Abstract
Background: Systemic maternal inflammation and neonatal hyperoxia arrest alveolarization in neonates. The aims were to test whether human mesenchymal stem cells (MSCs) reduce lung inflammation and improve lung development in perinatal inflammation- and hyperoxia-induced experimental bronchopulmonary dysplasia. Methods: Pregnant Sprague-Dawley rats were intraperitoneally injected with lipopolysaccharide (LPS, 0.5 mg/kg/day) on Gestational Days 20 and 21. Human MSCs (3×105 and 1×106 cells) in 0.03 ml normal saline (NS) were administered intratracheally on Postnatal Day 5. Pups were reared in room air (RA) or an oxygen-enriched atmosphere (O2) from Postnatal Days 1 to 14, and six study groups were obtained: LPS+RA+NS, LPS+RA+MSC (3×105 cells), LPS+RA+MSC (1×106 cells), LPS+O2+NS, LPS+O2+MSC (3×105 cells), and LPS+O2+MSC (1×106 cells). The lungs were excised for cytokine, vascular endothelial growth factor (VEGF) and connective tissue growth factor (CTGF) expression, and histological analyses on Postnatal Day 14. Results: Body weight was significantly lower in rats reared in hyperoxia than in those reared in RA. The LPS+O2+NS group exhibited a significantly higher mean linear intercept (MLI) and collagen density and a significantly lower vascular density than the LPS+RA+NS group did. Administering MSC to hyperoxia-exposed rats improved MLI and vascular density and reduced tumor necrosis factor-α and interleukin-6 levels and collagen density to normoxic levels. This improvement in lung development and fibrosis was accompanied by an increase and decrease in lung VEGF and CTGF expression, respectively. Conclusion: Human MSCs attenuated perinatal inflammation- and hyperoxia-induced defective alveolarization and angiogenesis and reduced lung fibrosis, likely through increased VEGF and decreased CTGF expression.
Keywords: Mesenchymal stem cells, hyperoxia, lipopolysaccharide, vascular endothelial growth factor, connective tissue growth factor
Introduction
Supplemental oxygen administered to newborn infants with respiratory disorders causes lung injury. In neonatal rats, prolonged exposure to hyperoxia reduces alveolar septation, increases terminal air space size, and increases lung fibrosis, similar to human bronchopulmonary dysplasia (BPD) [1]. Pulmonary inflammation and oxygen toxicity are believed to play major roles in the lung injury process, which causes the development of BPD [2]. Early systemic inflammation and reduced diversity of the respiratory microbiome in premature infants is associated with an increased risk of BPD [3]. Systemic maternal inflammation and neonatal hyperoxia in series arrest alveolarization, induce lung fibrosis, and impair lung mechanics in rodents [4,5]. Mesenchymal stem cells (MSCs) are multipotent stromal cells that self-renew and differentiate into various cell types including bone, cartilage, adipose tissue, muscle, and tendon cells [6]. MSCs have immunomodulatory, antiinflammatory, and regenerative effects [7]. Stem cell studies have revealed the potential of stem cells to repair damaged organs. Preclinical studies have provided evidence for the therapeutic benefit of bone marrow- and cord blood-derived MSCs in chronic oxygen-induced lung injury in rodents [8-21]. However, the effects of MSCs on BPD induced by maternal inflammation and postnatal hyperoxia are unknown. We hypothesized that the intratracheal administration of human MSCs on Postnatal Day 5 would attenuate experimental BPD in rats on Postnatal Day 14. The present study was performed to investigate the effects of human MSCs on lung inflammation and development in neonatal rats exposed to prenatal lipopolysaccharide (LPS) and postnatal hyperoxia.
Materials and methods
Isolation of human MSCs
Placental tissues were collected from eight healthy full-term placentas. Written informed consent was obtained from individual mothers before the study and the study was approved by the Ethics Committee of the Cardinal Tien hospital. The age range of the maternal donors was 20 to 45 years old. The placentas were kept at 4°C until they were placed into a biological safety cabinet. Placental-derived tissues were cut into small pieces, 1-2 mm3 in size, digested with 10 U/ml collagenase, 2.5 U/ml dispase, and 0.05% Trypsin-EDTA for 90 min at 37°C. Samples were then thoroughly washed in three changes of sterile phosphate-buffered saline (PBS). Tissue samples were then collected in 15 ml tubes and centrifuged at 800 rpm for 5 min. The cell pellet fraction was resuspended in α minimal essential medium (αMEM, Invitrogen, Carlsbad, CA, USA) with 10-15% fetal bovine serum (FBS, Invitrogen), 2 mM L-glutamine, 1 ng/ml basic fibroblast growth factor (FGF, PeproTech, Rocky Hill, NJ, USA), and penicillin, streptomycin, fungizone (PSF) (100 U/ml penicillin/100 mg/ml streptomycin/0.25 mg/ml Fungizone, Invitrogen), then plated in T75 flasks. Cultures were washed 3-5 times with PBS after 7 days to remove non-adherent cells from plastic-adherent colonies, which were further cultured up to 2 weeks with medium change every 3 days. The culture was maintained in αMEM, supplemented with 10-15% FBS, 2 mM L-glutamine, 1 ng/ml basic FGF, and PSF, at 37°C with saturated humidity and 5% CO2 throughout the culture period. Cells were passaged at approximately 70%-90% confluence. The stem cells were subcultured by treating with TrypLE™ (Life Technologies, Carlsbad, CA, USA) for 1 min at 37°C. The cells were washed and harvested by centrifugation at 1000 rpm for 5 min, then replated at a lower density (5,000 cells/cm2). The stem cells were maintained in αMEM, supplemented with 10% FBS, 2 mM L-glutamine, 1 ng/ml basic FGF, at 37°C, saturating humidity and 5% CO2. MSCs were characterized by analyzing the expression of CD markers (CD 44, CD73, CD90, CD105, CD11b, CD19, CD34, and CD45), and HLA-DR using flow cytometry (BD Stemflow™ hMSC Analysis Kit, BD, NC, USA) (Figure 1A). The capability of tri-lineage differentiation (osteocyte, chondrocyte and adipocyte) (Figure 1B) and the karyotyping result were also examined and demonstrated positive results (Figure 1C).
