–57 % [51, 52]. The reported prevalence rates of IWS in PICU patients who had received benzodiazepines and/or opioids for 5 or more days range from 35 to 57 % [53, 54].
Recommendation
The potential risk of opioid and/or benzodiazepine iatrogenic withdrawal syndrome should be considered after 5 days of continuous administration of these drugs (grade of recommendation = C).
Diagnosing withdrawal symptoms in NICU and PICU patients is complicated by the fact that these symptoms may overlap with clinical signs of pain or distress, respiratory distress, delirium and noise-induced stress [6, 55, 56]. These other factors must be excluded before the diagnosis can be confirmed. Regarding the fact that IWS may occur after 5 days, we recommend to continue assessment of withdrawal symptoms after the child has been discharged from the PICU.
Two instruments for assessing IWS in children have been sufficiently validated, namely the Withdrawal Assessment Tool version 1 (WAT-1) [57, 58] and the Sophia Observation withdrawal Symptoms-scale (SOS) [59, 60]. The WAT-1 is an 11-item scale and scores of 3 or higher (on a scale of 0–12) indicate that the child is suspected of experiencing withdrawal. The SOS consists of 15 items and is based on the underlying empirical structure of co-occurrences of withdrawal symptoms that experts considered relevant. A SOS score of 4 or higher reflects a high probability of withdrawal. Table 5 and supplementary material provide details on symptoms and the psychometric properties of these instruments, which are used in practice and in research.
Table 5.
IWS and delirium: summary of recommended assessment tools for critically ill children
| Withdrawal Assessment Tool version-1 (WAT-1) [57, 58] | Sophia Observation withdrawal Symptoms-scale (SOS) [59, 60] | Paediatric Confusion Assessment Method-Intensive Care Unit (pCAM-ICU) [66] | Cornell Assessment Paediatric-Delirium (CAP-D) [65, 71] | Sophia Observation withdrawal Symptoms-Paediatric Delirium scale (SOS-PD) [72, 73] | |
|---|---|---|---|---|---|
| Age range | Children 0–16 years | Children 0–16 years | 5–16 years | 0–21 years | 0–16 years |
| Variables assessed | Loose/watery stools Vomiting/retching/gagging Temperature > 37.8 °C State* Tremor Sweating Uncoordinated/repetitive movement Yawning of sneezing Startle to touch Muscle tone Time to gain calm state (SBS ≤ 0) |
Tachycardia Tachypnoea Fever (≥38.5 °C) Sweating Agitation Anxiety Tremors Increased muscle tension Inconsolable crying Grimacing Sleeplessness Motor disturbance Hallucinations Vomiting Diarrhoea |
Four features: 1. Acute change or fluctuation course of mental status 2. Inattention 3. Altered level of consciousness 4. Disorganized thinking |
Eye contact with caregiver Purposeful actions Awareness of surrounding Communicate needs Restless Inconsolable Underactive Response to interaction |
Agitation (restless), anxiety, eye contact, grimacing impaired attention Speech Tremors Muscle tone Purposeful actions Sleeplessness Hallucinations Disorientation Sweating Acute change/fluctuation Parents |
| Score range (cut off point) |
0–12 points ≥3 |
0–15 points ≥4 |
Features 1, 2 and 3 or 4a | 0–40 (9) |
0–15 (4) |
| Reliability data | + | + | + | + | ± |
| Forms of validity established | Content, construct, responsiveness | Face, construct | Criterion | Criterion | Face (criterion pilot) |
| Clinical utility | Feasibility and utility established at bedside | Feasibility and utility established at bedside | Feasibility | Utility established at bedside | Feasibility |
| Grade | A | A | B | A | C |
See supplementary material for detailed data regarding psychometric properties
aDelirium diagnosis using the Pediatric Confusion Assessment Method for the Intensive Care Unit requires positive features 1 and 2 with either positive feature 3 or 4
Recommendation
Use standardized IWS assessment instruments with proven clinical utility, validity and reliability in infants and children; WAT-1 or the SOS (grade of recommendation = A).
Delirium
Delirium is a neurocognitive disorder due to a somatic illness or its treatment. According to DSM-5 the core diagnostic criteria for delirium are (Table 1) (a) a disturbance of attention or awareness; (b) this disturbance is accompanied by changes in cognition that cannot be better accounted for by another pre-existing neurocognitive disorder (e.g. mental retardation, dementia); (c) the condition develops within hours or days, and often fluctuates during the day, typically worsening in the evening (‘sundowning’) and (d) there are indications from the patient’s history, examination or laboratory results that the disturbance is probably the result of a medical condition or its treatment [61]. The pathogenesis of delirium is largely unknown. The sufferers may be hyperactive, hypoactive or show signs of both states. Typical for the hypoactive delirium are slowed or sparse speech, hypoactive or slowed motor activity as well as lethargy, also described as reduced awareness or apathy. Adults and children largely show the same symptoms although hallucinations and hypoactive delirium are hard to observe in the very young children [62]. However, delirium has been described in infants below 1 year of age [63]. Delirium has not been described in neonates to date. Increasing evidence suggests there is a positive association between illness severity and paediatric delirium [64]. Many risk factors for delirium have been identified. These can be classified as patient-related, iatrogenic and environmental. Patient factors (e.g. infections, metabolic disorders, withdrawal from medications, restraints and sleep disturbance) and environmental factors may contribute to developing delirium [56].
The reported prevalence of paediatric delirium (PD) in PICU patients is 4–29 % [56, 65, 66]. Colville et al. found that 3 months after discharge one-third of PICU patients reported memories of psychotic features, including delusions and disturbing hallucinations, suggestive of delirium during PICU admission [67]. Adult delirium has been associated with higher mortality and morbidity and longer length of hospital stay [68]. PD, too, is associated with longer length of stay [69] and—as we suspect—increased morbidity. Thus, early recognition of this serious neuropsychiatric disorder is essential, and PICU nurses could facilitate this task.
