Abstract
Colon cancer stem cells (CCSCs) account for the tumorigenicity of colon cancer and promote its progression and metastasis. RSPO2, the agonist of canonical Wnt/beta-catenin pathway and serves as the growth factor of intestinal stem cells (ISCs), is considered playing an important role in CCSCs. However, the specific function of RSPO2 in CCSCs remains unclear. In this study, we demonstrated that RSPO2 was highly expressed in CCSCs-enriched HCT116 spheroid cells. Elevates the concentration of RSPO2 in medium in favor of enriching the LGR5+ cells and increasing the LGR5 expression in HCT116 spheroid cells, meanwhile silencing of RSPO2 by small interfering RNA inhibits LGR5 expression in HCT116 spheroid cells. In addition, RSPO2 promotes spheres formation but has little effect on the proliferation of HCT116 spheroid cells in vitro. Moreover, RSPO2 also promotes the invasion of HCT116 spheroid cells through enhancing Epithelial-mesenchymal transition (EMT). These findings suggests that RSPO2 is a potential growth factor for CCSCs, helps enriching the CCSCs by serum-free DMEM/F12 medium (SFM) culture and plays a vital role in the metastasis of colon cancer.
Keywords: Colon cancer stem cells (CCSCs), RSPO2, Wnt/beta-catenin pathway, intestinal stem cells (ISCs), epithelial-mesenchymal transition (EMT)
Introduction
The roof plate-specific spondin protein family (RSPO1-4) are typical secretory proteins could stimulate the canonical Wnt/beta-catenin signaling pathway by a positive feedback loop [1]. Since the WNT signaling plays an important role both in physiology and pathology of gastrointestinal tract, RSPOs are also implicated in biological process of gastrointestinal cells [2-4]. RSPO1 was first reported promoting the proliferation of the intestinal epithelia cells of mouse [5] and considered a potential growth factors of the intestinal stem cells (ISCs) [6,7], then RSPO2 were also disclosed as a potent mitogen for gastrointestinal epithelial cells [8]. Recently RSPO2 was reported closely related to the invasiveness and tumorigenic properties of mammary and pancreatic cancer [9,10], but the probably function of RSPO2 as drivers or inhibitors in colorectal cancer (CRC) still arguable [11-14]. In addition, the concept of cancer stem cells (CSCs) provides a new research angle in explaining the development, migration and recurrence of CRC [15]. Given most perspective suggesting CCSCs were derived from ISCs and share the certain characters with ISCs [16], RSPO2 probably plays an important role in CCSCs. We previously obtained the colon cancer stem-like spheroid cells (spheroid cells) and proved leucine-rich repeat-containing g-protein coupled receptor 5 (LGR5) is required for the maintenance of spheroid-derived CCSCs [17,18], as the specific ligands of LGR5 in Wnt pathway [19], RSPO2, its role in CCSCs and the relationship with LGR5 remains unclear.
In this study, we continued to use spheroid colon cancer cells models to assessed RSPO2’s role in CCSCs. We compared the expression of RSPO2 in spheroid colon cancer cells with its matched adherent cells, and try to disclose the relationship between RSPO2 and LGR5 in spheroid stem cells. In addition, we find RSPO2 could accelerate the sphere formation of CCSCs and promote its invasion and migration abilities through epithelial-mesenchymal transition (EMT).
Materials and methods
Cell culture
The HCT116 human colon cancer cell line (ATCC, CCL-247) and HT29 human colon cancer cell line (ATCC, HTB-38) were maintained in DMEM/F12 with 10% fetal bovine serum (serum added medium, SAM). For the spheroid cells culture, HCT116 and HT29 cells were were incubated in serum-free DMEM/F12 medium (SFM) containing 2% B27 (Invitrogen, Carlsbad, CA, USA), 20 ng/ml epidermal growth factor (EGF), 10 ng/ml basic fibroblast growth factor (bFGF) (both from Peprotech Inc. Rocky Hill, NJ, USA), 5 μg/ml routine insulin (Invitrogen) with humid atmosphere at 37°C with 5% CO2. Different levels of recombinant human RSPO2 protein (Peprotech) were added to each culture medium according to assays.
