Abstract
Introduction
The aim of our study was to determine and compare angiogenesis in benign prostatic hyperplasia (BPH), high-grade prostate intraepithelial neoplasia (HGPIN) and prostate cancer (Pca). Moreover, we evaluated its role as a prognostic factor for Pca.
Material and methods
We examined 39, 12 and 51 samples of BPH, HGPIN and Pca, respectively. Immunohistochemical methods were applied in order to evaluate the expression of VEGF and its receptors (VEGFR-1, VEGFR-2), while microvascular density (MVD) was determined using CD105. In Pca samples, we recorded stage, differentiation, perineural invasion, adjuvant radiotherapy and their correlation with angiogenesis.
Results
225 The expression of VEGF, VEGFR-1 and VEGFR-2 was significantly higher in Pca than compared to BPH (p <0.001, p <0.001 and p <0.001, respectively) and HGPIN (p <0.001, p <0.001 and p = 0.04, respectively), while there was no difference between BPH and HGPIN. MVD was higher in Pca compared to BPH (p <0.001) and HGPIN (p <0.01), while there was no difference between BPH and HGPIN. VEGF expression and MVD were significantly greater in Pca samples with poor differentiation (p = 0.044 and p = 0.038, respectively) and perineural invasion (p <0.001 and p = 0.019, respectively), while overexpression of VEGF was associated with advanced pathological stage (p = 0.047).
Conclusions
Angiogenesis is more prominent in Pca than in BPH and HGPIN, while there is no difference between BPH and HGPIN. Pharmaceutical inhibition of angiogenesis could be a valuable therapeutic option for Pca in the near future.
Keywords: VEGF, VEGF receptors, pathologic angiogenesis, CD105 antigen
INTRODUCTION
The prostate is a compound tubuloalveolar gland, which is commonly affected by two major processes; hyperplasia and malignancy. Benign prostate hyperplasia (BPH) is a common pathology in the aging male [1]. The cause is not completely understood, but there is little doubt that it is related to the action of androgens. Marshall et al. postulated that BPH with increased acinar and stromal cell proliferation stimulates increased vascularity (angiogenesis) [2].
Prostate cancer (Pca) is the most common malignancy in men in the USA, with an estimated 233,000 new cases diagnosed and 29,490 deaths in 2014 [3]. Solid tumor growth beyond the size of 2 mm is absolutely dependent on vascularization. Angiogenesis is a complex process involving multiple pathways that affect tumor growth, invasion and metastasis in Pca [4]. It is generally accepted that tumor angiogenesis is crucial both for the growth of a primary neoplastic tumor and also for the development of metastasis [5]. Angiogenic factors have been implicated in Pca development and progression, and measurements of tumor angiogenesis have been shown to correlate with metastasis in invasive prostate carcinoma [6].
High-grade prostatic intra-epithelial neoplasia (HGPIN) is considered a premalignant lesion which might progress to invasive carcinoma [7]. HGPIN affects the ducts and acini and is basically defined as a proliferation and anaplasia of the lumenal (or secretory) cells. The changes are not abrupt but increasingly greater, with the end of the spectrum being invasive adenocarcinoma [8].
Vascular endothelial growth factor (VEGF), originally known as vascular permeability factor, is considered as the most potent mediator of physiologic and pathologic angiogenesis and lymphangiogenesis [9]. VEGF is a 46 Kda heparin binding, homodimeric glycoprotein that shares sequence homologies with platelet-derived growth factor. Four isoforms of VEGF have been characterized. They are produced by alternative splicing, and consist of 206, 189, 165 and 121 amino acids [10]. VEGF exerts its action by binding to the specific cell surface receptors, fms-like tyrosine kinase 1 (FLT-1/VEGFR-1) and fetal liver kinase 1 (FLK-1/VEGFR-2). Once VEGF binds to VEGF receptors, receptor dimerization and autophosphorylation is induced and downstream signaling via several secondary messengers, including several protein kinases and phosphatases, is activated. This supports a proangiogenic phenotype [11]. The primary signaling receptor for VEGF is VEGFR-2, and activation of VEGFR-2 by VEGF on endothelial tip and stalk cells which directs the migration and extension of sprouting vessels, respectively [12, 13].
To understand the pathological significance and prognostic value of angiogenesis in malignancies, effective methods for determining cancer-related neovascularity in malignancies are essential. In human cancer tissues, the most common method for semiquantitative evaluation of angiogenesis is to measure microvessel density (MVD) using endothelial markers [14]. Endoglin (CD105), a receptor for transforming growth factor β (TGF-β), is considered a very sensitive and specific marker of MVD. CD105 is involved in normal vascular development, is highly expressed on the surface of proliferating vascular endothelial cells, and is highly expressed on endothelial cells during tumor angiogenesis [15]. TGF-β acts as a negative regulator for tumor angiogenesis via inhibition of proliferation and migration of endothelial cells and, thus, interrupting the formation of microvessels, whereas CD105 counteracts these actions [16]. In hypoxic conditions, CD105 is upregulated through induction of hypoxia-inducible factor 1-α [17]. CD105 is also upregulated on tumor endothelial cells after inhibition of the VEGF pathway [18]. CD105, which is an accessory receptor for diverse TGF-β family cytokines, has been implicated in prostate cancer cell migration and invasion [19].
The aim of our study was to record and compare the immunohistochemical expression of VEGF, VEGFR-1, VEGFR-2 and CD105 in BPH, PCa and HGPIN. In addition, we correlated the expression of these factors with pathological parameters of PCa such as pathological stage, differentiation, lymph node status and perineural invasion.