Recommendation
Search for potential sources of paediatric delirium and to take appropriate actions (grade of recommendation = D).
Delirium assessment
According to the literature, PD is underdiagnosed especially in young critically ill children [56]. A likely reason is that nurses and ICU physicians do not specifically focus on the symptoms of PD; and moreover, it is difficult to assess the symptoms in preverbal patients. Looking at behaviours has been suggested as an alternative [56, 63]. Taking into account the child’s developmental stages makes it possible to reliably and accurately interpret alterations in behaviour, communication and emotion in the critically ill child of any age [70]. A number of delirium symptoms overlap with those observed in other conditions, such as pain, distress and withdrawal syndrome [6]. Thus it would seem essential to use a reliable, validated and clinically useful bedside tool to screen delirium and guide treatment. This is an area of development but assessment instruments are already available. These are (1) the paediatric Confusion Assessment Method for ICU (pCAM-ICU) for children of 5 years or older [66]; (2) the Cornell Assessment Paediatric Delirium tool (CAP-D) for children of 0 up to 18 years of age [65, 71] and (3) the Sophia Observation withdrawal Symptoms-Paediatric Delirium scale [72, 73] (Table 5; supplementary material). In the lack of evidence, we recommend assessment of delirium at least once per shift or as indicated by the clinical condition of the child.
Recommendation
Use CAP-D as an instrument to assess paediatric delirium (grade of recommendation = A).
Together with the vital signs, delirium must be assessed and documented every 8–12 h (at least once per shift), 24–48 h after admission or as indicated by the delirium score of clinical condition of the child (grade of recommendation = D).
Pain and non-pain-related distress management protocols in relation to assessment
Effective pain and sedation management depends on the effectiveness of analgesics and sedatives as well as the use of assessment instruments to measure the effects and target of the administered drugs. A number of randomised controlled trials (RCTs) have provided evidence for the use of individual drugs such as morphine, midazolam, paracetamol, clonidine and dexmedetomidine [74–77]. The combined use of drugs in infants has also been evaluated, like fentanyl versus remifentanil combined with midazolam [78] or remifentanil versus midazolam [79]. The use of fentanyl or morphine is common practice around the world for postoperative analgesia in term newborns, infants and children, with recommended continuous infusions and dosages of 1–5 mcg/kg/h (fentanyl) and 10–40 mcg/kg/h (morphine), respectively [8]. Opioids and/or benzodiazepines are often given during artificial ventilation. The use of morphine as the drug of first choice for postoperative analgesia has been debated given the equipotency of intravenous paracetamol as the drug of first choice. With regards to sedation, Curley et al. failed to show beneficial effects of protocolized sedation versus usual care on length of artificial ventilation in a multicentre cluster randomised study of 31 PICUs in the USA [80]. Still, daily interruption of sedatives significantly improved short- and long-term outcomes in adults. All evidence indicates that the use of sedatives should be reduced. In children, daily interruption of sedation seems feasible and safe [81, 82]. However, the effectiveness needs to be demonstrated in large trials [83]. Following the evaluation of the level of evidence of analgesic and sedative drugs by Playfor [8], increased attention is being paid to optimal dosing of many of the drugs used routinely in the PICUs around the world. Studies have demonstrated that reassessment after an intervention is often neglected, although it is crucial in evaluating whether an intervention is effective or not [84, 85]. In summary, the overall aim of assessment of pain and non-pain-related distress in relation to treatment is to find the most appropriate dose for the individual patient to eliminate or reduce pain and discomfort to an acceptable level without side effects of therapy. Therefore, we recommend that the effect of a drug (e.g. increasing or decreasing of a pump, bolus) is re-evaluated depending on the drug’s half-life. One value outside the normal range of the score should not immediately result into a change in drug dosages. Strategies to reduce the incidence of IWS should begin by making efforts to reduce doses of benzodiazepines and/or opioids, and thereby preventing oversedation [44, 86].
Recommendation
The effect (e.g. increasing or decreasing of a pump, bolus) of a drug should be re-evaluated depending on the drug’s half-life (grade of recommendation = D).
A weaning strategy for gradual decreasing of opioid and/or benzodiazepine dosages is essential to prevent IWS. Strategy options include slowly tapering off the intravenous infusion rate or using an alternative route, like the enteral or subcutaneous route. However, the evidence of different strategies is scarce. At each step in the weaning process, possible withdrawal symptoms should be carefully monitored with the help of the WAT-1 or SOS.
Recommendation
Reassess for symptoms of withdrawal after treatment interventions (grade of recommendation = D).
Delirium in PICU patients has been treated with haloperidol and risperidone and both drugs demonstrated beneficial effects without significant side effects [56]. There remains a need for well-designed, randomised, placebo-controlled trials assessing the efficacy and safety of delirium drug therapy. Clinical pharmacological principles should go hand in hand with the daily use of validated assessment instruments with good psychometric properties. In this way optimal dosing and evaluation of specific behaviours of the individual critically ill patient will result in optimal synergy between care and cure.
Recommendation
Validated assessment tools for pain, sedation, withdrawal syndrome and delirium should be integrated in pain and non-pain-related treatment protocols (grade of recommendation = C).