Quantitative RT-PCR (qRT-PCR)
Total RNA was extracted using E.Z.N.A.® Total RNA Kit I (Omega Bio-Tek, Inc. Norcross, GA, USA) according to the manufacturer’s instructions. cDNAs were synthesized using PrimeScript RT Master Mix (Takara Bio, Inc. Shiga, Japan). The RT-PCR reaction was performed using ABI 7500 Fast (Applied Biosystems, Foster City, CA, USA) with SYBR Premix Ex TaqII (Takara). The cycle condition was: denaturation at 95°C for 30 sec, 40 amplification cycles at 95°C for 3 sec and 60°C for 30 sec. GAPDH was used as the control. Primer sequences used were: LGR5 forward, 5’-GAGGATCTGGTGAGCCTGAGAA-3’; and reverse, 5’-CATAAGTGATGCTGGAGCTGGTAA-3’, RSPO2 forward, 5’-AATGGGTGTAGCCGATGTCAA-3’; and reverse; 5’-CGGTGTCCATAGTACCCGGAT-3’. The results were analyzed by the 2-ΔΔct method.
Small interfering RNA (siRNA) transfection
siRNA were obtained from RiboBio Co. Ltd. (Guangzhou, China). Primer sequences of RSPO2-siRNA were: forward, 5’-GAAGAGAAGGGAUGCGCCAGUUGGUCAUUGGAGCGAAU-3’ and reverse, 5’-AGACGCAGUAAGCGAGCUAGACAAUGGGUGUAGCCGAU-3’, LGR5-siRNA was: forward, 5’-GCUCCAGCAUCACUUAUGATT-3’; and reverse, 5’-UCAUAAGUGAUGCUGGAGCTT-3’., scrambled siRNA was: 5’-UGGUUUACAUGUCGACUAA-3’. Dissociated spheroid cells (5×105) were seeded in 6-well plates in SFM. Twenty-four hours later, siRNA were transfected into spheroid cells at a final concentration of 100 nM using Lipofectamine RNAiMAX reagent (Invitrogen) according to the manufacturer’s instructions. Spheroid cells were either transfected or not transfected with scrambled siRNA and used as the blank or negative control (NC). The cells were collected for a series of experiments 48 h after transfection.
Western blotting
Cells were lysed in RIPA buffer with 10% phenylmethylsulfonyl fluoride. The cell extracts were loaded on 10% SDS-polyacrylamide gels and transferred onto polyvinylidene fluoride membranes. The membranes were blocked for 1 h at room temperature with 5% non-fat milk in TBST, and then incubated with antibodies: anti-LGR5 (diluted at 1:1000), anti-RSPO2 (diluted at 1:500) (Abcam, Cambridge, UK), anti-Snail (diluted at 1:1,000), anti-Twist (diluted at 1:1,000) anti-Vimentin (diluted at 1:1,000), anti-GAPDH (diluted at 1:1,000) (Cell Signaling Technology, Inc., Danvers, MA, USA) at 4°C overnight. Following incubation with HRP-conjugated secondary antibody (diluted at 1:2,000, Abcam), immuno-complexes were visualized by an enhanced chemiluminescence detection under FluorChem M System (ProteinSimple, San Jose, CA, USA). Endogenous GAPDH was used for normalization.
Detection of surface markers LGR5 by flow cytometry
Cells were dissociated and washed twice in PBS. Subsequently, cell suspensions were incubated with 1:50 PE-conjugated mouse anti-LGR5 Ab (OriGene, Rockville, MD, USA) for 20 min in the dark.The cells were then washed twice in cold PBS with 1% BSA and re-suspended in 300 μl cold PBS with 1% BSA for flow cytometric analysis within 1 h.