Figure 1.
Characterization of human MSCs. A. The expression of human MSC specific CD markers was analyzed by flow cytometry. BD Stemflow™ human MSC Analysis Kit was used to analyze MSC-specific surface markers (CD 44, CD73, CD90, CD105, CD11b, CD19, CD34, CD45, and HLA-DR). B. Tri-lineage differentiation (from left to right: osteocyte, chondrocyte, and adipocyte) was performed to show the differentiation potency of human MSC. C. The karyotype of human MSC was analyzed to ensure the chromosome stability of human MSC after in vitro expansion.
Animal model
Our study was approved by the Animal Care and Use Committee at Taipei Medical University (LAC-2014-0147). Time-dated pregnant Sprague-Dawley rats were housed in individual cages with 12-h light-dark cycles. Laboratory food and water were available ad libitum. The rats received LPS treatment that consisted of an intraperitoneal injection of LPS (0.5 mg/kg) from Escherichia coli serotype 0111:B4 (Sigma-Aldrich; St. Louis, MO, USA) in normal saline (NS) on Gestation Days 20 and 21. The rat dams were allowed to deliver vaginally at term. Within 12 h of birth, litters were pooled and randomly redistributed to the newly delivered mothers, and the pups were then randomly assigned to room air (RA) or oxygen-enriched atmosphere (O2) treatment. The pups in O2 treatment subgroups were reared in an atmosphere containing 85% O2 from Postnatal Days 1 to 14. The pups in RA control subgroups were reared in normal RA for 14 days. To avoid oxygen toxicity in the nursing mothers, they were rotated between the O2 treatment and RA control litters every 24 h. An oxygen-rich atmosphere was maintained in a transparent 40×50×60-cm plexiglass chamber receiving O2 continuously at 4 L/min. Oxygen levels were monitored using a ProOx P110 monitor (BioSpherix; Redfield, NY, USA).
Transplantation of human MSCs
Human MSCs (3×105 cells and 1×106 cells) in 0.03 ml of NS were administered intratracheally on Postnatal Day 5. For intratracheal transplantation, the rats were anesthetized with 1% isoflurane (Halocarbon Laboratories; River Edge, NJ, USA) and restrained on a board at a fixed angle. MSCs were administered into the trachea through a 30-gauge needle syringe. After the procedure, the animals were allowed to recover from anesthesia and were returned to their mothers. We obtained six study groups as follows: LPS+RA+NS, LPS+RA+MSC (3×105 cells), LPS+O2+NS, LPS+O2+MSC (3×105 cells), LPS +RA+MSC (1×106 cells), and LPS+O2+MSC (1×106 cells).
Pups from each group were deeply anesthetized with an overdose of isoflurane on Postnatal Day 14, and body and lung weights were recorded. Immediately after death, the left lung was ligated and the right lung was fixed by tracheal instillation of 10% buffered formalin at a pressure of 25 cm H2O for 10 min.
Cytokine levels
Lung tissue was homogenized in 1 ml of ice-cold lysis buffer containing 1% Nonidet P-40, 0.1% sodium dodecyl sulfate, 0.01 M deoxycholic acid, and a complete protease cocktail inhibitor. Cell extracts were centrifuged and the levels of tumor necrosis factor (TNF)-α and interleukin (IL)-6 in supernatants were measured using an enzyme-linked immunosorbent assay kit (Cloud-Clone Corp., Houston, TX, USA).
Western blotting
Lung tissues were homogenized, sonicated, and centrifuged at 500×g for 20 min at 4°C to remove cellular debris. Proteins (30 µg) were resolved in 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis in reduced conditions and electroblotted onto polyvinylidene difluoride membranes (Immobilon-P; Millipore, Bedford, MA, USA). After blocking with 5% nonfat dry milk, the membranes were incubated with antivascular endothelial growth factor (VEGF) and anticonnective tissue growth factor (CTGF) antibodies (1:1,000; Santa Cruz Biotechnology, Dallas, TX, USA) or anti-β-actin (1:5,000; Cell Signaling, Danvers, MA, USA), and then incubated with horseradish peroxidase-conjugated antirabbit or antigoat IgG antibody (Pierce Biotechnology, Rockford, IL, USA). Protein bands were detected using a SuperSignal Substrate from Pierce Biotechnology. Densitometric analysis was performed to measure the intensity of VEGF and CTGF expression and β-actin bands by using AIDA software. Data were normalized to β-actin for each animal.
