Abstract
Introduction
Multiparametric-MRI (mp-MRI) is an evolving noninvasive imaging modality that increases the accurate localization of prostate cancer at the time of MRI targeted biopsy, thereby enhancing clinical risk assessment, and improving the ability to appropriately counsel patients regarding therapy.
Material and methods
We used MEDLINE/PubMed to conduct a comprehensive search of the English medical literature. Articles were reviewed, data was extracted, analyzed, and summarized. In this review, we discuss the mp-MRI prostate exam, its role in targeted prostate biopsy, along with clinical applications and outcomes of MRI targeted biopsies.
Results
Mp-MRI, consisting of T2-weighted imaging, diffusion-weighted imaging, dynamic contrast-enhanced imaging, and possibly MR spectroscopy, has demonstrated improved specificity in prostate cancer detection as compared to conventional T2-weighted images alone. An MRI suspicion score has been developed and is depicted using an institutional Likert or, more recently, a standardized reporting scale (PI-RADS). Techniques of MRI-targeted biopsy include in-gantry MRI guided biopsy, TRUS-guided visual estimation biopsy, and software co-registered MRI-US guided biopsy (MRI-US fusion). Among men with no previous biopsy, MRI-US fusion biopsy demonstrates up to a 20% increase in detection of clinically significant cancers compared to systematic biopsy while avoiding a significant portion of low risk disease. These data suggest a potential role in reducing over-detection and, ultimately, over-treatment. Among men with previous negative biopsy, 72–87% of cancers detected by MRI targeted biopsy are clinically significant. Among men with known low risk cancer, repeat biopsy by MR-targeting improves risk stratification in selecting men appropriate for active surveillance secondarily reducing the need for repetitive biopsy during surveillance.
Conclusions
Use of mp-MRI for targeting prostate biopsies has the potential to reduce the sampling error associated with conventional biopsy by providing better disease localization and sampling. MRI-ultrasound fusion-targeted prostate biopsy may improve the identification of clinically significant prostate cancer while limiting detection of indolent disease, ultimately facilitating more accurate risk stratification. Literature supports the clinical applications of MRI-targeted biopsy in men who have never been biopsied before, those with a prior negative biopsy, and those with low risk disease considering active surveillance.
Keywords: multiparametric-MRI, targeted biopsy, MRI-targeted, image-guided, MRI-ultrasound fusion, prostate cancer, prostate biopsy
INTRODUCTION
Advances in multiparametric magnetic resonance imaging (mp-MRI) have demonstrated an improvement in detection and characterization of clinically significant prostate cancer [1]. Mp-MRI combines diffusion-weighted and, commonly, dynamic contrast-enhanced sequences, with conventional T2-weighted sequences. Introducing mp-MRI and MRI targeted biopsy as modalities to evaluate men at risk for prostate cancer may aid in better determining which men need a prostate biopsy and improve sampling in the performance of the biopsy, thereby allowing greater detection of clinically significant disease with fewer biopsy cores, more accurate risk stratification, and avoidance of the detection of indolent disease [2, 3]. MRI targeted biopsy may also allow for better risk stratification among men who are considering active surveillance. In this review we discuss limitations of the current prostate biopsy standard, components of the mp-MRI exam, techniques of MRI targeted biopsy, along with clinical applications and outcomes.
Current standard for prostate biopsy and limitations
The contemporary random 12-core systematic biopsy strategy relies on sampling efficiency for cancer detection and, as a result, is subject to sampling error. Although laterally directed cores within the peripheral zone increase detection, prostate cancers are frequently multifocal, small, intermingled with benign stroma, and not uniformly distributed within the gland [4]. Consequently, clinically significant cancers frequently go undetected when employing the standard 12-core biopsy template. Under-sampling of the cancer during conventional TRUS-guided biopsy also leads to incorrect risk stratification of clinically significant tumors as low volume or low grade. Nearly three quarters of men over the age of 50 years harbor clinically insignificant prostate cancer at autopsy. These clinically insignificant cancers are often identified by chance during a systematic biopsy, contributing, in part, to the problem of over-detection and over-treatment of indolent prostate cancer. Repeat biopsy, performed to overcome sampling error, further increases the detection of clinically insignificant prostate cancer. The recent trend of overcoming sampling error through increasing core number at the time of biopsy, also further escalates the risk of identifying small, indolent cancers which may have little to do with the patient's PSA elevation [4].
Components of a multiparametric prostate MRI exam
T2-Weighted imaging
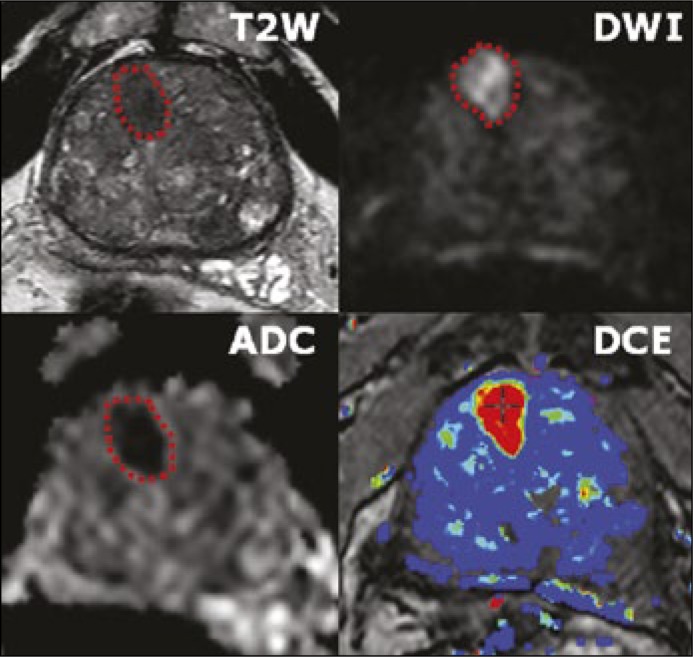
T2-weighted MR images, reflecting tissue water content, have high spatial resolution and clearly define the prostate's zonal anatomy, distinguishing the peripheral zone (high signal intensity) from the central zone (surrounding the ejaculatory ducts in the posterior prostate base and exhibiting decreased T2 signal intensity) and transition zones (surrounding the urethra, extending anteriorly and superiorly from the level of the verumontanum, and exhibiting heterogeneous, often swirled, signal-intensity) (Figure 1) [5]. Furthermore, this sequence aids tumor staging by facilitating evaluation of extra-prostatic extension, seminal vesicle invasion, and neurovascular bundle invasion by the tumor. The sensitivity of MRI in detecting extra-prostatic extension or seminal vesicle invasion has improved to a range of 73 to 80% with a high specificity of 97–100%. Extra-prostatic extension as small as 0.5 mm at histopathology has been accurately detected [6]. The peripheral zone of the prostate usually exhibits high signals on T2-weighted images. In contrast, peripheral zone tumors generally appear as round or oval foci of decreased signal using this sequence. The degree of intensity decrease differs with the Gleason score, with higher Gleason score components showing lower signal intensities [7]. T2-weighted imaging alone results in false positive findings, as low signal intensity can also be the consequence of benign abnormalities including acute and chronic prostatitis, atrophy, scars, post-irradiation or hormonal treatment effects, hyperplasia, and post-biopsy hemorrhage. Because post-biopsy changes can mimic prostate cancer on T2WI, it is generally recommended to delay mp-MRI for at least 8 to 12 weeks after biopsy [8]. Furthermore, low-grade tumors may be isointense to the high signal peripheral zone and therefore not well visualized using T2-weighted imaging. Cancer in the transition zone may be more difficult to discern than in the peripheral zone partly due to the heterogeneous appearance of BPH with areas of both increased and decreased signal intensity. In a recent meta-analysis, Tan et al. investigated the diagnostic performance of T2-weighted images alone in prostate tumor localization, demonstrating a sensitivity and specificity of 0.57–0.62 and 0.74–0.78, respectively [9]. These moderate performance characteristics highlight the need for use of additional sequences to achieve accurate tumor detection and localization.
