Abstract
Purpose
Conventional prostheses used for inguinal hernia repair are static and passive. This feasibility-study shows the features of a new 3D tension-free prosthesis in an experimental model.
Methods
This study was divided into two-phases: 1) aimed to test the physics intrinsic features and the anatomical adaptability of a new 3D designed mesh, and 2) aimed to evaluate the inflammatory reaction associated with different materials used. On phase-1 implantations were performed in pigs. During the first trial phase, the prostheses were also implanted on human cadavers. On phase-2, implantation was carried out on large swine. Follow-up was of 60-days, after which the animals were anaesthetized for laparoscopic assessment, and for sample collection of mesh implantation site for histological analysis.
Results
All animals showed good 3D mesh tolerance, and the follow-up period was uneventful. The laparoscopy showed no inflammatory lesions on the internal surface of the peritoneum. Macroscopic observation of implantation site revealed some local fibrosis and reorganization of tissue, no signs of infection, and no changes on original implant positioning. Histological analysis on phase-1 showed in most sample segments the deferent duct maintaining its central position and surrounded by vascular and nervous structures. On phase-2 differences in inflammatory lesion score could be found between subjects.
Conclusions
This new 3D mesh can be placed appropriately and from this preliminary animal study no untoward complications were noted over a 60 day period.
Keywords: Inguinal hernia, Dynamic design, 3D mesh, Experimental feasibility-study
Highlights
-
•
Feasibility-study to test a new-3D tension-free prosthesis in an experimental model.
-
•
All animals showed good mesh tolerance and the followup period was uneventful.
-
•
Endoscopic and histologic analysis were reported.
1. Introduction
Inguinal hernia repair is one of the most frequently performed surgical procedures. In the USA alone, over 800.000 hernia repairs are performed every year [1], [2], [3], [4], [5], [6]. By examining the literature, and drawing upon over 30 years of surgical experience, we began to question three key problems in inguinal hernia repair: 1) the complications consequent to invasive fixation, 2) the poor quality of tissue ingrowth induced by the mesh, and 3) the early mesh dislocation after implantation [7], [8], [9]. Working closely with a team of French-Spanish biomechanical engineers, we designed an implant that would translate outward visceral forces to cause a dynamic implant deformation that would induce greater gripping forces of the inguinal area. The main purpose of this preliminary experimental porcine and human cadaver study is to demonstrate the feasibility of implantation and the inflammation response of this new 3D mesh.
1.1. Material and methods
All surgical procedures were carried out at certified animal experimental facilities in Spain, after previous official approval by the internal Ethics and Animal Welfare Committee. All animals used in these experiments received humane care in accordance with the requirements of the European Parliament Directive 2010/63/UE, of 22nd September 2010, and the national Royal Decree 1201/2005 of 10th October, on protection of animals used for scientific research. This experimental study was divided in two phases, carried out 5 months apart. On the first phase (phase1) we aimed at testing a new 3D design of a mesh prosthesis intended for direct and indirect inguinal hernia repair by anterior open surgical approach. On the later phase (phase 2) the purposed was to determine if the mesh material of the prosthesis could modify the tissue inflammatory and foreign body reaction.
1.1.1. Study subjects
On phase 1 implantation was performed on 3 Large White intact male pigs, weighing an average of 74 ± 1,8 kg. During the first trial phase, 3D mesh prostheses were also implanted on two human cadavers, for evaluation of pliability and material intrinsic memory, as well as design adaptation to the human anatomy.
On phase 2, implantation was carried out on 5 intact Large White male swine, with an average weight of 102 ± 8,6 kg.
1.1.2. Anaesthesia and analgesia
Animals were firstly sedated by intramuscular injection of ketamine (20 mg/kg) in association with diazepam (0.5 mg/kg). Peripheral venous access was established through the insertion of a 20G catheter in the marginal vein of the ear. Anaesthetic induction was then achieved with propofol (3 mg/kg) administered intravenously (IV). Tracheal intubation and anaesthetic maintenance with sevoflurane (0,5 L/min, MAC 2,6) was possible with the use of a mechanical ventilator connected to the anaesthetic station [10], [11].
