Abstract
Our “tips and tricks” focuses on all aspects of upper tract endourology and we hope these will be of use to all trainees and consultants who perform ureteroscopy. We report an “expert consensus view” from experienced endourological surgeons, on all aspects of advanced ureteroscopic techniques, with a particular focus on avoiding and getting out of trouble while performing ureteroscopy. In this paper we provide a summary of placing ureteric access sheath, flexible ureteroscopy, intra renal stone fragmentation and retrieval, maintaining visual clarity and biopsy of ureteric and pelvicalyceal tumours.
Keywords: urolithiasis, ureteroscopy, stent, ureterorenoscopy
Placing a ureteric access sheath
The use of ureteric access sheaths prior to flexible ureterorenoscopy can be both a surgical preference and case-specific. They facilitate multiple passages of the ureterorenoscope, reduce intra-renal pressure and help improve irrigation flow [1]. However, the surgeon must be aware that access sheaths carry a risk of ureteric ischaemia and can lead to ureteric injury [2]. When using an access sheath, it is important to be aware of the size of the flexible ureteroscope, so that the access sheath of the correct size is used, since some of the new digital flexible ureteroscopes will only pass down certain-sized access sheaths (>10 Fr) [3].
We advocate performing an initial semi-rigid ureteroscopy before placement of the access sheath. This enables the UO to be optically dilated, ensures that any simultaneous ureteric stones are identified and gives an assessment as to whether the ureter is a “friendly”, capacious ureter or not. We do not advocate the use of ureteric balloons to dilate the ureter to aid sheath placement nor the use of other ureteric dilators. Although these might be an option in very particular circumstances, in most cases involving an unfavourable ureter, it is usually preferable to place a stent and return for the definitive operation at a later date
The decision to leave a safety wire outside an access sheath is one of personal preference. Some newer access sheaths enable a single wire to be used for placement and results in the wire being situated outside the sheath after placement [4]. Such sheaths offer the cost benefit of a single guidewire for the procedure (although this must be offset against the cost of the access sheath itself!).
Once the access sheath of the desired size and length has been chosen, pass the sheath over the guidewire using the Seldinger technique. The sheath passage should be monitored with pulsed fluoroscopy during ureteric passage, preventing possible buckling in the bladder or to identify early resistance and failure to progress. Pass the sheath slowly, feeling for resistance when placing over the wire. Ensure that the wire does not kink, or coil in the bladder, which will make advancement of the sheath impossible. If this should occur, gradual step-wise withdrawal of the wire, under close fluoroscopic control, is needed to straighten the wire, and then retry the insertion with particular attention to the sheath crossing the ureteric orifice and lowermost ureter (see Figure 1). If the access sheath progresses up the ureter well, consider placing it just below the PUJ, allowing the flexible scope to be fully deflectable. If you choose not to use an additional safety wire outside the access sheath, be careful when passing the flexible scope through the upper ureter/PUJ area to avoid unnecessary ureteric injury. When the procedure is completed, withdraw the ureteroscope and access sheath together with the tip of the ureteroscope placed just at the end of the sheath, watching the ureteric mucosa move past. Any ureteric injury can then be noted and stented accordingly. If a safety wire is not already in place, a wire can be pre-emptively inserted through the flexible ureterorenoscope to allow stent insertion if required.
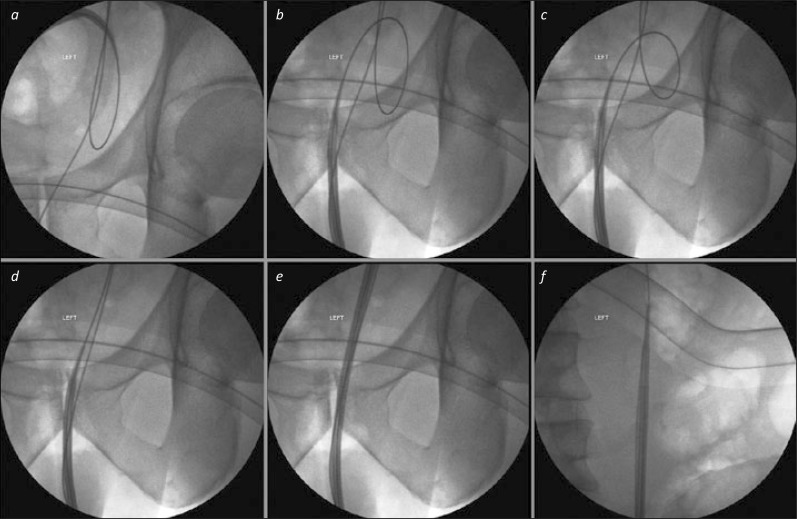
Figure 1.