Histology
Five-μm lung tissue sections were stained with hematoxylin and eosin and Masson’s trichrome, examined using light microscopy, and assessed for lung morphometry and fibrosis. Mean linear intercept (MLI), an indicator of mean alveolar diameter, was assessed in 10 Non-overlapping fields. Vascular density was determined in an unbiased manner in a minimum of four random lung fields stained with von Willebrand factor. Lung sections stained with Masson’s trichrome were assessed for the presence of collagen in 10 systematically sampled areas per section. Optical density values of collagen fibers were processed using Image Pro Plus 6.0 (Media Cybernetics; Bethesda, MD, USA).
Statistical analysis
All data are presented as mean ± standard deviation (SD). Statistical analyses were performed using two-way analysis of variance with a Bonferroni post hoc test for multiple group comparisons. The survival rate was evaluated using the Kaplan-Meier method, and log-rank test was used for intergroup comparisons. Differences were considered statistically significant when P < 0.05.
Results
Twenty LPS-treated dams gave birth to a total of 166 pups; 83 pups each were randomly distributed to RA and hyperoxia groups. Next, 23, 30, 30 pups and 26, 27, 30 pups were treated with NS, human MSCs (3×105 cells), and human MSCs (1×106 cells) in the RA and hyperoxia groups, respectively.
Survival
The rats reared in RA and treated with NS or MSCs (3×105 cells) all survived (Figure 2). One rat each reared in RA and treated with MSCs (1×106 cells) died on Postnatal Days 7 and 13, respectively. The rats reared in hyperoxia and receiving NS or MSCs exhibited a lower survival rate after Postnatal Day 5. Treatment with MSCs (1×106 cells) increased the survival rate from Postnatal Days 6 to 9. On Postnatal Day 14, the differences in the survival rate between rats treated with NS or MSCs were not significant.
Figure 2.
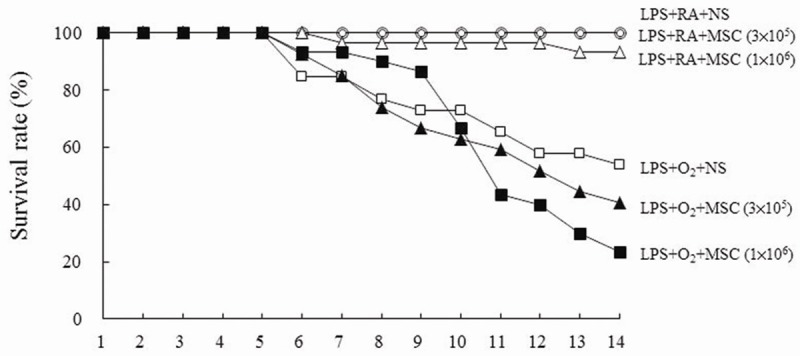
Effects of human MSCs on the survival rate on Postnatal Day 14.
Body weight, lung weight, and lung to body weight ratios
The rats reared in hyperoxia exhibited significantly lower body weights on Postnatal Day 14 than those reared in RA and treated with NS or MSCs (3×105 cells) (Table 1). Treatment with MSCs did not significantly influence body weights on Postnatal Day 14. Lung weights were comparable among rats treated with NS or MSCs. The LPS+O2+NS group exhibited a significantly higher lung to body weight ratio than the LPS+RA+NS and LPS+RA+MSC (3×105 cells) groups did.
Table 1.
Body weights, lung weights, and lung to body weight ratios in rat pups born to LPS-treated mothers on Postnatal Day 14
| Treatment | n | Body weight (g) | Lung weight (g) | Lung to body weight (%) |
|---|---|---|---|---|
| LPS+RA+NS | 23 | 25.50 ± 3.28 | 0.39 ± 0.05 | 1.53 ± 0.14 |
| LPS+RA+MSC (3×105 cells) | 30 | 26.27 ± 1.88 | 0.40 ± 0.04 | 1.51 ± 0.12 |
| LPS+RA+MSC (1×106 cells) | 28 | 24.55 ± 4.32 | 0.39 ± 0.05 | 1.62 ± 0.17 |
| LPS+O2+NS | 14 | 20.75 ± 5.76** | 0.38 ± 0.11 | 1.82 ± 0.22** |
| LPS+O2+MSC (3×105 cells) | 11 | 21.00 ± 5.57** | 0.34 ± 0.06 | 1.67 ± 0.47 |
| LPS+O2+MSC (1×106 cells) | 7 | 20.15 ± 1.15** | 0.34 ± 0.03 | 1.68 ± 0.21 |
Values are mean ± SD.
P < 0.01 vs. LPS+RA+NS and LPS+RA+MSC (3×105 cells).
Cytokine levels
The rats exposed to prenatal LPS and/or postnatal hyperoxia and treated with NS exhibited higher TNF-α and IL-6 levels in lung tissues on Postnatal Day 14 (Figure 3). Furthermore, the rats exposed to prenatal LPS and postnatal hyperoxia and treated with MSCs (1×106 cells) exhibited a significantly lower TNF-α level than those treated with NS did (Figure 3A). The administration of MSCs (3×105 and 1×106 cells) significantly reduced the IL-6 level in LPS- and hyperoxia-exposed rats than in NS-treated rats (Figure 3B).
