Abstract
Objective: In this study, the effects of survivin (SVV) on angiogenesis were evaluated in vitro and in vivo. Methods: The adenovirus (Ad)-mediated murine SVV gene was transfected into rat aortic endothelial cells (RAECs). RAECs expressing green fluorescent protein after transfection with Ad served as a negative control and those without transfection as a blank control. Then, the SVV mRNA was detected by quantitative real time RT-PCR. The SVV protein, cell cycle and apoptosis related proteins, and matrix metalloproteinase (MMPs) were detected by western blot assay. Immunofluorescence staining was conducted for proliferating cell nuclear antigen and MTT assay for cell viability. Transwell and matrigel chamber assay were employed to assess the migration and invasion of cells after transfection. TUNEL staining and flow cytometry were performed to detect the apoptotic REACs after treatment with anti-Fas antibody. Tube formation in matrigel membranes and matrigel plugs assay in nude mice were employed to confirm the angiogenic capacity in vitro and in vivo, respectively. Results: The mRNA and protein expressions of SVV increased significantly in SVV transfected cells. The SVV transfected cells showed increased cell proliferation, up-regulated expressions of cell cycle proteins, enhanced invasiveness and migration activities and increased expressions of MMP-2, 7 and 9. In addition, SVV protected against apoptosis of RAECs by inactivating caspase-3, 8 and 9. The tube formation and matrigel plugs assays showed SVV significantly increased blood vessels in vitro and in vivo. Conclusion: SVV may act as an angiogenic factor and used for therapeutic angiogenesis in peripheral arterial diseases.
Keywords: Survivin, therapeutic angiogenesis, peripheral arterial disease, rat aortic endothelial cell
Introduction
Peripheral arterial diseases (PAD) are often caused by atherosclerosis, which results in the progressive narrowing and occlusion of the peripheral arteries and inhibits the blood flow to the lower extremities. Critical limb ischemia (CLI) represents a complication of PAD and is characterized by rest pain, non-healing ulcers, and gangrene of the diseased leg. CLI is associated with high rates of limb loss and mortality worldwide. It has been reported that about 25% of CLI patients require major amputation within 6 months [1]. The reported mortality for patients with rest pain is approximately 25% at 1 year and >50% at 5 years [1], resulting in a significant health burden.
Restoration of blood flow to the ischemic limbs is crucial to prevent tissue injury after arterial occlusion. Therapeutic angiogenesis has emerged as a potential strategy for PAD patients to promote the growth of new vessels and thereby to supply sufficient blood flow to the ischemic limbs [2]. Angiogenesis, promoted by naked plasmid DNA or recombinant proteins, is a tightly regulated process that involves proteolytic digestion of the extracellular matrix (ECM), proliferation and invasion of vascular endothelial cells (ECs) and formation of functional capillaries [3]. Although angiogenic factors, such as vascular endothelial growth factor A (VEGF), hepatocyte growth factor and fibroblast growth factors, have provided encouraging results in the therapy of CLI , angiogenesis induced by these angiogenic factors are associated with vascular leakage, peripheral edema, non-reduction of amputation and non-improvement of peak walking time [4-6]. Therefore, new angiogenic factors should be identified and tested for angiogenic promotion in differentiated ECs to avoid these limitations.
As noted, therapeutic angiogenesis shares some endothelial growth factors and markers of angiogenesis with pathological angiogenesis [7], such as tumor angiogenesis. Survivin (SVV), a protein expressed in the embryonic tissues and malignancies, has been found to have highly pleiotropic functions and is involved in the regulation of tumor progression, metastasis and angiogenesis. SVV is a member of the family of inhibitor of apoptosis proteins (IAPs) and contains a single baculovirus IAP repeat domain that may interfere with vascular ECs apoptosis by interacting with caspase-3, 8, and 9. SVV has additional unique ability to promote cell survival in that it is essential for the mitosis and cell-cycle progression. The mechanism by which SVV enhances angiogenesis is likely related to its ability to inhibit ECs apoptosis, thereby enhancing cell survival [8]. In addition, previous investigations have shown that the expression of matrix metalloproteinase (MMPs) is up-regulated in the endometriosis and tumors in response to SVV expression [9,10]. MMPs play a crucial role in the degradation of ECM and basement membrane. Therefore, SVV is proposed to contribute to the invasion of endometriotic cells and tumor cells.
In view of the unique angiogenic properties of SVV in tumors, adenovirus -mediated SVV gene was transfected into rat aortic ECs (RAECs) to test the hypothesis that overexpression of SVV is able to inhibit RAECs apoptosis, promote their proliferation, migration and invasion, and induce angiogenesis which provides a new option for the therapeutic angiogenesis.
Materials and methods
Animals, cell lines, and adenoviral vectors
BALB/c nude mice were purchased from the Experimental Animal Center of Chongqing Medical University (Chongqing, China). All animal procedures were performed in acordance with a protocol approved by the Animal Care and Use Committee of Chongqing Medical University. RAECs were purchased from the American Type Culture Collection (ATCC; Manassas, USA) and maintained in Roswell Park Memorial Institute (RPMI)-1640 medium (Gibco, USA) supplemented with 10% fetal bovine serum (FBS) at 37°C in a humidified atmosphere containing 5% CO2.
The recombinant adenoviral vectors (Ad) expressing murine SVV and fused with green fluorescent protein gene (GFP, Ad-GFP-SVV) were constructed by Life Technologies Inc. (California, USA). The recombinant Ad expressing the GFP (Ad-GFP) alone was used as a control.
Adenovirus vector transfection
Cells were seeded into 6-well plates (5×104 cells/well) and incubated overnight. When RAECs reached 50-60% confluence, the medium was removed and replaced with RPMI-1640 containing Ad-GFP (5 PFU/cell) or Ad-GFP-SVV (5 PFU/cell). Then, these cells were divided into three groups: RAEC (blank control group, untreated), Ad-GFP (negative control group, transfected by Ad-GFP) and Ad-GFP/SVV group (transfected by Ad-GFP-SVV). Cells were incubated at 37°C for 12 h and transfection was confirmed by viewing cells under a fluorescent microscope.
Quantitative real-time RT-PCR (RT-qPCR)
To detect the mRNA expression of SVV in RAECs, RT-qPCR was performed at 12 h, 24 h, 48 h, and 72 h after transfection. Total RNA was collected using Trizol reagent (Invitrogen, CA) following the manufacturer’s instructions. The concentration and purity of total RNA were detected with the Nanodrop 2000 spectrophotometer (Thermo Scientific, USA). PCR was carried out with cDNA derived from 50 ng of RNA, 1 unit of Taq polymerase and reaction kits at a final volume of 20 μl. Conditions for PCR were as follows: denaturation at 95°C for 15 sec, annealing at 58°C for 20 sec and extension at 72°C for 20 sec. The relative expression of target gene was calculated with the comparative threshold cycle (Ct) method. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control. The primer sequences were as follows: 5’-TTTTGTGGCTTTGCTCTATTGT-3’ (sense), 5’-GGTAGGAGGACTCATCAGAAGGA-3’ (antisense) for SVV; 5’-GCAAGTTCAACGGCACAG-3’ (sense), 5’-GCCAGTAGACTCCACGACAT-3’ (antisense) for GAPDH.