Preoperative intravenous analgesia was accomplished with IV injection of ketorolac (1 mg/kg) in association with tramadol hydrochloride (1,5 mg/kg). During the entire procedure animals were infused with saline solution at a rate of 5 ml/kg/h. Before starting the surgical procedure and at sacrifice on both study phases, blood was drawn from the jugular vein, and blood analysis was conducted, in order to verify the animals' adequate general health condition.
Before initiating the procedure an intramuscular injection of amoxicillin-clavulanic acid (Synulox® – 1 ml/20 kg) was administered to each animal. At the end of the implantation and after closing both bilateral skin incisions, a slow release fentanyl patch (Duragesic®, Janssen Pharmaceuticals) was applied to the clean skin of the inner thigh. After recovery, buprenorphine (Buprex® – 1 ml/30 kg) was injected intramuscularly approximately 8 h after the beginning of implantation, in order to provide sufficient analgesia until initial release from the applied patch. All animals received additional anti-inflammatory therapy with meloxicam (Metacam® – 0.025 ml/kg) once a day for three consecutive days, and adjuvant antibiotics therapy with an oral formula of amoxicillin-clavulanic acid at a dose of 20 mg/kg every 12 h for five consecutive days.
With the conclusion of the surgical procedure, spontaneous ventilation was promoted by switching off the anaesthetic inflow of gas and maintaining mechanical inflow of O2. This inflow was interrupted when patients showed spontaneous respiratory efforts.
1.1.3. Implanted mesh prostheses
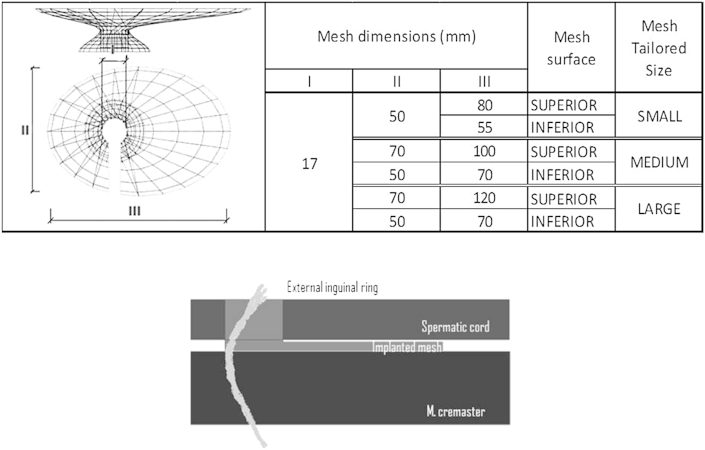
All 3D mesh prosthesis implanted on the first phase were Surgimesh™ Xlight™ (knitted polypropylene, 28 g/m2 Aspide Médical®, Lyon, France), and three mesh sizes were used (Fig. 1).
Fig. 1.
Dimensions of 3D phase 1 inguinal hernia mesh implants (up).Three-Dimensional schematic view of the prosthesis (above: elliptical portion, spermatic cord portion, below: small ring portion) (below).
On phase 2 different mesh materials were implanted (Table 1). According to the animals' own anatomy, the extent of mesh was sometimes shortened in its longitudinal axis. On each animal, a different mesh material was used, and fixation was achieved on the right side by three polypropylene sutures, applied to ventral, lateral and medial sides (Surgipro™ 2/0, 26 mm, partially absorbable material). As for the left groin no fixation was performed. The objective of this fixation (stitches) was to compare in the same animal the influence of various materials (mesh plus polypropylene stitches) on the inflammation response. Infact, in pigs, the inflammatory reaction is variables, therefore, in this way we decreased the variability of the results. In this study, we performed 3 stitches as maximum type of fixation. With the new 3D mesh the fixation is unnecessary, however, in this experimental model we tried adding polypropylene suture to achieve the maximal inflammatory reaction.
Table 1.