Discussion and conclusion
Providing comfort and minimizing anxiety, fear and distress in critically ill infants and children are an important part of the daily activities of intensive care nurses. These patients, who are unable to communicate their pain, discomfort, anxiety and fear, are at great risk of inadequate analgesia, sedation or delayed recognition of withdrawal syndrome and/or delirium. Just like all infants and children, this special population deserves consistent, on-going assessment and reassessment of interventions to confirm the best possible treatment for pain, distress, inadequate sedation, withdrawal syndrome and delirium. This position paper offers recommendations to this aim. To achieve the best possible outcome, interdisciplinary collaboration of nurses, physicians and hospital pharmacists/clinical pharmacologists is therefore warranted. Distress can be reduced by creating an optimal environment with little noise (<45 dB), favourable conditions for day-night (sleep) rhythm in combination with daylight, and family presence [87]. However, more research is needed to establish the effectiveness of non-pharmacological interventions in critically ill children. Furthermore, the nursing role includes providing information to parents, asking them about the nature and intensity of pain and distress of their infant or child and consequently listening to parents. This requires a particular awareness, knowledge of and insight into these phenomena. It may be difficult to discriminate between pain, distress, IWS and delirium in critically ill children, because the behavioural cues will overlap in part (Fig. 1). Pain frequently results in distress, but distress may have other causes than pain. Despite their close association, distinguishing between these concepts is clinically important as they are treated differently. A behavioural tool that is able to discriminate pain, sedation, IWS and delirium in all circumstances is not available. It could be challenging for clinicians to deal with all these different instruments. The decision to apply a particular instrument should always be driven by interpreting factors related to the context of the patient (e.g. use of sedatives, postoperative, prolonged administration of sedatives/opioids as a risk for IWS), environment and response to therapies (see Fig. 2). Combining this with the different scores then allows one to decide on the necessary action.
As the evidence for several recommendations is poor (e.g. grade D recommendations) further research is needed to strengthen these recommendations. Clinicians are recommended to select a validated and reliable assessment instrument and could be guided in the choice by the grade of recommendation. Furthermore, other factors should be considered like the ease of use, complexity of the tool and the time it takes to complete the assessment. All staff working on the NICU or PICU (physicians, nurses and nursing support staff) should be trained in the application of these instruments. Furthermore, assessment outcomes should be integrated in treatment decision trees with recommended dosages based on RCTs in paediatric patients. In addition to pain as the fifth vital sign, it may be time to also endorse non-pain-related distress in critically ill infants and children as the composite sixth vital sign [88].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors thank Ko Hagoort for carefully editing the manuscript.
Compliance with ethical standards
Conflicts of interest
All authors declare that they have no conflict of interest. All authors have completed and submitted the International Committee of Medical Journal Editors (ICMJE) form for disclosure of potential conflicts of interest.
Funding
This research received no specific funding.
Ethical approval
Not required.
Footnotes
The COMFORT scale was originally developed for assessing the level of distress in ventilated children. In combination with the use of the NRS pain the COMFORT-B scale is suitable to determine the need for analgesia or sedation.
ESPNIC position statement endorsed by ESPNIC (Medical and Nursing); Nursing Science section (Pain and Sedation study group) and Pharmacology section of ESPNIC.
References
- 1.Twite MD, Rashid A, Zuk J, Friesen RH. Sedation, analgesia, and neuromuscular blockade in the pediatric intensive care unit: survey of fellowship training programs. Pediatr Crit Care Med. 2004;5:521–532. doi: 10.1097/01.PCC.0000144710.13710.2E. [DOI] [PubMed] [Google Scholar]
- 2.Ng E, Taddio A, Ohlsson A (2012) Intravenous midazolam infusion for sedation of infants in the neonatal intensive care unit. Cochrane Database Syst Rev 6:CD002052 [DOI] [PubMed]
- 3.Carbajal R, Eriksson M, Courtois E, Boyle E, Avila-Alvarez A, Andersen RD, Sarafidis K, Polkki T, Matos C, Lago P, Papadouri T, Montalto SA, Ilmoja ML, Simons S, Tameliene R, van Overmeire B, Berger A, Dobrzanska A, Schroth M, Bergqvist L, Lagercrantz H, Anand KJ, EUROPAIN Survey Working Group Sedation and analgesia practices in neonatal intensive care units (EUROPAIN): results from a prospective cohort study. Lancet Respir Med. 2015;3:796–812. doi: 10.1016/S2213-2600(15)00331-8. [DOI] [PubMed] [Google Scholar]