Immunofluorescent staining
Spheroid cells were cytospun onto glass slides, fixed with 4% paraformaldehyde for 10 min, and permeablized with 0.1% Triton X-100 for 15 min. Adherent and differentiated cells were cultured on Glass Bottom dishes (Nest Scientific, Rahway, NJ, USA) for 48 h and fixed as described above. The cells were incubated with the primary anti-LGR5 (1:100) or anti-RSPO2 antibody (1:100) (Abcam) at 4°C overnight, followed by incubation with secondary DyLight-conjugated anti-rabbit antibody (Alexa Fluor 488, Abcam) for 1 h at room temperature. DAPI (Invitrogen) was used to counterstain the nuclei. Fluorescent images were captured by Zeiss confocal microscope (LSM-710; Zeiss, Jena, Germany).
Sphere formation assay
Cells were dissociated and seeded in 24-well ultra-low attachment plates (Corning Life Science, Oneonta, NY, USA) at a density of 2×103 cells/well in 500 μl SFM and then exogenous recombinant RSPO2 or RSPO2 -siRNA was added according to the assay. Spheres >50 μm were counted by microscope after 48 hour culture.
Proliferation assays
Cell proliferation was determined using Cell Counting kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan). All cells were dissociated and seeded in 96-well plates at a density of 2×103 cells/well in 100 μl matched medium overnight, and then treated with various concentrations of RSPO2 (0, 10, 20, 40 and 80 μg/ml). At 24 h, 48 h, 72 h after RSP02 added, 10 μl CCK-8 reagent was added to each well and the culture was incubated for a further 3 hours. The optical density (OD) value in each well was measured by a microplate reader at a wavelength of 450 nm. The siRNA interfering cells were dissociated and seeded in 96-well plates at a density of 2×103 cells/well in 100 ul SFM overnight, and then transfected with 100 nM of indicated siRNA. At 24 h, 48 h, 72 h after transfection, the OD value was measured as before.
Invasion assay
Invasion assay was performed using 24-well plate Transwell chambers with 8 μm-pore polycarbonate filter inserts (Corning Life Sciences). Cells were detached and 1×105 cells in 100 μl DMEM/F12 without FBS were seeded onto the upper chamber coated with Matrigel (BD Biosciences). Then, 600 μl DMEM/F12 medium containing 10% FBS was added into the lower chamber. After incubation for 36 h, the cells on the upper side of the membrane were removed and the cells that migrated to the underside were fixed in 4% paraformaldehyde, stained with 0.1% crystal violet and counted in five random fields under microscopy.
Statistical analysis
Results were presented as means ± SD. Statistical analysis was performed using one-way ANOVA or the Student’s t-test. The LSD method was used for multiple comparisons. P<0.05 was considered to be statistically significant.
Results
RSP02 was highly expressed in spheroid cells
RSPO2, is a member of the roof plate-specific spondin (RSPO) family, which are typical secreted agonists of canonical Wnt pathway by binding with LGRs. To obtain a stable and comprehensive RSPO2 expression profile in CCSCs, we cultured adherent HCT116 cells in SFM for 7 days, then debris removed by centrifugation and spheres disassociated by trypsin, until the spheres grew well after 3-4 times of passage (Figure 1A), then the mRNA expression and the protein expression of RSPO2 in both HCT116 spheroid cells and HT29 spheroid cells were elevated compared with adherent counterparts respectively (Figure 1B). The Immunofluorescent staining also confirmed that intracellular and extracellular RSPO2 expression got a significantly enhancement (Figure 1C). These findings suggested that the expression of R-Spondin2 was increased in spheroid cells.
Figure 1.