Wire problems (in bladder) for access sheath insertion. 1a. A straight safety wire is present, but the working wire, over which the access sheath is being passed, is substantially coiled in the bladder. Further advancement of the sheath will not only fail to access the ureter, but is likely to result in displacement of the working wire to the bladder. 1b. The access sheath has been withdrawn to the urethra. 1c. The working guide wire has been withdrawn slightly, such that the coiled loop is of smaller diameter. 1d. The working wire is now straight, and the tip of the access sheath has been moved along it towards the left ureteric orifice. 1e. Keeping the image intensifier over the lower third of the ureter, the access sheath can be advanced under fluoroscopic control to ensure there is no “buckling” in this part of the ureter. 1f. Once the lower third has been successfully traversed, the image intensifier can be moved to the proximal ureter to allow precise positioning of the tip of the sheath in the upper ureter.
If unable to pass the access sheath into the UO or much beyond the lower third of the ureter, despite prior normal rigid ureteroscopy, consider using only the inner obturator of the access sheath over your wire, enabling an initial ureteric dilatation by advancing the obturator to the upper ureter. Then, try repeating access sheath placement with both the sheath and inner component. If this is unsuccessful, one can consider using a stiff wire, rather than the standard guide wire, to aid sheath placement. Be aware that these wires can cause intra-renal bleeding if forced too hard or pushed through the urothelium. If you are still unable to place the sheath, discretion is much better than valour. Change tack, pass the flexible scope over the stone wire and perform your flexible ureteroscopy without a sheath (see below) or simply stent the patient and come back another day.
Flexible ureteroscope insertion via wire
Some surgeons prefer to place the flexible ureteroscope over a wire, without using an access sheath. In order to aid scope passage, one should use lubrication for the scope and urethra (such as Instillagel™) to reduce resistance, particularly in the male urethra.
One can consider using a double-tipped hydrophilic wire, thereby reducing damage to the fragile working channel of the flexible ureteroscope. When initially placing the ureteroscope, we would advocate having it free of all attachments (irrigation channel, light and camera leads), enabling smoother passage. It can then be passed over the wire, again using limited pulsed fluoroscopy to check progress.
If passage is obstructed at the level of the UO, as with semi-rigid ureteroscopy, rotate the flexi scope 180°, enabling the beak of the scope to pass through the UO more easily. If you are still unable to pass the flexible scope, stent the ureter with a view to performing a repeat procedure in approximately 2–6 weeks.
When manipulating the flexible ureteroscope using the dominant hand, with the thumb controlling deflection, consider holding the shaft of the ureterorenoscope just distal to the urethral opening/access sheath with the opposite hand (pincer grip technique), to aid scope advancement and rotation. This will also reduce torque and pressure on the flexible scope.
It is pertinent and useful to remember that the ureterorenoscope has 3 user inputs to manipulate the tip: in/out movement, thumb up/down and torque left/right. Trying to “waggle” the handle of the ureterorenoscope towards the patient's left or right leg in a movement similar to a car's windscreen wiper will not influence the tip of the scope. Deliberate, slow movements will get one around the whole collecting system faster than wild, fast movements. Once the ureterorenoscope is in the kidney, it is useful to orientate the endosopic view with a fluoroscopic “route map” of the collecting system, with a retrograde study performed via the scope. A “complete tour or walking the calyces” of the kidney can then be performed, visiting each calyx in turn, in much the same way that a diagnostic flexible cystoscopy is performed, i.e. in a methodical fashion that ensures complete coverage of the collecting system. As each calyx is entered, a “snap-shot” on the image intensifier, saved to the memory, will allow a confirmatory review during the case that all calyces were successfully seen. Furthermore, it can aid the rapid reintroduction of the scope towards the calyx of interest (stone or TCC bearing) later in the procedure (see Figure 2).