Western blot assay
The protein expressions of SVV, cellular cycle and apoptosis related proteins and MMPs in cells were detected by western blot assay. Cells were washed with cold phosphate buffer solution (PBS) and lysed in lysis buffer (pH 7.4, 150 mmol/l of NaCl, 50 mmol/l of Tris-HCl, 2 mmol/l of EDTA, 1% NP-40) for 30 min. The protein concentration was determined with Bradford dye-binding assay. GADPH or α-tubulin was used as an internal loading control. Total protein (20 mg/lane) was separated by 10% SDS-polyacrylamide gel and transferred onto a nitrocellulose membrane. The membrane was then blocked with primary polyclonal antibody at 4°C overnight. Afterward, the membranes were washed with tris buffer solution containing 0.05% Tween-20 and incubated with HRP-conjugated anti-mouse secondary antibody at 37°C for 1 h. After washing three times, band detection was conducted using enhanced chemiluminescence on a UVP gel imaging system (Upland, USA). The relative abundance of protein was quantified using the Quantity One software (Bio-Rad, Hercules, CA, USA).
Cell viability/proliferation assay
Cell viability was measured via the 3-(4,5-dmethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) method. Briefly, cells were seeded into 96-well plates at 1×104 cells/well and maintained at 37°C in a humidified atmosphere with 5% CO2. At the pre-designed time points, 20 μL of MTT in Hank’s balanced solution was added to each well at a final concentration of 5 μg/mL followed by incubation at 37°C for 4 h. The medium was then removed and 200 μL of dimethyl sulfoxide was added to dissolve the formazan crystals. Absorbance was measured at 490 nm using a microplate reader (Thermo Scientific, USA). Each experiment was repeated three times. As the proliferating cell nuclear antigen (PCNA) is highly correlated with cell proliferation, the immunofluorescence staining of PCNA was used to detect the proliferation of RAECs. RAECs were cultured on coverslips in plates and fixed in 4.0% paraformaldehyde in PBS (pH 7.4) for 15 min, permeabilized in 0.1% Triton X-100 in PBS for 5 min, and blocked with 1% bovine serum albumin (BSA) for 1 h. The cells were then incubated for 2 h at 37°C with rabbit PCNA monoclonal antibody (1:200, Abcam, UK). Then, cells were treated with Cy3-conjugated goat-anti-rabbit IgG (1:100, Abcam, UK) and nuclei were counterstained with DAPI. Images were acquired at a magnification of ×1000 under a Nikon microscope (Nikon Inc., Japan).
Cell migration and invasion assays
The 24-well transwell plate with 8-μm polyethylene terephthalate membrane filters was applied to test cell migration. Cells (2×105 cells/ml) were seeded in the upper chamber with 200 μl of serum-free medium, while 600 μl of RPMI-1640 with 10% FBS was added to the bottom chamber. The chamber was incubated at 37°C in a humidified environment with 5% CO2 for 24 h. Then, the non-migratory cells in the upper chamber were removed using a cotton swab, and cells in lower chamber were fixed in 4% paraformaldehyde for 10 min, stained with 0.1% crystal violet for 25 min and counted in three random fields under an inverted microscope. The cell invasion assay was essentially similar to the transwell migration assay except for the membrane filter being coated with matrigel matrix (BD Biosciences, USA) prior to the assay.
Cell apoptosis assay
The apoptosis of RAECs was induced by addition of anti-Fas antibody (0.5 mg/mL) and detected by TdT-mediated dUTP nick end labeling (TUNEL) staining and flow cytometry. The in situ cell death fluorescence detection kit was used for TUNEL staining according to the manufacturer’s instructions. DNA chromatin fragments were stained with DAPI. Apoptosis was defined by the appearance of apoptotic bodies and/or chromatin condensation. The cells after anti-Fas antibody treatment were collected and suspended in 500 μL of binding buffer, and then 5 μL of Annexin-V-fluorescein isothiocyanate and 5 μL of propidium iodide were added, followed by incubation for 15 min in dark. Flow cytometry (BD, Franklin Lakes, NJ, USA) was performed to detect apoptotic cells. Each experiment was repeated 3 times.
Tube formation assay
Tube formation assay in vitro was used to evaluate angiogenic activity of RAECs. 24-well plates were coated with 250 μL of growth factor-reduced matrigel (BD, Biosciences, USA). RAECs (5×104) were suspended in 500 μL of serum-free conditioned medium, then plated onto the polymerized matrigel and incubated at 37°C for 6 h. The capillary tube like structures formed by RAECs were photographed under a phase contrast inverted microscope, and the tubes and branches were counted with the Image Pro Plus 6.0 (Media Cybernetics, Atlanta, USA). The supernatant was collected after tube formation assay, and VEGF content was detected by enzyme-linked immunosorbent assay (R&D, USA) according to the manufacturer’s instructions. The experiment was repeated thrice.
In vivo matrigel plug assay
Six-week-old nude mice were divided into three groups with four animals (2 males and 2 females) in each group. Mice were injected subcutaneously injection of matrigel in Ad-transfected RAECs (250 μL of matrigel plus 250 μL of serum-free medium containing 2×106 cells) at the abdomen. Seven days later, mice were sacrificed and the Matrigel plugs were fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned. Then, 5 μm-thick sections were obtained and subjected to hematoxylin and eosin (H&E) staining, Masson’s trichrome staining and CD31 staining. The blood vessel formation in martigel plugs and capsules were quantified by three investigators blind to this study at a high magnification, and average was calculated.
Statistical analysis
Data are expressed as mean ± standard deviation (SD). The analysis of variance was used for comparisons among three groups, and a two-tailed Student t-test was used for comparisons between two groups. A value of P<0.05 was considered statistically. All statistical analyses were performed using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA).
Results
SVV expression in RAECs was up-regulated after Ad-GFP-SVV transfection
qRT-PCR was performed to detect the SVV mRNA expression in RAECs at 12 h, 24 h, 48 h, and 72 h after transfection with Ad-GFP-SVV. No SVV expression was found in blank and negative control groups. There were no significant difference in SVV mRNA expression in Ad-GFP-SVV group between 48 h and 72 h. Therefore, 48 h was determined as the optimal transfection time. Then, western blot assay was applied to detect the SVV protein expression in RAECs at 48 h. Results showed that SVV protein expression in Ad-GFP/SVV group was significantly enhanced (Figure 1).
Figure 1.