- 4.Kidder C. Reestablishing health: factors influencing the child’s recovery in pediatric intensive care. J Pediatr Nurs. 1989;4:96–103. [PubMed] [Google Scholar]
- 5.Anand KJ, Willson DF, Berger J, Harrison R, Meert KL, Zimmerman J, Carcillo J, Newth CJ, Prodhan P, Dean JM, Nicholson C. Tolerance and withdrawal from prolonged opioid use in critically ill children. Pediatrics. 2010;125:e1208–e1225. doi: 10.1542/peds.2009-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ista E, van Dijk M, Gamel C, Tibboel D, de Hoog M. Withdrawal symptoms in children after long-term administration of sedatives and/or analgesics: a literature review. “Assessment remains troublesome”. Intensive Care Med. 2007;33:1396–1406. doi: 10.1007/s00134-007-0696-x. [DOI] [PubMed] [Google Scholar]
- 7.Barr J, Fraser GL, Puntillo K, Ely EW, Gelinas C, Dasta JF, Davidson JE, Devlin JW, Kress JP, Joffe AM, Coursin DB, Herr DL, Tung A, Robinson BR, Fontaine DK, Ramsay MA, Riker RR, Sessler CN, Pun B, Skrobik Y, Jaeschke R. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 8.Playfor S, Jenkins I, Boyles C, Choonara I, Davies G, Haywood T, Hinson G, Mayer A, Morton N, Ralph T, Wolf A. Consensus guidelines on sedation and analgesia in critically ill children. Intensive Care Med. 2006;32:1125–1136. doi: 10.1007/s00134-006-0190-x. [DOI] [PubMed] [Google Scholar]
- 9.Chanques G, Nelson J, Puntillo K. Five patient symptoms that you should evaluate every day. Intensive Care Med. 2015;41:1347–1350. doi: 10.1007/s00134-015-3729-x. [DOI] [PubMed] [Google Scholar]
- 10.Vet NJ, Brussee JM, de Hoog M, Mooij MG, Verlaat CW, Jerchel IS, van Schaik RH, Koch BC, Tibboel D, Knibbe CA, de Wildt SN, SKIC (2016) Inflammation and organ failure severely affect midazolam clearance in critically ill children. Am J Respir Crit Care Med. doi:10.1164/rccm.201510-2114OC [DOI] [PubMed]
- 11.Gelinas C, Chanques G, Puntillo K. In pursuit of pain: recent advances and future directions in pain assessment in the ICU. Intensive Care Med. 2014;40:1009–1014. doi: 10.1007/s00134-014-3299-3. [DOI] [PubMed] [Google Scholar]
- 12.Hadjistavropoulos T, Craig KD, Duck S, Cano A, Goubert L, Jackson PL, Mogil JS, Rainville P, Sullivan MJ, de C Williams A, Vervoort T, Fitzgerald TD. A biopsychosocial formulation of pain communication. Psychol Bull. 2011;137:910–939. doi: 10.1037/a0023876. [DOI] [PubMed] [Google Scholar]
- 13.Westcott C. The sedation of patients in intensive care units: a nursing review. Intensive Crit Care Nurs. 1995;11:26–31. doi: 10.1016/S0964-3397(95)81210-5. [DOI] [PubMed] [Google Scholar]
- 14.Ista E, van Dijk M, Tibboel D, de Hoog M. Assessment of sedation levels in pediatric intensive care patients can be improved by using the COMFORT “behavior” scale. Pediatr Crit Care Med. 2005;6:58–63. doi: 10.1097/01.PCC.0000149318.40279.1A. [DOI] [PubMed] [Google Scholar]
- 15.Cong X, McGrath JM, Cusson RM, Zhang D. Pain assessment and measurement in neonates: an updated review. Adv Neonatal Care. 2013;13:379–395. doi: 10.1097/ANC.0b013e3182a41452. [DOI] [PubMed] [Google Scholar]
- 16.van Dijk M, Tibboel D. Update on pain assessment in sick neonates and infants. Pediatr Clin North Am. 2012;59:1167–1181. doi: 10.1016/j.pcl.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Crellin DJ, Harrison D, Santamaria N, Babl FE. Systematic review of the face, legs, activity, cry and consolability scale for assessing pain in infants and children: is it reliable, valid, and feasible for use? Pain. 2015;156:2132–2151. doi: 10.1097/j.pain.0000000000000305. [DOI] [PubMed] [Google Scholar]
- 18.Dorfman TL, Rempel GR, Scott SD, Hartling L. Pain assessment in mechanically ventilated PICU patients: it’s complicated. Pediatric Pain Lett. 2014;16:3–7. [Google Scholar]
- 19.Dutch Institute for Healthcare Improvement (CBO) (2007) Evidence-based guideline development. Manual for workgroup members. Dutch Institute for Healthcare Improvement (Kwaliteitsinstituut voor de Gezondheidszorg CBO), Utrecht
- 20.Puntillo KA, Morris AB, Thompson CL, Stanik-Hutt J, White CA, Wild LR. Pain behaviors observed during six common procedures: results from Thunder Project II. Crit Care Med. 2004;32:421–427. doi: 10.1097/01.CCM.0000108875.35298.D2. [DOI] [PubMed] [Google Scholar]
- 21.Roofthooft DW, Simons SH, Anand KJ, Tibboel D, van Dijk M. Eight years later, are we still hurting newborn infants? Neonatology. 2014;105:218–226. doi: 10.1159/000357207. [DOI] [PubMed] [Google Scholar]
- 22.Carbajal R, Rousset A, Danan C, Coquery S, Nolent P, Ducrocq S, Saizou C, Lapillonne A, Granier M, Durand P, Lenclen R, Coursol A, Hubert P, de Saint Blanquat L, Boelle PY, Annequin D, Cimerman P, Anand KJ, Breart G. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA. 2008;300:60–70. doi: 10.1001/jama.300.1.60. [DOI] [PubMed] [Google Scholar]
- 23.Stevens BJ, Pillai Riddell RR, Oberlander TF, Gibbins S. Assessment of pain in neonates and infants. In: Anand KJ, Stevens BJ, McGrath PJ, editors. Pain in neonates and infants. New York: Elsevier; 2007. pp. 67–86. [Google Scholar]