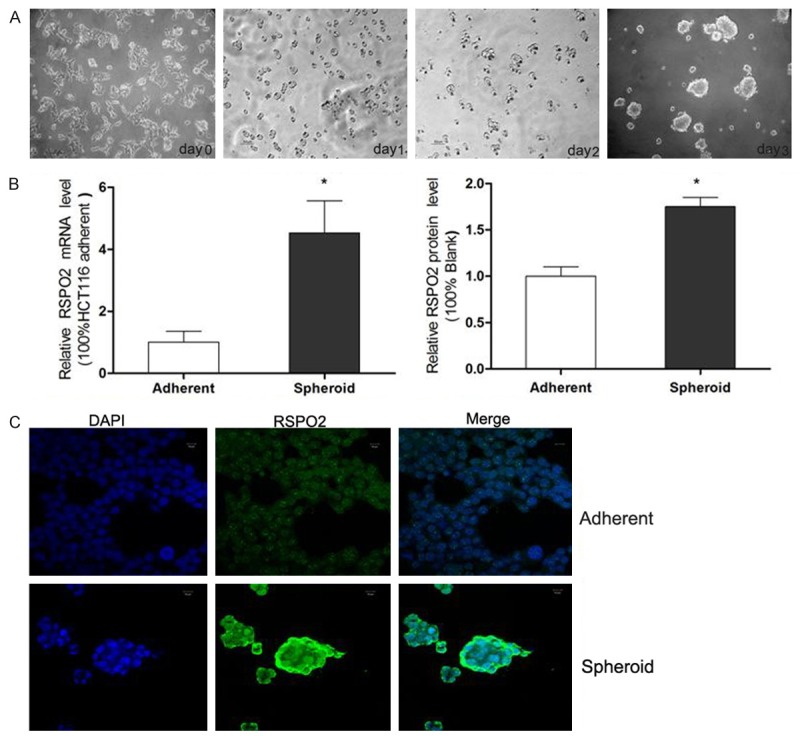
RSPO2 expression in spheroid HCT116 cells. (A) Morphology of HCT116 adherent and spheroid cells. The HCT116 cells grew in medium containing FBS (serum added medium, SAM) was typical monolayer adherent cells (DAY 0), when the HCT116 cells subcultured in serum-free DMEM/F12 medium (SFM), they became suspended and turned into large spheres (DAY1-3) (×100). (B) RSPO2 mRNA expression and protein expression level in HCT116 adherent and spheroid cells, detected by qRT-PCR and western blotting. (C) Immunofluorescent staining of RSPO2 in HCT116 adherent (A) and spheroid cells (B). RSPO2 staining is green and nuclei are stained in blue.
RSPO2 associated with the sphere formation and proliferation of HCT116 cells
Since the spheroid cells had a higher RSPO2 expression than corresponding adherent cells, RSPO2 might in favor of the enrichment of CCSCs. The spheres formation test showed that HCT116 cells subcultured into SFM formed more spheres (sphere diameter>50 μm) co-cultured with RSPO2, HCT116 adherent cells co-cultured with the 40 ng/ml RSPO2 group had the most spheres (10.3±2.6) than cells with 0 ng/ml RSPO blank group (5.8±1.2).Meanwhile when the RSPO2 expression of established spheroid cells was silenced by siRNA transfection, the formed spheres also significantly decreased in the RSPO2-siRNA group (1.5±1.1) compared to the NC group (4.8±1.7). CCK-8 assay showed an unexpected curve that spheroid cells’ growth had no significant change when RSPO2 added in the SFM. However, the RSPO2-siRNA group proliferated faster than the NC and blank control group (Figure 2).
Figure 2.