Figure 1.
Multiparametric MRI imaging incorporates T2-weighted, diffusion-weighted, and dynamic contrast-enhanced imaging to identify areas in the prostate suspicious for cancer based on anatomic and functional characteristics.
Diffusion-weighted imaging
Diffusion-weighted imaging (DWI) assesses the diffusion of water molecules within different tissues (Figure 1). Normal prostate glandular tissue has a higher water diffusion rate than cancer tissue owing to restricted diffusion in tightly packed cancer cells. Changing the amplitude of the diffusion-weighting gradients varies the degree of diffusion weighting, referred to as a ‘b-value’, for a given DWI sequence. At low b-values, DWI predominantly reflects water movement over larger length scales, mainly secondary to capillary perfusion, whereas at higher b-values, DWI interrogates movement over small length scales, such as within cells or intracellular organelles [10, 11]. There is a debate regarding the most appropriate b value to be used for DWI of the prostate. Historically, b values up to 800–1000 s/mm2 were used. However, recent studies report improved lesion detection using even higher b values of up to 2000 s/mm2 [12]. In addition, the actual diffusion coefficient of water cannot be directly measured by MRI. Rather diffusion-weighted images must be acquired using at least two different b values, from which a diffusion coefficient can be calculated. The most widely applied coefficient in clinical practice is the apparent diffusion coefficient (ADC). The ADC map better reflects the diffusivity of water molecules than the directly acquired trace b value images themselves, which in fact reflect a combination of both diffusion weighting and T2 weighting (Figure 1). Prostate tumors generally demonstrate increased signal on high b value DWI as well as decreased ADC, given the association between tumor cellularity and restricted diffusion. ADC values derived from DWI images are inversely correlated with the Gleason score of lesions at biopsy or surgery [13] however, the confidence intervals are widely overlapping, limiting the ability to use ADC as a surrogate to the Gleason score. The value of DWI for prostate cancer imaging has been established in numerous publications in recent years [14].
Perfusion imaging
During dynamic contrast-enhanced (DCE) MRI, a bolus of intravenous contrast medium (gadolinium) is injected and serial, rapid sequences are obtained (Figure 1). The objective is to demonstrate the increased enhancement of the prostate cancer compared with normal prostatic tissue, which correlates with tumor angiogenesis. Given the serial rapid imaging of the prostate, DCE-MRI allows assessment of contrast kinetics within focal lesions. Prostate tumors release factors that promote vessel formation and capillary permeability, thereby leading to more rapid enhancement than surrounding normal tissue [15]. Prostate cancer typically enhances faster and to a greater extent than surrounding prostate, and will also show a more rapid washout of contrast in a fraction of cases. Typically, images are acquired sequentially at a rate of at least every 10 seconds and preferably <7 seconds and for a total duration of at least 2 minutes [15, 16]. Even though prostatitis-related enhancement is usually diffuse and non-focal in nature, and BPH-related enhancement is often well-encapsulated and spherical, the non-specific nature of these patterns limits the utility of DCE findings in isolation, resulting in DCE often being applied largely as an adjunct to interpretations based primarily on findings on T2WI and DWI.
Multiparametric-MRI
Although individual imaging sequences have utility in the detection of prostate cancer, results are optimized by multiparametric (mp) MRI, which combines all of the sequences in an integrated fashion to improve specificity (Figure 1). Mp-MRI offers superior diagnostic power for prostate cancer detection and can assist risk stratification based on lesion size, extent and ADC value [17]. In one study, mp-MRI sensitivity exceeded 80% for detecting 0.2 cm3 of Gleason 4 + 3 or above and 0.5 cm3 of ≥ Gleason 3 + 4 [18]. Adding DCE and/or DW imaging to T2-weighted MRI has been demonstrated to significantly improve sensitivity from 63% to 79–81% in the peripheral zone, while maintaining a stable specificity [19]. A recent pooled data meta-analysis showed a specificity of 88%, sensitivity of 74%, with a negative predictive value of 65% to 94% when employing mp-MRI to detect prostate cancer [20]. Furthermore, additional studies have highlighted the combined use of DW, DCE, and T2-weighted imaging to increase accuracy in detection of transition zone cancer compared to T2WI alone, from 64% to 79% [21]. Mp-MRI has also shown to have greater accuracy in detection of recurrent prostate cancer after radiation [22]. Nevertheless, given moderate specificity, mp-MRI findings require biopsy to confirm the presence of tumor and assess Gleason score [17].
Multiparametric-MRI prostate lesion scoring system
With the advent of mp-MRI as a tool to identify suspicious areas within the prostate, many grading schemes have evolved in order to assess the likelihood of cancer based on image characteristics. The Prostate Imaging Reporting and Data System (PI-RADS), recently updated to PI-RADS version 2.0, is a reporting scheme based on specific features of T2-weighted, DWI, and DCE sequences aimed to standardize prostate MRI reporting and grade suspicious regions of the prostate on a reproducible scale to represent their likelihood of harboring cancer foci (Table 1) [16, 23]. For interpretation, PI-RADS provides explicit criteria for assigning a region of interest (ROI) a score from 1 to 5 for each sequence (T2WI, DWI, and DCE). These scores reflect the ROI's degree of abnormality on each particular sequence. An overall score from 1 to 5 is also assigned, reflecting the likelihood that the ROI reflects a clinically significant cancer. The prostate is divided into discrete anatomic regions (either 16 or 27 regions, based on models recommended by the ESUR/PI-RADS guidelines), and any identified individual lesions are assigned to these various regions.