Weight of the meshes used on phase 2 implantation.
| Animal | Mesh material | Weight g/m2 |
|---|---|---|
| 1 | Surgimesh™ WN non woven non knitted | 80 |
| 2 | Surgimesh™ 1 knitted | 75 |
| 3 | Surgimesh™ XB silicone PP layer | 300 |
| 4 | Surgimesh™ Xlight™ knitted polypropylene | 28 |
| 5 | Surgimesh™ absorbable (Polip + PLA) | 45 |
1.1.4. Surgical procedure
All subjects were placed in dorsal recumbency, and skin as well as subcutaneous incision was performed on the left groin. Muscle layers and external inguinal ring were later incised. Dissection at this point was performed respecting inguinal and genitofemoral nerves. Procedure was then carried on with the dissection of the spermatic cord and enlargement of the pre peritoneal space so that the mesh could be correctly spread against the peritoneum. A constrained 3D prosthesis was then inserted and its small ring was placed in the pre peritoneal area, surrounding the spermatic cord. Superior aspect of the mesh (elliptical portion) stayed unfolded above inguinal floor and below the spermatic cord (Fig. 1). After implantation, suture of surgical planes was completed in standard fashion [5]. Any stitches was applied to fix the prosthesis.
All pre and intra operative aspects of phase 2 implantation were similar to the first phase of the study, and post-operative treatment followed as previously described.
1.1.5. Follow-up
Follow up for both study phases lasted for 60 days, after which the animals were anaesthetized for laparoscopic assessment of intra abdominal lesions and macroscopic analysis. Sample collection at implantation site was completed and samples sent for posterior histological analysis. Macroscopic examination at implantation site was carried out after incision of the previously operated area and before removal of spermatic cord and surrounding tissue. Observation of signs of inflammation, infection or mesh displacement was considered at explantation. Animals were sacrificed by an intravenous injection of potassium chloride, after sample collection, and while still under anaesthesia. Histological analysis and lesion scoring were performed by a pathologist in a single-blind manner (Table 2).
Table 2.
Histological grading lesion score. (TRI and PSR: histological colorations).
| 0 | 1 | 2 | 3 | 4 | |
|---|---|---|---|---|---|
| Deferent duct lesions | Normal | Wall reduction | Flattening of epithelium and wall reduction | Moderate lesions | Severe lesions with dilation and loss of structures |
| Inflammation (PMN, eosinophils, lymphocytes, plasma cells, macrophages 40x) |
0 | 1–5 cells | 5–10 cells | Important | Concentrated cells |
| Granulomas | 0 | 1 | 1–2 | 3–5 | >5 |
| Giant cells | 0 | 1–2 | 3–5 | Moderate | Severe |
| Necrosis | 0 | Minimal | Mild | Moderate | Severe |
| Neovascularization | 0 | 1–3 capillaries | 4–7 capillaries and connective tissue | Capillary bands and connective tissue | Major Neovascularization areas |
| Fibrosis (TRI) | 0 | Thin bands | Moderate bands | Dense bands | Major fibrosis areas |
| Presence of hyaline substance (PSR) | 0 | Minimal | Moderate | Marked | Very severe |
1.1.6. Histological analysis
For histological analysis, different strategies were adopted to obtain the appropriate tissue sample according to the mesh design applied on each phase. Thus, on phase 1, we retrieved a major sample for each groin, which included foreign mesh material and the entire structure of the spermatic cord external to the peritoneum. Afterwards this main sample was divided into three distinct segments: one anterior, one medial which included the central part of the 3D implant (through which passes the deferent duct and spermatic vessels), and one posterior segment. On phase 2, we considered that there was no need to divide the main sample in three different portions, and so we obtained only two segments of each sample for analysis: one anterior (which corresponds with the medial segment of phase 1) and another one posterior.
Staining was performed with hematoxylin-eosin and, for collagen deposition, samples were stained with picrosirius red and Masson's trichrome. Inflammatory lesion scoring was completed according to an adaptation of the Annex E of the ISO 10993-6, in which the pathologist added new parameters concerning deferent duct lesion and foreign body reaction (presence of granulomas and giant multinucleated cells). Score variation for each parameter (deferent duct lesion, presence of inflammatory cells infiltrate, presence of granulomas, presence of giant multinucleated cells, presence of necrosis, level of neovascularization, degree of fibrosis, and amount of hyaline substance) goes from 0 to 4, with a maximum lesion score of 32 points for each sample segment (Table 2).
1.1.7. Statistical analysis
All data is presented as mean ± standard deviation if not otherwise mentioned. Statistical analysis was carried out using SPSS™ v15.0 for Windows (SPSS, Inc., IBM Corporation, Chicago, USA. P values of less than 0,05 were considered statistically significant.