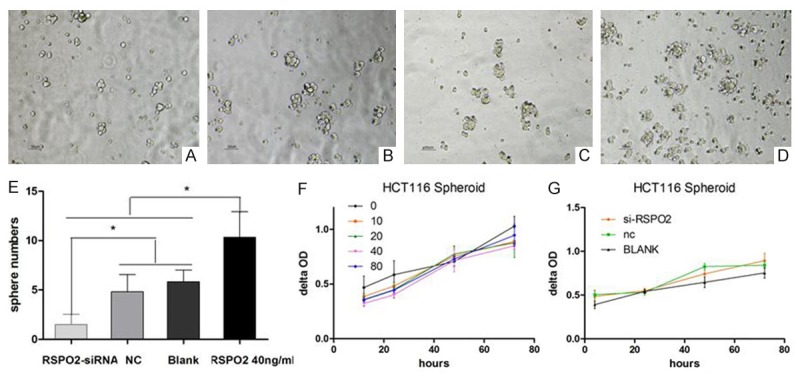
RSPO2’s role in the sphere formation and proliferation of spheroid HCT116 cells. A: Morphology of HCT116 spheres with treatment of RSPO2-siRNA (×100); B: Morphology of HCT116 spheres in NC (×100); C: Morphology of HCT116 spheres in blank (×100); D: Morphology of HCT116 spheres with treatment of RSPO2 40 ng/ml (×100); E: More spheres were formed when RSPO2 ‘s concentration in SFM was changed by recombinant protein or si-RNA silence. NC and blank were as controls *P<0.05 vs corresponding groups; F: The growth curve of HCT116 spheroid cells (0, 10, 20, 40 and 80 μg/ml recombinant RPSO2 was added in SFM); G: The growth curve of HCT116 spheroid cells (blank control, negative control (NC), RPSO2-small interfering RNA (siRNA) transfected spheroid cells).
Expression of LGR5 is increased following exogenous RSPO2 protein stimulation
LGR5, also known as GPR49, is a member of the G protein coupled receptor (GPCR) family of proteins, a leading candidate marker of CCSCs and also played an important role in the maintenance of CCSCs. We first detected that the maximum relative LGR5 mRNA expression of HCT116 adherent cells cultured in SAM was 6 hours after 10 ng/ml RSPO2 added, then we tried with different RSPO2 concentrations (0 ng/ml, 5 ng/ml, 10 ng/ml, 20 ng/ml, 40 ng/ml, 80 ng/ml) to reveal that the optimal LGR5 mRNA expression was around the 40 ng/ml RSPO2 stimulation. Therefore, 40 ng/ml RSPO2 and 12 hours were chose as the action concentration and time to detect the extra-and intracellular expression of LGR5 protein in HCT116 cells. Flow cytometric analysis revealed that the proportion of HCT116 LGR5+ cells in SAM had a significant rise from (3.08±1.65) to (19.61±1.16) after RSPO2 stimulation, while the LGR5+ spheroid cells was increased from (37.97±2.70) to (40.77±13.79) in SFM culture, respectively (Figure 2A), Western blot showed nearly 1 fold higher LGR5 protein expression of HCT116 adherent cells after RSPO2 stimulation (Figure 3). Similarly, stronger cytoplastic LGR5 immunofluorescent staining was also observed by confocal microscope (Figure 4). Above findings showed that RSPO2 could enhance the expression of LGR5 of HCT116 adherent cells.
Figure 3.
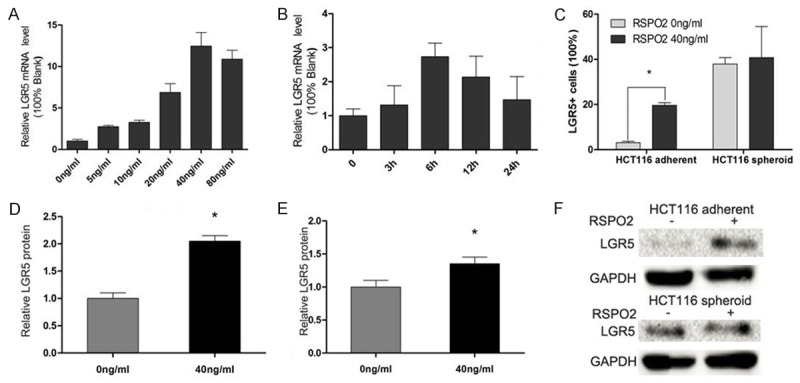
Recombinant RSPO2 up-regulated the expression of LGR5 in HCT116 spheroid cells. A and B: LGR5 mRNA expression of HCT116 spheroid cells when recombinant RSPO2 added was a time- and dose- dependent manner, detected by quantitative RT-PCR (qRT-PCR). C: LGR5+ cells were detected by flow cytometry. One of three similar experiments is shown. *P<0.05 vs corresponding group. D-F: LGR5 protein expression level in HCT116 adherent and spheroid cells after recombinant RSPO2 added, detected by western blotting.