Table 1.
PI-RADS 2.0 scoring criteria
| Assessment category | Peripheral zone | Transition zone | ||||
|---|---|---|---|---|---|---|
| DWI | T2W | DCE | DWI | T2W | DCE | |
| 1 | 1 | Any | Any | Any | 1 | Any |
| 2 | 2 | Any | Any | Any | 2 | Any |
| 3 | 3 | Any | (-) | ≤ 4 | 3 | Any |
| 4 | 3 | Any | (+) | 5 | 3 | Any |
| 4 | Any | Any | Any | 4 | Any | |
| 5 | 5 | Any | Any | Any | 5 | Any |
Adapted from: American College of Radiology. MR Prostate Imaging Reporting and Data System version 2.0. Accessed June 2015, from http://www.acr.org/Quality-Safety/Resources/PIRADS/
Studies evaluating PI-RADS criteria through targeted biopsy have shown strong predictive ability in identifying the likelihood of prostate cancer, as well as correlation with Gleason score [24, 25, 26]. Despite this, several institutions reporting outcomes of targeted biopsy have alternatively used Likert scales to assess pre-biopsy disease risk [2, 27]. While heterogeneous, most have generally been shown to predict the overall likelihood of cancer and likelihood of high-grade disease. Meng et al. demonstrated a significant stepwise increase in risk of Gleason ≥7 disease with increasing MRI suspicion score on a 5-point Likert scale [3]. Salami et al. compared the predictive capability of mp-MRI scored on a Likert scale to the Prostate Cancer Prevention Trial risk calculator, and demonstrated a higher area under the curve for mp-MRI compared to the risk calculator [28]. Several studies have also directly compared PI-RADS scoring of lesions to Likert scale scoring of the same mp-MRI studies, showing no difference in performance [29, 30, 31]. The recent release of PI-RADS version 2.0 gives criteria for devising an overall score from 1–5 based on findings from the different sequences, thereby helping reproducibility in terms of overall suspicion scores, and may encourage more widespread adoption of this standardized reporting. Moreover, it has been recently shown that the second version of PI-RADS is only moderately reproducible [32]. On average, it shows good correlation with histopathologic results and high sensitivity for clinically significant disease, but specificity is low. In a recent review, no recommendations regarding which PI-RADS suspicion score lesions require biopsy and which can be observed could be provided because of heterogeneity of the studies reported throughout the literature [26]. PI-RADS will continue to evolve as more experience is gained.
Technique of MRI targeted biopsy
Currently three techniques of MRI guidance are available for targeted prostate biopsy: visual estimation TRUS-guided biopsy (also referred to as cognitive fusion), in-bore MRI guided biopsy, and software based co-registration guided biopsy with MRI to ultrasound fusion. Each method possesses its own advantages and disadvantages but to date, no prospective comparison of the three methods has been made. Visual estimation TRUS-guided biopsy, in which the ultrasound operator simply aims the biopsy needle at the prostate area where the previously reviewed MRI demonstrates a lesion, allows rapid adaption of MRI-targeted biopsy into clinical practice and requires no additional equipment beyond the MRI and a conventional transrectal ultrasound. The technique does carry a learning curve in that there is no real-time feedback regarding needle placement accuracy and the biopsy is prone to human error without actual image overlay. The effectiveness of visual estimation targeted biopsy in detecting prostate cancer varies between studies, likely due to investigator experience and variable practices in imaging approach. However, a prospective study by Wysock et al. comparing MRI-US fusion versus visual estimation demonstrated that fusion biopsy was more often histologically informative than visual targeting, revealing more nonbenign pathology than, but did not increase cancer detection [33]. A trend toward increased detection with fusion biopsy was observed across all study subsets.
In bore MRI-guided biopsy is performed within the MRI gantry, by a radiologist, who plans the biopsy based upon an acquired MRI and confirms biopsy needle localization under repetitive MRI sequences. Typically only a few targeted cores are taken and systematic sampling is not readily performed, leaving normal appearing prostate tissue unsampled. The advantages of this method are fewer sampled cores, visual feedback regarding the accuracy of needle placement, and, in theory, the reduced detection of insignificant tumors. However, the biopsy requires a longer procedure time (1–2 hours), has a high cost, and is resource-intensive in that it requires prolonged access to a MRI scanner.
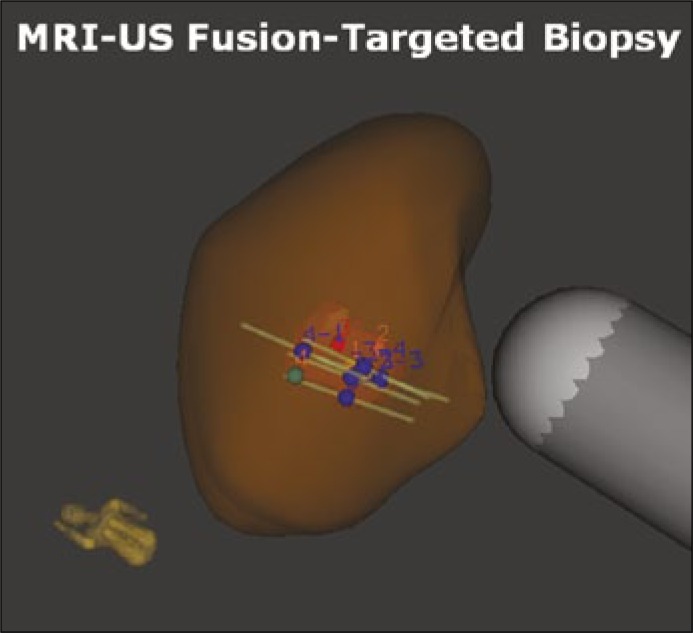
Software co-registered MRI targeted TRUS biopsy allows the operator to image the prostate using ultrasound, while a previously performed and annotated MRI is fused with the real-time ultrasound using a digital overlay, creating a three-dimensional reconstruction of the prostate, on which the previously marked ROI are identified. Spatial tracking of the ultrasound probe through mechanical or electromagnetic means allows accurate placement of the needle guide relative to the three-dimensional reconstruction (Figure 2). MRI-US-fusion biopsy potentially has greater reproducibility due to less operator dependence by providing real time feedback of actual biopsied locations. The disadvantages include the cost of an additional device and the requirement for specialized operator training.