Figure 3.
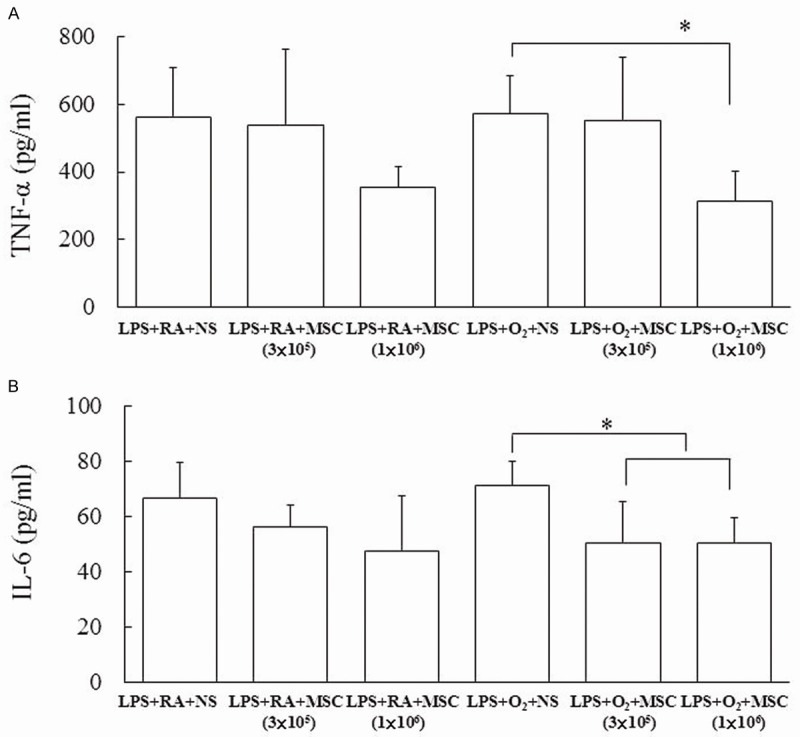
TNF-α (A) and IL-6 (B) levels in lung tissues of 14-day-old rats exposed to prenatal LPS and postnatal RA or hyperoxia and treated with NS or MSCs on Postnatal Day 5. The rats exposed to prenatal LPS and/or postnatal hyperoxia and treated with NS exhibited higher TNF-α and IL-6 levels on Postnatal Day 14. The rats exposed to prenatal LPS and postnatal hyperoxia and treated with MSCs (1×106 cells) exhibited a significantly lower TNF-α level than those treated with NS did. The administration of MSCs (3×105 and 1×106 cells) significantly reduced the IL-6 level in LPS- and hyperoxia-exposed rats than in NS-treated rats. *P < 0.05.
Histology results
Representative lung sections stained with hematoxylin and eosin from prenatal LPS- and postnatal hyperoxia-exposed rats on Postnatal Day 14 are shown in Figure 4A. The rats reared in hyperoxia and treated with NS exhibited a significantly higher MLI than those reared in RA and treated with NS or MSCs did (Figure 4B). Treatment with MSCs significantly decreased the hyperoxia-induced increase in the MLI. The rats exposed to prenatal LPS and postnatal hyperoxia and treated with NS exhibited significantly lower vascular density than those reared in RA and treated with NS or MSCs did (Figure 5). By contrast, the administration of MSCs to hyperoxia-exposed rats improved vascular density to normoxic levels.
Figure 4.
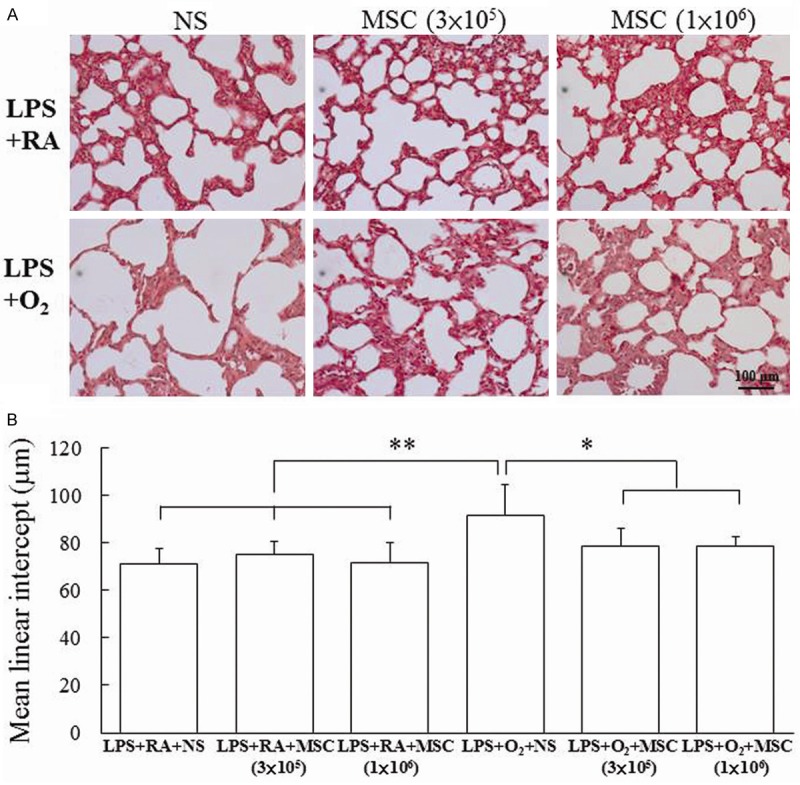
(A) Representative histology and (B) mean linear intercept in 14-day-old rats exposed to prenatal LPS and postnatal RA or hyperoxia and treated with NS or MSCs on Postnatal Day 5. The rats reared in hyperoxia and treated with NS exhibited a significantly higher MLI than those reared in RA and treated with NS or MSCs did. Treatment with MSCs significantly decreased the hyperoxia-induced increase in the MLI. *P < 0.05, **P < 0.01.