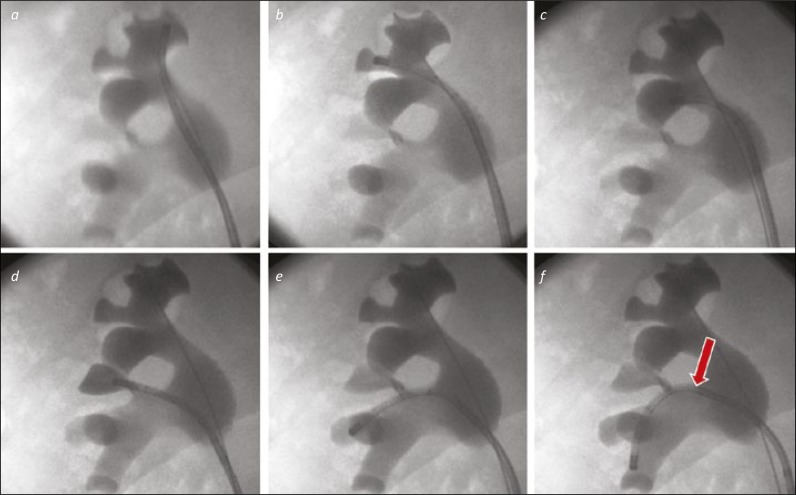
Figure 2.
A “Complete Tour” of the right kidney. 2a. The ureterorenoscope is directed to the upper medial calyx as the starting point for an anti-clockwise tour of the collecting system. 2b. The upper middle calyx will be visualised (where the safety wire is located) as the scope is moved to the upper lateral calyx. 2c. The scope is seen in the posterior upper pole calyx. 2d. The interpole, not well seen on the images 3a-c is filled with contrast via the scope to confirm it has been visualised. 2e. The scope is placed in the next calyces down, in the lateral part of the lower pole. Both calyces can be inspected in turn before moving to the lowermost calyx. 2f. The ureterorenoscope is deflected into the lower medial calyx. This required use of the secondary (passive) deflection from the renal pelvis / infundibulum to the interpole (arrow).
Laser fragmentation and basket technique
Before performing laser fragmentation in the kidney, consider repositioning the stone into a more favourable position (upper calyx or even upper ureter if feasible). The aim is to keep the ureteroscope as straight as possible while fragmenting, reducing the risk of damage to the flexible ureterorenoscope (see Figure 3). It is important to be aware of the individual properties of the different intra-renal baskets available, and especially to understand the pros and cons of the preferred basket. It is to be noted that increasing the size of the basket (corresponding to shaft diameter) will significantly reduce irrigant flow [1].
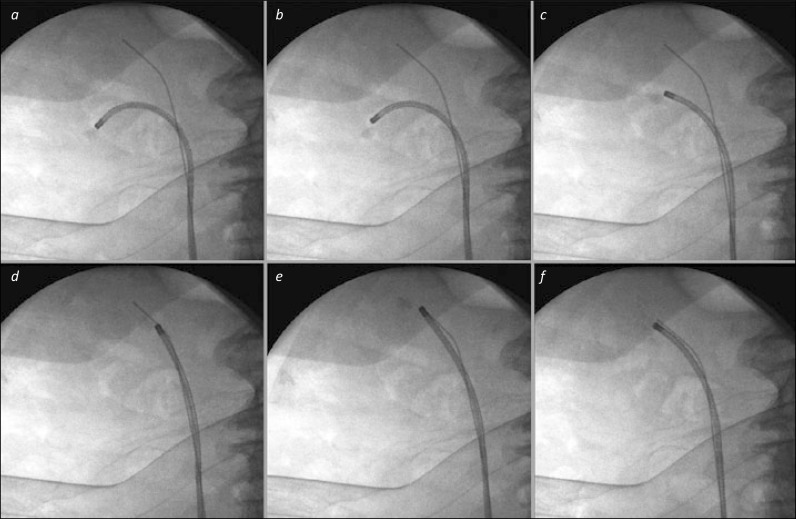
Figure 3.