Figure 2.
3D reconstructed prostate model showing suspicious area identified on mpMRI, allowing targeted sampling of abnormalities in the prostate.
Hardware and software platforms for MRI-US fusion
A number of commercial platforms have become available for MRI-US-fusion. These applications vary by method of co-registration (mechanical, electromagnetic, or real-time) and utilize different hardware platforms for spatial tracking of the probe within the co-registered image (Table 2). The general workflow of all platforms first requires segmentation of a pre-biopsy diagnostic MRI in axial and sagittal frames to allow construction of the 3-dimensional model. Each suspicious ROI is likewise segmented and annotated within the model. Then, depending on the particular platform, targets are delineated before or after MRI data have been loaded onto the software platform. The co-registration of the MRI to the US image can be performed either by rigid, elastic, or both methods, depending on the platform. Rigid fusion involves overlay of images allowing translation and rotation without change to the images themselves. Elastic fusion accounts for the addition of local deformation by stretching the image volumes and targeted lesion(s) resulting in matched borders [34]. TRUS-guided biopsy of the prostate is performed and MRI and real-time TRUS images are superimposed and displayed side-by-side, thus creating an easily navigable three-dimensional prostate reconstruction. Because MRI and US images have been co-localized and co-registered, they allow blending back and forth between MRI and TRUS.
Table 2.
Summary of MRI-US fusion platforms
| System trade name (manufacturer) | US image acquisition | Image registration | Biopsy route | Tracking mechanism |
|---|---|---|---|---|
| UroNav (Philips, In Vivo) | Manual sweep from base to apex | Rigid | Transrectal | Electromagnetic tracking |
| Artemis (Eigen) | Manual rotation along fixed axis | Rigid and elastic | Transrectal | Mechanical arm with encoders |
| Urostation (Koelis) | Automatic probe rotation | Elastic | Transrectal | Image-based (TRUS-TRUS) registration |
| HI-RVS (Hitachi) | Real-time biplanar TRUS | Rigid | Transrectal or transperineal | External magnetic field generator |
| BioJet (DK Technologies) | Manual sweep | Rigid | Transrectal or transperineal | Mechanical arm with encoders, stepper |
| BiopSee (MedCom) | Manual sweep with biplanar probe | Manual, marker based, or automatic | Transperineal | Stepper with encoders |
Clinical applications
No previous biopsy
In men presenting for first biopsy, the potential advantages of MRI-targeted biopsy are twofold: improving detection of high-grade cancer by reducing the false-negative rate of biopsy, and avoiding detection of low-grade disease by selectively targeting tumor foci which are more likely to be clinically significant. Several studies reporting outcomes of combined MRI-targeted biopsy and systematic biopsy among biopsy naive men have suggested the potential to achieve these goals using mp-MRI in the primary biopsy setting (Table 3) [35, 39]. Delongchamps et al. compared outcomes of targeted and systematic biopsy among 391 men presenting for first biopsy. The investigators reported improved detection of high-grade cancer using targeted biopsy, which missed only 2/63 (3%) Gleason ≥7 cancers detected by systematic biopsy while detecting an additional 17 high grade cancers [36, 37]. Additionally, 39 Gleason 6 cancers identified on systematic biopsy were avoided by targeted biopsy. Pokorny et al. similarly observed that MRI-targeted biopsy increased the detection of intermediate/high-risk disease by 18% and decreased the diagnosis of low-risk cancer by 89% in a prospective trial of 223 men [38]. Mendhiratta et al. observed a 15% increase in Gleason score ≥7 cancers by MRI-US fusion targeted biopsy as compared to systematic biopsy among 382 consecutive biopsy naive men [39]. Additionally, the majority of cancers missed by targeted biopsy were clinically insignificant by Epstein (62%) and UCSF-CAPRA (82%) criteria, suggesting that systematic biopsy largely contributes to the detection of low-risk disease among biopsy naive men undergoing both targeted and systematic biopsies [39].
Table 3.
Summary of trials of MRI-US fusion-targeted biopsy compared to systematic biopsy
| Investigators | Study size | Biopsy history | TB technique | SB technique | Definition of clinically significant cancer |
Overall CDR (TB) | Overall CDR (SB) | Clinically significant CDR (TB) |
Clinically significant CDR (SB) |
|---|---|---|---|---|---|---|---|---|---|
| Meng et al. [3] | 601 | 49% BN 29% PNB 22% AS |
Transrectal | 12-core TRUS | GS ≥3+4 | 39% | 40% | 26% | 19% |
| Mendhiratta et el. [39] | 382 | 100% BN | Transrectal | 12-core TRUS | GS ≥3+4 | 24% | 18% | 16% | 9% |
| Mozer et al. [63] | 152 | 100% BN | Transrectal | 12-core TRUS | CCL ≥4 mm or GS ≥3+4 | 54% | 57% | 43% | 37% |
| Salami et al. [42] | 140 | 100% BN | Transrectal | 12-core TRUS | Epstein criteria [64] | 52% | 49% | 48% | 31% |
| Siddiqui et al. [65] | 1003 | 20% BN 43% PNB 37% AS |
Transrectal | 12-core TRUS | GS ≥4+3 | 46% | 47% | 17% | 12% |
| Sonn et al. [66] | 171 | 38% PNB 62% AS |
Transrectal | 12-core TRUS | GS ≥3+4 | 35% | 44% | 13% | 12% |
| Rastinehad et al. [67] | 105 | 33% BN 67% PNB |
Transrectal | 12-core TRUS | Epstein criteria [64] | 51% | 49% | 45% | 32% |
| Wysock et al. [27] | 125 | 54% BN 27% PNB 19% AS |
Transrectal | 12-core TRUS | GS ≥3+4 | 36% | NR | 23% | NR |
| Kuru et al. [68] | 347 | 51% BN 49% PNB |
Transperineal | 24-core Transperineal | NCCN criteria (intermediate or high risk) | 51% | 50% | 41% | 38% |
| Delongchamps et al. [36] (Rigid fusion) |
131 | 100% BN | Transrectal | 12-core TRUS | CCL ≥ 4 mm or GS ≥ 3+4 | 49% | 46% | NR | NR |
| Delongchamps et al. [36] (Elastic fusion) |
133 | 100% BN | Transrectal | 12-core TRUS | CCL ≥ 4 mm or GS ≥ 3+4 | 47% | 33% | NR | NR |
| Fiard et al. [69] | 30 | 43% BN 57% PNB |
Transrectal | 12-core TRUS | ≥ 10 mm cancer or GS ≥ 3+4 | 55% | 43% | 50% | 33% |
| Sonn et al. [41] | 105 | 100% PNB | Transrectal | 12-core TRUS | CCL ≥ 4 mm or GS ≥ 3+4 | 24% | 28% | 22% | 15% |
| Rud et al. [70] | 90 | 12% BN 88% Repeat* |
Transrectal | 12-core TRUS | GS ≥ 3+4 | 68% (54/80) |
14% (6/42) |
46% (37/80) |
5% (2/42) |
TB – MR-targeted biopsy; SB: systematic biopsy; BN – biopsy naive; PNB – prior negative biopsy; AS: active surveillance; CCL – cancer-core length; GS – Gleason score
62/90 (69%) men presented for repeat biopsy without prior treatment for cancer, while 17/90 (19%) men had biochemical recurrence after radiation therapy for previously diagnosed cancer.