MATERIAL AND METHODS
Tissues and clinical parameters
A total of 102 paraffin embedded archival specimens were obtained from the Department of Pathology of the University Hospital of Ioannina. Our material consisted of 51 radical prostatectomy specimens with prostate adenocarcinoma of various Gleason scores, 39 specimens of BPH and 12 specimens of HGPIN, obtained from retropubic or transurethral prostatectomy. All hematoxylin-eosin-stained sections were reviewed, the quality of the material was checked and the best slides from each specimen were selected. In PCa patients we selected slides showing central and peripheral areas of the tumor, avoiding areas with necrosis. Patients’ records were reviewed, and clinicopathological characteristics and follow-ups were noted.
Immunochemistry
We used the EnVision System and the monoclonal antibodies VEGF (Ab-3 JH121,Neomarkers, USA), VEGFR-1 (RP 077,Diagnostic Biosystems, USA), VEGFR-2 (RP 076, Diagnostic Biosystems, USA) and CD105 (Clone SN6h, DAKO, DENMARK). Briefly, 5-µm-thick histological sections from formalin-fixed paraffin embedded blocks of tumor tissue were dewaxed in xylene, rehydrated through graded alcohols, immersed in 10mM Tris and 0.5 M EDTA, pH 9.0 and microwaved twice for 5 minutes each. Subsequently, the sections were incubated with 0.3% H2O2 for 30 minutes to block endogenous peroxidase activity. The sections were then incubated overnight at 4°C with the primary antibodies (dilutions: VEGF, 1:30; VEGFR-1, 1:30; VEGFR-2, 1:30; and CD105, 1:5).
VEGF, VEGFR-1 and VEGFR-2 quantification
The percentage of epithelial cells that exhibited a positive cytoplasmic immunoreactivity to VEGF, VEGFR-1 and VEGFR-2 was determined by counting at least 500 epithelial cells in each case. The intensity of the staining was recorded as follows: 0; no staining, 1; mild staining, 2; moderate staining, 3; intense staining.
Microvessel detection and counting
Two investigators (NG and AG) determined MVD in each slide independently and blindly to patients’ outcomes. Vascularity was measured by the average number of CD105 positive vessels. Slides were examined by light microscopy and three areas with the highest number of stained microvessel were identified (‘hot spots’). Microvessels were counted in each of the three ‘hot spots’ using a 200x magnification field (x20 objective lens, x10 ocular lens) and the total number was divided by three. The area of the optical field in the microscope used for the counts was 0.7386 mm2. Determining microvessel density was expressed as the number of stained microvessels per optical field. Although in most of the counted vessels a lumen was identified, this was not necessary. Any cell or cell cluster showing positive CD105 staining was counted as a vessel, as described in the Weidner method [20]. No counts were performed in areas of necrosis and inflammation. Sections, in which three ‘‘hot spots’’ could not be identified, were excluded from further analysis. If the two investigators had differences higher than ten microvessels per high-power optical field, sections were reviewed again until they reached a consensus.
Statistical analysis
Analyses were performed by using SPSS Version 14.0 (SPSS Inc, Chicago, IL, USA). For comparison between variables we used the Kruskal-Wallis test and Pearson Chi-Square test while multiple regressions models were used to assess correlation with intensity. VEGF and MVD were compared in the PCa group with respect to the Gleason score with a students t-test. In the cancer specimens, in order to examine if the pathological parameters were affected by the angiogenetic factors within the same group, a multifactor ANOVA or Bonferroni multiple comparison test were implemented assuming no interactions between the factors. P-values of less than 0.05 were considered statistically significant with 95% confidence intervals.
RESULTS
Clinicopathological data
The mean age of the patients was 71, 71 and 67 for BPH, HGPIN and Pca, respectively. In Pca cases thirty-seven patients (72%) were classified as pathological stage T2 and 14 (28%) as stage pT3. One patient had a well differentiated tumor (Gleason score 5) while 19 (37%) and 31 (61%) had moderate (Gleason score 6) and poor (Gleason score 7–10) differentiated tumors, respectively. Two patients (4%) had a detectable metastasis in at least one lymph node at the time of diagnosis while perineural invasion was detected in 24 patients (47%). Twelve patients (24%) were submitted to adjuvant radiotherapy after radical prostatectomy.
VEGF expression in BPH, HGPIN and PCa
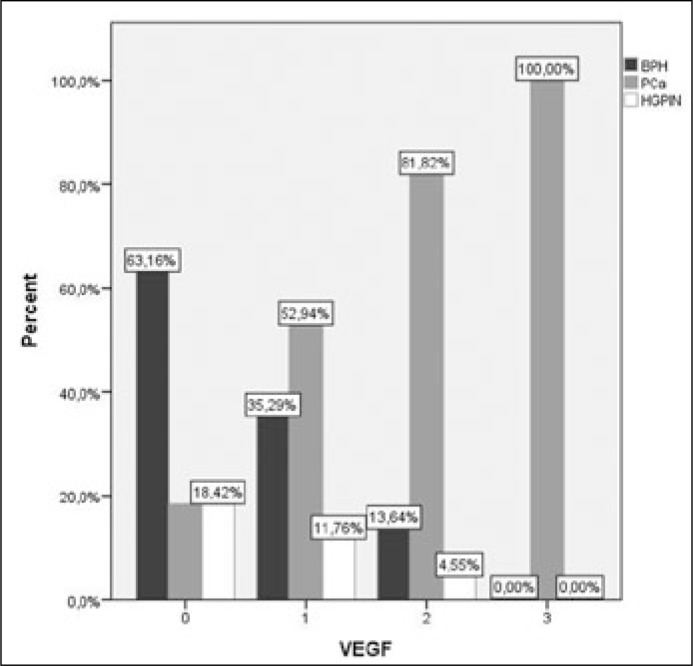
VEGF cytoplasmic staining was statistically significantly higher in PCa (56.27%) compared to BPH (9%) and HGPIN (9%) (p <0.01 and p <0.01, respectively) while no difference was observed between BPH and HGPIN (p = 1.00). Moreover, in PCa intensity of VEGF staining was significantly higher when compared to BPH (p <0.01) and HGPIN (p = 0.013), while there was no difference between BPH and HGPIN (p = 0.853) (Figures 1, 2).