Basket relocation of lower pole stone. 3a. The stone has been identified in the lower pole, and is grasped in a basket to prepare for relocation. 3b and c. The ureterorenoscope is manoeuvred out of the lower calyx towards the renal pelvis. 3d. The scope is advanced into the patient to the upper pole – the presence of the safety wire in the upper calyx can aid this both under endoscopic and fluoroscopic control. 3e. The stone is then released from the basket, which is withdrawn from the scope, and replaced with an appropriate laser fibre for stone fragmentation. 3f. The stone has been successfully broken into small pieces. It will be easier to re-pass the ureterorenoscope back and forth to this upper calyx for basket stone retrieval than to direct it repeatedly to the lower calyx where the stone was originally, particularly as the safety wire “guides the way”.
There is debate amongst surgeons whether to laser the stone to dust or fragment and retrieve intra-renal fragments, and sometimes the best results are achieved by a combination of the two techniques. Sometimes, it will not be feasible to remove multiple fragments (such as with a tight ureter and no access sheath or safety wire in a “wireless” ureterorenoscopy), so it is important for all endourologists to be aware of the different stone fragmentation effects, when varying the energy and frequency settings. The different laser settings and their effect on stone fragmentation have been highlighted in a recent publication [5]. Again, personal preference will dictate one's practice. It is helpful to liaise with the anaesthetist to control the patient's respiratory movement during stone fragmentation in the kidney. This will not only increase efficacy of lasertripsy but also reduce the risk of compromising the view from bleeding (through increased accuracy of laser onto the stone as opposed to the urothelium), and both factors will help reduce the overall operating time as well.
When the laser fibre is inserted, ensure that the ureterorenoscope is straight in a non-deflected, neutral position – one of the advantages of having an additional wire in the kidney is maintaining a straight ureterorenoscope. If the wire is straight, and can be seen running parallel with the direction of vision of the scope, then it stands to reason that the scope is also straight – a quick “flash” of the image intensifier (regardless of the presence of the additional wire) will confirm this. Maintaining the scope straight as the laser fibre is passed reduces the risk of working channel damage, avoiding costly repairs. Reusable laser fibres can result in small microfractures, which then contribute to flexible ureterorenoscope damage. The use of disposable laser fibres is increasing, and they have been shown to be cost-effective and reduce ureteroscope damage [6].
Improving vision in the kidney
Vision is key to achieving good fragmentation and stone-free rates, particularly in the kidney. Whilst the use of contrast improves vision (clears dust/blood) it can cause refractory errors if saline is introduced. Ureteric access sheaths enable improved irrigation rates, with larger access sheaths (12/14Fr) offering better flow compared to smaller sheaths (10/12Fr) [1]. However, as noted above, it is important to note that larger access sheaths can potentially cause ureteric ischaemia and even direct ureteric trauma including perforation.
Simple hand held pump devices can be used and will help increase irrigant flow. They are best used judiciously as they transiently increase intra-renal pressure. Blood will affect vision and may result in a premature end to the procedure. With minor bleeding, increasing the irrigation for a few minutes may help improve the view. Aspiration of the collecting system may result in further bleeding; therefore, be patient and wait for the vision to improve.
Other factors that can affect the quality of vision include the focusing of optical scope, adjusting the brightness of light, correct white balancing and appropriate use of vision enhancement features on the stacking system. Correct adjustment of these factors aids vision and results in successfully completed procedures.
Digital ureterorenoscopes use ”chip-on-tip” technology and are increasingly commonplace in endourology operating theatres. In vitro assessment has shown improved image resolution and colour reproducibility without compromising depth of field, image distortion, grayscale imaging and ureteroscope deflection. Whilst field of view may be reduced in some comparisons, this is counteracted by an increase in image size [7]. Improved optical characteristics translate to improved clinical outcomes with significant improvements in mean operative time, flexible ureterorenoscopy time and efficiency of stone fragmentation [8, 9].