Figure 4.
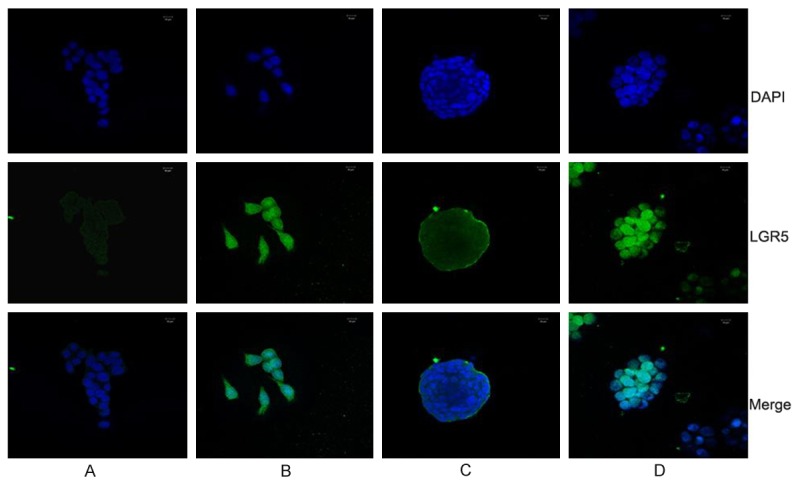
Immunofluorescent staining of LGR5 in HCT116 cells. A and B were HCT116 adherent cells with 0 ng/ml or 40 ng/ml recombinant RSPO2, C and D were HCT116 spheroid cells with 0 ng/ml or 40 ng/ml recombinant RSPO2; LGR5 staining is green and nuclei are stained in blue.
Expression of LGR5 is decreased following downregulation of RSPO2 expression
Then RSPO2-siRNA was used to silence the mRNA expression of RSPO2 in HCT116 spheroid cells. RSPO2 mRNA was downregulated by 68.2% in HCT116 spheroid cells at 48 h after RSPO2-siRNA transfection compared to the control. Moreover, western blotting confirmed that the RSPO2 protein expression was also markedly reduced (Figure 5). 48 hours post-transfection, immunofluorescent staining revealed that the percentages of LGR5+ cells were decreased in the RSPO2-siRNA group, as compared to the NC group and blank group (Figure 6). These data reveal that RSPO2 plays a key role in sustaining the LGR5+ CCSCs.
Figure 5.