Figure 1.
Intensity of VEGF staining was significant higher in PCa compared to BPH (p <0.01) and HGPIN (p = 0.013).
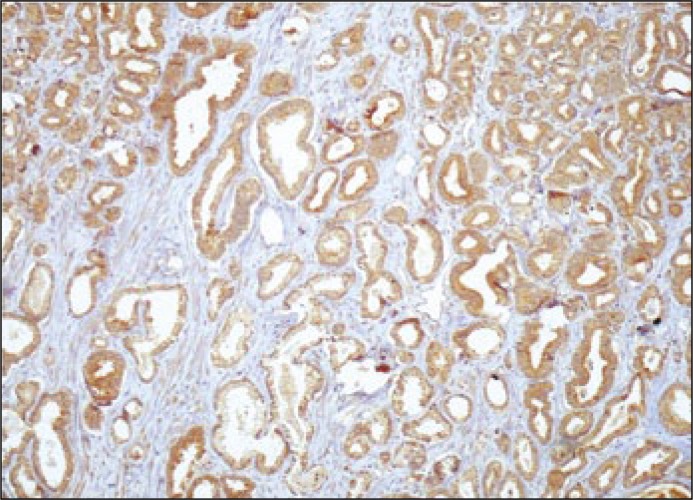
Figure 2.
VEGF immunoreactivity in prostate cancer cells (magnification x100).
VEGFR-1 and VEGFR-2 expression in BPH, HGPIN and PCa
The percentage of cancer specimens positive for VEGFR-1 and VEGFR-2 was 37% and 60%, respectively. There was a statistically significant difference in both VEGFR-1 and VEGFR-2 expression between PCa and BPH (p <0.001 and p <0.001, respectively) and PCa compared to HGPIN (p <0.001 and p = 0.04, respectively). There was no statistically significant difference (p = 0.981) in the VEGFR-1 immunoreactivity in epithelial cells of BPH (8.9%) and HGPIN (10.4%). Similarly, no significant difference (p = 0.492) was observed in the VEGFR-2 immuno-reactivity between BPH (8%) and HGPIN (28%).
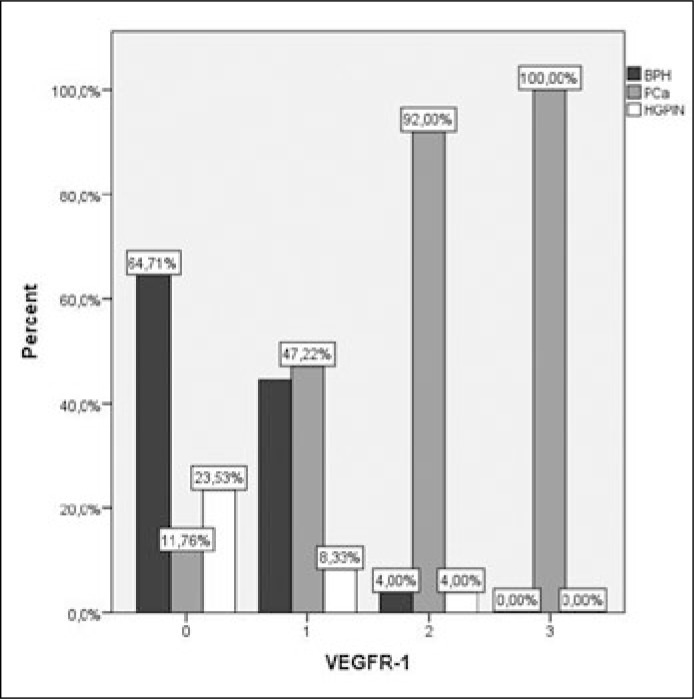
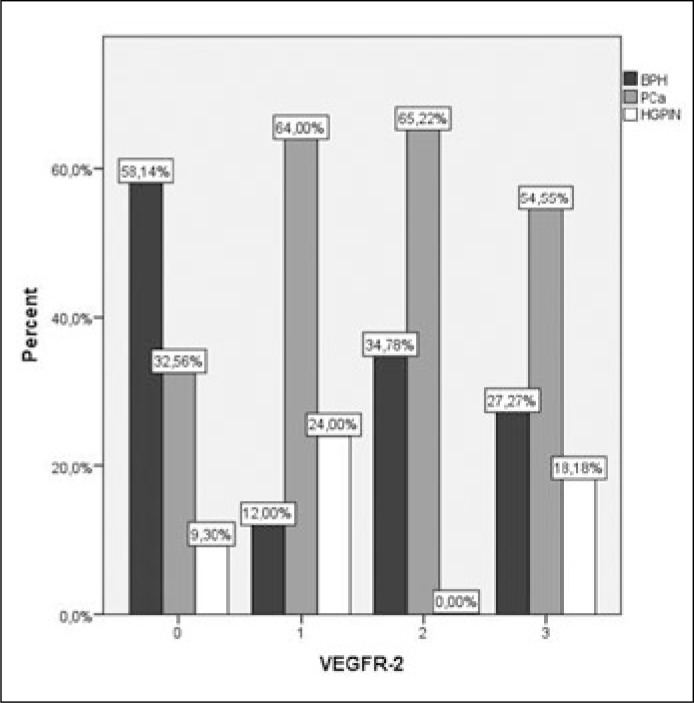
In Pca the intensity of VEGFR-1 staining was significantly higher compared to BPH (p <0.001) and HGPIN (p <0.01), while there was no difference between BPH and HGPIN (p = 0.379) (Figures 3, 4). Intensity of VEGFR-2 staining in prostate cancer was significantly higher compared to BPH (p <0.01) while there was no difference between PCa and HGPIN (p = 0.134). VEGFR-2 expression was significant higher in HGPIN when compared to BPH (p <0.01) (Figures 5, 6).