- 24.Pillai Riddell RR, Stevens BJ, McKeever P, Gibbins S, Asztalos L, Katz J, Ahola S, Din L. Chronic pain in hospitalized infants: health professionals’ perspectives. J Pain. 2009;10:1217–1225. doi: 10.1016/j.jpain.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 25.Ambuel B, Hamlett KW, Marx CM, Blumer JL. Assessing distress in pediatric intensive care environments: the COMFORT scale. J Pediatr Psychol. 1992;17:95–109. doi: 10.1093/jpepsy/17.1.95. [DOI] [PubMed] [Google Scholar]
- 26.van Dijk M, de Boer JB, Koot HM, Tibboel D, Passchier J, Duivenvoorden HJ. The reliability and validity of the COMFORT scale as a postoperative pain instrument in 0 to 3-year-old infants. Pain. 2000;84:367–377. doi: 10.1016/S0304-3959(99)00239-0. [DOI] [PubMed] [Google Scholar]
- 27.van Dijk M, Bouwmeester NJ, Duivenvoorden HJ, Koot HM, Tibboel D, Passchier J, de Boer JB. Efficacy of continuous versus intermittent morphine administration after major surgery in 0–3-year-old infants; a double-blind randomized controlled trial. Pain. 2002;98:305–313. doi: 10.1016/S0304-3959(02)00031-3. [DOI] [PubMed] [Google Scholar]
- 28.van Dijk M, de Boer JB, Koot HM, Duivenvoorden HJ, Passchier J, Bouwmeester N, Tibboel D. The association between physiological and behavioral pain measures in 0- to 3-year-old infants after major surgery. J Pain Symptom Manage. 2001;22:600–609. doi: 10.1016/S0885-3924(01)00288-3. [DOI] [PubMed] [Google Scholar]
- 29.Bai J, Hsu L, Tang Y, van Dijk M. Validation of the COMFORT behavior scale and the FLACC scale for pain assessment in Chinese children after cardiac surgery. Pain Manag Nurs. 2012;13:18–26. doi: 10.1016/j.pmn.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Boerlage AA, Ista E, de Jong M, Tibboel D, van Dijk M. The COMFORT behavior scale: is a shorter observation period feasible? Pediatr Crit Care Med. 2012;13:e124–e125. doi: 10.1097/PCC.0b013e3182192d92. [DOI] [PubMed] [Google Scholar]
- 31.Boerlage AA, Ista E, Duivenvoorden HJ, de Wildt SN, Tibboel D, van Dijk M. The COMFORT behaviour scale detects clinically meaningful effects of analgesic and sedative treatment. Eur J Pain. 2015;19:473–479. doi: 10.1002/ejp.569. [DOI] [PubMed] [Google Scholar]
- 32.Johansson M, Kokinsky E. The COMFORT behavioural scale and the modified FLACC scale in paediatric intensive care. Nurs Crit Care. 2009;14:122–130. doi: 10.1111/j.1478-5153.2009.00323.x. [DOI] [PubMed] [Google Scholar]
- 33.Valkenburg AJ, Boerlage AA, Ista E, Duivenvoorden HJ, Tibboel D, van Dijk M. The COMFORT-behavior scale is useful to assess pain and distress in 0- to 3-year-old children with Down syndrome. Pain. 2011;152:2059–2064. doi: 10.1016/j.pain.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Merkel SI, Voepel-Lewis T, Shayevitz JR, Malviya S. The FLACC: a behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997;23:293–297. [PubMed] [Google Scholar]
- 35.Voepel-Lewis T, Zanotti J, Dammeyer JA, Merkel S. Reliability and validity of the face, legs, activity, cry, consolability behavioral tool in assessing acute pain in critically ill patients. Am J Crit Care. 2010;19:55–61. doi: 10.4037/ajcc2010624. [DOI] [PubMed] [Google Scholar]
- 36.Ramelet AS, Rees N, McDonald S, Bulsara M, Abu-Saad HH. Development and preliminary psychometric testing of the multidimensional assessment of pain scale: MAPS. Paediatr Anaesth. 2007;17:333–340. doi: 10.1111/j.1460-9592.2006.02115.x. [DOI] [PubMed] [Google Scholar]
- 37.Ramelet AS, Rees NW, McDonald S, Bulsara MK, Huijer Abu-Saad H. Clinical validation of the multidimensional assessment of pain scale. Paediatr Anaesth. 2007;17:1156–1165. doi: 10.1111/j.1460-9592.2007.02325.x. [DOI] [PubMed] [Google Scholar]
- 38.de Jong A, Baartmans M, Bremer M, van Komen R, Middelkoop E, Tuinebreijer W, van Loey N. Reliability, validity and clinical utility of three types of pain behavioural observation scales for young children with burns aged 0–5 years. Pain. 2010;150:561–567. doi: 10.1016/j.pain.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 39.Dorfman TL, Sumamo Schellenberg E, Rempel GR, Scott SD, Hartling L. An evaluation of instruments for scoring physiological and behavioral cues of pain, non-pain related distress, and adequacy of analgesia and sedation in pediatric mechanically ventilated patients: a systematic review. Int J Nurs Stud. 2014;51:654–676. doi: 10.1016/j.ijnurstu.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 40.Stevens BJ, Gibbins S, Yamada J, Dionne K, Lee G, Johnston C, Taddio A. The premature infant pain profile-revised (PIPP-R): initial validation and feasibility. Clin J Pain. 2014;30:238–243. doi: 10.1097/AJP.0b013e3182906aed. [DOI] [PubMed] [Google Scholar]
- 41.Gibbins S, Stevens BJ, Yamada J, Dionne K, Campbell-Yeo M, Lee G, Caddell K, Johnston C, Taddio A. Validation of the premature infant pain profile-revised (PIPP-R) Early Hum Dev. 2014;90:189–193. doi: 10.1016/j.earlhumdev.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Carnevale FA, Razack S. An item analysis of the COMFORT scale in a pediatric intensive care unit. Pediatr Crit Care Med. 2002;3:177–180. doi: 10.1097/00130478-200204000-00016. [DOI] [PubMed] [Google Scholar]