Figure 5.
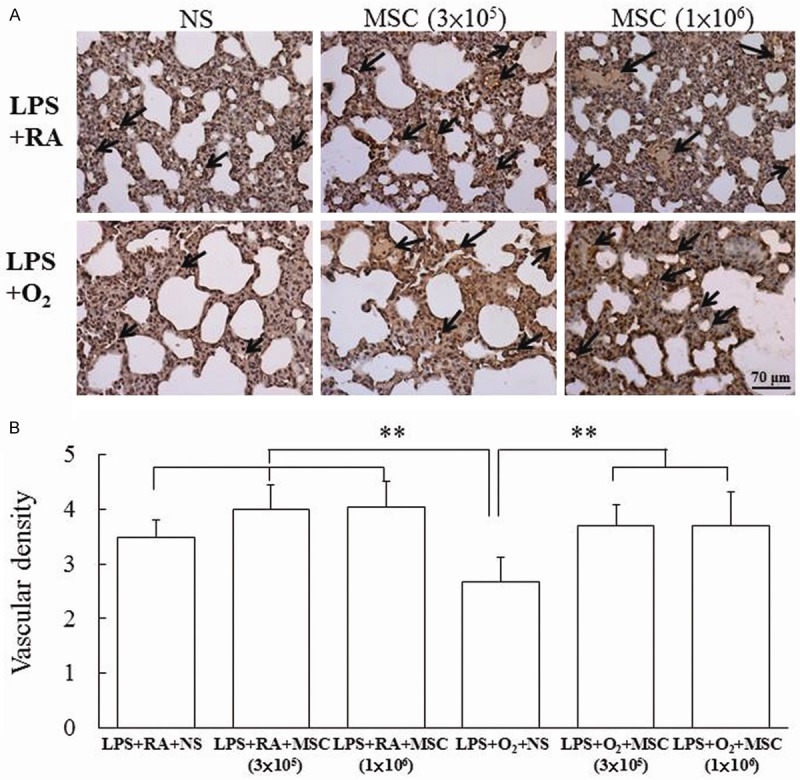
(A) Representative histology and (B) vascular density in 14-day-old rats exposed to prenatal LPS and postnatal RA or hyperoxia and treated with NS or MSCs on Postnatal Day 5. The rats exposed to prenatal LPS and postnatal hyperoxia and treated with NS exhibited significantly lower vascular density than those reared in RA and treated with NS or MSCs did. The administration of MSCs to hyperoxia-exposed rats improved vascular density to normoxic levels. **P < 0.01.
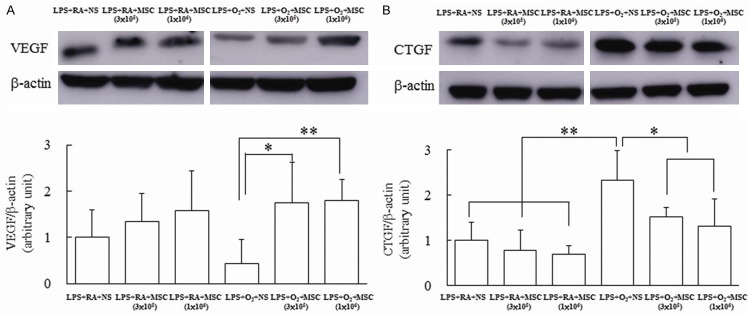
VEGF and CTGF protein expression
The rats exposed to prenatal LPS and postnatal RA and treated with NS exhibited lower VEGF protein expression, and treatment with MSCs increased VEGF expression, but the differences were not statistically significant (Figure 6A). The rats exposed to prenatal LPS and postnatal hyperoxia exhibited decreased VEGF expression and treatment with MSCs significantly increased VEGF expression in these rats compared with the NS-treated rats. Treatment with MSCs decreased CTGF expression in the rats exposed to prenatal LPS and postnatal RA, but the differences were not statistically significant (Figure 6B). The rats exposed to prenatal LPS and postnatal hyperoxia and treated with NS exhibited higher CTGF protein expression than RA-exposed rats did, and treatment with MSCs (3×105 and 1×106 cells) significantly reduced CTGF protein expression.
Figure 6.
