Abstract
Basigin, also called CD147 or EMMPRIN, is a transmembrane glycoprotein that belongs to the immunoglobulin superfamily. Basigin has isoforms; the common form (basigin or basigin-2) has two immunoglobulin domains, and the extended form (basigin-1) has three. Basigin is the receptor for cyclophilins, S100A9 and platelet glycoprotein VI, whereas basigin-1 serves as the receptor for the rod-derived cone viability factor. Basigin tightly associates with monocarboxylate transporters and is essential for their cell surface translocation and activities. In the same membrane plane, basigin also associates with other proteins including GLUT1, CD44 and CD98. The carbohydrate portion of basigin is recognized by lectins, such as galectin-3 and E-selectin. These molecular recognitions form the basis for the role of basigin in the transport of nutrients, migration of inflammatory leukocytes and induction of matrix metalloproteinases. Basigin is important in vision, spermatogenesis and other physiological phenomena, and plays significant roles in the pathogenesis of numerous diseases, including cancer. Basigin is also the receptor for an invasive protein RH5, which is present in malaria parasites.
Keywords: basigin, cyclophilins, glycoproteins, immunoglobulin superfamily, monocarboxylate transporters
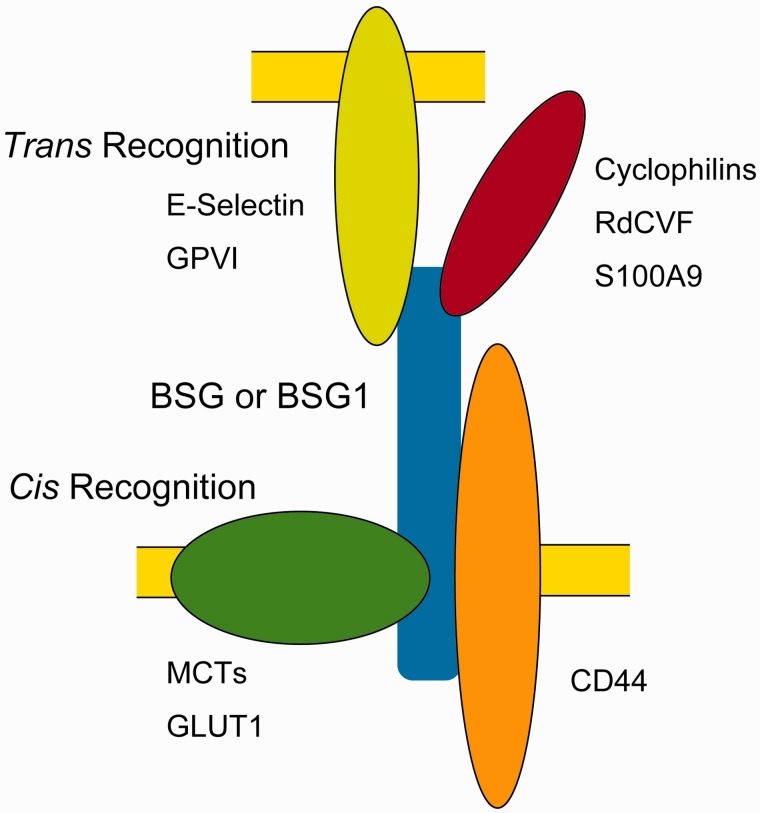
The immunoglobulin (Ig) superfamily consists of proteins with at least one Ig domain and plays essential roles in intercellular communication (1). Basigin (BSG) (2), also called CD147 or EMMPRIN (3), is a member of the Ig superfamily and is important in numerous physiological and pathological phenomena. It is a highly glycosylated transmembrane protein and recognizes molecules in the same cells, especially in the same membrane (cis-recognition), and those located extracellularly (trans-recognition) (Fig. 1).
Fig. 1.
BSG recognizes various molecules in both cis and trans manners. BSG or BSG1 is illustrated in a blue colour, and representative binding partners are shown. In trans-recognition, either a soluble protein (red) or protein on an adjacent cell (pale green) binds to BSG. In cis-recognition, BSG binds to proteins in the same cell, especially in the same membrane, such as a transporter (green) or receptor (orange). Names of some of these proteins are written in this figure.
Reflecting its pleiotrophic functions, BSG was found independently from various viewpoints and was given many different names, such as gp42, BSG, HT7, neurothelin, OX-47, M6, 5A11 and EMMPRIN (4–7). Furthermore, the Ok(a) blood group is carried by BSG (8). The symbol of the human BSG gene provided by Human Genome Organisation is BSG, and the gene and protein name is basigin (Ok blood group). As a leukocyte differentiation antigen, the Cluster of Differentiation Nomenclature gave CD147 to BSG.
BSG and two other transmembrane proteins, embigin (9, 10) and neuroplastin (11), have closely related structures and form a distinct family in the Ig superfamily. Although the founding member of this family is embigin (9), it is appropriate to call this family the BSG family because BSG has been studied in more detail from physiological and pathological aspects.
The present review intends to provide concise and up-to-date knowledge on BSG. An emphasis is placed on molecular interactions between BSG and its binding partners, as well as the roles of these interactions in various physiological and pathological processes. Previous reviews on BSG are also available (4–7).
Structure
BSG is located on chromosome 19 at p13.3 (12) and consists of ten exons spanning approximately 12 kb. The BSG gene has been detected in all vertebrates examined to date and is also present in Drosophila melanogaster (13) and Schistosoma. Although the BSG family in mammals consists of three members, BSG is the sole member in D.melanogaster (13).
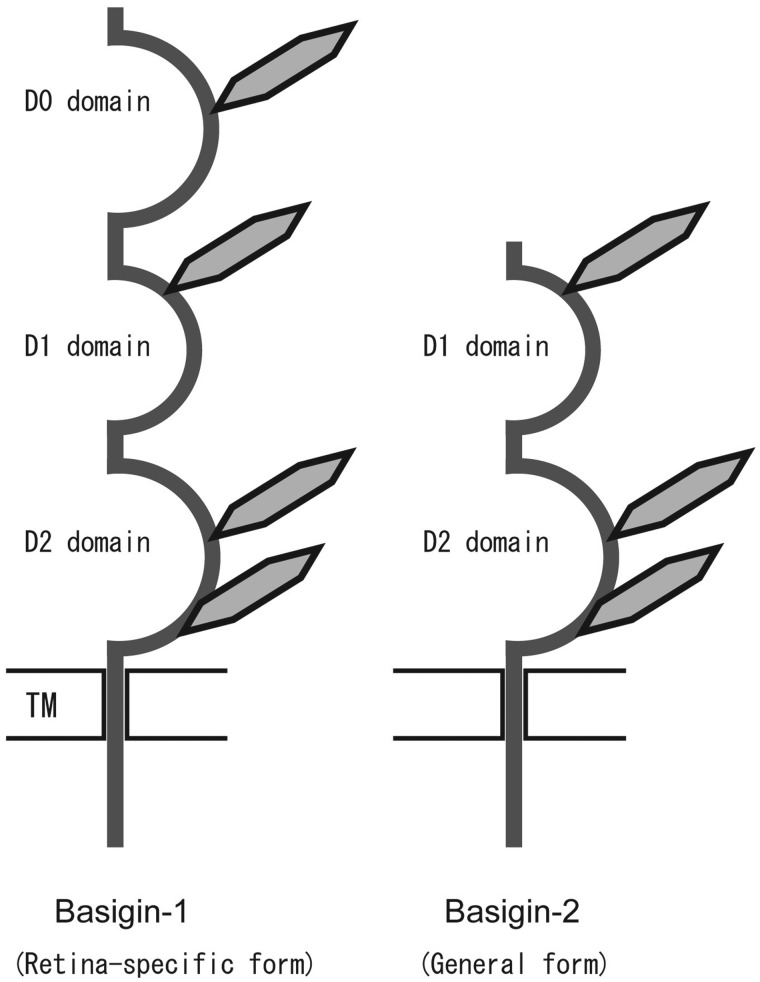
There are isoforms in human BSG (14, 15) generated by differential splicing and differences in transcription initiation sites. Basigin-1 (BSG1) has three Ig domains and has been identified as the retina-specific form (16). Basigin-2 (BSG2) is the common form and has two Ig domains (Fig. 2). Due to its wide distribution, BSG2 is simply referred to as BSG in this review.