- 43.Purser L, Warfield K, Richardson C. Making pain visible: an audit and review of documentation to improve the use of pain assessment by implementing pain as the fifth vital sign. Pain Manag Nurs. 2014;15:137–142. doi: 10.1016/j.pmn.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 44.Vet NJ, Ista E, de Wildt SN, van Dijk M, Tibboel D, de Hoog M. Optimal sedation in pediatric intensive care patients: a systematic review. Intensive Care Med. 2013;39:1524–1534. doi: 10.1007/s00134-013-2971-3. [DOI] [PubMed] [Google Scholar]
- 45.Marx CM, Smith PG, Lowrie LH, Hamlett KW, Ambuel B, Yamashita TS, Blumer JL. Optimal sedation of mechanically ventilated pediatric critical care patients. Crit Care Med. 1994;22:163–170. doi: 10.1097/00003246-199401000-00029. [DOI] [PubMed] [Google Scholar]
- 46.Curley MA, Harris SK, Fraser KA, Johnson RA, Arnold JH. State behavioral scale: a sedation assessment instrument for infants and young children supported on mechanical ventilation. Pediatr Crit Care Med. 2006;7:107–114. doi: 10.1097/01.PCC.0000200955.40962.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kudchadkar SR, Yaster M, Punjabi NM. Sedation, sleep promotion, and delirium screening practices in the care of mechanically ventilated children: a wake-up call for the pediatric critical care community. Crit Care Med. 2014;42:1592–1600. doi: 10.1097/CCM.0000000000000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Best KM, Boullata JI, Curley MA. Risk factors associated with iatrogenic opioid and benzodiazepine withdrawal in critically ill pediatric patients: a systematic review and conceptual model. Pediatr Crit Care Med. 2015;16:175–183. doi: 10.1097/PCC.0000000000000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jenkins IA, Playfor SD, Bevan C, Davies G, Wolf AR. Current United Kingdom sedation practice in pediatric intensive care. Paediatr Anaesth. 2007;17:675–683. doi: 10.1111/j.1460-9592.2006.02180.x. [DOI] [PubMed] [Google Scholar]
- 50.Sfoggia A, Fontela PS, Moraes A, da Silva F, Sober RB, Noer RB, Bruno F, Einloft P, Garcia PC, Piva JP. Sedation and analgesia in children submitted to mechanical ventilation could be overestimated? J Pediatr (Rio J) 2003;79:343–348. doi: 10.1590/S0021-75572003000400013. [DOI] [PubMed] [Google Scholar]
- 51.Arnold JH, Truog RD, Orav EJ, Scavone JM, Hershenson MB. Tolerance and dependence in neonates sedated with fentanyl during extracorporeal membrane oxygenation. Anesthesiology. 1990;73:1136–1140. doi: 10.1097/00000542-199012000-00011. [DOI] [PubMed] [Google Scholar]
- 52.Franck LS, Vilardi J, Durand D, Powers R. Opioid withdrawal in neonates after continuous infusions of morphine or fentanyl during extracorporeal membrane oxygenation. Am J Crit Care. 1998;7:364–369. [PubMed] [Google Scholar]
- 53.Fonsmark L, Rasmussen YH, Carl P. Occurrence of withdrawal in critically ill sedated children. Crit Care Med. 1999;27:196–199. doi: 10.1097/00003246-199901000-00052. [DOI] [PubMed] [Google Scholar]
- 54.Katz R, Kelly HW, Hsi A. Prospective study on the occurrence of withdrawal in critically ill children who receive fentanyl by continuous infusion. Crit Care Med. 1994;22:763–767. doi: 10.1097/00003246-199405000-00009. [DOI] [PubMed] [Google Scholar]
- 55.Playfor SD, Thomas DA, Choonara II. Recall following paediatric intensive care. Paediatr Anaesth. 2000;10:703–704. doi: 10.1046/j.1460-9592.2000.ab01ad.x. [DOI] [PubMed] [Google Scholar]
- 56.Schieveld JN, Leroy PL, van Os J, Nicolai J, Vos GD, Leentjens AF. Pediatric delirium in critical illness: phenomenology, clinical correlates and treatment response in 40 cases in the pediatric intensive care unit. Intensive Care Med. 2007;33:1033–1040. doi: 10.1007/s00134-007-0637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Franck LS, Harris SK, Soetenga DJ, Amling JK, Curley MA. The withdrawal assessment tool-1 (WAT-1): an assessment instrument for monitoring opioid and benzodiazepine withdrawal symptoms in pediatric patients. Pediatr Crit Care Med. 2008;9:573–580. doi: 10.1097/PCC.0b013e31818c8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Franck LS, Scoppettuolo LA, Wypij D, Curley MA. Validity and generalizability of the withdrawal assessment tool-1 (WAT-1) for monitoring iatrogenic withdrawal syndrome in pediatric patients. Pain. 2012;153:142–148. doi: 10.1016/j.pain.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ista E, van Dijk M, de Hoog M, Tibboel D, Duivenvoorden HJ. Construction of the Sophia observation withdrawal symptoms-scale (SOS) for critically ill children. Intensive Care Med. 2009;35:1075–1081. doi: 10.1007/s00134-009-1487-3. [DOI] [PubMed] [Google Scholar]
- 60.Ista E, de Hoog M, Tibboel D, Duivenvoorden HJ, van Dijk M. Psychometric evaluation of the Sophia observation withdrawal symptoms scale in critically Ill children. Pediatr Crit Care Med. 2013;14:761–769. doi: 10.1097/PCC.0b013e31829f5be1. [DOI] [PubMed] [Google Scholar]
- 61.American Psychiatric Association . Diagnostic and statistical manual of mental disorders, fifth edition (DSM-5) Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 62.Turkel SB, Tavare CJ. Delirium in children and adolescents. J Neuropsychiatry Clin Neurosci. 2003;15:431–435. doi: 10.1176/jnp.15.4.431. [DOI] [PubMed] [Google Scholar]