Figure 3.
Intensity of VEGFR-1 staining was significant higher in PCa compared to BPH (p <0.001) and HGPIN (p <0.01), while there was no difference between BPH and HGPIN (p = 0.379).
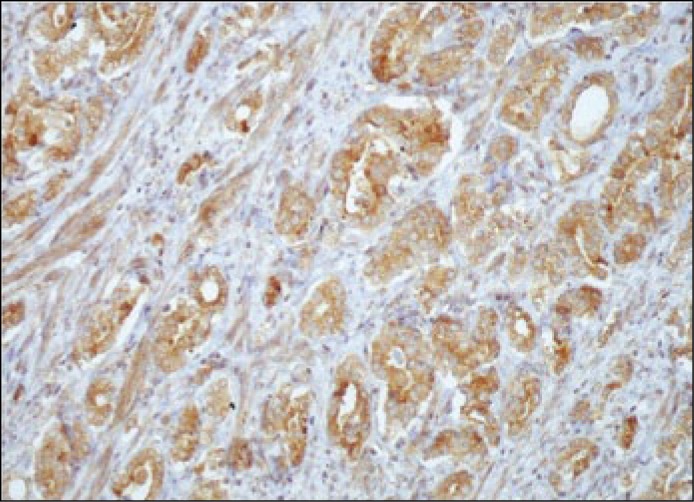
Figure 4.
VEGFR-1 immunoreactivity in prostate cancer cells (magnification x100).
Figure 5.
Intensity of VEGFR-2 staining was significant higher compared to BPH (p <0.01) and HGPIN (p = 0.013).
Figure 6.
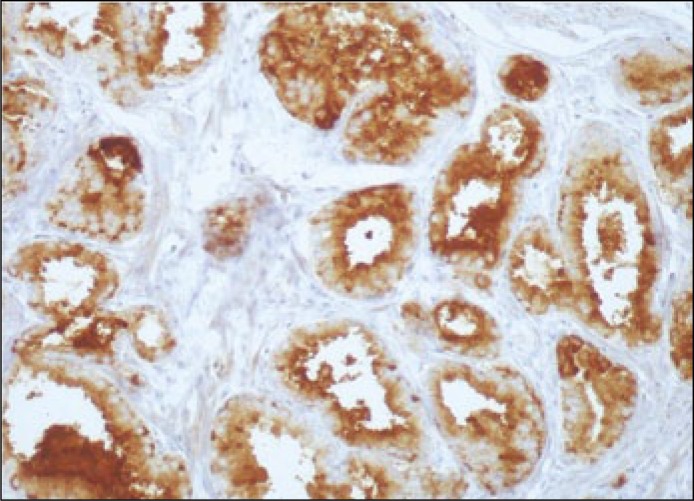
VEGFR-2 immunoreactivity in prostate cancer cells (magnification x100).
Microvessel density
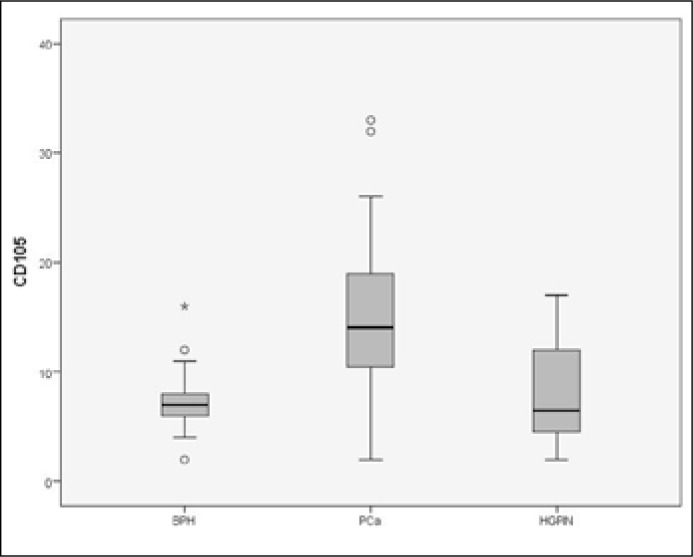
CD105 was used for the determination of microvessel density. We observed statistically significant differences in the number of capillaries and the distribution of the microcirculation in the different morphologic patterns. Median MVD was 29.1 in PCa, while the corresponding values were 14.46 and 15.84 in BPH and HGPIN, respectively (Figure 7). The vascular networks, as highlighted by immunohistochemistry, appeared more disorganized in malignant than in normal prostate. MVD was a statistically significant in PCa when compared to BPH (p <0.01) and HGPIN (p <0.01) while no difference was observed between BPH and HGPIN (p = 0.95) (Figure 8).
Figure 7.
Box-plot of microvessel density for BPH, HGPIN and PCa demonstrating an increased MVD in PCa.