Ureteric stenting
The placement of a ureteric stent, particularly in an emergency setting, such as relieving obstruction in an infected system, can be a daunting task. Problems might arise with guide wire placement, but hopefully the tips discussed earlier will aid this. When placing a stent it is useful to try and deploy the proximal coil (especially multi-length stent) in the upper calyx, thus enabling a smaller component of the stent in the bladder. Of course, it is important not to leave the distal end too short! These can migrate into the ureter and be tricky to reposition.
Consider the use of a ureteric catheter or tethered stent if feasible, for short-term drainage. One must always consider whether a stent is really needed, as they have associated morbidity. If considering leaving a stent, good preoperative counselling of the patient is vital. Patients will need to be aware that they might have some pain or discomfort postoperatively. This will help reduce unnecessary readmissions for stent related symptoms.
When placing the stent, if one is having difficulty with buckling at the UO, bring the cystoscope closer to the UO and push slowly under vision. If the stent is not moving, use fluoroscopy to check the wire placement in the collecting system. Excess wire in the renal end can equally hamper progress. By pulling back the wire slightly under fluoroscopy (monitoring the renal end), the stent can then be advanced. Do not forget to check that your assistant is maintaining adequate stiffness of the wire whilst the stent is being inserted. Some surgeons prefer to place the stents under radiological guidance, using fluoroscopy alone with a “freehand” technique, as opposed to inserting the stent via a cystoscope under direct vision. In such cases, care must be taken to ensure the distal end of the stent is not pushed too high in the ureter. It is best to visualise and ensure its position in the bladder before sending the patient to recovery – if there is any doubt from the final fluoroscopic image, it is best to be sure by passing the cystoscope and having a look! It is important to remember that bilateral ureteroscopy may result in bilateral stent insertion.
Finally, regardless of the circumstances leading to stent placement, it is important to ensure that an appropriate postoperative plan is in place to track and remove the stent. Depending on the local set-up, the use of stent registers, or tracking emails or web-based applications might be useful to avoid these ‘missed stents’.
Ureteric and renal tumors
Diagnostic ureterorenoscopy and biopsy has been recommended for cases of upper tract tumors [3, 10]. Selective urine cytology is an important aspect of this procedure. A pre-instrumented ureteric sample is preferable. This can be achieved by administrating 20 mg of furosemide in the anaesthetic room. After a diagnostic cystoscopy, a previously saline-filled ureteric catheter can be placed in the ureter to collect selective cytology from one or both ureters relatively quickly.
So-called ”wireless” diagnostic ureteroscopy has been advocated as a technique [10]. This reduces the chance of mucosal trauma, therefore reducing the risk of unnecessary biopsy. This technique can be technically challenging and is not universally practised. For safety reasons, one may prefer to use a guidewire in diagnostic cases.
When performing a ureteric/renal tumor biopsy there are two common methods used. One biopsy technique is by performing a cold cup technique biopsy, utilising a tip-less stone basket. The base of the tumor can be snared, and then avulsed with some traction. The second technique utilises single use biopsy devices. Once tumor specimens are taken and still in the grasper, consider pushing the biopsy device forward to gently avulse the biopsy. If one pulls back on the device, the graspers may slip and offer a degraded specimen. It pays to be careful when avulsing tissue as perforation of the collecting system may occur. Ureteroscopic biopsies have been shown to yield a 90% diagnostic rate for tumor grade, with low false negative rates [11]. Recent evidence suggests that placing the biopsies in Bouin's solution may offer better preservation of nuclear detail [12].
The use of routine access sheaths for biopsies and laser fragmentation of tumours is discouraged, as any minor trauma of the ureter may theoretically result in tumor seeding, but this might be necessary in selected cases where multiple passes need to be made for biopsy from the pelvicalyceal system. In addition, the presence of an access sheath will result in lower intra-renal pressure, and allow better irrigation, both of which are valuable in cases of TCC, which, unlike stones, bleed with compromised views under low irrigation flow rates. Newer techniques such as PDD, NBI or SPIES might be increasingly used for greater diagnostic accuracy in these cases.