1.2. Results
1.2.1. Macroscopic findings
All animals showed good tolerance of the surgical procedure, and the follow-up period was uneventful, with no signs of local ischemia or pain due to surgery. Haemogram and blood biochemical indicators showed no deviation from normal values, neither before nor after surgery or at follow-up.
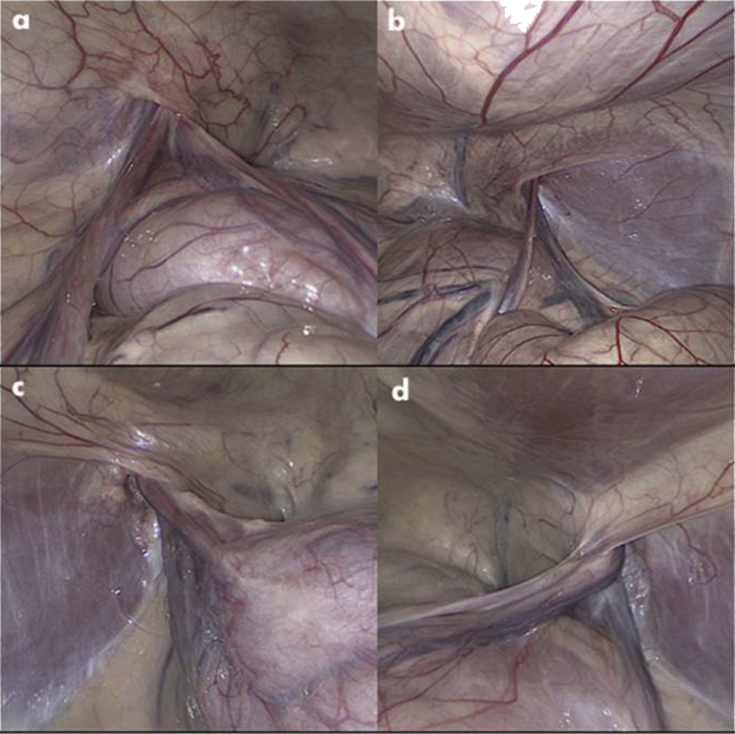
Internal images obtained by laparoscopy showed no inflammatory lesions on the internal surface of the peritoneum around the area of the internal inguinal ring. There were also no adhesions or signs of infection ((Fig. 2(a, b, c, d)).
Fig. 2.
a,b,c,d: Laparoscopic images of the internal inguinal area.
Macroscopic observation of implantation site revealed presence of local fibrosis and signs of tissue reorganization, no macroscopic signs of infection, and no changes on original implant positioning. Superficial inguinal nodes were found enlarged in most cases. On the first, second and third animal of phase 2, and all three animals used on phase 1, direct observation of mesh material was possible.
1.2.2. Mesh design assessment
Cadaver implantation on phase 1 was successful and uneventful. Implanted prosthesis showed high degree of malleability, and 3D design led the surgeon to intuitive adequate pre peritoneal placement of the mesh (small ring) around the spermatic cord.
In order to determine the influence of mesh design, we compared the six medial sample segments' degree of inflammatory lesion (21,17 ± 2,56) from phase 1, with the two anterior sample segments of the fourth animal implanted on phase 2 (19,50 ± 2,12). No statistically significant differences were found between lesion scores.
1.2.3. Histological findings
On Table 3 the numeric and the histological grading score of inflammatory lesion for each sample can be consulted.
Table 3.
*Inflammatory lesion scores for each sample. With anterior term we considered the more internal portion where the mesh is placed (around the spermatic cord). With the posterior, we considered the more external portion where the mesh is placed.