Fig. 2.
Schematic presentation of two BSG isoforms, basigin-1 (BSG1) and basigin-2 (BSG) (14–18). TM, transmembrane region; hexagons, carbohydrates presumed to be linked to Asn-glycosylation sites.
Ig domains are classified into V-set, C1- and C2-sets and I-set, which is intermediate of V- and C-sets. The steric structure of the extracellular portion of BSG was determined by X-ray crystallography and NMR spectroscopy (17–19). Consequently, the Ig domains of BSG (Fig. 2) were assigned as follows: D0, I-set; D1, C2-set; D2, I-set.
The transmembrane region consisting of 23 amino acids is highly conserved among species and among members of the BSG family (13, 20) (Fig. 3). The complete conservation of Glu in the middle of the transmembrane region is noteworthy. This feature implies that intramembrane association between BSG and the neighbouring molecule is important for BSG function.
Fig. 3.
Deduced protein sequence of human BSG precursor (20). An arrow indicates the cleavage site of the signal sequence (3). A broad line indicates the transmembrane region, whereas thin lines show putative disulphide bridges. Open stars ( ), amino acids conserved between human and Drosophila BSG (13); closed stars (★), amino acids conserved between human, mouse, and Drosophila BSG, mouse embigin and rat neuroplastin; open circles (◯), amino acids conserved between human and mouse BSG, mouse embigin and rat neuroplastin, but not in Drosophila BSG (10, 13). The juxtamembrane cytoplasmic region conserved between human and Drosophila BSG is involved in regulation of the cytoskeletal structure (48).
), amino acids conserved between human and Drosophila BSG (13); closed stars (★), amino acids conserved between human, mouse, and Drosophila BSG, mouse embigin and rat neuroplastin; open circles (◯), amino acids conserved between human and mouse BSG, mouse embigin and rat neuroplastin, but not in Drosophila BSG (10, 13). The juxtamembrane cytoplasmic region conserved between human and Drosophila BSG is involved in regulation of the cytoskeletal structure (48).
Except for the transmembrane and juxtamembrane cytoplasmic regions, human and D.melanogaster BSG have only moderate homology, and many amino acids conserved between the BSG family members from a certain mammalian species are not conserved between human and D.melanogaster BSG (Fig. 3).
Molecular Interactions
Monocarboxylate transporters
The finding of tight associations of BSG with monocarboxylate transporters (MCTs) was a breakthrough in BSG research. MCTs catalyse the transport of substituted short-chain fatty acids, such as lactate, pyruvate and ketone bodies, across the plasma membrane. There are four isoforms (MCT1–MCT4) with different modes of expression and with distinct substrate and inhibitor affinities (21).
MCTs directly bind to BSG and its family members in the same membrane. MCT1, MCT3 and MCT4 use BSG as the ancillary protein (21, 22), whereas MCT2 uses embigin (23), neuroplastin (11), and in the spermatozoa, BSG (24). Embigin also binds to MCT1 when BSG is absent, as has been reported in rat erythrocytes (25). Evidence for binding has been obtained using indirect immunoprecipitation, chemical crosslinking and immunostaining. The transmembrane and cytoplasmic regions of BSG are essential and sufficient for association with MCT1 (22). Fluorescent resonance energy transfer studies have revealed that BSG forms a dimer when it binds to MCT1 (26).
As shown in co-transfection experiments, BSG serves as a chaperone required for the plasma membrane translocation of MCTs (22). BSG also has been concluded to be necessary for the expression of MCT activities, as the target of an MCT inhibitor, p-chloromercuribenzene sulphonate, is not MCTs, but BSG (23). As described above, BSG plays critical roles in energy metabolism by associating with MCTs. The tight association between BSG and MCTs is consistent with the high level of expression of BSG in metabolically active cells, such as tumour cells and activated lymphocytes (22).
The physiological significance of MCT–BSG interactions was confirmed in studies using BSG-deficient mice, in which both cone and rod visual functions were severely affected from an early age (27). However, photoreceptor cells retained the normal morphology until 8 weeks after birth. Thereafter, they degenerated gradually and mostly disappeared by 41 weeks of age. Thus, functional abnormalities preceded morphological abnormalities. The cause of this unusual phenotype was clarified by analysing MCTs.
Muller glial cells in the retina depend primarily on glycolysis and export lactate, which is taken up by photoreceptor cells as an important energy source. This intercellular cooperation is mediated by MCTs. In BSG-deficient mice, MCT1 was almost completely lost from the retina, with trace amounts remaining in intracellular aggregates (28). In these mice, MCT3 also disappeared from the site of its restricted location, namely, the basolateral membrane of the retinal pigment epithelium. Thus, the loss of BSG results in deficits in the cell-surface translocation of MCTs, leading to their degradation. The marked decreases in MCTs cause deficits in visual functions through energy depletion, followed by the death of the affected cells.
Rod-derived cone viability factor and GLUT1
In the retina, rod photoreceptors secrete rod-derived cone viability factor (RdCVF), which is a truncated thioredoxin-like protein without enzymatic activity and enhances the survival of cone photoreceptors. As patients with retinitis pigmentosa suffer gradual degeneration of cone photoreceptors, RdCVF has the potential to become a therapeutic for this disease. Recently, the receptor for RdCVF has been found to be BSG1, the extended form specific for the retina (29). Furthermore, BSG1 binds to GLUT1, which is a glucose transporter with 12 membrane-spanning regions (29). Importantly, RdCVF-mediated glucose uptake in cone-enriched cultures was shown to be suppressed by the knockdown of BSG1. Based on these findings, the following mechanism of RdCVF action has come out. RdCVF binds to BSG1 on the surface of cone photoreceptors and enhances the activity of GLUT1, thereby promoting the survival of these cells because of the abundance of energy source in the cell. The mode of action of RdCVF fits the molecular characteristics of BSG.
CD44 and epidermal growth factor receptor
CD44, the major hyaluronan receptor, is present in a supramolecular complex containing BSG and MCTs (30). Furthermore, the enhanced expression of BSG triggers the formation of a lipid raft-associated supramolecular complex containing BSG, CD44 and epidermal growth factor (EGF) receptor (6, 31). Hyaluronan promotes invasive properties of tumour cells, and appears to stabilize and enhance the activity of the supramolecular complex (6). Indeed, the inhibition of hyaluronan functions by hyaluronan oligosaccharides was reported to suppress functions of MCTs and EGF receptor in the supramolecular complex (6, 30, 31).