Prior negative biopsy
Men with prior negative biopsies often present for repeat biopsy as a consequence of the limited negative predictive value of a negative systematic biopsy [40]. Among this population of men, the advantage of MRI-targeted biopsy is in identifying areas of suspicion within the prostate which would otherwise be missed by repeat systematic sampling, as several recent series have demonstrated (Table 3) [41, 42, 43]. Sonn et al. observed that targeted biopsy detected more clinically significant cancers and fewer clinically insignificant cancers than systematic biopsy in a series of 105 consecutive men [41]. Among 140 men, Salami et al. observed higher detection rates of clinically significant cancer by targeted biopsy compared to systematic biopsy (48% vs. 31%), and additionally noted that only 3.5% of clinically significant cancers would have been missed by targeted biopsy alone in this cohort. In a study of 161 men with a prior negative biopsy, Mendhiratta et al. found MRI fusion targeted biopsy to detect more Gleason score ≥7 disease (14.9% compared to systematic (9.35, p = 0.02) [44]. Using UCSF-CAPRA criteria, only one man was re-stratified from low-risk to higher risk based on systematic results compared to MRI fusion targeted biopsy alone. In light of evidence suggesting a clinical benefit of targeted biopsy for men with prior negative biopsies, the NCCN guidelines for prostate cancer detection have suggested that men with two or more prior negative biopsies who present for repeat biopsy should undergo pre-biopsy mp-MRI to identify areas of occult disease [45].
Prior positive biopsy
Prostate mp-MRI may have two applications in men with a prior positive prostate biopsy. First, it improves risk stratification for selecting appropriate candidates for active surveillance (AS) by allowing the identification and targeting of MRI lesions with biopsy, ultimately ruling out clinically significant disease. Secondly, it reduces the need for repetitive biopsy through non-invasive serial monitoring for those on active surveillance.
Including mp-MRI into disease characterization strategies improves risk stratification by identifying disease typically missed on standard biopsy (Table 3) [1, 46, 47]. The presence of mp-MRI lesions significantly increased the probability of Gleason score upgrade on repeat systematic biopsy [48, 49]. Furthermore, several series report disease reclassification rates of 17–36% when mp-MRI with targeted biopsy is utilized for surveillance biopsy on men enrolled on active surveillance by prior Epstein criteria [48, 50, 51, 52, 53]. Findings on mp-MRI predicting disease reclassification include number of lesions, MRI suspicion score and lesion density [53, 54]. Stamatakis et al. have published a nomogram predicting probability of meeting AS criteria based upon MRI characteristics [53]. It has also been shown that MRI may reasonably decrease the number of repeat biopsies in patients on active surveillance by as much as 68% [55]. Conversely, disease reclassification for men on AS with normal mp-MRI appears to be very low with negative predictive value ranges from 81–90% [49, 51, 56]. These findings provide compelling rationale for the incorporation of mp-MRI and targeted biopsy into active surveillance selection criteria.
Observable changes in disease characteristics on mp-MRI would offer a novel, non-invasive method for following disease progression. Early data regarding serial imaging of lesions report that 45.4% of men with lesions at baseline will demonstrate progression on imaging (increase in lesion volume or conspicuity; defined as DWI of lesion/DWI of normal prostate). In contrast, only 17% of those without visible lesions demonstrated radiographic progression [57]. Another study reported a similar finding of three times overall risk of cancer progression for men with abnormal findings on mp-MRI [58]. The time interval for changes in mp-MRI was evaluated in a cohort of men with small index lesions (≤7 or ≤5 mm). No change in size was noted over a period of approximately 2 years [59]. Data from the MAPPED study may provide additional insight into temporal changes in MRI findings [60]. Finally, early data on serial mp-MRI with targeted biopsy amongst men on AS demonstrated a negative predictive value of 80% for stable MRI findings [61]. MRI-US fusion biopsy techniques allow for disease mapping and provide templates for repeat biopsy both in locations of disease diagnosed with targeted biopsy but also with standard biopsy. Initial data regarding repeat fusion biopsy of mp-MRI targets demonstrated increased cancer detection when compared to standard repeat biopsy [62]. In summary, mp-MRI with targeted biopsy can provide improved risk stratification for selecting appropriate candidates for AS. The impact of mp-MRI and further targeted biopsy for those on active surveillance remains to be determined.
CONCLUSIONS
Use of mp-MRI for targeting prostate biopsies has the potential to reduce the sampling error associated with conventional biopsy by providing better disease localization and sampling. MRI-US targeted prostate biopsy may improve the detection of clinically significant prostate cancer while limiting detection of indolent disease, ultimately increasing accurate risk stratification. Literature supports the clinical applications of MRI-targeted biopsy in men who are biopsy naïve, those with a prior negative biopsy, and those on with low risk disease considering active surveillance.
CONFLICTS OF INTEREST
Samir S. Taneja – consultant Hitachi-Aloka; scientific investigator – Trod, advisory board – Opko, Royalties – Elsevier.