| Phase 1 | Animal 1 | Left side | Anterior | 15* | Phase 2 | Animal 1 | Left side | Anterior | 16 |
| Medial | 20 | Posterior | 17 | ||||||
| Posterior | 11 | Right side | Anterior | 15 | |||||
| Right side | Anterior | 16 | Posterior | 13 | |||||
| Medial | 19 | Animal 2 | Left side | Anterior | 10 | ||||
| Posterior | 1 | Posterior | 12 | ||||||
| Animal 2 | Left side | Anterior | 19 | Right side | Anterior | 17 | |||
| Medial | 18 | Posterior | 10 | ||||||
| Posterior | 18 | Animal 3 | Left side | Anterior | 6 | ||||
| Right side | Anterior | 21 | Posterior | 6 | |||||
| Medial | 24 | Right side | Anterior | 12 | |||||
| Posterior | 8 | Posterior | 12 | ||||||
| Animal 3 | Left side | Anterior | 17 | Animal 4 | Left side | Anterior | 18 | ||
| Medial | 22 | Posterior | 22 | ||||||
| Posterior | 10 | Right side | Anterior | 21 | |||||
| Right side | Anterior | 24 | Posterior | 20 | |||||
| Medial | 24 | Animal 5 | Left side | Anterior | 9 | ||||
| Posterior | 13 | Posterior | 9 | ||||||
| Right side | Anterior | 14 | |||||||
| Posterior | 11 | ||||||||
Histological analyses of the 6 samples (18 segments) on phase 1 were similar. They showed the deferent duct maintaining its central position and surrounded by vascular and nervous structures, in most animals and most sample segments. A granulomatous inflammatory reaction was present externally to these structures and a small degree of fibrosis and a high amount of hyaline substance were observed, indicating acute scarring and foreign body reaction. Major neo-vascularization occurred in many segments, and in 30% of obtained samples a certain degree of duct lesion could be assessed. Necrosis in variable degrees was present in two of the sample segments.
On the other hand, on phase 2, inflammatory lesion differences could be found between subjects. On the first implanted animal, granulomas appeared large in size, forming a defined band around the spermatic cord. Inflammatory cells around and inside cavities of foreign material were abundant and included giant multinucleated cells. Angiogenesis did not appear notable. Areas of dense fibrosis could be observed, alternating with areas of hyaline degeneration.
On the second animal, small size granulomas could be found surrounding the entire spermatic cord. These granulomas were formed by several cell layers around empty cavities, and there was a high degree of angiogenesis, especially in anterior segments. Spermatozoids were observed inside the deferent duct of this animal.
The third implant was the one which generated the smallest inflammatory response. A large cystic cavity could be observed close to the spermatic cord, with some macrophages and giant cells in its walls.
Inflammatory infiltrates were scarce, as was the degree of neovascularization. There was a predominance of fibrosis with abundant collagen.
For the fourth implantation, we used the same mesh material as in the first phase of this study. In these samples, a large number of medium size granulomas could be found surrounding empty cavities adjacent to the spermatic cord. Infiltrating inflammatory cells were scarce, but granulomas were highly cellular with an elevated percentage of giant multinucleated cells. Neovascularization was severe, with large areas of numerous vessels. Collagen and hyaline substance deposition was found variable, with the latter being more intense in both segments of the right spermatic cord.
On the fifth animal, mesh material was made of a new combination of polypropylene and polylactic acid, with semi absorbable characteristics. These samples showed a low number of small size granulomas around the spermatic cord, mostly formed of only one to two cell layers of macrophages, lymphocytes and few giant cells. Fibrosis with collagen deposition was more significant than the presence of hyaline degeneration.
On phase 2, we proceeded with the comparison of the different mesh materials. A Kruskal-Wallis test was used and applied to the sum of anterior and posterior lesion score of each groin, and no statistical differences were found. With the same test, we compared anterior lesion scores, and on a third trial posterior lesion scores, with no statistical significance between groups. Lesion scores between analyzed sample segments showed great variation when individually observed, according to each prosthesis composition (Table 2). Relationship between the inflammatory reaction and the different material used is summarized in the Table 4. Fig. 3, show the histological findings with the new 3D mesh under trial.
Table 4.