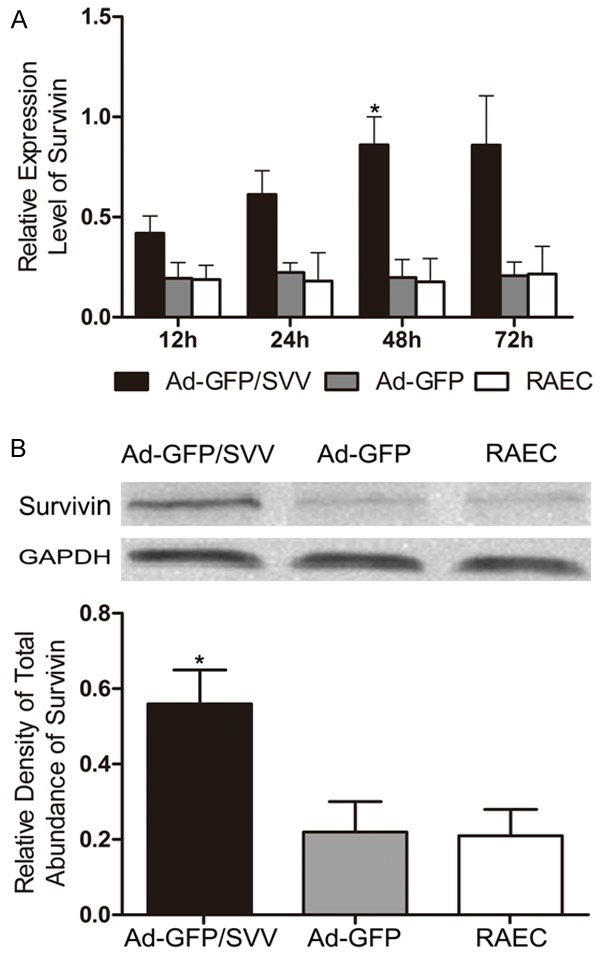
mRNA (A) and protein (B) expressions of SVV in RAECs significantly increased at 48 h after SVV transfection (Ad-GFP/SVV ) as compared to negative control group (Ad-GFP) and blank control group (RAECs, *P<0.05). GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
SVV enhanced RAECs proliferation
PCNA is a marker of cell proliferation. The PCNA expression was detected by immunofluorescence staining. As shown in (Figure 2A and 2B), SVV over-expression in RAECs significantly increased PCNA expression. Then, the effect of SVV on cell growth was explored by MTT assay. As shown in (Figure 2C), SVV overexpression significantly enhanced the growth of RAECs, but there were no marked difference between blank control group and negative control group in cell growth. Meanwhile, western blot assay indicated that the protein expressions of cyclins (cyclin B1, cyclin D1, and cyclin E) and CKDs (CDC2, CDK 4 and CDK2) were significantly increased in Ad-GFP/SVV group (Figure 3). These findings suggest that SVV significantly elevates the proliferation of RAECs by promoting cell cycle progression.
Figure 2.
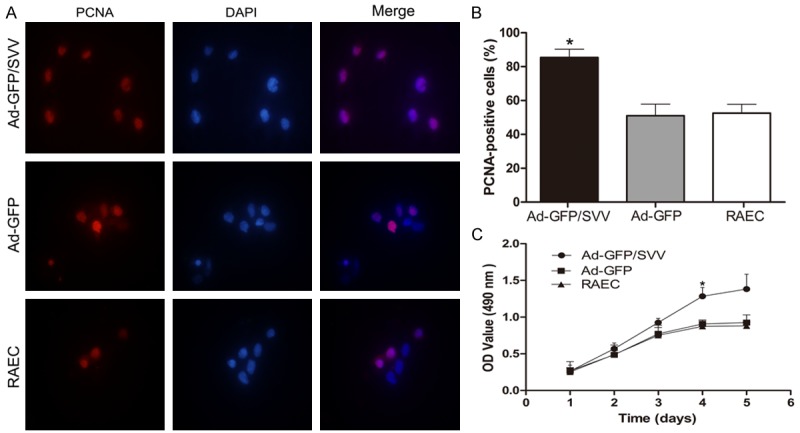
Effect of SVV on the proliferation of RAECs. A. Immunofluorescence staining for PCNA in RAECs after adenovirus transfection (original magnification: 1000×). B. Percentage of PCNA-positive cells after transfection. C. MTT assay. Proliferation of cells in Ad-GFP/SVV group significantly increased as compared to two control groups (Ad-GFP and RAECs, *P<0.05). SVV significantly enhanced the proliferation of RAECs. PCNA, proliferating cell nuclear antigen.
Figure 3.
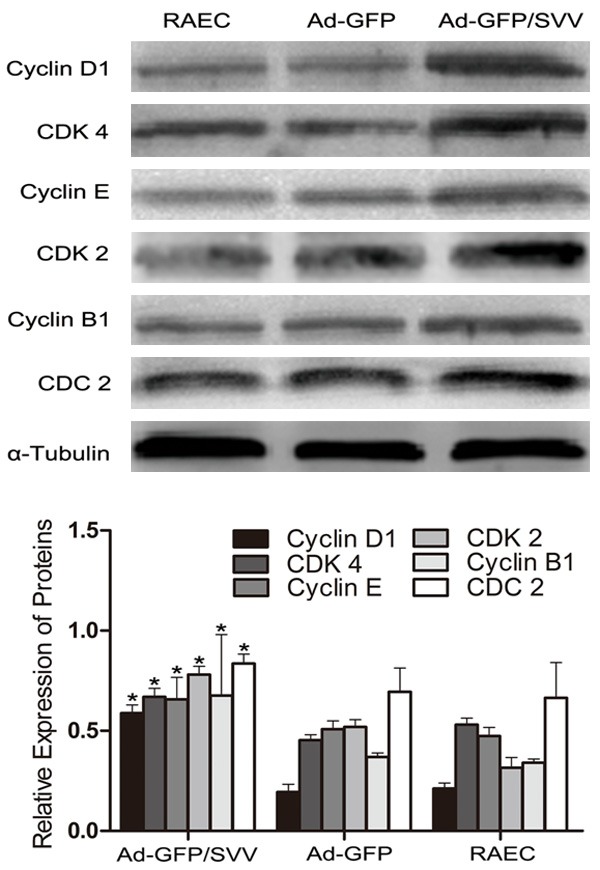
Expressions of cell cycle related proteins in RAECs after transfection. Results showed the expressions of cyclin D1, cyclin E, cyclin B, CDK 4, CDK 2 and CDC 2 increased significantly in Ad-GFP/SVV group when compared with two control groups (Ad-GFP and RAECs, *P<0.05).
SVV promotes RAECs invasion and migration
The effect of SVV on the RAECs migration was examined by transwell assay. The number of cells migrating through the membrane in Ad-GFP/SVV group significantly increased as compared to negative and blank control groups (Figure 4). These indicate that SVV significantly enhances the migration of RAECs. To investigate the effect of SVV on the RAECs invasion, a matrigel chamber assay was carried out. Results showed cells with SVV over-expression degraded the matrigel basement membrane matrix and the number of cells migrating through the membrane significantly increased in Ad-GFP/SVV group as compared to remaining groups. Moreover, the transient transfection of SVV significantly increased the expressions of MMP-2, 7 and 9 in AD-GFP/SVV group when compared with two control groups (Figure 5).
Figure 4.