Integrins, CD43, CD98, γ-secretase, NOD2 and γ-catenin
Interactions with integrins are particularly important in considering BSG signalling. Integrins α3β1 and α6β1 were co-immunoprecipitated with BSG from cell lysates and also colocalized with BSG at the cell surface (32). However, it remained unclear whether BSG binds to integrins directly or indirectly through other molecules. Although a binding site was detected between BSG and β1-integrin, this site was favourable for trans-binding, and not for cis-binding (33).
At least four molecules can mediate interactions between BSG and integrins: CD98, CD43, MCT4 and galectin-3. BSG directly binds to CD98 (34), which covalently binds to amino acid transporters and associates with integrins. Therefore, BSG can interact with integrins through CD98. Functional interactions between CD98, integrins and BSG were also proposed following an analysis of antibody-induced cell aggregation (35). On the other hand, a sialoglycoprotein, CD43, associates with BSG and LFA-1, a leukocyte integrin (36). Furthermore, MCT4, but not MCT1 binds β1-integrin in epithelial cell lines (37). Thus, MCT4 can mediate an indirect association between BSG and integrins. Galectin-3, a lectin, will be described later.
The functional interactions between integrins and BSG are quite evident in D.melanogaster. Notably, abnormalities in intracellular structures caused by BSG mutations in flies are enhanced by integrin mutations (13). Furthermore, BSG cooperates with an integrin in apposition of extraembryonic membranes (38).
Other molecules which interact with BSG in a cis manner include the γ-secretase complex (39) and NOD2 (40). The γ-secretase complex cleaves the β-amyloid precursor protein within the plasma membrane, leading to the production of amyloid β-peptides, which accumulate in amyloid plaques found in patients with Alzheimer’s disease. The depletion of BSG by an RNA interference enhances the production of amyloid β-peptides. Thus, the role of BSG in this complex is to suppress the enzymatic activity (39). An external signal to BSG is expected to alter the suppressive activity. NOD2, a cytoplasmic protein, is a component of the innate immune system, and the effect of BSG on this protein is also suppressive (40).
Finally, in vascular endothelial cells, the cytoplasmic tail of BSG binds to γ-catenin, which associates with Nm23, a nucleotide diphosphate kinase capable of producing ATP (41). In BSG-deficient mice, endothelial junctions were found to be altered, partly due to impairment of actomyosin contractions caused by lower ATP levels.
Cyclophilins
Among the molecules that bind to BSG extracellularly and transmit their signals, cyclophilin A has been examined in the most detail (5). This protein was originally found as a receptor for an immunosuppressive drug, cyclosporin A. Cyclophilin A is present intracellularly and is also secreted in response to immunological stimuli. The secreted cyclophilin A exhibits chemotactic activity for neutrophils, eosinophils and T cells. BSG was found to be the major signalling receptor for cyclophilin A and its related molecule, cyclophilin B (5, 42). Binding between BSG and cyclophilin A is not strong (18) and appears to be transitional in nature. Cyclophilin A typically binds to heparan sulphate, a heparin-like polysaccharide, and then to BSG (5, 42). In a subset of T cells, syndecan-1, a proteoglycan with heparan sulphate, binds to BSG in a cis manner (43). A pretreatment with an antibody to syndecan-1 or the knockdown of syndecan-1 markedly reduced cellular responsiveness to cyclophilin B. Therefore, syndecan-1 is important in BSG signalling and appears to form a receptor complex with BSG. The downstream signalling system after cyclophilin–BSG binding most likely involves interactions with integrins because the main response observed after cyclophilin treatments is enhancement of cell migration.
GPVI, S100A9 and apolipoprotein D
Other proteins also utilize BSG as their receptors. Platelet glycoprotein VI (GPVI) binds to BSG with a Kd of 88 nM (44). The rolling of ADP-stimulated platelets is significantly enhanced on immobilized BSG-Fc than on Fc. Therefore, the BSG–GPVI interaction may be important for platelet rolling and adhesion.
S100A9 is a component of the heterodimeric protein, calprotectin, which is released during tissue damage and is implicated in inflammation and metastasis. The receptor for S100A9 has been found to be BSG (45). Blockage of BSG expression suppressed the effects of S100A9 in melanoma cells, namely, the promotion of cell migration and enhanced secretion of cytokines and matrix metalloproteinases (MMPs). Therefore, the tumour-promoting effects of calprotectin are mediated by BSG (45). The downstream signalling system utilizes tumour necrosis factor receptor-associated factor 2.
Moreover, apolipoprotein D, which transports small lipophilic molecules, binds to BSG for internalization (46).
Homophilic interactions
The members of the Ig superfamily, to which BSG belongs, frequently exhibit hemophilic associations. However, co-immunoprecipitation of tagged BSG revealed that homophilic association of BSG occurs only in the same membrane (cis-recognition) and is mediated by the D1 domain (47). Nevertheless, transfection with D.melanogester BSG leads to cell aggregation of the recipient cells (48). Interaction of BSG with external BSG (trans-recognition) might happen when BSG forms a dimer (14) or cooperates with another molecule, such as galectin-3. In contrast to conventional BSG, BSG1 appears to exhibit homophilic trans-associations; the Kd value is approximately 40 µM, which is typical for interactions between membrane proteins (49).
Carbohydrate recognition
BSG has three Asn glycosylation sites (20), to which high mannose-type and complex-type glycans are linked (50). High mannose-type glycans are composed of mannose and N-acetylglucosamine, while complex-type glycans have outer chains containing sialic acid, galactose and N-acetylglucosamine. Importantly, poly-N-acetyllactosamine (a repeating structure of galactose and N-acetylglucosamine) is present in an outer chain, the so-called β1,6-branch, of BSG glycans (51). Galectin-3 recognizes poly-N-acetyllactosamine, which is located not only in glycans of BSG but also in those of other proteins including β1-integrin. Therefore, galectin-3 can associate BSG with BSG itself, or with other proteins such as β1-integrin, in both cis and trans manners, and is important for BSG signalling (52, 53).
In the kidney, BSG serves as a major carrier of a carbohydrate ligand for E-selectin, which initiates the first step of neutrophil recruitment (54). BSG also carries a glycan involved in spermatogenesis, which is discussed in the next section (55). Furthermore, the lectin domain of endo180 recognizes glycans of BSG, probably the high mannose-type one (56). Glycans in BSG are also important for the cell-surface expression of BSG (50).
Physiological Activities
Reproduction and development
BSG plays important roles in reproduction and development. Male and female BSG-deficient mice are both sterile (57, 58). Regarding male sterility, most spermatocytes in the deficient mice are arrested at the metaphase of the first meiosis and degenerate. A few spermatocytes differentiate into early spermatids, but none to sperm (57). The molecular basis for this deficit has not been elucidated in detail, and may include impaired MCT actions and failed integrin signalling. Interestingly, BSG is a carrier of a carbohydrate ligand with exposed N-acetylglucosamine (55). This ligand mediates germ cell adhesion to Sertoli cells and is essential for spermatogenesis (59). In BSG-deficient mice, this ligand is greatly decreased (55), raising a possibility that the decrease is a significant reason of male sterility.
Female sterility has been attributed to impaired ability of fertilization and implantation (58). In support of the above conclusion, BSG is strongly expressed in cumulus cells, which aid the function of oocytes, as well as in the endometrial epithelium of the uterus and the trophectoderm of blastocysts, both of which interact at the time of implantation (57, 58). Furthermore, BSG changes gene expression profiles in uterine stromal cells, which are important for implantation (60).