(A) Representative Western blots and (B) quantitative data determined using densitometry for VEGF and CTGF protein expression in lung tissues on Postnatal Day 14. The rats exposed to prenatal LPS and postnatal hyperoxia exhibited decreased VEGF expression and treatment with MSCs significantly increased VEGF expression in these rats compared with the NS-treated rats. The rats exposed to prenatal LPS and postnatal hyperoxia and treated with NS exhibited higher CTGF protein expression than RA-exposed rats did, and treatment with MSCs (3×105 and 1×106 cells) significantly reduced CTGF protein expression. *P < 0.05, **P < 0.01.
Collagen density
The rats reared in hyperoxia exhibited widespread collagen deposition in both the peribronchial and parenchymal portions of the lung than those reared in RA did (Figure 7A). By contrast, collagen deposition in MSC-treated rats was significantly decreased. The rats reared in hyperoxia and treated with NS exhibited significantly higher collagen density than those reared in RA and treated with MSCs did (Figure 7B). Treatment with MSCs significantly reduced the hyperoxia-induced increase in the collagen density.
Figure 7.
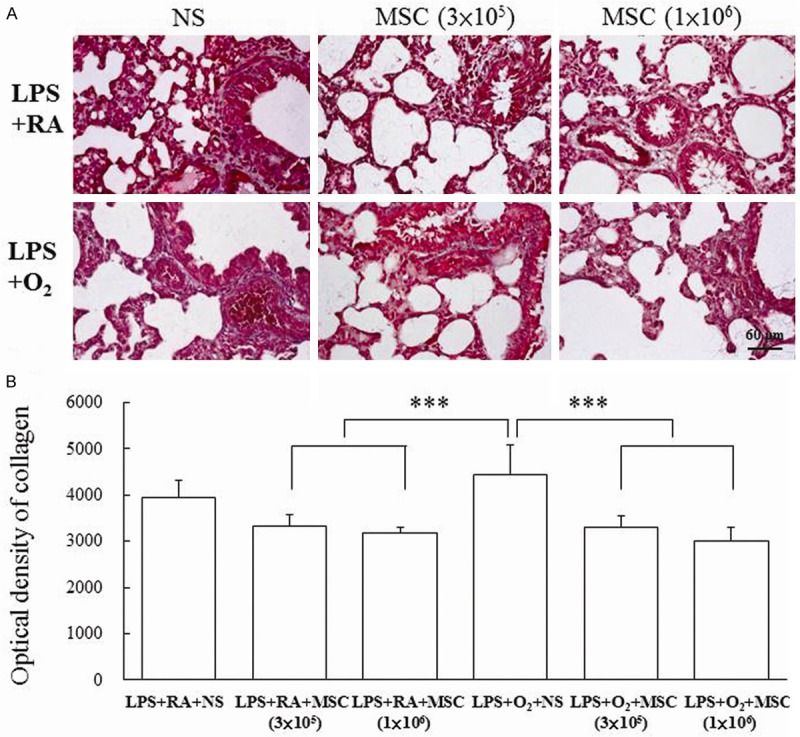
(A) Masson’s trichrome staining and (B) collagen density in 14-day-old rats exposed to prenatal LPS and postnatal RA or hyperoxia conditions and treated with NS or MSCs on Postnatal Day 5. The rats reared in hyperoxia and treated with NS exhibited significantly higher collagen density than those reared in RA and treated with MSCs did. Treatment with MSCs significantly reduced the hyperoxia-induced increase in the collagen density. ***P < 0.001.
Discussion
Our in vivo model revealed that prenatal LPS and postnatal hyperoxia exposure arrested alveolarization, reduced angiogenesis, and increased collagen density in the lungs of rat offspring on Postnatal Day 14. The decreased angiogenesis and increased collagen density was associated with decreased VEGF and increased CTGF expression. Intratracheal administration of MSCs on Postnatal Day 5 improved alveolarization and angiogenesis and reduced collagen density. The improvement in angiogenesis and collagen density was accompanied by an increase and decrease in lung VEGF and CTGF protein expression, respectively. The major findings are that the intratracheal administration of human MSCs reduced lung fibrosis by reducing CTGF expression in an animal model of BPD. These results suggested that human MSCs attenuated experimental BPD likely through increased VEGF and decreased CTGF expression.
Our study demonstrated that rats reared in hyperoxia and treated with NS or MSCs exhibited significantly lower body weights on Postnatal Day 14 than those reared in RA and treated with NS or MSCs did. Lung weights were comparable among the rats reared in RA or hyperoxia and treated with NS or MSCs. Rats reared in hyperoxia and treated with NS exhibited a high percentage of lung hemorrhage, and thus significantly higher lung to body weight ratios than those reared in RA and treated with NS or MSCs did (3×105 cells). These results suggested that body weight was mainly influenced by hyperoxia, MSC treatment did not influence body weight, and lung growth was arrested during a 2-week exposure to 85% O2.
Supplemental oxygen administered to newborn infants with respiratory distress can increase oxidative stress and cause cytokine production. The role of cytokines in BPD has been supported by human and animal studies that showed increased cytokine levels and inflammatory cells are associated with the development of BPD [3,22]. In this study, we found comparable cytokine levels in the rats reared in RA and hyperoxia. These results are consistent with our previous findings that maternal LPS treatment similarly increased lung IFN-γ, IL-1β, and TNF-α levels in neonatal rats reared in RA or hyperoxia [4]. The administration of MSCs reduced the maternal LPS- and postnatal hyperoxia-induced increase in TNF-α and IL-6 levels. These reduction effects of human MSCs on cytokines are consistent with previous studies [10,11,23]. These results suggested that the therapeutic effects of MSCs on the developing lungs are partially mediated through the inhibition of proinflammatory cytokine production.