References
- 1.Bjurlin MA, Meng X, Le Nobin J, et al. Optimization of prostate biopsy: the role of magnetic resonance imaging targeted biopsy in detection, localization and risk assessment. J Urol. 2014;192:648–658. doi: 10.1016/j.juro.2014.03.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. Jama. 2015;313:390–397. doi: 10.1001/jama.2014.17942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meng X, Rosenkrantz AB, Mendhiratta N, et al. Relationship Between Prebiopsy Multiparametric Magnetic Resonance Imaging (MRI), Biopsy Indication, and MRI-ultrasound Fusion-targeted Prostate Biopsy Outcomes. Eur Urol. 2015 doi: 10.1016/j.eururo.2015.06.005. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjurlin MA, Carter HB, Schellhammer P, Cookson MS, Gomella LG, Troyer D, et al. Optimization of initial prostate biopsy in clinical practice: sampling, labeling and specimen processing. J Urol. 2013;189:2039–2046. doi: 10.1016/j.juro.2013.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hricak H, Dooms GC, McNeal JE, et al. MR imaging of the prostate gland: normal anatomy. AJR Am J Roentgenol. 1987;148:51–58. doi: 10.2214/ajr.148.1.51. [DOI] [PubMed] [Google Scholar]
- 6.Heijmink SW, Futterer JJ, Hambrock T, et al. Prostate cancer: body-array versus endorectal coil MR imaging at 3 T--comparison of image quality, localization, and staging performance. Radiology. 2007;244:184–195. doi: 10.1148/radiol.2441060425. [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Mazaheri Y, Zhang J, Ishill NM, Kuroiwa K, Hricak H. Assessment of biologic aggressiveness of prostate cancer: correlation of MR signal intensity with Gleason grade after radical prostatectomy. Radiology. 2008;246:168–176. doi: 10.1148/radiol.2461070057. [DOI] [PubMed] [Google Scholar]
- 8.Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22:746–757. doi: 10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan CH, Hobbs BP, Wei W, Kundra V. Dynamic contrast-enhanced MRI for the detection of prostate cancer: meta-analysis. AJR Am J Roentgenol. 2015;204:W439–448. doi: 10.2214/AJR.14.13373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta RT, Kauffman CR, Polascik TJ, Taneja SS, Rosenkrantz AB. The state of prostate MRI in 2013. Oncology. 2013;27:262–270. [PubMed] [Google Scholar]
- 11.Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol. 2007;188:1622–1635. doi: 10.2214/AJR.06.1403. [DOI] [PubMed] [Google Scholar]
- 12.Lim HK, Kim JK, Kim KA, Cho KS. Prostate cancer: apparent diffusion coefficient map with T2-weighted images for detection-a multireader study. Radiology. 2009;250:145–151. doi: 10.1148/radiol.2501080207. [DOI] [PubMed] [Google Scholar]
- 13.De Cobelli F, Ravelli S, Esposito A, et al. Apparent diffusion coefficient value and ratio as noninvasive potential biomarkers to predict prostate cancer grading: comparison with prostate biopsy and radical prostatectomy specimen. AJR American J Roentgenol. 2015;204:550–557. doi: 10.2214/AJR.14.13146. [DOI] [PubMed] [Google Scholar]
- 14.Jie C, Rongbo L, Ping T. The value of diffusion-weighted imaging in the detection of prostate cancer: a meta-analysis. Eur Radiol. 2014;24:1929–1941. doi: 10.1007/s00330-014-3201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verma S, Turkbey B, Muradyan N, et al. Overview of dynamic contrast-enhanced MRI in prostate cancer diagnosis and management. AJR Am J Roentgenol. 2012;198:1277–1288. doi: 10.2214/AJR.12.8510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radiology ACo. PI-RADS™Prostate Imaging and Reporting and Data System v2. http://wwwacrorg/~/media/ACR/Documents/PDF/QualitySafety/Resources/PIRADS/PIRADSV2pdf. 2015.
- 17.Turkbey B, Choyke PL. Multiparametric MRI and prostate cancer diagnosis and risk stratification. Curr Opin Urol. 2012;22:310–315. doi: 10.1097/MOU.0b013e32835481c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villers A, Puech P, Mouton D, Leroy X, Ballereau C, Lemaitre L. Dynamic contrast enhanced, pelvic phased array magnetic resonance imaging of localized prostate cancer for predicting tumor volume: correlation with radical prostatectomy findings. Urol. 2006;176:2432–2437. doi: 10.1016/j.juro.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Delongchamps NB, Rouanne M, Flam T, et al. Multiparametric magnetic resonance imaging for the detection and localization of prostate cancer: combination of T2-weighted, dynamic contrast-enhanced and diffusion-weighted imaging. BJU Int. 2011;107:1411–1418. doi: 10.1111/j.1464-410X.2010.09808.x. [DOI] [PubMed] [Google Scholar]
- 20.de Rooij M, Hamoen EH, Futterer JJ, Barentsz JO, Rovers MM. Accuracy of multiparametric MRI for prostate cancer detection: a meta-analysis. AJR Am J Roentgenol. 2014;202:343–351. doi: 10.2214/AJR.13.11046. [DOI] [PubMed] [Google Scholar]
- 21.Yoshizako T, Wada A, Hayashi T, et al. Usefulness of diffusion-weighted imaging and dynamic contrast-enhanced magnetic resonance imaging in the diagnosis of prostate transition-zone cancer. Acta Radiol. 2008;49:1207–1213. doi: 10.1080/02841850802508959. [DOI] [PubMed] [Google Scholar]
- 22.Donati OF, Jung SI, Vargas HA, et al. Multiparametric prostate MR imaging with T2-weighted, diffusion-weighted, and dynamic contrast-enhanced sequences: are all pulse sequences necessary to detect locally recurrent prostate cancer after radiation therapy? Radiology. 2013;268:440–450. doi: 10.1148/radiol.13122149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22:746–757. doi: 10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tewes S, Hueper K, Hartung D, et al. Targeted MRI/TRUS fusion-guided biopsy in men with previous prostate biopsies using a novel registration software and multiparametric MRI PI-RADS scores: first results. World J Urol. 2015;33:1707–1714. doi: 10.1007/s00345-015-1525-4. [DOI] [PubMed] [Google Scholar]
- 25.Delongchamps NB, Lefevre A, Bouazza N, Beuvon F, Legman P, Cornud F. Detection of significant prostate cancer with magnetic resonance targeted biopsies--should transrectal ultrasound-magnetic resonance imaging fusion guided biopsies alone be a standard of care? J Urol. 2015;193:1198–1204. doi: 10.1016/j.juro.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Hamoen EH, de Rooij M, Witjes JA, Barentsz JO, Rovers MM. Use of the Prostate Imaging Reporting and Data System (PI-RADS) for Prostate Cancer Detection with Multiparametric Magnetic Resonance Imaging: A Diagnostic Meta-analysis. Eur Urol. 2015;67:1112–1121. doi: 10.1016/j.eururo.2014.10.033. [DOI] [PubMed] [Google Scholar]