A large portion of BSG-deficient embryos are lethal, and BSG-deficient mice, when born, are small and frequently die before reaching adulthood (57). The major cause of embryonic lethality appears to be failed implantation (57). This deficit is influenced by the genetic background of the deficient embryos (61).
BSG is also important for the development of the meibomian gland, which contributes to maintain the condition of the ocular surface (62), and cell interactions during tooth development necessary for enamel mineralization (63).
The sensory and nervous systems
BSG plays important roles in the function of the sensory and nervous systems. As described before, BSG is essential for the survival and function of photoreceptors through the plasma membrane expression of MCTs in photoreceptors (27, 28) and enhancement of GLUT1 activity in cone photoreceptors (29). Furthermore, the destruction of BSG genes in mice (64) and D.melanogaster (13) leads to insensitivity to irritating odours.
BSG-deficient mice also display various behavioural abnormalities, some, but not all of which can be explained by deficits in sensory organs (65). This finding is consistent with strong expression of BSG in subregions of the brain, including the hippocampal formation and amygdala (66). As the molecular basis of BSG action in the nervous system, the association with MCTs is expected to be also important, as neurons and glial cells cooperate in energy metabolism through the efflux and uptake of lactate. Furthermore, in the synapses of D.melanogaster, BSG is known to be essential for regulating the distribution of synaptic vesicles and vesicle release (48). In BSG mutant flies, these regulatory functions are impaired, with concomitant disturbances in the actin network. A short segment of the juxtamembrane cytoplasmic region (Fig. 3) is required for this BSG function.
Immunological responses
BSG is involved in the regulation of immune responses, as suggested by earlier observations that BSG became strongly expressed in activated lymphocytes (5). Anti-BSG antibodies and BSG-knock down reagents show various effects on lymphocyte activities in vitro (5). Importantly, lymphocytes from BSG-deficient mice are more active in mixed lymphocyte reactions, indicating the suppressive role of BSG in this process (64). Recent studies have revealed that BSG suppresses T-cell receptor-dependent activation of T cells in general and is also involved in T-cell development in the thymus (67). BSG exerts the suppressive effects by inhibiting nuclear factor of activated T-cells (68). Consistent with its suppressive role, BSG is a marker for a subpopulation of activated human regulatory T cells with highly suppressive activity (69). BSG is also important for the recruitment of neutrophils to injured tissues because it is the major receptor for cyclophilins (5) and acts as a carrier of the E-selectin ligand (54).
Diseases
Malignant tumours
BSG is overexpressed in a broad range of human malignant tumours (6, 70). Furthermore, BSG activities in tumour cells are considered to be enhanced by the tumour-associated overexpression of glycans with binding activity to galectin-3 (71), hyaluronan, which is abundant in the tumour microenvironment and is recognized by CD44 (6), and MCT4, which is preferentially expressed in tumour cells and binds to integrins (37). BSG promotes invasive properties, proliferation, and survival of tumour cells (6). Thus, the overexpression of BSG in tumours is generally regarded as an unfavourable prognostic marker (6, 7, 70).
BSG acts through multiple molecular mechanisms in tumour cells. Firstly, it is required for the expression of MCT activities (22), and is also likely to be involved in MCT activation by receiving signals from CD44 and hyaluronan (6). The enhanced activity of MCTs, especially MCT4, is necessary for tumour cells, as they heavily rely on aerobic glycolysis and need to rapidly remove the resulting lactate in order to maintain intracellular pH. Thus, enhanced MCT activity accounts for a significant portion of the growth-, survival- and invasion-promoting activities of BSG in tumour cells (6, 72, 73).
Furthermore, BSG enhances the production of MMPs (74), vascular endothelial growth factor (VEGF) (75) and hyaluronan (6) by tumour cells, contributing to increased migration and proliferation of these cells and angiogenesis. These activities of BSG most likely depend on cis-interaction with integrins and the downstream signalling systems (33) and/or interactions with EGF receptor and the downstream signalling cascade (6, 31).
A detailed study has been performed on the role of BSG in myeloma cells (76). B-cell malignancies such as multiple myeloma frequently colonize bone marrow. This colonization is mediated by cyclophilin A and BSG. Thus, cyclophilin A secreted by endothelial cells in the blood vessels of bone marrow attracts myeloma cells, which strongly express BSG, the major cyclophilin A receptor. In support of the above conclusion, an anti-BSG antibody was found to suppress colonization and proliferation of multiple myeloma cells in an in vivo scaffold system.
BSG in tumour cells is also involved in epithelial mesenchymal transition, the formation of invadopodia and chemoresistance (6). The interaction between BSG and P-glycoprotein may be important for chemoresistance (77).
BSG not only acts within tumour cells but also to adjacent normal cells. As was originally reported, BSG induces the production of MMPs in mesenchymal cells in order to assist tumour invasion (3). It also induces VEGF and its receptor in endothelial cells, thereby enhancing angiogenesis (75, 78). The major active principle in these cases may be a BSG dimer or oligomers (14), which are either shed from the cells after proteolytic cleavage (79) or are present in extracellular vesicles (80). The cell-surface receptor for external BSG has not been established; it may be BSG itself (14) or integrins (33). Furthermore, some other molecules such as galectin-3 (52, 53) may play important roles in the recognition.
As BSG is important for growth, survival and invasion of tumour cells, anti-BSG reagents, such as anti-BSG antibodies, peptide fragments of BSG and siRNAs directed to BSG are being explored as anti-tumour therapeutics (7, 70)
Infectious diseases
BSG also plays critical roles in certain infectious diseases, such as malaria. Erythrocyte invasion is central to the pathogenesis of malaria by Plasmodium falciparum, the most lethal malaria parasite, and is mediated by several molecular interactions between membrane proteins on the parasite and those on erythrocytes (7). RH5 is the only parasite protein that is essential to invasion by all strains of the parasite examined to date. The binding partner of RH5 has been discovered to be BSG (81). The precise structure of the complex between RH5 and BSG has been elucidated, revealing amino acid residues in RH5 essential for binding to BSG (82). This knowledge will assist in developing a potent vaccine to prevent malaria. Furthermore, an anti-BSG antibody may become a therapeutic for drug-resistant malaria, as it was shown to rapidly clear an established P.falciparum blood-stage infection without any overt toxicity in an in vivo infection model (83).
Neisseria meningitidis is a bacterium with the potential to cause meningitis epidemics. After entering the bloodstream, this bacterium may rapidly induce fatal septic shock. Its adhesion to vascular endothelial cells is the initial step in its invasion into the bloodstream and is mediated by interactions between the pilus components of the bacterium and BSG on endothelial cells (84).
BSG also facilitates HIV-1 infection by interacting with cyclophilin A associated with the virus and serves as a receptor for measles virus on epithelial cells by binding to cyclophilin B in the virions (5, 7). Furthermore, BSG enhances the intracellular invasion of Listeria monocytogenes by suppressing NOD2 (40).
Other diseases
Using experimental models, BSG has been shown to be involved in many other diseases, such as rheumatoid arthritis, myocardial infarction, multiple sclerosis and renal fibrosis (5, 7, 85). The most frequently observed route is through the cyclophilin–BSG axis (5), which enhances the migration of inflammatory leukocytes. In addition, the enhanced adhesion of platelets to vascular walls mediated by the GPVI–BSG interaction leads to vascular inflammation and then to atherosclerosis through monocyte recruitment (44). The E-selectin ligand on BSG also contributes to enhanced inflammation (54). Importantly, antibodies to BSG frequently suppress the development of the above-mentioned diseases in models, and thus are promising as therapeutics for these diseases (5, 7).