VEGF is a potent endothelial cell mitogen, which is essential for vasculogenesis and angiogenesis during embryonic development [24]. Angiogenesis is essential for alveolarization during normal lung development [25]. Here, we demonstrated that prenatal LPS and postnatal exposure to hyperoxia reduced vascular density, and treatment with MSCs on Postnatal Day 5 increased VEGF expression and restored vascular density in the lungs of rat offspring. These findings are consistent with those of Chang et al. who revealed that MSC transplantation on Postnatal Day 3 increased lung VEGF levels in hyperoxia-induced lung injury [23]. CTGF is crucial in the pathogenesis of hyperoxia-induced lung fibrosis [1]. In the present study, we also demonstrated that prenatal LPS and postnatal exposure to hyperoxia increased collagen and CTGF protein expression in lung tissues on Postnatal Day 14; however, the administration of MSCs reduced collagen and CTGF expression. These results suggested that MSCs exert paracrine effects and increase VEGF expression and reduce CTGF expression in lung tissues.
In this study, although the survival rate was not significantly improved, the administration of human MSCs to the rats exposed to prenatal LPS and postnatal hyperoxia significantly improved lung development in the surviving animals. The rats reared in hyperoxia exhibited a lower survival rate after Postnatal Day 6. Treatment with MSCs (1×106 cells) improved the survival rate from Postnatal Days 6 to 9. The differences in the survival rates between rats treated with NS or MSCs were not significant on Postnatal Day 14. These results suggested that an additional dose of MSCs is required to maintain the survival rate.
Conclusion
The intratracheal administration of human MSCs attenuated experimental BPD by enhancing lung development and reducing lung fibrosis, as indicated by decreasing MLI and collagen expression and increasing vascular density. These beneficial effects of human MSCs on BPD are mediated by increasing VEGF expression and decreasing cytokine and CTGF expression. Additional studies are required to examine the exact mechanisms responsible for the therapeutic effects of human MSCs in reducing hyperoxia-induced lung fibrosis.
Acknowledgements
This study was supported by a grant from Meridigen Biotech Co., Ltd. Taipei, Taiwan (2014-RD-VIV-003).
Disclosure of conflict of interest
None.
References
- 1.Chen CM, Wang LF, Chou HC, Lan YD, Lai YP. Up-regulation of connective tissue growth factor in hyperoxia-induced lung fibrosis. Pediatr Res. 2007;62:128–133. doi: 10.1203/PDR.0b013e3180987202. [DOI] [PubMed] [Google Scholar]
- 2.Welty SE. Is there a role for antioxidant therapy in bronchopulmonary dysplasia? J Nutr. 2001;131:947S–950S. doi: 10.1093/jn/131.3.947S. [DOI] [PubMed] [Google Scholar]
- 3.Lohmann P, Luna RA, Hollister EB, Devaraj S, Mistretta TA, Welty SE, Versalovic J. The airway microbiome of intubated premature infants: characteristics and changes that predict the development of bronchopulmonary dysplasia. Pediatr Res. 2014;76:294–301. doi: 10.1038/pr.2014.85. [DOI] [PubMed] [Google Scholar]
- 4.Su CL, Chou HC, Huang LT, Yeh TF, Chen CM. Combined effects of maternal inflammation and neonatal hyperoxia on lung fibrosis and RAGE expression in newborn rats. Pediatr Res. 2014;75:273–280. doi: 10.1038/pr.2013.222. [DOI] [PubMed] [Google Scholar]
- 5.Velten M, Heyob KM, Rogers LK, Welty SE. Deficits in lung alveolarization and function after systemic maternal inflammation and neonatal hyperoxia exposure. J Appl Physiol. 2010;108:1347–1356. doi: 10.1152/japplphysiol.01392.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–4. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 7.Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–9. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aslam M, Baveja R, Liang OD, Fernandez-Gonzalez A, Lee C, Mitsialis SA, Kourembanas S. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med. 2009;180:1122–1130. doi: 10.1164/rccm.200902-0242OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Haaften T, Byrne R, Bonnet S, Rochefort GY, Akabutu J, Bouchentouf M, Rey-Parra GJ, Galipeau J, Haromy A, Eaton F, Chen M, Hashimoto K, Abley D, Korbutt G, Archer SL, Thébaud B. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am J Respir Crit Care Med. 2009;180:1131–1142. doi: 10.1164/rccm.200902-0179OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H, Fang J, Su H, Yang M, Lai W, Mai Y, Wu Y. Bone marrow mesenchymal stem cells attenuate lung inflammation of hyperoxic newborn rats. Pediatr Transplant. 2012;16:589–598. doi: 10.1111/j.1399-3046.2012.01709.x. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Wang H, Shi Y, Peng W, Zhang S, Zhang W, Xu J, Mei Y, Feng Z. Role of bone marrow-derived mesenchymal stem cells in the prevention of hyperoxia-induced lung injury in newborn mice. Cell Biol Int. 2012;36:589–594. doi: 10.1042/CBI20110447. [DOI] [PubMed] [Google Scholar]