- 27.Wysock JS, Rosenkrantz AB, Huang WC, et al. A prospective, blinded comparison of magnetic resonance (MR) imaging-ultrasound fusion and visual estimation in the performance of MR-targeted prostate biopsy: The profus trial. Eur Urol. 2014;66:343–551. doi: 10.1016/j.eururo.2013.10.048. [DOI] [PubMed] [Google Scholar]
- 28.Salami SS, Vira MA, Turkbey B, et al. Multiparametric magnetic resonance imaging outperforms the Prostate Cancer Prevention Trial risk calculator in predicting clinically significant prostate cancer. Cancer. 2014;120:2876–2882. doi: 10.1002/cncr.28790. [DOI] [PubMed] [Google Scholar]
- 29.Renard-Penna R, Mozer P, Cornud F, et al. Prostate Imaging Reporting and Data System and Likert Scoring System: Multiparametric MR Imaging Validation Study to Screen Patients for Initial Biopsy. Radiology. 2015;275:458–468. doi: 10.1148/radiol.14140184. [DOI] [PubMed] [Google Scholar]
- 30.Vache T, Bratan F, Mege-Lechevallier F, Roche S, Rabilloud M, Rouviere O. Characterization of prostate lesions as benign or malignant at multiparametric MR imaging: comparison of three scoring systems in patients treated with radical prostatectomy. Radiology. 2014;272:446–455. doi: 10.1148/radiol.14131584. [DOI] [PubMed] [Google Scholar]
- 31.Rosenkrantz AB, Kim S, Lim RP, et al. Prostate cancer localization using multiparametric MR imaging: comparison of Prostate Imaging Reporting and Data System (PI-RADS) and Likert scales. Radiology. 2013;269:482–492. doi: 10.1148/radiol.13122233. [DOI] [PubMed] [Google Scholar]
- 32.Muller BG, Shih JH, et al. Prostate Cancer: Interobserver Agreement and Accuracy with the Revised Prostate Imaging Reporting and Data System at Multiparametric MR Imaging. Radiology. 2015:741–750. doi: 10.1148/radiol.2015142818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wysock JS, Rosenkrantz AB, Huang WC, et al. A Prospective, Blinded Comparison of Magnetic Resonance (MR) Imaging-Ultrasound Fusion and Visual Estimation in the Performance of MR-targeted Prostate Biopsy: The PROFUS Trial. Eur Urol. 2013;66:343–351. doi: 10.1016/j.eururo.2013.10.048. [DOI] [PubMed] [Google Scholar]
- 34.Ukimura O, Desai MM, Palmer S, et al. 3-Dimensional elastic registration system of prostate biopsy location by real-time 3-dimensional transrectal ultrasound guidance with magnetic resonance/transrectal ultrasound image fusion. J Urol. 2012;187:1080–1086. doi: 10.1016/j.juro.2011.10.124. [DOI] [PubMed] [Google Scholar]
- 35.Haffner J, Lemaitre L, Puech P, et al. Role of magnetic resonance imaging before initial biopsy: comparison of magnetic resonance imaging-targeted and systematic biopsy for significant prostate cancer detection. BJU Int. 2011;108:E171–178. doi: 10.1111/j.1464-410X.2011.10112.x. [DOI] [PubMed] [Google Scholar]
- 36.Delongchamps NB, Peyromaure M, Schull A, et al. Prebiopsy magnetic resonance imaging and prostate cancer detection: comparison of random and targeted biopsies. J Urol. 2013;189:493–499. doi: 10.1016/j.juro.2012.08.195. [DOI] [PubMed] [Google Scholar]
- 37.Mozer P, Roupret M, Le Cossec C, et al. First round of targeted biopsies using magnetic resonance imaging/ultrasonography fusion compared with conventional transrectal ultrasonography-guided biopsies for the diagnosis of localised prostate cancer. BJU Int. 2015;115:50–57. doi: 10.1111/bju.12690. [DOI] [PubMed] [Google Scholar]
- 38.Pokorny MR, de Rooij M, Duncan E, et al. Prospective study of diagnostic accuracy comparing prostate cancer detection by transrectal ultrasound-guided biopsy versus magnetic resonance (MR) imaging with subsequent MR-guided biopsy in men without previous prostate biopsies. Eur Urol. 2014;66:22–29. doi: 10.1016/j.eururo.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Mendhiratta N, Rosenkrantz AB, Meng X, et al. Magnetic Resonance Imaging-Ultrasound Fusion-Targeted Prostate Biopsy in a Consecutive Cohort of Men with No Previous Biopsy: Reduction of Over-Detection through Improved Risk Stratification. J Urol. 2015;194:1601–1606. doi: 10.1016/j.juro.2015.06.078. [DOI] [PubMed] [Google Scholar]
- 40.Abraham NE, Mendhiratta N, Taneja SS. Patterns of repeat prostate biopsy in contemporary clinical practice. J Urol. 2015;193:1178–1184. doi: 10.1016/j.juro.2014.10.084. [DOI] [PubMed] [Google Scholar]
- 41.Sonn GA, Chang E, Natarajan S, et al. Value of targeted prostate biopsy using magnetic resonance-ultrasound fusion in men with prior negative biopsy and elevated prostate-specific antigen. Eur Urol. 2014;65:809–815. doi: 10.1016/j.eururo.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salami SS, Ben-Levi E, Yaskiv O, et al. In patients with a previous negative prostate biopsy and a suspicious lesion on magnetic resonance imaging, is a 12-core biopsy still necessary in addition to a targeted biopsy? BJU Int. 2015;115:562–570. doi: 10.1111/bju.12938. [DOI] [PubMed] [Google Scholar]
- 43.Mendhiratta N, Meng X, Rosenkrantz AB, et al. Pre-Biopsy MRI and MRI-Ultrasound Fusion-Targeted Prostate Biopsy in Men with Previous Negative Biopsies: Improved Cancer Detection and Risk Stratification; American Urological Association Annual Meeting; May 17, 2015; New Orleans, LA. [Google Scholar]
- 44.Mendhiratta N, Meng X, Rosenkrantz AB, et al. Pre-Biopsy MRI and MRI-Ultrasound Fusion-Targeted Prostate Biopsy in Men with Previous Negative Biopsies: Impact on Repeat Biopsy Strategies. Urology. 2015;86:1192–1199. doi: 10.1016/j.urology.2015.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carroll PR, Parsons JK, Andriole G, et al. Prostate cancer early detection, version 1.2014. Featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2014;12:1211–1219. doi: 10.6004/jnccn.2014.0120. [DOI] [PubMed] [Google Scholar]
- 46.Rastinehad AR, Turkbey B, Salami SS, et al. Improving detection of clinically significant prostate cancer: magnetic resonance imaging/transrectal ultrasound fusion guided prostate biopsy. J Urol. 2014;191:1749–1754. doi: 10.1016/j.juro.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ouzzane A, Renard-Penna R, Marliere F, et al. Magnetic Resonance Imaging Targeted Biopsy Improves Selection of Patients Considered for Active Surveillance for Clinically Low-Risk Prostate Cancer Based on Systematic Biopsies. J Urol. 2015;194:350–356. doi: 10.1016/j.juro.2015.02.2938. [DOI] [PubMed] [Google Scholar]