On the other hand, BSG inhibits the pathogenesis of lupus nephritis by suppressing the development of interleukin-17-producing T cells (86).
Concluding Remarks
BSG serves as receptors for several molecules through trans-recognition using its Ig domains and also interacts with numerous molecules through cis-recognition, which is largely intramembranous. A typical mode of action of BSG is receiving a signal through trans-recognition, and transducing it through cis-recognition, as in the case for the action of RdCVF through BSG1, and probably in the action of cyclophilins. The key to BSG functions is cis-recognition, as illustrated by tight associations with MCTs. Concerning cis-recognition, still much remain to be elucidated, including components of the supramolecular complex containing BSG and the dynamics of their associations.
Funding
The author’s work cited in this review was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and from Japan Society for the Promotion of Science.
Conflict of Interest
None declared.
Glossary
Abbreviations
- BSG
basigin
- EGF
epidermal growth factor
- GLUT
glucose transporter
- GPVI
platelet glycoprotein VI
- Ig
immunoglobulin
- MCT
monocarboxylate transporter
- MMP
matrix metalloproteinase
- RdCVF
rod-derived cone viability factor
- VEGF
vascular endothelial growth factor
References
- 1.Williams A.F., Barclay A.N. (1988) The immunoglobulin superfamily––domains for cell surface recognition. Annu. Rev. Immunol. 6, 381–405 [DOI] [PubMed] [Google Scholar]
- 2.Miyauchi T., Kanekura T., Yamaoka A., Ozawa M., Miyazawa S., Muramatsu T. (1990) Basigin, a new, broadly distributed member of the immunoglobulin superfamily, has strong homology with both the immunoglobulin V domain and the β-chain of major histocompatibility complex class II antigen. J. Biochem. 107, 316–323 [DOI] [PubMed] [Google Scholar]
- 3.Biswas C., Zhang Y., DeCastro R., Guo H., Nakamura T., Kataoka H., Nabeshima K. (1995) The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 55, 434–439 [PubMed] [Google Scholar]
- 4.Muramatsu T., Miyauchi T. (2003) Basigin (CD147): a multifunctional transmembrane protein involved in reproduction, neural function, inflammation and tumor invasion. Histol. Histopathol. 18, 981–987 [DOI] [PubMed] [Google Scholar]
- 5.Yurchenko V., Constant S., Eisenmesser E., Bukrinsky M. (2010) Cyclophilin-CD147 interactions: a new target for anti-inflammatory therapeutics. Clin. Exp. Immunol. 160, 305–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grass G.D., Dai L., Qin Z., Parsons C., Toole B.P. (2014) CD147: regulator of hyaluronan signaling in invasiveness and chemoresistance. Adv. Cancer Res. 123, 351–373 [DOI] [PubMed] [Google Scholar]
- 7.Muramatsu T. (2012) Basigin: a multifunctional membrane protein with an emerging role in infections by malaria parasites. Expert Opin. Ther. Targets 16, 999–1011 [DOI] [PubMed] [Google Scholar]
- 8.Spring F.A., Holmes C.H., Simpson K.L., Mawby W.J., Mattes M.J., Okubo Y., Parsons S.F. (1997) The Oka blood group antigen is a marker for the M6 leukocyte activation antigen, the human homolog of OX-47 antigen, basigin and neurothelin, an immunoglobulin superfamily molecule that is widely expressed in human cells and tissues. Eur. J. Immunol. 27, 891–897 [DOI] [PubMed] [Google Scholar]
- 9.Ozawa M., Huang R.P., Furukawa T., Muramatsu T. (1988) A teratocarcinoma glycoprotein carrying a developmentally regulated carbohydrate marker is a member of the immunoglobulin gene superfamily. J. Biol. Chem. 263, 3059–3062 [PubMed] [Google Scholar]
- 10.Tachikui H., Kurosawa N., Kadomatsu K., Muramatsu T. (1999) Genomic organization and promoter activity of embigin, a member of the immunoglobulin superfamily. Gene 240, 325–332 [DOI] [PubMed] [Google Scholar]
- 11.Beesley P.W., Herrera-Molina R., Smalla K.H., Seidenbecher C. (2014) The Neuroplastin adhesion molecules: key regulators of neuronal plasticity and synaptic function . J. Neurochem. 131, 268–283 [DOI] [PubMed] [Google Scholar]
- 12.Kaname T., Miyauchi T., Kuwano A., Matsuda Y., Muramatsu T., Kajii T. (1993) Mapping basigin (BSG), a member of the immunoglobulin superfamily, to 19p13.3 . Cytogenet. Cell Genet. 64, 195–197 [DOI] [PubMed] [Google Scholar]
- 13.Curtin K.D., Meinertzhagen I.A., Wyman R.J. (2005) Basigin (EMMPRIN/CD147) interacts with integrin to affect cellular architecture . J. Cell Sci. 118, 2649–2660 [DOI] [PubMed] [Google Scholar]
- 14.Belton R.J., Jr., Chen L., Mesquita F.S., Nowak R.A. (2008) Basigin-2 is a cell surface receptor for soluble basigin ligand . J. Biol. Chem. 283, 17805–17814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao C.G., Kong L.M., Song F., Xing J.L., Wang L.X., Sun Z.J., Tang H., Yao H., Zhang Y., Wang L., Wang Y., Yang X.M., Li Y., Chen Z.N. (2011) Characterization of basigin isoforms and the inhibitory function of basigin-3 in human hepatocellular carcinoma proliferation and invasion. Mol. Cell. Biol. 31, 2591–2604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ochrietor J.D., Moroz T.P., van Ekeris L., Clamp M.F., Jefferson S.C., deCarvalho A.C., Fadool J.M., Wistow G., Muramatsu T., Linser P.J. (2003) Retina-specific expression of 5A11/Basigin-2, a member of the immunoglobulin gene superfamily. Invest. Ophthalmol. Vis. Sci. 44, 4086–4096 [DOI] [PubMed] [Google Scholar]
- 17.Yu X.L., Hu T., Du J.M., Ding J.P., Yang X.M., Zhang J., Yang B., Shen X., Zhang Z., Zhong W.D., Wen N., Jiang H., Zhu P., Chen Z.N. (2008) Crystal structure of HAb18G/CD147: implications for immunoglobulin superfamily homophilic adhesion. J. Biol. Chem. 283, 18056–18065 [DOI] [PubMed] [Google Scholar]
- 18.Schlegel J., Redzic J.S., Porter C.C., Yurchenko V., Bukrinsky M., Labeikovsky W., Armstrong G.S., Zhang F., Isern N.G., DeGregori J., Hodges R., Eisenmesser E.Z. (2009) Solution characterization of the extracellular region of CD147 and its interaction with its enzyme ligand cyclophilin A. J. Mol. Biol. 391, 518–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Redzic J.S., Armstrong G.S., Isern N.G., Jones D.N., Kieft J.S., Eisenmesser E.Z. (2011) The retinal specific CD147 Ig0 domain: from molecular structure to biological activity. J. Mol. Biol. 411, 68–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyauchi T., Masuzawa Y., Muramatsu T. (1991) The basigin group of the immunoglobulin superfamily: complete conservation of a segment in and around transmembrane domains of human and mouse basigin and chicken HT7 antigen. J. Biochem. 110, 770–774 [DOI] [PubMed] [Google Scholar]