Figure 8.
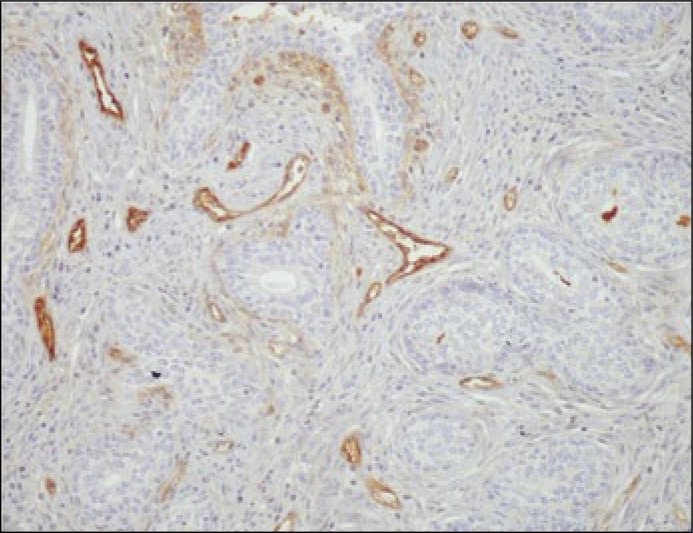
Microvessels within a hot spot area, with endothelial cells heavily stained with CD105, in prostate cancer cells (magnification x100).
We recorded no relationship between the expression of VEGF, its receptors and MVD both in BPH and HGPIN. However, in PCa statistically we recorded significant correlation between the expressions of VEGF and MVD (p = 0.04) as well as between VEGF and VEGFR-2 (p = 0.01). Similarly, there was a significant relationship between VEGFR-1 and VEGFR-2 (p = 0.03).
Correlations of VEGF, VEGFR-1, VEGFR-2 and CD105 with pathological parameters in PCa
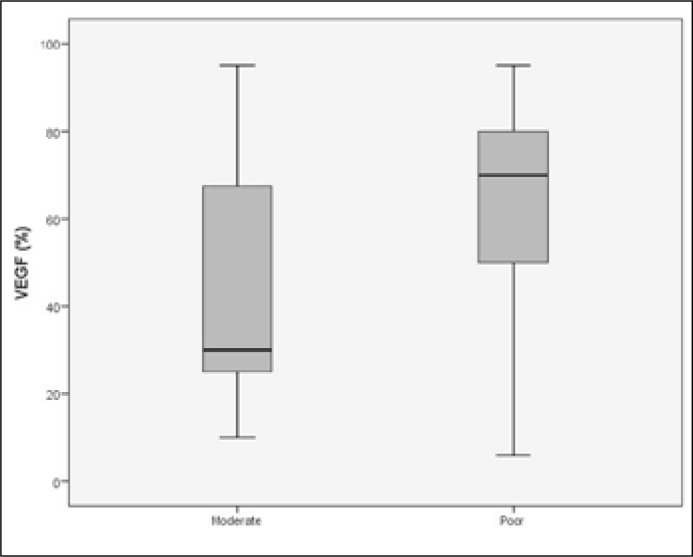
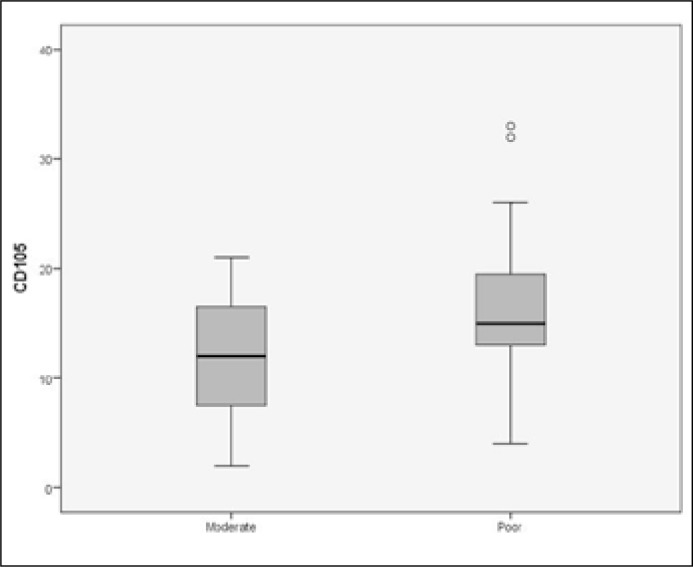
There was no statistically significant correlation between the expression of VEGFR-1, VEGFR-2, CD105 and the pathological stage (p = 0.482, p = 0.861, p = 0.298). On the other hand, higher VEGF expression was correlated with an adverse pathological stage (T3) (p = 0.04). Moreover, high expression of VEGF and CD105 was significantly correlated with poor differentiation (p = 0.04 and p = 0.03, respectively) and perineural invasion (p = 0.03 and p = 0.01) (Figures 9, 10). No correlation was found between the above angiogenic factors and the usage of adjuvant radiotherapy.
Figure 9.
Patients with poorly differentiated tumors had higher expression of VEGF (p = 0.04).
Figure 10.
Patients with poorly differentiated tumors had higher expression of CD105 (p = 0.03).