- 63.Silver GH, Kearney JA, Kutko MC, Bartell AS. Infant delirium in pediatric critical care settings. Am J Psychiatry. 2010;167:1172–1177. doi: 10.1176/appi.ajp.2010.09111606. [DOI] [PubMed] [Google Scholar]
- 64.Silver G, Traube C, Gerber LM, Sun XM, Kearney J, Patel A, Greenwald B. Pediatric delirium and associated risk factors: a single-center prospective observational study. Pediatr Crit Care Med. 2015;16:303–309. doi: 10.1097/PCC.0000000000000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Silver G, Traube C, Kearney J, Kelly D, Yoon MJ, Moyal WN, Gangopadhyay M, Shao HB, Ward MJ. Detecting pediatric delirium: development of a rapid observational assessment tool. Intens Care Med. 2012;38:1025–1031. doi: 10.1007/s00134-012-2518-z. [DOI] [PubMed] [Google Scholar]
- 66.Smith HA, Boyd J, Fuchs DC, Melvin K, Berry P, Shintani A, Eden SK, Terrell MK, Boswell T, Wolfram K, Sopfe J, Barr FE, Pandharipande PP, Ely EW. Diagnosing delirium in critically ill children: validity and reliability of the pediatric confusion assessment method for the intensive care unit. Crit Care Med. 2011;39:150–157. doi: 10.1097/CCM.0b013e3181feb489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Colville G, Kerry S, Pierce C. Children’s factual and delusional memories of intensive care. Am J Respir Crit Care Med. 2008;177:976–982. doi: 10.1164/rccm.200706-857OC. [DOI] [PubMed] [Google Scholar]
- 68.Ely EW, Gautam S, Margolin R, Francis J, May L, Speroff T, Truman B, Dittus R, Bernard R, Inouye SK. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27:1892–1900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smeets IA, Tan EY, Vossen HG, Leroy PL, Lousberg RH, van Os J, Schieveld JN. Prolonged stay at the paediatric intensive care unit associated with paediatric delirium. Eur Child Adolesc Psychiatry. 2010;19:389–393. doi: 10.1007/s00787-009-0063-2. [DOI] [PubMed] [Google Scholar]
- 70.Silver G, Kearney J, Traube C, Hertzig M. Delirium screening anchored in child development: the Cornell assessment for pediatric delirium. Palliat Support Care. 2015;13:1005–1011. doi: 10.1017/S1478951514000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Traube C, Silver G, Kearney J, Patel A, Atkinson TM, Yoon MJ, Halpert S, Augenstein J, Sickles LE, Li C, Greenwald B. Cornell assessment of pediatric delirium: a valid, rapid, observational tool for screening delirium in the PICU. Crit Care Med. 2014;42:656–663. doi: 10.1097/CCM.0b013e3182a66b76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Dijk M, Knoester H, van Beusekom BS, Ista E. Screening pediatric delirium with an adapted version of the Sophia Observation withdrawal Symptoms scale (SOS) Intensive Care Med. 2012;38:531–532. doi: 10.1007/s00134-011-2434-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ista E, Te Beest H, De Hoog M, Tibboel D, Van Dijk M. A preliminary validation of a screening tool for pediatric delirium. Arch Dis Child. 2014;99(Suppl 2):A84–A85. doi: 10.1136/archdischild-2014-307384.226. [DOI] [Google Scholar]
- 74.Ceelie I, de Wildt SN, van Dijk M, van den Berg MM, van den Bosch GE, Duivenvoorden HJ, de Leeuw TG, Mathot R, Knibbe CA, Tibboel D. Effect of intravenous paracetamol on postoperative morphine requirements in neonates and infants undergoing major noncardiac surgery: a randomized controlled trial. JAMA. 2013;309:149–154. doi: 10.1001/jama.2012.148050. [DOI] [PubMed] [Google Scholar]
- 75.Anand KJ, Hall RW, Desai N, Shephard B, Bergqvist LL, Young TE, Boyle EM, Carbajal R, Bhutani VK, Moore MB, Kronsberg SS, Barton BA, NEOPAIN Trial Investigators Group Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet. 2004;363:1673–1682. doi: 10.1016/S0140-6736(04)16251-X. [DOI] [PubMed] [Google Scholar]
- 76.Hunseler C, Balling G, Rohlig C, Blickheuser R, Trieschmann U, Lieser U, Dohna-Schwake C, Gebauer C, Moller O, Hering F, Hoehn T, Schubert S, Hentschel R, Huth RG, Muller A, Muller C, Wassmer G, Hahn M, Harnischmacher U, Behr J, Roth B, Clonidine Study G Continuous infusion of clonidine in ventilated newborns and infants: a randomized controlled trial. Pediatr Crit Care Med. 2014;15:511–522. doi: 10.1097/PCC.0000000000000151. [DOI] [PubMed] [Google Scholar]
- 77.Carney L, Kendrick J, Carr R. Safety and effectiveness of dexmedetomidine in the pediatric intensive care unit (SAD-PICU) Can J Hosp Pharm. 2013;66:21–27. doi: 10.4212/cjhp.v66i1.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Welzing L, Link F, Junghaenel S, Oberthuer A, Harnischmacher U, Stuetzer H, Roth B. Remifentanil-induced tolerance, withdrawal or hyperalgesia in infants: a randomized controlled trial. RAPIP trial: remifentanil-based analgesia and sedation of paediatric intensive care patients. Neonatology. 2013;104:34–41. doi: 10.1159/000348790. [DOI] [PubMed] [Google Scholar]
- 79.Rigby-Jones AE, Priston MJ, Sneyd JR, McCabe AP, Davis GI, Tooley MA, Thorne GC, Wolf AR. Remifentanil-midazolam sedation for paediatric patients receiving mechanical ventilation after cardiac surgery. Br J Anaesth. 2007;99:252–261. doi: 10.1093/bja/aem135. [DOI] [PubMed] [Google Scholar]