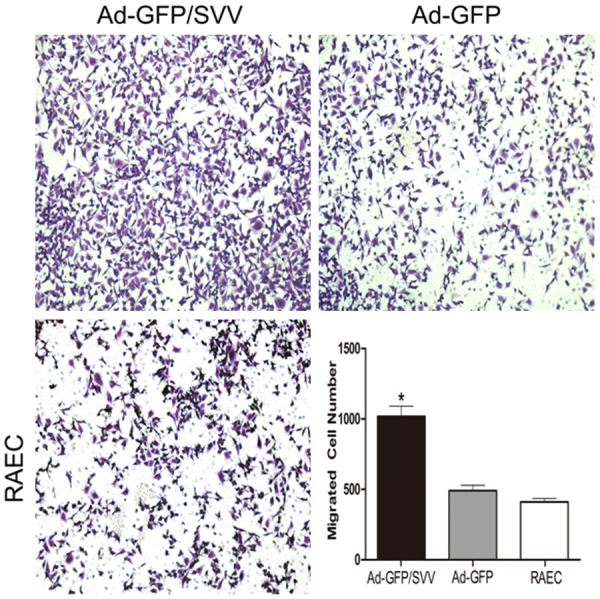
Transwell cell migration assay in RAECs infected with SVV and GFP (original magnification: 200×). The number of cells migrating through the membrane in Ad-GFP/SVV group significantly increased when compared with two control groups (Ad-GFP and RAECs, *P<0.05).
Figure 5.
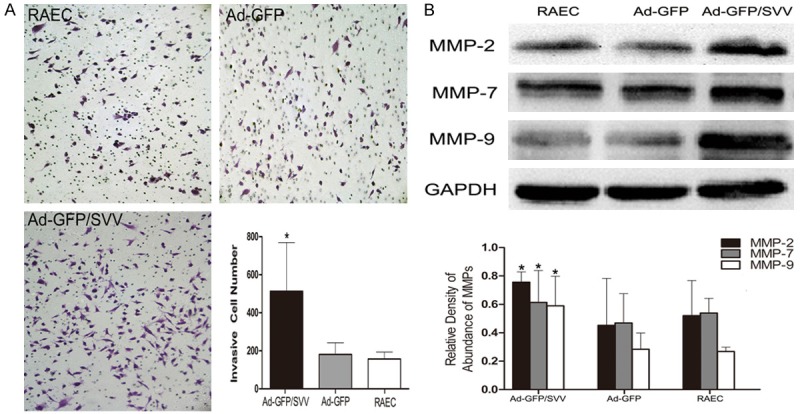
Matrigel cell invasion assay (A. Original magnification: 200×) and Western blot assay of MMPs expression (B) in RAECs after transient transfection with SVV and GFP. Results showed the number of invasive cells and the expressions of MMP-2, 7, and 9 increased dramatically in Ad-GFP/SVV group (*P<0.05). MMPs, matrix metalloproteinases; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
SVV inhibits RACEs apoptosis by suppressing caspases
TUNEL- and DAPI-positive RAECs after SVV transfection are shown in (Figure 6). The amount of TUNEL- and DAPI-positive RAECs was significantly decreased as compared two control groups. As shown in (Figure 7, 5.48% of RAECs became apoptotic after addition of anti-Fas antibody in Ad-GFP/SVV group. In negative and blank control groups, the proportions of apoptotic RAECs were 19.3% and 20.74%, respectively (P<0.05 vs Ad-GFP/SVV group). Furthermore, the expressions of apoptosis related proteins (cleaved-caspase-3, 8 and 9) were detected via immunohistochemistry (Figure 8). As expected, the expressions of cleaved-caspase -3, 8 and 9 significantly decreased in RAECs of Ad-GFP/SVV group as compared to two control groups. Altogether, these indicate that SVV protects against RAECs apoptosis induced by anti-Fas antibody.
Figure 6.
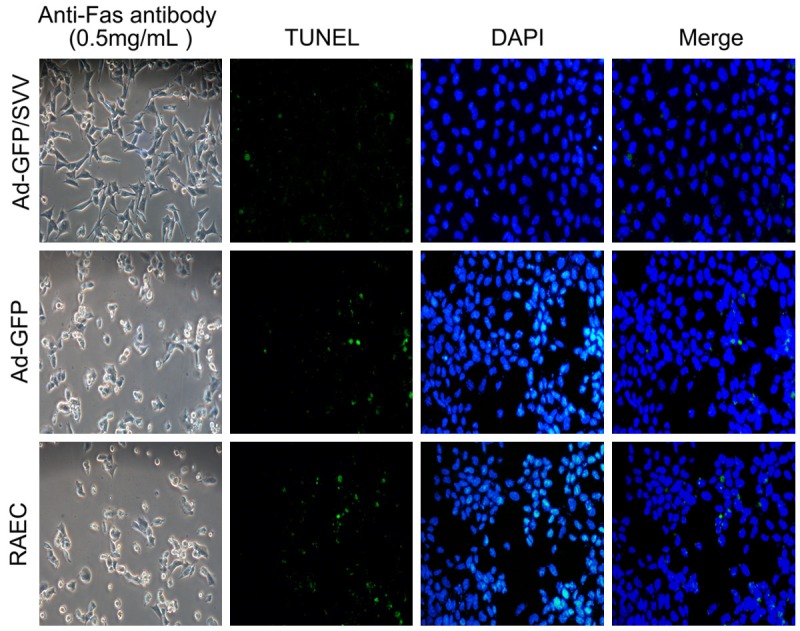
Detection of apoptotic cells after anti-Fans antibody treatment via TUNEL staining and DAPI staining (original magnification: 200×). Representative photomicrographs of TUNEL staining showed apoptotic cells after anti-Fans antibody treatment in RAECs undergoing SVV transfection. SVV transfection effectively inhibited the apoptosis of RAECs, as evidenced by few TUNEL-positive cells.
Figure 7.
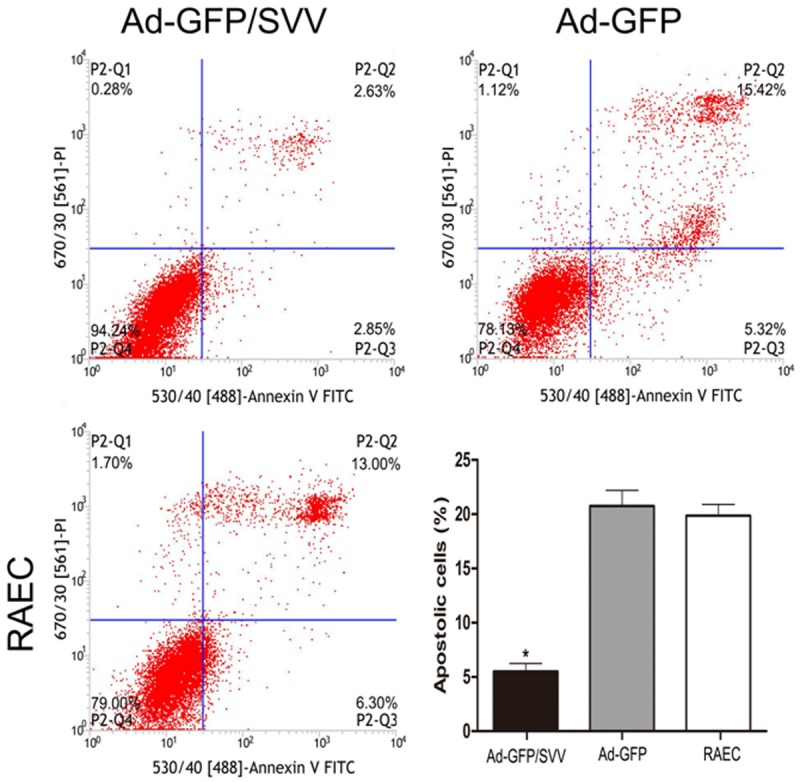
Detection of apoptotic cells after anti-Fans antibody treatment via flow cytometry. Cells in the upper right and lower right quadrants were early apoptotic cells and late apoptotic cells, respectively. SVV over-expression effectively inhibited the apoptosis of RAECs (*P<0.05).