DISCUSSION
It has been proposed that neovascularization begins in BPH and that it keeps progressing in a stepwise manner in the subsequent proliferative premalignant and malignant stages [21]. Studies examining the association of VEGF immunohistochemical expression and angiogenesis with clinicopathological factors and outcomes in BPH, HGPIN and PCa have yielded inconclusive and conflicting results. These discrepancies may be caused by differences in the tumor location, the choice of antibodies, and cutoffs as well as variability in immunochemistry protocols. As the interest for the use of angiogenic inhibitors in BPH (5α-reductase inhibitors) and PCa (anti-VEGF, anti-endoglin) increases rapidly, it is necessary to have valid conclusions for the importance of microvessel counts and VEGF expression in order to address their impact on prognosis and their use as surrogates [22, 23, 24].
Recent reports in the literature have addressed the issue of the importance of the VEGF system in the development of normal prostate, prostatic hyperplasia and carcinoma [25]. Our observations confirm earlier studies supporting that the VEGF and its receptors are expressed in PCa in significantly higher rates than BPH [26]. Moreover, it was found that the expression of VEGFR-2 is strongest in HGPIN when compared with hyperplasia. In contrast no difference was observed between BPH and HGPIN regarding the expression of VEGF and VEGFR-1. Other studies have shown that the expression of VEGF in HGPIN is significantly higher than in normal prostate, without ever comparing with BPH [27]. In accordance to our results, Woolard et al have shown that in epithelial cells, VEGF and VEGFR-1 expression was higher in tumor tissue when compared to benign tissue. VEGF-D and VEGFR-3 expression was significantly higher in benign tissue when compared to tumor in the stroma and the endothelium of lymphatic and blood vessels [28]. In addition, the frequency of blood vessels was lower in tumor tissue when compared with benign tissue. Very recently Nordby et al. have shown that after radical prostatectomy high expression of VEGFR-2 in either stroma or epithelium was independently associated with a higher incidence of prostate cancer relapse and high combined expression of either VEGF, VEGFR-2 or both in stroma was independently associated with a higher incidence of biochemical failure [29]. Together with previous studies showing efficiency of targeting VEGFR-2 in prostate cancer, this study highlights its potential as a target for therapy, and may aid in future selection of prostate cancer patients for novel anti-angiogenic treatment.
Using immunohistochemical staining and rapid colorimetric in situ hybridization, Kuniyasu et al. found that VEGF expression and the Gleason score were closely linked [30]. In addition, other studies have reported that a higher VEGF level was significantly associated with a worse outcome in PCa patients, but other studies did not show any significant link between VEGF and survival in these patients [31]. Liu et al. in their recent meta-analysis demonstrated that high VEGF expression level was a significant marker for predicting OS, CSS and BF in PCa patients [32].
Also, significant is the observation that in PCa overexpression of VEGF and VEGR-1 there was an associated overexpression of VEGFR-2. Several studies have also shown that the complex VEGF/VEGFR-2 induces proliferation of cancer cells having an autocrine action both in solid tumors such as PCa as well as in hematological malignancies such as leukemia [33, 34]. These investigations also showed that VEGF/VEGFR-2 blockade leads to a significant reduction in the proliferation of tumor cells.
MVD has been widely used for the evaluation of angiogenesis in many previous studies, and it is recognized as one of the most useful prognostic markers for tumor progression and survival in patients with various different types of cancer. Immunohistochemistry and antibodies against the endothelial cell markers CD31, CD34, and CD105 have frequently been used in the previous studies of cancer tissues. It was suggested that CD105 might be the optimal antibody for the identification of microvessels to preclude disturbances caused by the low accuracy of the panendothelial markers (CD31, CD34, and von Willebrand factor) used so far [35]. There are many recent reports that have used these antibodies to investigate the pathological significance of MVD in PCa tissues [36]. In the statistical analysis of our data we observed a higher mean value of CD105 in PCa than in BPH and HGPIN, consistent with most reports [37]. It has been observed that tumors vary in ‘angiogenic ability’ of cancer cells. MVD varies among different tumors as well as between different regions within the same tumor.
Our data showed that increased MVD was correlated with a higher Gleason score in accordance to other studies demonstrating that in Pca MVD is different between clinically localized low vs. high Gleason score disease reaching, in the latter instance, levels similar to those observed in Pca with bone metastases [38]. Miyata et al. recently identified that CD105-MVD is a significant and independent predictor of biochemical recurrence in prostate cancer patients who underwent radical prostatectomy with neoadjuvant hormone treatment [37]. Karzai et al. have recently applied an anti-CD105 antibody (TRC105) in metastatic castration-resistant prostate cancer with favorable results regarding tumor growth, progression and metastases in prostate cancer [39].
Approved antiangiogenic drugs such as bevacizumab, sorafenib, sunitinib, and pazopanib primarily target the VEGF signaling pathway and are associated with modest survival advantages in localized PCa and various malignancies such as renal, breast, colorectal, lung cancer and glioblastoma. Unfortunately, well established anti-angiogenic therapies have failed to improve survival outcomes in advanced PCa [40].
Limitations
Some limitations of our study should be discussed. First, the sample size was limited and did not allowed the performance of a multivariate analysis. Such an analysis would have confirmed observations of the univariate analysis for the predictive ability of the angiogenic factors overexpression on the patients’ outcome and on the aggressive behavior of the prostate cancer. Second, the co-overexpression of the protein and its receptor was not observed in all of the tumor samples; and thus, a potential autocrine action of the examined factors might not be a general property of prostate carcinoma. More studies, preferentially prospective, are required to determine whether angiogenic expression will aid patient evaluation beyond traditional prognostic factors in an individual basis.