CONCLUSIONS
The indications for endoluminal surgery continue to widen, mainly through technological advances, such that this branch of urological practice continues to increase year on year. As with most forms of surgery, meticulous preoperative planning will lead to a more successful outcome. If one tries to envisage the potential problems preoperatively, appropriate solutions can be considered beforehand. Despite the majority of cases being relatively straightforward, many potential confounding factors can affect the success rates of these procedures. We hope the above “tips and tricks” will enable the practitioner to offer safe, effective and successful upper tract surgery.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Turk C, Knoll T, Petrik A, et al. EAU Guidelines on Interventional Treatment for Urolithiasis. Eur Urol. 2015 doi: 10.1016/j.eururo.2015.07.041. [DOI] [PubMed] [Google Scholar]
- 2.Turney BW, Reynard JM, Noble JG, Keoghane SR. Trends in urological stone disease. BJU Int. 2012;109:1082–1087. doi: 10.1111/j.1464-410X.2011.10495.x. [DOI] [PubMed] [Google Scholar]
- 3.Somani BK, Aboumarzouk O, Srivastava A, Traxer O. Flexible ureterorenoscopy: Tips and tricks. Urol Ann. 2013;5:1–6. doi: 10.4103/0974-7796.106869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ulvik Ø, Rennesund K, Gjengstø P, Wentzel-Larsen T, Ulvik NM. Ureteroscopy with and without safety guide wire: should the safety wire still be mandatory? J Endourol. 2013;27:1197–1202. doi: 10.1089/end.2013.0248. [DOI] [PubMed] [Google Scholar]
- 5.Dickstein RJ, Kreshover JE, Babayan RK, Wang DS. Is a safety wire necessary during routine flexible ureteroscopy? J Endourol. 2010;24:1589–1592. doi: 10.1089/end.2010.0145. [DOI] [PubMed] [Google Scholar]
- 6.Song T, Liao B, Zheng S, Wei Q. Meta-analysis of postoperatively stenting or not in patients underwent ureteroscopic lithotripsy. Urol Res. 2012;40:67–77. doi: 10.1007/s00240-011-0385-7. [DOI] [PubMed] [Google Scholar]
- 7.Haleblian G, Kijvikai K, de la Rosette J, Preminger G. Ureteral stenting and urinary stone management: a systematic review. J Urol. 2008;179:424–430. doi: 10.1016/j.juro.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 8.Nabi G, Cook J, N'Dow J, McClinton S. Outcomes of stenting after uncomplicated ureteroscopy: systematic review and meta-analysis. BMJ. 2007;334:e572. doi: 10.1136/bmj.39119.595081.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes KT, Bing MT, Tracy CR. Do ureteric stent extraction strings affect stent-related quality of life or complications after ureteroscopy for urolithiasis: a prospective randomised control trial. BJU Int. 2014;113:605–609. doi: 10.1111/bju.12541. [DOI] [PubMed] [Google Scholar]
- 10.Ng YH, Somani BK, Dennison A, Kata SG, Nabi G, Brown S. Irrigant flow and intrarenal pressure during flexible ureteroscopy: the effect of different access sheaths, working channel instruments, and hydrostatic pressure. J Endourol. 2010;24:1915–1920. doi: 10.1089/end.2010.0188. [DOI] [PubMed] [Google Scholar]
- 11.Traxer O, Thomas A. Prospective Evaluation and Classification of Ureteral Wall Injuries Resulting from Insertion of a Ureteral Access Sheath During Retrograde Intrarenal Surgery. J Urol. 2013;189:580–584. doi: 10.1016/j.juro.2012.08.197. [DOI] [PubMed] [Google Scholar]
- 12.Al-Qahtani SM, Letendre J, Thomas A, Natalin R, Saussez T, Traxer O. Which ureteral access sheath is compatible with your flexible ureteroscope? J Endourol. 2014;28:286–290. doi: 10.1089/end.2013.0375. [DOI] [PubMed] [Google Scholar]