Figure 8.
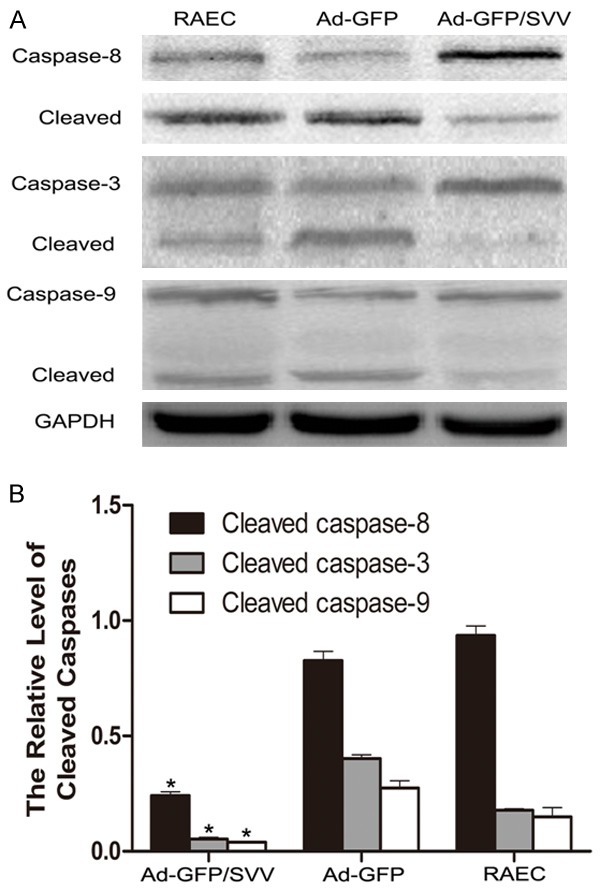
The expressions of apoptosis proteins in RAECs. A. The represented protein bands from Western blot assay. B. The quantification of protein bands. The protein expressions of cleaved-caspase-8, 3, and 9 decreased significantly in Ad-GFP/SVV group as compared to two control groups (Ad-GFP and RAECs, *P<0.05). GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
SVV enhances tube formation in RAECs
SVV plays a key role in the tumor angiogenesis, and therefore, matrigel-coated tube formation assay was performed in RAECs after SVV transfection. After SVV transfection, more tubes and branches were formed as compared to cells in control groups. Moreover, the VEGF expression in Ad-GFP/SVV group was significantly higher than in two control groups. These suggest that SVV contributes to an angiogenic environment (Figure 9).
Figure 9.
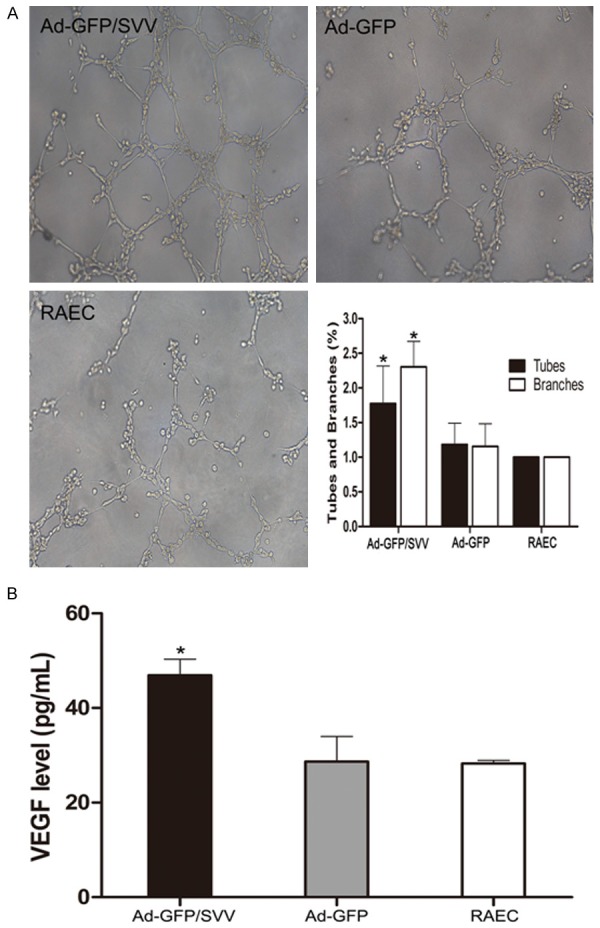
Effects of SVV on tube formation and VEGF expression in RAECs. A. Representative photographs from tube formation assay (original magnification: 200×). The mean number of tubes and branches in Ad-GFP/SVV group significantly increased when compared with control groups (Ad-GFP and RAECs, *P<0.05). B. Enzyme-linked immunosorbent assay for VEGF in the cell supernatant. VEGF content in Ad-GFP/SVV group was markedly higher than in control groups (Ad-GFP and RAECs, *P<0.05).
SVV promotes angiogenesis in vivo
To determine whether SVV affects angiogenesis in vivo, matrigel plug assay was performed in 12 nude mice. As shown in Figure 10A, the plugs were harvested for H&E staining, Masson’s trichrome staining and CD31 staining and the angiogenesis was evaluated. Results showed that SVV trasnfection significantly increased the number of neovessels in the plugs and capsules as compared to control groups (Figure 10B). These indicate that SVV, a novel apoptosis inhibitor, exhibits potent angiogenic promotion.
Figure 10.