- 48.Da Rosa MR, Milot L, Sugar L, et al. A prospective comparison of MRI-US fused targeted biopsy versus systematic ultrasound-guided biopsy for detecting clinically significant prostate cancer in patients on active surveillance. J Magn Reson Imaging. 2015;41:220–225. doi: 10.1002/jmri.24710. [DOI] [PubMed] [Google Scholar]
- 49.Mullins JK, Bonekamp D, Landis P, et al. Multiparametric magnetic resonance imaging findings in men with low-risk prostate cancer followed using active surveillance. BJU Int. 2013;111:1037–1045. doi: 10.1111/j.1464-410X.2012.11641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siddiqui MM, Rais-Bahrami S, Truong H, et al. Magnetic Resonance Imaging/Ultrasound-Fusion Biopsy Significantly Upgrades Prostate Cancer Versus Systematic 12-core Transrectal Ultrasound Biopsy. Eur Urol. 2013;64:713–719. doi: 10.1016/j.eururo.2013.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Margel D, Yap SA, et al. Impact of multiparametric endorectal coil prostate magnetic resonance imaging on disease reclassification among active surveillance candidates: a prospective cohort study. J Urol. 2012;187:1247–1252. doi: 10.1016/j.juro.2011.11.112. [DOI] [PubMed] [Google Scholar]
- 52.Hu JC, Chang E, Natarajan S, et al. Targeted prostate biopsy in select men for active surveillance: do the Epstein criteria still apply? J Urol. 2014;192:385–390. doi: 10.1016/j.juro.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stamatakis L, Siddiqui MM, Nix JW, Logan J, Rais-Bahrami S, Walton-Diaz A, et al. Accuracy of multiparametric magnetic resonance imaging in confirming eligibility for active surveillance for men with prostate cancer. Cancer. 2013;119:3359–3366. doi: 10.1002/cncr.28216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yerram NK, Volkin D, Turkbey B, et al. Low suspicion lesions on multiparametric magnetic resonance imaging predict for the absence of high-risk prostate cancer. BJU Int. 2012;110:E783–788. doi: 10.1111/j.1464-410X.2012.11646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siddiqui MM, Truong H, Rais-Bahrami S, et al. Clinical Implications of a Multiparametric MRI Based Nomogram Applied to Prostate Cancer Active Surveillance. J Urol. 2015;193:1943–1949. doi: 10.1016/j.juro.2015.01.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park BH, Jeon HG, Choo SH, et al. Role of multiparametric 3.0-Tesla magnetic resonance imaging in patients with prostate cancer eligible for active surveillance. BJU Int. 2014;113:864–870. doi: 10.1111/bju.12423. [DOI] [PubMed] [Google Scholar]
- 57.Stevens DJ MC, Ahmed H, Ahmed HU, et al. 1096 the natural history of untreated prostate MRI lesions in an active surveillance prostate cancer population–260 patient-years. Eur Urol Suppl. 2012;11:e1096–e1096a. [Google Scholar]
- 58.Fradet V, Kurhanewicz J, Cowan JE, et al. Prostate cancer managed with active surveillance: role of anatomic MR imaging and MR spectroscopic imaging. Radiology. 2010;256:176–183. doi: 10.1148/radiol.10091147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rais-Bahrami S, Turkbey B, Rastinehad AR, et al. Natural history of small index lesions suspicious for prostate cancer on multiparametric MRI: recommendations for interval imaging follow-up. Diagn Interv Radiol. 2014;20:293–298. doi: 10.5152/dir.2014.13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robertson NL, Moore CM, Ambler G, et al. MAPPED study design: a 6 month randomised controlled study to evaluate the effect of dutasteride on prostate cancer volume using magnetic resonance imaging. Contemp Clin Trials. 2013;34:80–89. doi: 10.1016/j.cct.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 61.Walton Diaz A, Shakir NA, George AK, et al. Use of serial multiparametric magnetic resonance imaging in the management of patients with prostate cancer on active surveillance. Urol Oncol. 2015;33:202 e1–7. doi: 10.1016/j.urolonc.2015.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sonn GA, Filson CP, Chang E, et al. Initial experience with electronic tracking of specific tumor sites in men undergoing active surveillance of prostate cancer. Urol Oncol. 2014;32:952–957. doi: 10.1016/j.urolonc.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mozer P, Rouprêt M, Le Cossec C, et al. First round of targeted biopsies using magnetic resonance imaging/ultrasonography fusion compared with conventional transrectal ultrasonography-guided biopsies for the diagnosis of localised prostate cancer. BJU Int. 2015;115:50–57. doi: 10.1111/bju.12690. [DOI] [PubMed] [Google Scholar]
- 64.Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994;271:368–374. [PubMed] [Google Scholar]
- 65.Siddiqui M, Rais-Bahrami S, Turkbey B, et al. Comparison of mr/ultrasound fusion–guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA. 2015;313:390–397. doi: 10.1001/jama.2014.17942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sonn GA, Chang E, Natarajan S, et al. Value of targeted prostate biopsy using magnetic resonance-ultrasound fusion in men with prior negative biopsy and elevated prostate-specific antigen. Eur Urol. 2014;65:809–815. doi: 10.1016/j.eururo.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rastinehad AR, Turkbey B, Salami SS, et al. Improving detection of clinically significant prostate cancer: Magnetic resonance imaging/transrectal ultrasound fusion guided prostate biopsy. J Urol. 2014;191:1749–1754. doi: 10.1016/j.juro.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuru TH, Roethke MC, Seidenader J, et al. Critical evaluation of magnetic resonance imaging targeted, transrectal ultrasound guided transperineal fusion biopsy for detection of prostate cancer. J Urol. 2013;190:1380–1386. doi: 10.1016/j.juro.2013.04.043. [DOI] [PubMed] [Google Scholar]
- 69.Fiard G, Hohn N, Descotes JL, Rambeaud JJ, Troccaz J, Long JA. Targeted MRI-guided prostate biopsies for the detection of prostate cancer: Initial clinical experience with real-time 3-dimensional transrectal ultrasound guidance and magnetic resonance/transrectal ultrasound image fusion. Urology. 2013;81:1372–1378. doi: 10.1016/j.urology.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 70.Rud E, Baco E, Eggesbø HB. MRI and ultrasound-guided prostate biopsy using soft image fusion. Anticancer Res. 2012;32:3383–3389. [PubMed] [Google Scholar]