- 80.Curley MA, Wypij D, Watson RS, Grant MJ, Asaro LA, Cheifetz IM, Dodson BL, Franck LS, Gedeit RG, Angus DC, Matthay MA, RESTORE Study Investigators. Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network Protocolized sedation vs usual care in pediatric patients mechanically ventilated for acute respiratory failure: a randomized clinical trial. JAMA. 2015;313:379–389. doi: 10.1001/jama.2014.18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gupta K, Gupta VK, Jayashree M, Singhi S. Randomized controlled trial of interrupted versus continuous sedative infusions in ventilated children. Pediatr Crit Care Med. 2012;13:131–135. doi: 10.1097/PCC.0b013e31820aba48. [DOI] [PubMed] [Google Scholar]
- 82.Wildschut ED, Hanekamp MN, Vet NJ, Houmes RJ, Ahsman MJ, Mathot RA, de Wildt SN, Tibboel D. Feasibility of sedation and analgesia interruption following cannulation in neonates on extracorporeal membrane oxygenation. Intensive Care Med. 2010;36:1587–1591. doi: 10.1007/s00134-010-1931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vet NJ, de Wildt SN, Verlaat CW, Knibbe CA, Mooij MG, Hop WC, van Rosmalen J, Tibboel D, de Hoog M, Skic Daily interruption of sedation in critically ill children: study protocol for a randomized controlled trial. Trials. 2014;15:55. doi: 10.1186/1745-6215-15-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ceelie I, de Wildt SN, de Jong M, Ista E, Tibboel D, van Dijk M. Protocolized post-operative pain management in infants; do we stick to it? Eur J Pain. 2012;16:760–766. doi: 10.1002/j.1532-2149.2011.00056.x. [DOI] [PubMed] [Google Scholar]
- 85.Aukes DI, Roofthooft DW, Simons SH, Tibboe D, van Dijk M. Pain management in neonatal intensive care: evaluation of the compliance with guidelines. Clin J Pain. 2015;31:830–835. doi: 10.1097/AJP.0000000000000168. [DOI] [PubMed] [Google Scholar]
- 86.Tobias JD. Tolerance, withdrawal, and physical dependency after long-term sedation and analgesia of children in the pediatric intensive care unit. Crit Care Med. 2000;28:2122–2132. doi: 10.1097/00003246-200006000-00079. [DOI] [PubMed] [Google Scholar]
- 87.Wang D, Aubertin C, Barrowman N, Moreau K, Dunn S, Harrold J. Examining the effects of a targeted noise reduction program in a neonatal intensive care unit. Arch Dis Child Fetal Neonatal Ed. 2014;99:F203–F208. doi: 10.1136/archdischild-2013-304928. [DOI] [PubMed] [Google Scholar]
- 88.Fraser GL, Riker RR. Monitoring sedation, agitation, analgesia, and delirium in critically ill adult patients. Crit Care Clin. 2001;17:967–987. doi: 10.1016/S0749-0704(05)70189-5. [DOI] [PubMed] [Google Scholar]
- 89.Carr DB, Goudas LC. Acute pain. Lancet. 1999;353:2051–2058. doi: 10.1016/S0140-6736(99)03313-9. [DOI] [PubMed] [Google Scholar]
- 90.Jonsdottir RB, Kristjansdottir G. The sensitivity of the premature infant pain profile—PIPP to measure pain in hospitalized neonates. J Eval Clin Pract. 2005;11:598–605. doi: 10.1111/j.1365-2753.2005.00603.x. [DOI] [PubMed] [Google Scholar]
- 91.McNair C, Ballantyne M, Dionne K, Stephens D, Stevens B. Postoperative pain assessment in the neonatal intensive care unit. Arch Dis Child Fetal Neonatal Ed. 2004;89:F537–F541. doi: 10.1136/adc.2003.032961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stevens B, Johnston C, Petryshen P, Taddio A. Premature infant pain profile: development and initial validation. Clin J Pain. 1996;12:13–22. doi: 10.1097/00002508-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 93.Hummel P, Lawlor-Klean P, Weiss MG. Validity and reliability of the N-PASS assessment tool with acute pain. J Perinatol. 2010;30:474–478. doi: 10.1038/jp.2009.185. [DOI] [PubMed] [Google Scholar]
- 94.Hummel P, Puchalski M, Creech SD, Weiss MG. Clinical reliability and validity of the N-PASS: neonatal pain, agitation and sedation scale with prolonged pain. J Perinatol. 2008;28:55–60. doi: 10.1038/sj.jp.7211861. [DOI] [PubMed] [Google Scholar]
- 95.van Dijk M, Roofthooft DWE, Anand KJS, Guldemond F, de Graaf J, Simons S, de Jager Y, van Goudoever JB, Tibboel D. Taking up the challenge of measuring prolonged pain in (premature) neonates: the COMFORTneo scale seems promising. Clin J Pain. 2009;25:607–616. doi: 10.1097/AJP.0b013e3181a5b52a. [DOI] [PubMed] [Google Scholar]
- 96.Johansson M, Kokinsky E. The COMFORT behavioural scale and the modified FLACC scale in paediatric intensive care. Nurs Crit Care. 2009;14:122–130. doi: 10.1111/j.1478-5153.2009.00323.x. [DOI] [PubMed] [Google Scholar]
- 97.Ramelet AS, Rees NW, McDonald S, Bulsara MK, Huijer Abu-Saad H. Clinical validation of the multidimensional assessment of pain scale. Paediatr Anaesth. 2007;17:1156–1165. doi: 10.1111/j.1460-9592.2007.02325.x. [DOI] [PubMed] [Google Scholar]
- 98.Bear LA, Ward-Smith P. Interrater reliability of the COMFORT scale. Pediatr Nurs. 2006;32:427–434. [PubMed] [Google Scholar]
- 99.Nolent P, Nanquette MC, Carbajal R, Renolleau S. Which sedation scale should be used in the paediatric intensive care unit? A comparative prospective study. Arch Pediatr. 2006;13:32–37. doi: 10.1016/j.arcped.2005.09.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.