Lesion score of the different materials used.
| Order | Animal | Mesh | Average lesion score | Left side | Right side |
|---|---|---|---|---|---|
| 1 | P11-0858 | Surgimesh 1 knited | 12,25 | 11 | 13,5 |
| 2 | P11-0856 | Surgimesh xlight knited polipropylene | 20,25 | 20 | 20,5 |
| 3 | P11-0857 | Surgimesh XB silicona PP layer | 9 | 6 | 12 |
| 4 | P11-0804 | Surgimesh WN non woven non knited | 15,25 | 16,5 | 14 |
| 5 | P11-0803 | Surgimesh absorbable (polip + PLA) | 10,75 | 9 | 12,5 |
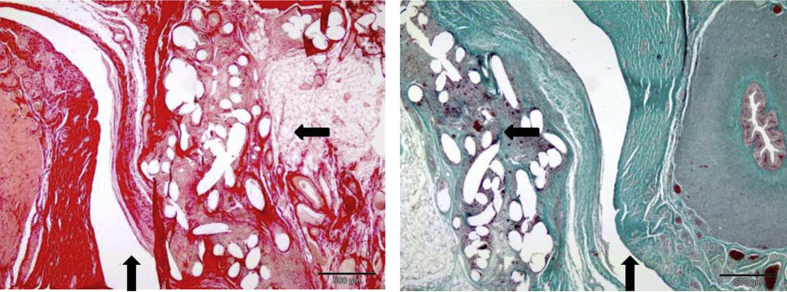
Fig. 3.
Histological image ((picrosirius red (left) and Masson's trichrome (right)) of the novel 3D mesh under trial. Spermatic duct (⇑) and the granulomatous tissue reaction (⇦).
A Kruskal-Wallis test was used and significant differences were found between the five groups. After applying a Mann-Whitney U test to each paired sampled we obtained significant differences between meshes 1, 3, 4 and 5, also between meshes 2 and 4, meshes 3 and 4, and finally between meshes 4 and 5.
2. Discussion
This novel 3D inguinal mesh is different from the others for two main reasons: 1) the intrinsic structure (lightweight) is quite different from the others meshes: knitted polypropylene, 28 g/m2; 2) the 3D shape of this mesh is original (Figure), to achieve a high degree of malleability, led the surgeon to intuitive and easy pre-peritoneal placement of the mesh. Therefore, considering these new important features, we decided to evaluate the mesh in animal and human cadavers first. Thus, has permitted to modify the final features of the mesh, achieving a prosthesis easier to manipulate and easier to implant.
The mesh recently achieved the CE mark and is commercialized for human use. A clinical human study is in progress (till now 400 patients were included in 3 countries).
Based upon the literature, some complications in hernia repair such as bleeding, hematoma, and mesh dislocation are usually caused by invasive fixation of implants [5], [10], [12]. Reduction of fixation and improvement in tissue growth could lead to a reduction in complication rates in inguinal hernia [16], [17], [18]. To achieve an auto-adjusting implant, the authors worked with biomechanical engineers to design an implant that would specifically translate visceral expulsion forces into radial gripping forces. This allows the prosthesis to be deployed in a constrained state and, after deploying, constantly applies an outward force to the boundary of the defect, maintaining the implant firmly in situ. The 3D mesh ensures the implant compresses in the vertical axis when intra-abdominal pressure rises [19], [21], [22]. This buffering effect acts to protect against expulsion, especially during the delicate phase prior to the initial tissue ingrowth [19], [21]. After the first weeks, the implant is firmly integrated within the groin.
Following on from extensive physical modelling, the earlier discussed results show that the authors were indeed able to implant the device without fixation and demonstrated no migration of the implant. In fact, the inflammatory reaction with our mesh was optimal to fix and stabilize the mesh potentially reducing the risks of hernia recurrence. This is important as the elimination of the conventional fixation may decrease many of the complications associated with inguinal hernia, such as chronic pain through nerve entrapment by sutures [23], [24], [25], [26]. What is critical in the above results is that the design of this implant appears to have achieved this “auto adaptation” without excessive and constant radial force. This is clearly seen in the histology samples and related to the macroscopic results regarding lack of damage to the surrounding tissues.
Furthermore, another point of this study was to demonstrate that a 3D prosthetic scaffold material, enhances the biological response, improves tissue incorporation, and reduces shrinkage [27], [28], [29], [30], [31].
Despite the limits (small series, low statistical power, and the short follow-up) of this experimental feasibility study, the results showed that: 1) lack of fixation may potentially decrease the post-operative pain, 2) the prosthesis has a 3D anatomic shape, so the physiological strength of the abdominal wall may “stabilize” and maintain the mesh in the good position during the inflammatory process, 3) and finally, the inflammatory reaction due to material used is optimal to consolidate the fixation.