- 21.Halestrap A.P. (2012) The monocarboxylate transporter family––structure and functional characterization. IUBMB Life 64, 1–9 [DOI] [PubMed] [Google Scholar]
- 22.Kirk P., Wilson M.C., Heddle C., Brown M.H., Barclay A.N., Halestrap A.P. (2000) CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J. 19, 3896–3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson M.C., Meredith D., Fox J.E., Manoharan C., Davies A.J., Halestrap A.P. (2005) Basigin (CD147) is the target for organomercurial inhibition of monocarboxylate transporter isoforms 1 and 4: the ancillary protein for the insensitive MCT2 is EMBIGIN (gp70). J. Biol. Chem. 280, 27213–27221 [DOI] [PubMed] [Google Scholar]
- 24.Mannowetz N., Wandernoth P., Wennemuth G. (2012) Basigin interacts with both MCT1 and MCT2 in murine spermatozoa. J. Cell. Physiol. 227, 2154–2162 [DOI] [PubMed] [Google Scholar]
- 25.Poole R.C., Halestrap A.P. (1997) Interaction of the erythrocyte lactate transporter (monocarboxylate transporter 1) with an integral 70-kDa membrane glycoprotein of the immunoglobulin superfamily. J. Biol. Chem. 272, 14624–14628 [DOI] [PubMed] [Google Scholar]
- 26.Wilson M.C., Meredith D., Halestrap A.P. (2002) Fluorescence resonance energy transfer studies on the interaction between the lactate transporter MCT1 and CD147 provide information on the topology and stoichiometry of the complex in situ. J. Biol. Chem. 277, 3666–3672 [DOI] [PubMed] [Google Scholar]
- 27.Hori K., Katayama N., Kachi S., Kondo M., Kadomatsu K., Usukura J., Muramatsu T., Mori S., Miyake Y. (2000) Retinal dysfunction in basigin deficiency. Invest. Ophthalmol. Vis. Sci. 41, 3128–3133 [PubMed] [Google Scholar]
- 28.Philp N.J., Ochrietor J.D., Rudoy C., Muramatsu T., Linser P.J. (2003) Loss of MCT1, MCT3, and MCT4 expression in the retinal pigment epithelium and neural retina of the 5A11/basigin-null mouse. Invest. Ophthalmol. Vis. Sci. 44, 1305–1311 [DOI] [PubMed] [Google Scholar]
- 29.Ait-Ali N., Fridlich R., Millet-Puel G., Clerin E., Delalande F., Jaillard C., Blond F., Perrocheau L., Reichman S., Byrne L.C., Olivier-Bandini A., Bellalou J., Moyse E., Bouillaud F., Nicol X., Dalkara D., van Dorsselaer A., Sahel J.A., Leveillard T. (2015) Rod-derived cone viability factor promotes cone survival by stimulating aerobic glycolysis. Cell 161, 817–832 [DOI] [PubMed] [Google Scholar]
- 30.Slomiany M.G., Grass G.D., Robertson A.D., Yang X.Y., Maria B.L., Beeson C., Toole B.P. (2009) Hyaluronan, CD44, and emmprin regulate lactate efflux and membrane localization of monocarboxylate transporters in human breast carcinoma cells. Cancer Res. 69, 1293–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grass G.D., Tolliver L.B., Bratoeva M., Toole B.P. (2013) CD147, CD44, and the epidermal growth factor receptor (EGFR) signaling pathway cooperate to regulate breast epithelial cell invasiveness. J. Biol. Chem. 288, 26089–26104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berditchevski F., Chang S., Bodorova J., Hemler M.E. (1997) Generation of monoclonal antibodies to integrin-associated proteins. Evidence that α3β1 complexes with EMMPRIN/basigin/OX47/M6. J. Biol. Chem. 272, 29174–29180 [DOI] [PubMed] [Google Scholar]
- 33.Li Y., Wu J., Song F., Tang J., Wang S.J., Yu X.L., Chen Z.N., Jiang J.L. (2012) Extracellular membrane-proximal domain of HAb18G/CD147 binds to metal ion-dependent adhesion site (MIDAS) motif of integrin β1 to modulate malignant properties of hepatoma cells. J. Biol. Chem. 287, 4759–4772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu D., Hemler M.E. (2005) Metabolic activation-related CD147-CD98 complex. Mol. Cell. Proteomics 4, 1061–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho J.Y., Fox D.A., Horejsi V., Sagawa K., Skubitz K.M., Katz D.R., Chain B. (2001) The functional interactions between CD98, β1-integrins, and CD147 in the induction of U937 homotypic aggregation. Blood 98, 374–382 [DOI] [PubMed] [Google Scholar]
- 36.Khunkaewla P., Schiller H.B., Paster W., Leksa V., Cermak L., Andera L., Horejsi V., Stockinger H. (2008) LFA-1-mediated leukocyte adhesion regulated by interaction of CD43 with LFA-1 and CD147. Mol. Immunol. 45, 1703–1711 [DOI] [PubMed] [Google Scholar]
- 37.Gallagher S.M., Castorino J.J., Philp N.J. (2009) Interaction of monocarboxylate transporter 4 with β1-integrin and its role in cell migration. Am. J. Physiol. Cell. Physiol. 296, C414–C421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reed B.H., Wilk R., Schock F., Lipshitz H.D. (2004) Integrin-dependent apposition of Drosophila extraembryonic membranes promotes morphogenesis and prevents anoikis. Curr. Biol. 14, 372–380 [DOI] [PubMed] [Google Scholar]
- 39.Zhou S., Zhou H., Walian P.J., Jap B.K. (2005) CD147 is a regulatory subunit of the γ-secretase complex in Alzheimer’s disease amyloid β-peptide production. Proc. Natl Acad. Sci. U S A 102, 7499–7504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Till A., Rosenstiel P., Brautigam K., Sina C., Jacobs G., Oberg H.H., Seegert D., Chakraborty T., Schreiber S. (2008) A role for membrane-bound CD147 in NOD2-mediated recognition of bacterial cytoinvasion. J. Cell Sci. 121, 487–495 [DOI] [PubMed] [Google Scholar]
- 41.Moreno V., Gonzalo P., Gomez-Escudero J., Pollan A., Acin-Perez R., Breckenridge M., Yanez-Mo M., Barreiro O., Orsenigo F., Kadomatsu K., Chen C.S., Enriquez J.A., Dejana E., Sanchez-Madrid F., Arroyo A.G. (2014) An EMMPRIN-γ-catenin-Nm23 complex drives ATP production and actomyosin contractility at endothelial junctions. J. Cell Sci. 127, 3768–3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yurchenko V., Zybarth G., O’Connor M., Dai W.W., Franchin G., Hao T., Guo H., Hung H.C., Toole B., Gallay P., Sherry B., Bukrinsky M. (2002) Active site residues of cyclophilin A are crucial for its signaling activity via CD147. J. Biol. Chem. 277, 22959–22965 [DOI] [PubMed] [Google Scholar]