- 12.Chang YS, Oh W, Choi SJ, Sung DK, Kim SY, Choi EY, Kang S, Jin HJ, Yang YS, Park WS. Human umbilical cord blood-derived mesenchymal stem cells attenuate hyperoxia-induced lung injury in neonatal rats. Cell Transplant. 2009;18:869–886. doi: 10.3727/096368909X471189. [DOI] [PubMed] [Google Scholar]
- 13.Chang YS, Choi SJ, Sung DK, Kim SY, Oh W, Yang YS, Park WS. Intratracheal transplantation of human umbilical cord blood-derived mesenchymal stem cells dose-dependently attenuates hyperoxia-induced lung injury in neonatal rats. Cell Transplant. 2011;20:1843–1854. doi: 10.3727/096368911X565038. [DOI] [PubMed] [Google Scholar]
- 14.Pierro M, Ionescu L, Montemurro T, Vadivel A, Weissmann G, Oudit G, Emery D, Bodiga S, Eaton F, Péault B, Mosca F, Lazzari L, Thébaud B. Short-term, long-term and paracrine effect of human umbilical cord-derived stem cells in lung injury prevention and repair in experimental bronchopulmonary dysplasia. Thorax. 2013;68:475–484. doi: 10.1136/thoraxjnl-2012-202323. [DOI] [PubMed] [Google Scholar]
- 15.Vosdoganes P, Hodges RJ, Lim R, Westover AJ, Acharya RY, Wallace EM, Moss TJ. Human amnion epithelial cells as a treatment for inflammation-induced fetal lung injury in sheep. Am J Obstet Gynecol. 2011;205:156, e26–e33. doi: 10.1016/j.ajog.2011.03.054. [DOI] [PubMed] [Google Scholar]
- 16.Balasubramaniam V, Ryan SL, Seedorf GJ, Roth EV, Heumann TR, Yoder MC, Ingram DA, Hogan CJ, Markham NE, Abman SH. Bone marrow-derived angiogenic cells restore lung alveolar and vascular structure after neonatal hyperoxia in infant mice. Am J Physiol Lung Cell Mol Physiol. 2010;298:L315–L323. doi: 10.1152/ajplung.00089.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansmann G, Fernandez-Gonzalez A, Aslam M, Vitali SH, Martin T, Mitsialis SA, Kourembanas S. Mesenchymal stem cell-mediated reversal of bronchopulmonary dysplasia and associated pulmonary hypertension. Pulm Circ. 2012;2:170–181. doi: 10.4103/2045-8932.97603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutsko RP, Young KC, Ribeiro A, Torres E, Rodriguez M, Hehre D, Devia C, McNiece I, Suguihara C. Long-term reparative effects of mesenchymal stem cell therapy following neonatal hyperoxia-induced lung injury. Pediatr Res. 2013;73:46–53. doi: 10.1038/pr.2012.152. [DOI] [PubMed] [Google Scholar]
- 19.Waszak P, Alphonse R, Vadivel A, Ionescu L, Eaton F, Thébaud B. Preconditioning enhances the paracrine effect of mesenchymal stem cells in preventing oxygen-induced neonatal lung injury in rats. Stem Cells Dev. 2012;21:2789–2797. doi: 10.1089/scd.2010.0566. [DOI] [PubMed] [Google Scholar]
- 20.Tropea KA, Leder E, Aslam M, Lau AN, Raiser DM, Lee JH, Balasubramaniam V, Fredenburgh LE, Alex Mitsialis S, Kourembanas S, Kim CF. Bronchioalveolar stem cells increase after mesenchymal stromal cell treatment in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2012;302:L829–L837. doi: 10.1152/ajplung.00347.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Reilly M, Thébaud B. Stem cells for the prevention of neonatal lung disease. Neonatology. 2015;107:360–364. doi: 10.1159/000381135. [DOI] [PubMed] [Google Scholar]
- 22.Ambalavanan N, Carlo WA, D’Angio CT, McDonald SA, Das A, Schendel D, Thorsen P, Higgins RD. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Cytokines associated with bronchopulmonary dysplasia or death in extremely low birth weight infants. Pediatrics. 2009;123:1132–1141. doi: 10.1542/peds.2008-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang YS, Ahn SY, Jeon HB, Sung DK, Kim ES, Sung SI, Yoo HS, Choi SJ, Oh WI, Park WS. Critical role of vascular endothelial growth factor secreted by mesenchymal stem cells in hyperoxic lung injury. Am J Respir Cell Mol Biol. 2014;51:391–399. doi: 10.1165/rcmb.2013-0385OC. [DOI] [PubMed] [Google Scholar]
- 24.Peters KG, De Vries C, Williams LT. Vascular endothelial growth factor receptor expression during embryogenesis and tissue repair suggests a role in endothelial differentiation and blood vessel growth. Proc Natl Acad Sci U S A. 1993;90:8915–8919. doi: 10.1073/pnas.90.19.8915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jakkula M, Le Cras TD, Gebb Sarah, Hirth KP, Tuder RM, Voelkel NF, Abman SH. Inhibition of angiogenesis decreases alveolization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol. 2000;279:L600–L607. doi: 10.1152/ajplung.2000.279.3.L600. [DOI] [PubMed] [Google Scholar]