3. Conclusion
This mesh can be placed appropriately and from this preliminary animal no untoward complications were noted over a 60 day period. We acknowledge that this is a preliminary experimental study, we think that the use of complex 3D implants could offer clinical benefits if translated into human cases. Only a large prospective human trial and long-term follow-up may answer the questions about the recurrence rates and the chronic pain.
Financial support
None.
Ethical approval
All animals used in these experiments received humane care in accordance with the requirements of the European Parliament Directive 2010/63/UE, of 22nd September 2010, and the national Royal Decree 1201/2005 of 10th October, on protection of animals used for scientific research.
Author contribution
Fabrizio PANARO: study design, data collections, data analysis, writing.
Ana Maria MATOS-AZEVEDO: data collections data analysis, writing.
José Antonio FATAS: data collections.
Juan MARIN: data collections, data analysis.
Francis NAVARRO: study design, data analysis.
Cristobal ZARAGOZA-FERNANDEZ: study design, data analysis, Writing.
Conflicts of interest
None.
Guarantor
F PANARO, MD.
ISRCTN
NA.
Acknowledgements
None.
References
- 1.Rutkow I.M. Demographic and socioeconomic aspects of hernia repair in the United States in 2003. Surg Clin North Am. 2003;83:1045–1051. doi: 10.1016/S0039-6109(03)00132-4. [DOI] [PubMed] [Google Scholar]
- 2.Lichtenstein I., Shulman A. Ambulatory outpatient hernia surgery. Including a new concept, introducing tension-free repair. Int Surg. 1986;71:1–4. [PubMed] [Google Scholar]
- 3.Schwab R., Conze J., Willms A., Klinge U., Becker H.P., Schumpelick V. Management of recurrent inguinal hernia after previous mesh repair: a challenge. Chirurg. 2006;77:523–530. doi: 10.1007/s00104-006-1158-7. [DOI] [PubMed] [Google Scholar]
- 4.Hernia E.U., Trialist Collaboration Mesh compared with nonmesh methods on open groin hernia repair. Systematic review of randomized controlled trial. Br J Surg. 2000;87:854–859. doi: 10.1046/j.1365-2168.2000.01539.x. [DOI] [PubMed] [Google Scholar]
- 5.Amid P.K. Lichtenstein tension-free hernioplasty: its inception, evolution, and principles. Hernia. 2004;8:1–7. doi: 10.1007/s10029-003-0160-y. [DOI] [PubMed] [Google Scholar]
- 6.Debord J.R. The historical development of prosthetics in hernia surgery. Surg Clin North Am. 1998;78:973–1006. doi: 10.1016/S0039-6109(05)70365-0. [DOI] [PubMed] [Google Scholar]
- 7.Rutkow I.M., Robbins A.W. Mesh plug hernia repair: a follow-up report. Surgery. 1995;117:597–598. doi: 10.1016/s0039-6060(05)80263-6. [DOI] [PubMed] [Google Scholar]
- 8.Awad Z.T., Puri V., LeBlanc K. Mechanisms of ventral vernia recurrence after mesh repair and a new proposed classification. J Am Coll Surg. 2005;201:132–140. doi: 10.1016/j.jamcollsurg.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 9.Penttinen R., Grönroos J.M. Mesh repair of common abdominal hernias: a review on experimental and clinical studies. Hernia. 2008;12:337–344. doi: 10.1007/s10029-008-0362-4. [DOI] [PubMed] [Google Scholar]
- 10.Scheidbach H., Tamme C., Tannapfel A., Lippert H., Köckerling F. In vivo studies comparing the biocompatibility of various polypropylene meshes and their handling properties during endoscopic total extraperitoneal (TEP) patchplasty: an experimental study in pigs. Surg Endosc. 2004;18:211–220. doi: 10.1007/s00464-003-8113-1. [DOI] [PubMed] [Google Scholar]