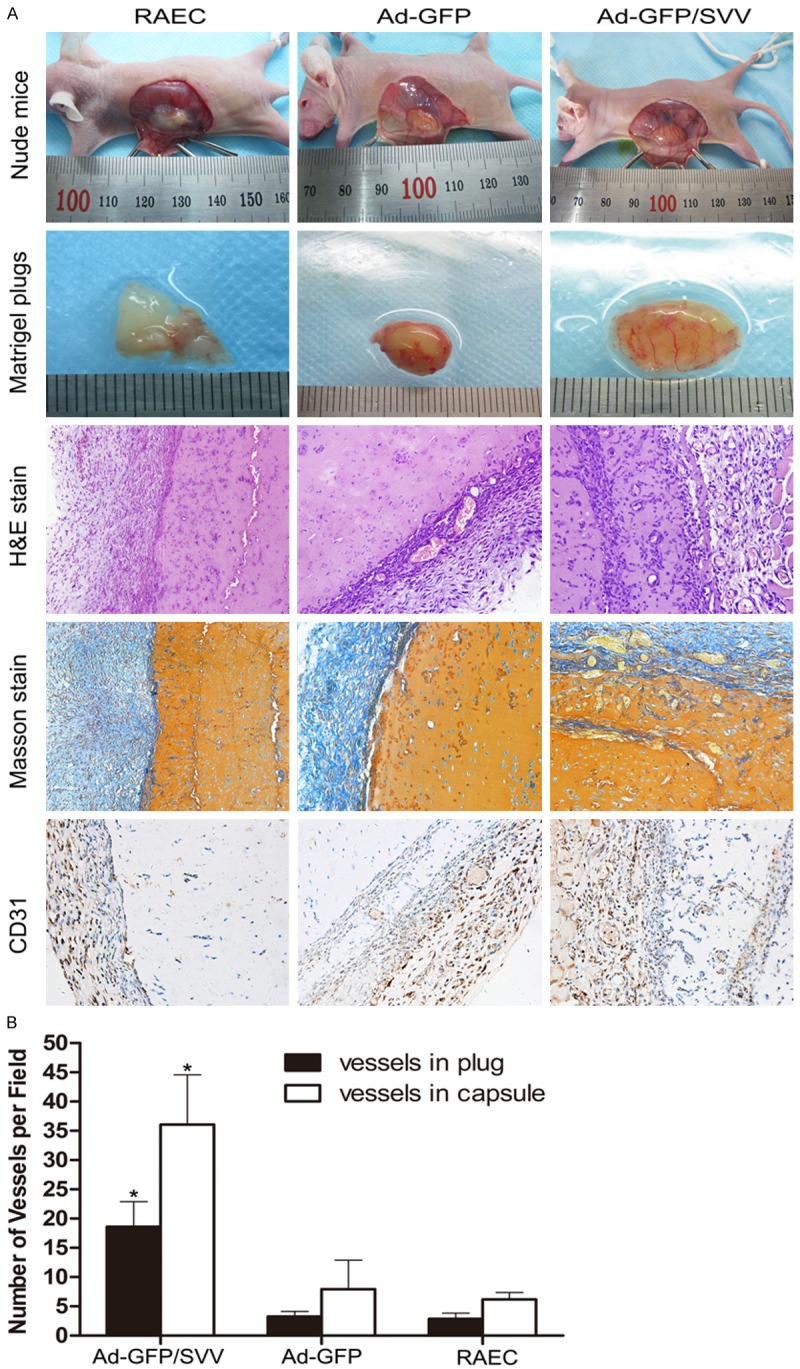
Effect of SVV on the angiogenesis of RAECs in vivo. A. Nude mice were subcutaneously injected with matrigel mixed with RAECs after transfection. Seven days later, animals were sacrificed and the plugs were collected and photographed. Sections of matrigel plugs were processed for H&E staining, Masson’s trichrome staining and CD31 staining (original magnification: 200×). B. Quantification of angiogenesis in the martigel plugs and capsules. The number of vessels in the plugs and capsules significantly increased in Ad-GFP/SVV group as compared to two control groups (Ad-GFP and RAECs, *P<0.05).
Discussion
One of the cornerstone requirements for angiogenesis is the continuous preservation of endothelial viability, a multifaceted process that involves the regulation of cell division and expression of specialized anti-apoptotic genes [11]. One such target gene is SVV which is an inhibitor of apoptosis, can promote cell proliferation, and is associated with the angiogenesis in tumors [12]. In present study, our results demonstrated that SVV over-expression could enhance the proliferation, migration, invasion, and apoptosis resistance of RAECs. Moreover, SVV activated the expressions of angiogenesis-related molecules in RACEs and robustly promoted angiogenesis in vitro and in vivo. All these findings suggest that SVV can be used to promote the angiogenesis in PAD.
Cellular division is ultimately dependent on the progression of cell cycle, in which cells transit through the G0/G1 phase to the S-phase, and eventually to the G2-M phase [13]. The crucial role of SVV in cellular division is unanimously accepted. In cell division, SVV acts as a regulator of polymerization and movement of spindle microtubules and cytokinesis in the metaphase and anaphase of mitosis [14-16]. Moreover, earlier studies also indicate that SVV expression increases during G1 phase, and reaches a peak in G2/M phase, suggesting that it affects cell cycle progression [17]. In the present study, RAECs were successfully transfected with SVV gene, demonstrated by the elevated expressions of SVV at mRNA and protein levels. MTT assay indicated SVV over-expression promoted cell viability, which attributed to the expressions of cell cycle-related proteins. The cell cycle is regulated by a complex network of cyclins and cyclin-dependent kinases (CDKs), whose formation and activation are able to promote cell cycle progression. The cyclin-CDK complexes, cyclin-D1/CDK4 and cyclin-E/CDK2 control the G1 to S transition through phosphorylating the retinoblastoma protein and increasing PCNA expression, while cyclin B1 and Cdc2 regulate the progression into G2/M phase [18]. In present study, the expressions of cyclins (cyclin B1, cyclin D1,and cyclin E) and CKDs (CDC2, CDK 4 and CDK2) were significantly increased after SVV transfection. Moreover, the expression of PCNA, a subunit of DNA polymerase and a biomarker for the evaluation of proliferative activity of cells, was increased significantly in SVV transfection group. These were consistent with previous findings indicating that SVV expression is associated with cell proliferation reported in embryonic tissues and cancers [19,20].
Ischemic diseases are the consequence of endothelial damage, and the main causes of this damage are inflammation and oxidativestress [21,22], which have been reported to impair endothelial growth and angiogenesis, leading to cell apoptosis [23]. Therefore, to prevent ECs from apoptosis is crucial for the angiogenesis [7]. Apoptosis is regulated by a family of cysteine proteases termed caspases. The caspase family members can be divided into initiator (such as caspase-8, and 10) and effector (such as caspase-3, and 9) caspases. SVV is known as an inhibitor of caspases and able to block cell apoptosis. In Fas/Fas ligand (FasL) apoptotic pathway, binding of FasL to Fas results in the activation caspase-8 and 10, ultimately leading to the activation of caspase-3 and 9. In our study, anti-Fas antibody was used to induce the apoptosis of RAECs, and the expressions of caspases in Fas/FasL death pathway were detected by Western blot assay. Results showed the number of apoptotic cells in RAECs after SVV transfection was significantly lower than in two control groups, as shown by flow cytometry and TUNEL staining. Furthermore, the expressions of cleaved-caspases 8, 3 and 9 were significantly suppressed in RAECs after SVV transfection. The suppression of caspases activity in SVV over-expressing RAECs shown in the present study was consistent with the general function of IAP proteins as caspase inhibitors in tumors.