- 43.Pakula R., Melchior A., Denys A., Vanpouille C., Mazurier J., Allain F. (2007) Syndecan-1/CD147 association is essential for cyclophilin B-induced activation of p44/42 mitogen-activated protein kinases and promotion of cell adhesion and chemotaxis. Glycobiology 17, 492–503 [DOI] [PubMed] [Google Scholar]
- 44.Seizer P., Borst O., Langer H.F., Bultmann A., Munch G., Herouy Y., Stellos K., Kramer B., Bigalke B., Buchele B., Bachem M.G., Vestweber D., Simmet T., Gawaz M., May A.E. (2009) EMMPRIN (CD147) is a novel receptor for platelet GPVI and mediates platelet rolling via GPVI-EMMPRIN interaction. Thromb. Haemost. 101, 682–686 [DOI] [PubMed] [Google Scholar]
- 45.Hibino T., Sakaguchi M., Miyamoto S., Yamamoto M., Motoyama A., Hosoi J., Shimokata T., Ito T., Tsuboi R., Huh N.H. (2013) S100A9 is a novel ligand of EMMPRIN that promotes melanoma metastasis. Cancer Res. 73, 172–183 [DOI] [PubMed] [Google Scholar]
- 46.Najyb O., Brissette L., Rassart E. (2015) Apolipoprotein D internalization is a basigin-dependent mechanism. J. Biol. Chem. 290, 16077–16087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshida S., Shibata M., Yamamoto S., Hagihara M., Asai N., Takahashi M., Mizutani S., Muramatsu T., Kadomatsu K. (2000) Homo-oligomer formation by basigin, an immunoglobulin superfamily member, via its N-terminal immunoglobulin domain. Eur. J. Biochem. 267, 4372–4380 [DOI] [PubMed] [Google Scholar]
- 48.Besse F., Mertel S., Kittel R.J., Wichmann C., Rasse T.M., Sigrist S.J., Ephrussi A. (2007) The Ig cell adhesion molecule Basigin controls compartmentalization and vesicle release at Drosophila melanogaster synapses. J. Cell Biol. 177, 843–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanna S.M., Kirk P., Holt O.J., Puklavec M.J., Brown M.H., Barclay A.N. (2003) A novel form of the membrane protein CD147 that contains an extra Ig-like domain and interacts homophilically. BMC Biochem. 4, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang W., Luo W.J., Zhu P., Tang J., Yu X.L., Cui H.Y., Wang B., Zhang Y., Jiang J.L., Chen Z. N. (2013) Modulation of CD147-induced matrix metalloproteinase activity: role of CD147 N-glycosylation. Biochem. J. 449, 437–448 [DOI] [PubMed] [Google Scholar]
- 51.Tang W., Chang S.B., Hemler M.E. (2004) Links between CD147 function, glycosylation, and caveolin-1. Mol. Biol. Cell 15, 4043–4050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Priglinger C.S., Szober C.M., Priglinger S.G., Merl J., Euler K.N., Kernt M., Gondi G., Behler J., Geerlof A., Kampik A., Ueffing M., Hauck S.M. (2013) Galectin-3 induces clustering of CD147 and integrin-β1 transmembrane glycoprotein receptors on the RPE cell surface. PLoS One 8, e70011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mauris J., Woodward A.M., Cao Z., Panjwani N., Argueso P. (2014) Molecular basis for MMP9 induction and disruption of epithelial cell-cell contacts by galectin-3. J. Cell Sci. 127, 3141–3148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kato N., Yuzawa Y., Kosugi T., Hobo A., Sato W., Miwa Y., Sakamoto K., Matsuo S., Kadomatsu K. (2009) The E-selectin ligand basigin/CD147 is responsible for neutrophil recruitment in renal ischemia/reperfusion. J. Am. Soc. Nephrol. 20, 1565–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bi J., Li Y., Sun F., Saalbach A., Klein C., Miller D.J., Hess R., Nowak R.A. (2013) Basigin null mutant male mice are sterile and exhibit impaired interactions between germ cells and Sertoli cells. Dev. Biol. 380, 145–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodriguez-Teja M., Gronau J.H., Minamidate A., Darby S., Gaughan L., Robson C., Mauri F., Waxman J., Sturge J. (2015) Survival outcome and EMT suppression mediated by a lectin domain interaction of Endo180 and CD147. Mol. Cancer Res. 13, 538–547 [DOI] [PubMed] [Google Scholar]
- 57.Igakura T., Kadomatsu K., Kaname T., Muramatsu H., Fan Q.W., Miyauchi T., Toyama Y., Kuno N., Yuasa S., Takahashi M., Senda T., Taguchi O., Yamamura K., Arimura K., Muramatsu T. (1998) A null mutation in basigin, an immunoglobulin superfamily member, indicates its important roles in peri-implantation development and spermatogenesis. Dev. Biol. 194, 152–165 [DOI] [PubMed] [Google Scholar]
- 58.Kuno N., Kadomatsu K., Fan Q.W., Hagihara M., Senda T., Mizutani S., Muramatsu T. (1998) Female sterility in mice lacking the basigin gene, which encodes a transmembrane glycoprotein belonging to the immunoglobulin superfamily. FEBS Lett. 425, 191–194 [DOI] [PubMed] [Google Scholar]
- 59.Akama T.O., Nakagawa H., Sugihara K., Narisawa S., Ohyama C., Nishimura S., O’Brien D.A., Moremen K.W., Millan J.L., Fukuda M.N. (2002) Germ cell survival through carbohydrate-mediated interaction with Sertoli cells. Science 295, 124–127 [DOI] [PubMed] [Google Scholar]
- 60.Chen L., Belton R.J., Jr., Nowak R.A. (2009) Basigin-mediated gene expression changes in mouse uterine stromal cells during implantation. Endocrinology 150, 966–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen S., Kadomatsu K., Kondo M., Toyama Y., Toshimori K., Ueno S., Miyake Y., Muramatsu T. (2004) Effects of flanking genes on the phenotypes of mice deficient in basigin/CD147. Biochem. Biophys. Res. Commun. 324, 147–153 [DOI] [PubMed] [Google Scholar]
- 62.Mauris J., Dieckow J., Schob S., Pulli B., Hatton M.P., Jeong S., Bauskar A., Gabison E., Nowak R., Argueso P. (2015) Loss of CD147 results in impaired epithelial cell differentiation and malformation of the meibomian gland. Cell Death Dis. 6, e1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khaddam M., Huet E., Vallee B., Bensidhoum M., Le Denmat D., Filatova A., Jimenez-Rojo L., Ribes S., Lorenz G., Morawietz M., Rochefort G.Y., Kiesow A., Mitsiadis T.A., Poliard A., Petzold M., Gabison E.E., Menashi S., Chaussain C. (2014) EMMPRIN/CD147 deficiency disturbs ameloblast-odontoblast cross-talk and delays enamel mineralization. Bone 66, 256–266 [DOI] [PubMed] [Google Scholar]
- 64.Igakura T., Kadomatsu K., Taguchi O., Muramatsu H., Kaname T., Miyauchi T., Yamamura K., Arimura K., Muramatsu T. (1996) Roles of basigin, a member of the immunoglobulin superfamily, in behavior as to an irritating odor, lymphocyte response, and blood-brain barrier. Biochem. Biophys. Res. Commun. 224, 33–36 [DOI] [PubMed] [Google Scholar]