CONCLUSIONS
In conclusion, angiogenesis was significantly higher in Pca when compared to BPH and HGPIN, while there was no difference between BPH-HGPIN. Angiogenesis correlates with a more aggressive potential in patients with PCa. Also, VEGF expression and MVD should be examined in the context of other proposed angiogenic molecules, and the expression of VEGF receptors should also be considered. Our observations, strengthen the case for novel research avenues on VEGF and other angiogenesis-inducing molecules, which may become useful prognostic markers and serve as a basis for the development of antiangiogenic strategies within the therapeutic interventions in prostatic cancer
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Cindolo L, Pirozzi L, Sountoulides P, et al. Patient's adherence on pharmacological therapy for benign prostatic hyperplasia (BPH)-associated lower urinary tract symptoms (LUTS) is different: is combination therapy better than monotherapy? BMC Urol. 2015;15:96. doi: 10.1186/s12894-015-0090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marshall S, Narayan P. Treatment of prostatic bleeding: suppression of angiogenesis by androgen deprivation. J Urol. 1993;149:1553–1554. doi: 10.1016/s0022-5347(17)36446-7. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 4.Aragon-Ching JB, Madan RA, Dahut WL. Angiogenesis inhibition in prostate cancer: current uses and future promises. J Oncol. 2010;2010:1–7. doi: 10.1155/2010/361836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 6.Weidner N, Carroll PR, Flax J, Blumenfeld W, Folkman J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993;143:401–409. [PMC free article] [PubMed] [Google Scholar]
- 7.Pallares J, Rojo F, Iriarte J, Morote J, Armadans LI, de Torres I. Study of microvessel density and the expression of the angiogenic factors VEGF, bFGF and the receptors Flt-1 and FLK-1 in benign, premalignant and malignant prostate tissues. Histol Histopathol. 2006;21:857–865. doi: 10.14670/HH-21.857. [DOI] [PubMed] [Google Scholar]
- 8.Tamboli P, Amin MB, Xu HJ, Linden MD. Inmunohistochemical expression of retinoblastoma and p53 tumor supressors genes in prostatic intraepitelial neoplasia: comparison with prostatic adenocarcinoma and benign prostate. Mod Pathol. 1998;11:247–252. [PubMed] [Google Scholar]
- 9.Mori R, Dorff TB, Xiong S, et al. The relationship between proangiogenic gene expression levels in prostate cancer and their prognostic value for clinical outcomes. Prostate. 2010;70:1692–1700. doi: 10.1002/pros.21204. [DOI] [PubMed] [Google Scholar]
- 10.Pallares J, Rojo F, Iriarte J, Morote J, Armadans LI, de Torres I. Study of microvessel density and the expression of the angiogenic factors VEGF, bFGF and the receptors Flt-1 and FLK-1 in benign, premalignant and malignant prostate tissues. Histol Histopathol. 2006;21:857–865. doi: 10.14670/HH-21.857. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi H, Shibuya M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin Sci (Lond) 2005;109:227–241. doi: 10.1042/CS20040370. [DOI] [PubMed] [Google Scholar]
- 12.Gille H, Kowalski J, Li B, et al. Analysis of biological effects and signaling properties of Flt-1 (VEGFR-1) and KDR (VEGFR-2). A reassessment using novel receptor-specific vascular endothelial growth factor mutants. J Biol Chem. 2001;276:3222–3230. doi: 10.1074/jbc.M002016200. [DOI] [PubMed] [Google Scholar]
- 13.Gerhardt H, Golding M, Fruttiger M, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu L, Li X, Ye L, et al. Vascular endothelial growth inhibitor 174 is a negative regulator of aggressiveness and microvascular density in human clear cell renal cell carcinoma. Anticancer Res. 2014;34:715–722. [PubMed] [Google Scholar]
- 15.Dallas NA, Samuel S, Xia L, Fan F, Gray MJ, Lim SJ, Ellis LM. Endoglin (CD105): a marker of tumor vasculature and potential target for therapy. Clin Cancer Res. 2008;14:1931–1937. doi: 10.1158/1078-0432.CCR-07-4478. [DOI] [PubMed] [Google Scholar]
- 16.El-Gohary YM, Silverman JF, Olson PR, et al. Endoglin (CD105) and vascular endothelial growth factor as prognostic markers in prostatic adenocarcinoma. Am J Clin Pathol. 2007;127:572–579. doi: 10.1309/X6NXYE57DLUE2NQ8. [DOI] [PubMed] [Google Scholar]
- 17.Rosen LS, Hurwitz HI, Wong MK, et al. A phase I first-in-human study of TRC105 (anti-endoglin antibody) in patients with advanced cancer. Clin Cancer Res. 2012;18:4820–4829. doi: 10.1158/1078-0432.CCR-12-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis DW, Inoue K, Dinney CP, Hicklin DJ, Abbruzzese JL, McConkey DJ. Regional effects of an antivascular endothelial growth factor receptor monoclonal antibody on receptor phosphorylation and apoptosis in human 253J B-V bladder cancer xenografts. Cancer Res. 2004;64:4601–4610. doi: 10.1158/0008-5472.CAN-2879-2. [DOI] [PubMed] [Google Scholar]