- 11.Klinge U., Klosterhalfen B., Müller M., Ottinger A.P., Schumpelick V. Shrinking of polypropylene mesh in vivo: an experimental study in dogs. Eur J Surg. 1998;164:965–969. doi: 10.1080/110241598750005156. [DOI] [PubMed] [Google Scholar]
- 12.Aasvang E., Kehlet H. Surgical management of chronic pain after inguinal hernia repair. Br J Surg. 2005;92:795–801. doi: 10.1002/bjs.5103. [DOI] [PubMed] [Google Scholar]
- 16.Woloson S.K., Greisler H.P. Biochemistry, immunology, and tissue response to prosthetic material. In: Bendavid R., Abrahamson J., Arregui M.E., Flament J.B., Phillips E.H., editors. Abdominal Wall Hernias, Principles and Management. Springer-Verlag; New York: 2001. pp. 201–207. [Google Scholar]
- 17.LeBlanc K.A. Tack hernia: a new entity. JSLS. 2003;7:383–387. [PMC free article] [PubMed] [Google Scholar]
- 18.Tozzi P. The physiology of blood flow and artery wall. In: Tozzi P., editor. Sutureless Anastomoses. Steinkopff, Springer; Darmstadt: 2001. pp. 12–24. [Google Scholar]
- 19.O'Dwyer P.J., Kingsnorth A.N., Mohillo R.G., Small P.K., Lammers B., Horeysee G. Randomized clinical trial assessing impact of a lightweight or heavyweight on chronic pain after inguinal hernia repair. Br J Surg. 2005;92:166–170. doi: 10.1002/bjs.4833. [DOI] [PubMed] [Google Scholar]
- 21.Klosterhalfen B., Klinge U., Schumpelick V. Functional and morphological evaluation of different polypropylene-mesh modifications for abdominal wall repair. Biomaterials. 1998;19:2235–2246. doi: 10.1016/s0142-9612(98)00115-x. [DOI] [PubMed] [Google Scholar]
- 22.Klosterhalfen B., Junge K., Klinge U. The lightweight and large porous mesh concept for hernia repair. Expert Rev Med Devices. 2005;2:103–117. doi: 10.1586/17434440.2.1.103. [DOI] [PubMed] [Google Scholar]
- 23.Amato G., Romano G., Salamone G. Nerve degeneration in inguinal hernia specimens. Hernia. 2011;15:53–58. doi: 10.1007/s10029-010-0735-3. [DOI] [PubMed] [Google Scholar]
- 24.Amid P.K. Causes, prevention, and surgical treatment of postherniorrhaphy neuropathic inguinodynia: triple neurectomy with proximal end implantation. Hernia. 2004;8:343–349. doi: 10.1007/s10029-004-0247-0. [DOI] [PubMed] [Google Scholar]
- 25.Kincaid J.C., Stewart J.D. Focal peripheral neuropathies. J Clin Neuromuscul Dis. 1999;1:113. doi: 10.1097/00131402-199912000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Conze J., Kingsnorth A.N., Flament J.B., Simmermacher R., Arlt G., Langer C. Randomized clinical trial comparing lightweight composite mesh with polyester or polypropylene mesh for incisional hernia repair. Br J Surg. 2005;92:1488–1493. doi: 10.1002/bjs.5208. [DOI] [PubMed] [Google Scholar]
- 27.Hardy M.A. The biology of scar formation in physical therapy. Phys Ther. 1989;69:1014–1024. doi: 10.1093/ptj/69.12.1014. [DOI] [PubMed] [Google Scholar]
- 28.Kumar V., Abbas A.K., Fausto N. Saunders; Philadelphia, PA: 2005. Blood Vessel. Robbins & Cotran Pathologic Basis of Disease; pp. 513–515. [Google Scholar]
- 29.Cobbs W.S., Kercher K.W., Heniford B.T. The argument for lightweight polypropylene mesh in hernia repair. Surg Innov. 2005;12:63–69. doi: 10.1177/155335060501200109. [DOI] [PubMed] [Google Scholar]
- 30.Cobbs W.S., Peindl R.M., Zerey M., Carbonell A.M., Heniford B.T. Mesh terminology 101. Hernia. 2009;3:1–6. doi: 10.1007/s10029-008-0428-3. [DOI] [PubMed] [Google Scholar]
- 31.Porrero J.L., Cano-Valderrama O., Castillo M.J., Alonso M.T. Proposed technique for inguinal hernia repair with self-gripping mesh: avoiding fixation to undesired structures. Hernia. 2014 Dec 6 doi: 10.1007/s10029-014-1315-8. PMID: 25480125 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]