Migration and invasion of vascular ECs are essential events in the process of angiogenesis. Vascular ECs need to loose their mutual contact and interact with basement membrane. Various proteins (such as MMPs and VEGF) influence angiogenesis by destabilizing the vascular structure and degrading the extracellular matrix, thus favoring EC sprout from pre-existing capillaries. The mutual regulation in expressions of VEGF and MMPs accelerate the proliferation and migration of ECs in the angiogenesis [24]. In addition, previous studies have indicated that the SVV induced cell migration has involvement of the formation of SVV-XIAP (X-linked IAP) complex, which may promote the nuclear factor-κB-dependent activation of a process that promotes cell motility, including enhanced production and deposition of fibronectin [25,26]. In the present study, transwell assay and matrigel chamber assay were employed to investigate the migration and invasion of ECs after SVV transfection, respectively. The numbers of migrating and invasive cells significantly increased in SVV transfection group as compared to two control groups. Meanwhile, the expressions of MMP-2, 7, and 9 in these cells were significantly enhanced. The matrigel matrix is a solubilized basement membrane preparation extracted from the mouse sarcoma, which is composed of laminin, heparin sulfate proteoglycans, and collagen IV etc [27]. MMP-7 participates in the degradation of ECM components, such as elastin, type IV collagen, proteoglycans, fibronectin, vitronectin and aggrecan [28]; MMP-2 is also known as ‘72 kD type IV collagenase’ and MMP-9 as ‘92 kD type IV collagenase’ [29]. Our results demonstrated that SVV transfection promoted the invasion of RAECs by increasing MMPs expression.
Angiogenesis occurs in stages, which orchestrate a network of cooperative interactions. To confirm the angiogenesis capacity of SVV in vitro and in vivo, tube formation assay and matrigel plug assay were used in the present study. Results showed the capillary-like tubes in vitro and the neovessels in plugs and capsules in vivo in SVV group significantly increased, which may be attributed to two reasons: first, SVV transfected cells were incorporated into pre-existing capillaries. Our results showed that SVV promoted RAECs proliferation and invasion in vitro, and further to increase the formation of capillary-like tube in the matrigel basement membrane. Thus, the cells may directly participate in angiogenesis, and SVV transfection promotes the angiogenic effect. Second, the soluble factors in local microenvironment, such as VEGF, may enhance the angiogenic capacity of ECs in pre-existing capillaries. Our results demonstrated that SVV over-expression could increase the VEGF expression in local microenvironment. Meanwhile, the capillaries density in matrigel plug capsule originating from subcutaneous blood vessels of nude mice significantly increased in SVV group. This suggests that SVV induced angiogenesis in vitro and in vivo may occur simultaneously through a paracrine dependent manner. This conclusion is consistent with previous findings that SVV may regulate angiogenesis not only via controlling ECs proliferation, but via increasing secretion of VEGF as in tumor cells [30-32].
Conclusions
Collectively, our study demonstrates that SVV can promote angiogenesis in vivo by enhancing the proliferation, migration, invasion, and apoptosis resistance of vascular ECs, which suggests the potential of SVV as a therapeutic intervention for PAD. However, there are several limitations in the present study. Previous investigations have shown that an enhanced SVV protein expression was detectable in granulation tissues and participated in the angiogenesis by increasing ECs viability [33]. Moreover, recent studies also define a regulatory role of survivin in normal adult cells, such as polymerphonuclear cells, hematopoietic cells [34-36]. These findings in normal tissues indicate that SVV expression is not tumor specific, and the tumorigenicity of SVV should be further investigate to ensure the safety. Furthermore, animal PAD models should be established to investigate the angiogenic capacity of SVV in the ischemic environment, because the roles of apoptosis, proliferation, migration and invasion of vascular ECs in such environment are different from those in normal tissues with good blood perfusion. These limitations will be resolved in our future studies.
Acknowledgements
This study was supported by the National Key Clinical Specialties Construction Program of China and the National Natural Science Foundation of China (No. 81200230). The authors thank Di Qi for her assistance in this study.
Disclosure of conflict of interest
None.
References
- 1.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45(Suppl S):S5–67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 2.Gupta R, Tongers J, Losordo DW. Human studies of angiogenic gene therapy. Circ Res. 2009;105:724–736. doi: 10.1161/CIRCRESAHA.109.200386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang JJ, Shi YQ, Li RL, Hu A, Lu ZY, Weng L, Wang SQ, Han YP, Zhang L, Li B, Hao CN, Duan JL. Angiogenesis effect of therapeutic ultrasound on HUVECs through activation of the PI3K-Akt-eNOS signal pathway. Am J Transl Res. 2015;7:1106–1115. [PMC free article] [PubMed] [Google Scholar]
- 4.Kusumanto YH, van Weel V, Mulder NH, Smit AJ, van den Dungen JJ, Hooymans JM, Sluiter WJ, Tio RA, Quax PH, Gans RO, Dullaart RP, Hospers GA. Treatment with intramuscular vascular endothelial growth factor gene compared with placebo for patients with diabetes mellitus and critical limb ischemia: a doubleblind randomized trial. Hum Gene Ther. 2006;17:683–691. doi: 10.1089/hum.2006.17.683. [DOI] [PubMed] [Google Scholar]
- 5.Muona K, Makinen K, Hedman M, Manninen H, Yla-Herttuala S. 10-year safety follow-up in patients with local VEGF gene transfer to ischemic lower limb. Gene Ther. 2012;19:392–395. doi: 10.1038/gt.2011.109. [DOI] [PubMed] [Google Scholar]
- 6.Rajagopalan S, Mohler ER 3rd, Lederman RJ, Mendelsohn FO, Saucedo JF, Goldman CK, Blebea J, Macko J, Kessler PD, Rasmussen HS, Annex BH. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: a phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation. 2003;108:1933–1938. doi: 10.1161/01.CIR.0000093398.16124.29. [DOI] [PubMed] [Google Scholar]
- 7.Karamysheva AF. Mechanisms of angiogenesis. Biochemistry (Moscow) 2008;73:751–762. doi: 10.1134/s0006297908070031. [DOI] [PubMed] [Google Scholar]
- 8.Altieri DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene. 2003;22:8581–8589. doi: 10.1038/sj.onc.1207113. [DOI] [PubMed] [Google Scholar]
- 9.Gao F, Zhang Y, Yang F, Wang P, Wang W, Su Y, Luo W. Survivin promotes the invasion of human colon carcinoma cells by regulating the expression of MMP7. Mol Med Rep. 2014;9:825–830. doi: 10.3892/mmr.2014.1897. [DOI] [PubMed] [Google Scholar]
- 10.Ueda M, Yamashita Y, Takehara M, Terai Y, Kumagai K, Ueki K, Kanda K, Yamaguchi H, Akise D, Hung YC, Ueki M. Survivin gene expression in endometriosis. J Clin Endocrinol Metab. 2002;87:3452–3459. doi: 10.1210/jcem.87.7.8682. [DOI] [PubMed] [Google Scholar]