- 65.Naruhashi K., Kadomatsu K., Igakura T., Fan Q.W., Kuno N., Muramatsu H., Miyauchi T., Hasegawa T., Itoh A., Muramatsu T., Nabeshima T. (1997) Abnormalities of sensory and memory functions in mice lacking BSG gene. Biochem. Biophys. Res. Commun. 236, 733–737 [DOI] [PubMed] [Google Scholar]
- 66.Fan Q.W., Yuasa S., Kuno N., Senda T., Kobayashi M., Muramatsu T., Kadomatsu K. (1998) Expression of basigin, a member of the immunoglobulin superfamily, in the mouse central nervous system. Neurosci. Res. 30, 53–63 [DOI] [PubMed] [Google Scholar]
- 67.Yao H., Teng Y., Sun Q., Xu J., Chen Y.T., Hou N., Cheng X., Yang X., Chen Z.N. (2014) Important functional roles of basigin in thymocyte development and T cell activation. Int. J. Biol. Sci. 10, 43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ruiz S., Castro-Castro A., Bustelo X.R. (2008) CD147 inhibits the nuclear factor of activated T-cells by impairing Vav1 and Rac1 downstream signaling. J. Biol. Chem. 283, 5554–5566 [DOI] [PubMed] [Google Scholar]
- 69.Solstad T., Bains S.J., Landskron J., Aandahl E.M., Thiede B., Tasken K., Torgersen K.M. (2011) CD147 (Basigin/Emmprin) identifies FoxP3+CD45RO+CTLA4+-activated human regulatory T cells. Blood 118, 5141–5151 [DOI] [PubMed] [Google Scholar]
- 70.Kanekura T., Chen X. (2010) CD147/basigin promotes progression of malignant melanoma and other cancers. J. Dermatol. Sci. 57, 149–154 [DOI] [PubMed] [Google Scholar]
- 71.Partridge E.A., Le Roy C., Di Guglielmo G.M., Pawling J., Cheung P., Granovsky M., Nabi I.R., Wrana J.L., Dennis J.W. (2004) Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science 306, 120–124 [DOI] [PubMed] [Google Scholar]
- 72.Baba M., Inoue M., Itoh K., Nishizawa Y. (2008) Blocking CD147 induces cell death in cancer cells through impairment of glycolytic energy metabolism. Biochem. Biophys. Res. Commun. 374, 111–116 [DOI] [PubMed] [Google Scholar]
- 73.Le Floch R., Chiche J., Marchiq I., Naiken T., Ilc K., Murray C.M., Critchlow S.E., Roux D., Simon M.P., Pouyssegur J. (2011) CD147 subunit of lactate/H+ symporters MCT1 and hypoxia-inducible MCT4 is critical for energetics and growth of glycolytic tumors. Proc. Natl. Acad. Sci. U S A 108, 16663–16668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun J., Hemler M. E. (2001) Regulation of MMP-1 and MMP-2 production through CD147/extracellular matrix metalloproteinase inducer interactions. Cancer Res. 61, 2276–2281 [PubMed] [Google Scholar]
- 75.Tang Y., Nakada M.T., Kesavan P., McCabe F., Millar H., Rafferty P., Bugelski P., Yan L. (2005) Extracellular matrix metalloproteinase inducer stimulates tumor angiogenesis by elevating vascular endothelial cell growth factor and matrix metalloproteinases. Cancer Res. 65, 3193–3199 [DOI] [PubMed] [Google Scholar]
- 76.Zhu D., Wang Z., Zhao J.J., Calimeri T., Meng J., Hideshima T., Fulciniti M., Kang Y., Ficarro S.B., Tai Y.T., Hunter Z., McMilin D., Tong H., Mitsiades C.S., Wu C.J., Treon S.P., Dorfman D.M., Pinkus G., Munshi N.C., Tassone P., Marto J.A., Anderson K.C., Carrasco R.D. (2015) The Cyclophilin A-CD147 complex promotes the proliferation and homing of multiple myeloma cells. Nat. Med. 21, 572–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang W.J., Li Q.Q., Xu J.D., Cao X.X., Li H.X., Tang F., Chen Q., Yang J.M., Xu Z.D., Liu X.P. (2008) Interaction between CD147 and P-glycoprotein and their regulation by ubiquitination in breast cancer cells. Chemotherapy 54, 291–301 [DOI] [PubMed] [Google Scholar]
- 78.Bougatef F., Quemener C., Kellouche S., Naimi B., Podgorniak M.P., Millot G., Gabison E.E., Calvo F., Dosquet C., Lebbe C., Menashi S., Mourah S. (2009) EMMPRIN promotes angiogenesis through hypoxia-inducible factor-2α-mediated regulation of soluble VEGF isoforms and their receptor VEGFR-2. Blood 114, 5547–5556 [DOI] [PubMed] [Google Scholar]
- 79.Hatanaka M., Higashi Y., Fukushige T., Baba N., Kawai K., Hashiguchi T., Su J., Zeng W., Chen X., Kanekura T. (2014) Cleaved CD147 shed from the surface of malignant melanoma cells activates MMP2 produced by fibroblasts. Anticancer Res. 34, 7091–7096 [PubMed] [Google Scholar]
- 80.Redzic J.S., Kendrick A.A., Bahmed K., Dahl K.D., Pearson C.G., Robinson W.A., Robinson S.E., Graner M.W., Eisenmesser E.Z. (2013) Extracellular vesicles secreted from cancer cell lines stimulate secretion of MMP-9, IL-6, TGF-β1 and EMMPRIN. PLoS One 8, e71225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Crosnier C., Bustamante L.Y., Bartholdson S.J., Bei A.K., Theron M., Uchikawa M., Mboup S., Ndir O., Kwiatkowski D.P., Duraisingh M.T., Rayner J.C., Wright G.J. (2011) Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature 480, 534–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wright K.E., Hjerrild K.A., Bartlett J., Douglas A.D., Jin J., Brown R.E., Illingworth J.J., Ashfield R., Clemmensen S.B., de Jongh W.A., Draper S.J., Higgins M.K. (2014) Structure of malaria invasion protein RH5 with erythrocyte basigin and blocking antibodies. Nature 515, 427–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zenonos Z.A., Dummler S.K., Muller-Sienerth N., Chen J., Preiser P.R., Rayner J.C., Wright G.J. (2015) Basigin is a druggable target for host-oriented antimalarial interventions. J. Exp. Med. 212, 1145–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bernard S.C., Simpson N., Join-Lambert O., Federici C., Laran-Chich M.P., Maissa N., Bouzinba-Segard H., Morand P.C., Chretien F., Taouji S., Chevet E., Janel S., Lafont F., Coureuil M., Segura A., Niedergang F., Marullo S., Couraud P.O., Nassif X., Bourdoulous S. (2014) Pathogenic Neisseria meningitidis utilizes CD147 for vascular colonization. Nat. Med. 20, 725–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kato N., Kosugi T., Sato W., Ishimoto T., Kojima H., Sato Y., Sakamoto K., Maruyama S., Yuzawa Y., Matsuo S., Kadomatsu K. (2011) Basigin/CD147 promotes renal fibrosis after unilateral ureteral obstruction. Am. J. Pathol. 178, 572–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maeda K., Kosugi T., Sato W., Kojima H., Sato Y., Kamimura D., Kato N., Tsuboi N., Yuzawa Y., Matsuo S., Murakami M., Maruyama S., Kadomatsu K. (2015) CD147/Basigin limits lupus nephritis and Th17 cell differentiation in mice by inhibiting the interleukin-6/STAT-3 pathway. Arthritis Rheum. 67, 2185–2195 [DOI] [PubMed] [Google Scholar]