- 19.Romero D, O'Neill C, Terzic A, et al. Endoglin regulates cancer-stromal cell interactions in prostate tumors. Cancer Res. 2011;71:3482–3493. doi: 10.1158/0008-5472.CAN-10-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis-correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 21.Mazzucchelli R, Montironi R, Santinelli A, Lucarini G, Pugnaloni A, Biagini G. Vascular endothelial growth factor expression and capillary architecture in high-grade PIN and prostate cancer in untreated and androgen-ablated patients. Prostate. 2000;45:72–79. doi: 10.1002/1097-0045(20000915)45:1<72::aid-pros9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 22.Memis A, Ozden C, Ozdal OL, Guzel O, Han O, Seckin S. Effect of finasteride treatment on suburethral prostatic microvessel density in patients with hematuria related to benign prostate hyperplasia. Urol Int. 2008;80:177–180. doi: 10.1159/000112610. [DOI] [PubMed] [Google Scholar]
- 23.Karzai FH, Apolo AB, Cao L, et al. A phase I study of TRC105 anti-endoglin (CD105) antibody in metastatic castration-resistant prostate cancer. BJU Int. 2015;116:546–555. doi: 10.1111/bju.12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qi WX, Fu S, Zhang Q, Guo XM. Efficacy and toxicity of anti-VEGF agents in patients with castration-resistant prostate cancer: a meta-analysis of prospective clinical studies. Asian Pac J Cancer Prev. 2014;15:8177–8182. doi: 10.7314/apjcp.2014.15.19.8177. [DOI] [PubMed] [Google Scholar]
- 25.Hrouda D, Nicol DL, Gardiner RA. The role of angiogenesis in prostate development and the pathogenesis of prostate cancer. Urol Res. 2003;30:347–355. doi: 10.1007/s00240-002-0287-9. [DOI] [PubMed] [Google Scholar]
- 26.Pallares J, Rojo F, Iriarte J, Morote J, Armadans LI, de Torres I. Study of microvessel density and the expression of the angiogenic factors VEGF, bFGF and the receptors Flt-1 and FLK-1 in benign, premalignant and malignant prostate tissues. Histol Histopathol. 2006;21:857–865. doi: 10.14670/HH-21.857. [DOI] [PubMed] [Google Scholar]
- 27.Mazzucchelli R, Montironi R, Santinelli A, et al. Vascular endothelial growth factor expression and capillary architecture in high-grade PIN and prostate cancer in untreated and androgen-ablated patients. Prostate. 2000;45:72–79. doi: 10.1002/1097-0045(20000915)45:1<72::aid-pros9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 28.Woollard DJ, Opeskin K, Coso S, Wu D, Baldwin ME, Williams ED. Differential expression of VEGF ligands and receptors in prostate cancer. Prostate. 2013;73:563–572. doi: 10.1002/pros.22596. [DOI] [PubMed] [Google Scholar]
- 29.Nordby Y, Andersen S, Richardsen E, et al. Stromal expression of VEGF-A and VEGFR-2 in prostate tissue is associated with biochemical and clinical recurrence after radical prostatectomy. Prostate. 2015;75:1682–1693. doi: 10.1002/pros.23048. [DOI] [PubMed] [Google Scholar]
- 30.Kuniyasu H, Troncoso P, Johnston D, et al. Relative expression of type IV collagenase, E-cadherin, and vascular endothelial growth factor/vascular permeability factor in prostatectomy specimens distinguishes organ-confined from pathologically advanced prostate cancers. Clin Cancer Res. 2000;6:2295–2308. [PubMed] [Google Scholar]
- 31.Mori R, Dorff TB, Xiong S, et al. The relationship between proangiogenic gene expression levels in prostate cancer and their prognostic value for clinical outcomes. Prostate. 2010;70:1692–1700. doi: 10.1002/pros.21204. [DOI] [PubMed] [Google Scholar]
- 32.Liu ZQ, Fang JM, Xiao YY, et al. Prognostic role of vascular endothelial growth factor in prostate cancer: a systematic review and meta-analysis. Int J Clin Exp Med. 2015;8:2289–2298. [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson MW, Roberts JS, Heckford SE, et al. A potential autocrine role for vascular endothelial growth factor in prostate cancer. Cancer Res. 2002;62:854–859. [PubMed] [Google Scholar]
- 34.Dias S, Hattori K, Heissig B, et al. Inhibition of both paracrine and autocrine VEGF/ VEGFR-2 signaling pathways is essential to induce long-term remission of xenotransplanted human leukemias. Proc Natl Acad Sci USA. 2001;98:10857–10862. doi: 10.1073/pnas.191117498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fonsatti E, Altomonte M, Nicotra MR, Natali PG, Maio M. Endoglin (CD105): a powerful therapeutic target on tumor-associated angiogenetic blood vessels. Oncogene. 2003;22:6557–6563. doi: 10.1038/sj.onc.1206813. [DOI] [PubMed] [Google Scholar]
- 36.Miyata Y, Ohba K, Matsuo T, et al. Tumor-associated stromal cells expressing E-prostanoid 2 or 3 receptors in prostate cancer: Correlation with tumor aggressiveness and outcome by angiogenesis and lymphangiogenesis. Urology. 2013;81:136–142. doi: 10.1016/j.urology.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 37.Miyata Y, Mitsunari K, Asai A, Takehara K, Mochizuki Y, Sakai H. Pathological significance and prognostic role of microvessel density, evaluated using CD31, CD34, and CD105 in prostate cancer patients after radical prostatectomy with neoadjuvant therapy. Prostate. 2015;75:84–91. doi: 10.1002/pros.22894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bigler SA, Deering RE, Brawer MK. Comparison of microscopic vascularity in benign and malignant prostate tissue. Hum Pathol. 1993;24:220–226. doi: 10.1016/0046-8177(93)90304-y. [DOI] [PubMed] [Google Scholar]
- 39.Karzai FH, Apolo AB, Cao L, et al. A phase I study of TRC105 anti-CD105 (endoglin) antibody. BJU Int. 2014;116:546–555. doi: 10.1111/bju.12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fay AP, Bellmunt J. Is angiogenesis still an attractive target in metastatic castration-resistant prostate cancer? BJU Int. 2015;116:500–501. doi: 10.1111/bju.13070. [DOI] [PubMed] [Google Scholar]