- 11.Mesri M, Morales-Ruiz M, Ackermann EJ, Bennett CF, Pober JS, Sessa WC, Altieri DC. Suppression of vascular endothelial growth factor-mediated endothelial cell protection by survivin targeting. Am J Pathol. 2001;158:1757–1765. doi: 10.1016/S0002-9440(10)64131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez JG, Rodriguez DA, Valenzuela M, Calderon C, Urzua U, Munroe D, Rosas C, Lemus D, Diaz N, Wright MC, Leyton L, Tapia JC, Quest AF. Survivin expression promotes VEGF-induced tumor angiogenesis via PI3K/Akt enhanced beta-catenin/Tcf-Lef dependent transcription. Mol Cancer. 2014;13:209. doi: 10.1186/1476-4598-13-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Onumah OE, Jules GE, Zhao Y, Zhou L, Yang H, Guo Z. Overexpression of catalase delays G0/G1- to S-phase transition during cell cycle progression in mouse aortic endothelial cells. Free Radic Biol Med. 2009;46:1658–1667. doi: 10.1016/j.freeradbiomed.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sampath SC, Ohi R, Leismann O, Salic A, Pozniakovski A, Funabiki H. The chromosomal passenger complex is required for chromatin-induced microtubule stabilization and spindle assembly. Cell. 2004;118:187–202. doi: 10.1016/j.cell.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 15.Giodini A, Kallio MJ, Wall NR, Gorbsky GJ, Tognin S, Marchisio PC, Symons M, Altieri DC. Regulation of microtubule stability and mitotic progression by survivin. Cancer Res. 2002;62:2462–2467. [PubMed] [Google Scholar]
- 16.Rivadeneira DB, Caino MC, Seo JH, Angelin A, Wallace DC, Languino LR, Altieri DC. Survivin promotes oxidative phosphorylation, subcellular mitochondrial repositioning, and tumor cell invasion. Sci Signal. 2015;8:ra80. doi: 10.1126/scisignal.aab1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li F, Ambrosini G, Chu EY, Plescia J, Tognin S, Marchisio PC, Altieri DC. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature. 1998;396:580–584. doi: 10.1038/25141. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki A, Hayashida M, Ito T, Kawano H, Nakano T, Miura M, Akahane K, Shiraki K. Survivin initiates cell cycle entry by the competitive interaction with Cdk4/p16 (INK4a) and Cdk2/cyclin E complex activation. Oncogene. 2000;19:3225–3234. doi: 10.1038/sj.onc.1203665. [DOI] [PubMed] [Google Scholar]
- 19.Muschol-Steinmetz C, Friemel A, Kreis NN, Reinhard J, Yuan J, Louwen F. Function of survivin in trophoblastic cells of the placenta. PLoS One. 2013;8:e73337. doi: 10.1371/journal.pone.0073337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryan BM, O’Donovan N, Duffy MJ. Survivin: a new target for anti-cancer therapy. Cancer Treat Rev. 2009;35:553–562. doi: 10.1016/j.ctrv.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Tao L, Bei Y, Zhang H, Xiao J, Li X. Exercise for the heart: signaling pathways. Oncotarget. 2015;6:20773–20784. doi: 10.18632/oncotarget.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dreger H, Westphal K, Wilck N, Baumann G, Stangl V, Stangl K, Meiners S. Protection of vascular cells from oxidative stress by proteasome inhibition depends on Nrf2. Cardiovasc Res. 2010;85:395–403. doi: 10.1093/cvr/cvp279. [DOI] [PubMed] [Google Scholar]
- 23.Ding Z, Liu S, Wang X, Khaidakov M, Dai Y, Mehta JL. Oxidant stress in mitochondrial DNA damage, autophagy and inflammation in atherosclerosis. Sci Rep. 2013;3:1077. doi: 10.1038/srep01077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deryugina EI, Quigley JP. Tumor angiogenesis: MMP-mediated induction of intravasation- and metastasis-sustaining neovasculature. Matrix Biol. 2015;44-46:94–112. doi: 10.1016/j.matbio.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehrotra S, Languino LR, Raskett CM, Mercurio AM, Dohi T, Altieri DC. IAP regulation of metastasis. Cancer Cell. 2010;17:53–64. doi: 10.1016/j.ccr.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dohi T, Okada K, Xia F, Wilford CE, Samuel T, Welsh K, Marusawa H, Zou H, Armstrong R, Matsuzawa S, Salvesen GS, Reed JC, Altieri DC. An IAP-IAP complex inhibits apoptosis. J Biol Chem. 2004;279:34087–34090. doi: 10.1074/jbc.C400236200. [DOI] [PubMed] [Google Scholar]
- 27.Kleinman HK, McGarvey ML, Liotta LA, Robey PG, Tryggvason K, Martin GR. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982;21:6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- 28.Han JC, Li XD, Du J, Xu F, Wei YJ, Li HB, Zhang YJ. Elevated matrix metalloproteinase-7 expression promotes metastasis in human lung carcinoma. World J Surg Oncol. 2015;13:5. doi: 10.1186/1477-7819-13-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sillat T, Saat R, Pollanen R, Hukkanen M, Takagi M, Konttinen YT. Basement membrane collagen type IV expression by human mesenchymal stem cells during adipogenic differentiation. J Cell Mol Med. 2012;16:1485–1495. doi: 10.1111/j.1582-4934.2011.01442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tu SP, Jiang XH, Lin MC, Cui JT, Yang Y, Lum CT, Zou B, Zhu YB, Jiang SH, Wong WM, Chan AO, Yuen MF, Lam SK, Kung HF, Wong BC. Suppression of survivin expression inhibits in vivo tumorigenicity and angiogenesis in gastric cancer. Cancer Res. 2003;63:7724–7732. [PubMed] [Google Scholar]
- 31.Li QX, Zhao J, Liu JY, Jia LT, Huang HY, Xu YM, Zhang Y, Zhang R, Wang CJ, Yao LB, Chen SY, Yang AG. Survivin stable knockdown by siRNA inhibits tumor cell growth and angiogenesis in breast and cervical cancers. Cancer Biol Ther. 2006;5:860–866. doi: 10.4161/cbt.5.7.2893. [DOI] [PubMed] [Google Scholar]
- 32.Wang P, Zhen H, Zhang J, Zhang W, Zhang R, Cheng X, Guo G, Mao X, Wang J, Zhang X. Survivin promotes glioma angiogenesis through vascular endothelial growth factor and basic fibroblast growth factor in vitro and in vivo. Mol Carcinog. 2012;51:586–595. doi: 10.1002/mc.20829. [DOI] [PubMed] [Google Scholar]
- 33.O’Connor DS, Schechner JS, Adida C, Mesri M, Rothermel AL, Li F, Nath AK, Pober JS, Altieri DC. Control of apoptosis during angiogenesis by survivin expression in endothelial cells. Am J Pathol. 2000;156:393–398. doi: 10.1016/S0002-9440(10)64742-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Altznauer F, Martinelli S, Yousefi S, Thurig C, Schmid I, Conway EM, Schoni MH, Vogt P, Mueller C, Fey MF, Zangemeister-Wittke U, Simon HU. Inflammation-associated cell cycleindependent block of apoptosis by survivin in terminally differentiated neutrophils. J Exp Med. 2004;199:1343–1354. doi: 10.1084/jem.20032033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gurbuxani S, Xu Y, Keerthivasan G, Wickrema A, Crispino JD. Differential requirements for survivin in hematopoietic cell development. Proc Natl Acad Sci U S A. 2005;102:11480–11485. doi: 10.1073/pnas.0500303102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobayashi Y, Yukiue H, Sasaki H, Fukai I, Yokoyama T, Kiriyama M, Yamakawa Y, Maeda M, Fujii Y. Developmentally regulated expression of survivin in the human thymus. Hum Immunol. 2002;63:101–107. doi: 10.1016/s0198-8859(01)00369-x. [DOI] [PubMed] [Google Scholar]