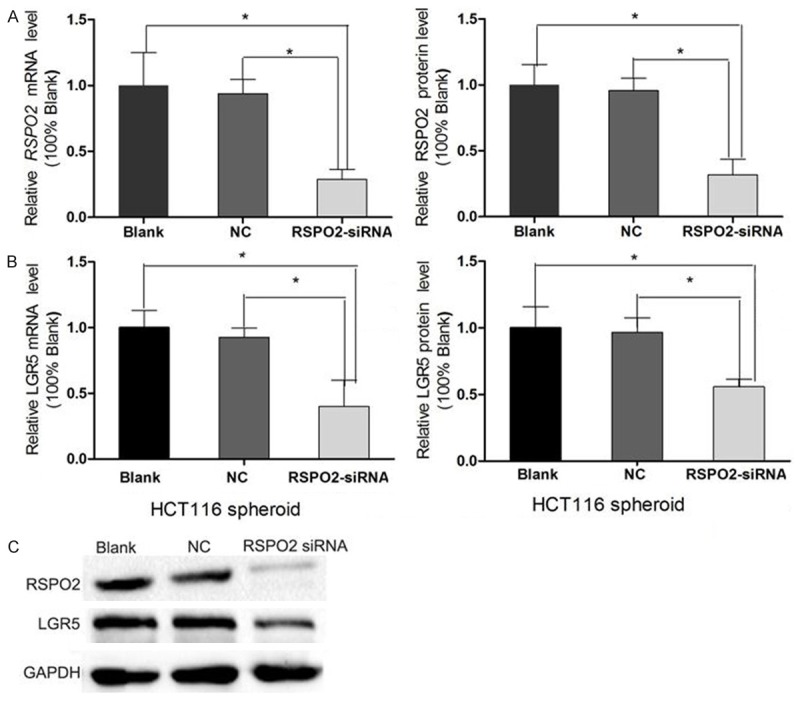
LGR5 expression was down-regulated after RSPO2 knockdown in HCT116 spheroid cells. A. Relative RSPO2 mRNA level and protein expression of HCT116 spheroid cells at 48 h after small interfering RNA (siRNA) transfection was determined by quantitative RT-PCR (qRT-PCR) and western blotting (WB) respectively to test the efficacy of RSPO2-siRNA knockdown. B. LGR5 mRNA level and protein expression of HCT116 spheroid cells at 48 h after RSPO2-siRNA transfection was determined by qRT-PCR and WB. C. WB result. NC: negative control.
Figure 6.
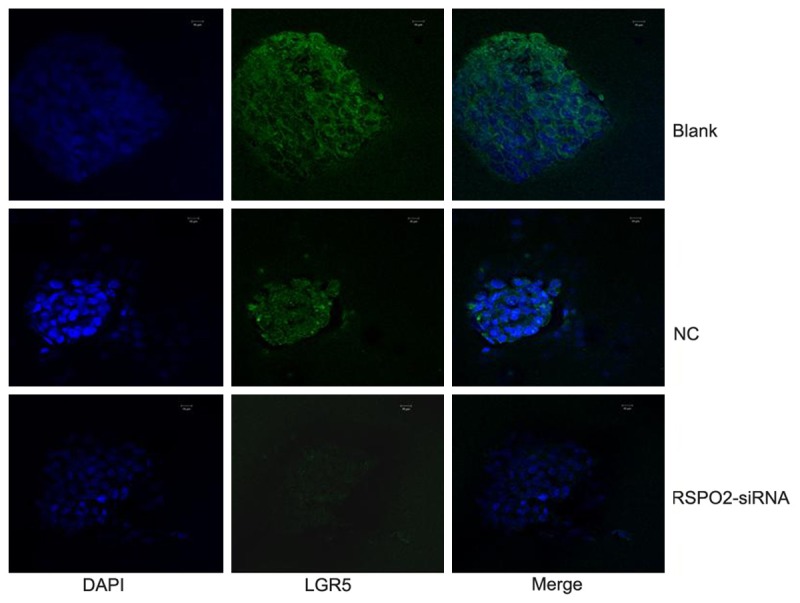
Immunofluorescent staining of LGR5 in HCT116 spheroid cells, LGR5 staining is green and nuclei are stained in blue.
RSPO2 promotes the invasive and migrate ability of HCT116 spheroid cells through EMT procedure
Results of Transwell matrigel invasion assays showed that the cell number of HCT116 spheroid cells in 40 ng/ml exogenous RSPO2 group invaded the underside of the membrane was (36.7±5.1), the RSPO2-siRNA group was (4.6 ±0.4), Blank group was (13.5±1.4) and the NC group was (12.0±1.3) respectively. The qRT-PCR and WB detection showed RSPO2 can improve the mRNA expression of snail, Twist, Vimentin, and N-cadherin in HCT116 spheroid cells, and the protein level of snail, Twist, Vimentin, N-cadhein also got a significant increase (Figure 7). These results confirmed that RSPO2 activated EMT procedure to promote the invasion and migration of spheroid cells.
Figure 7.
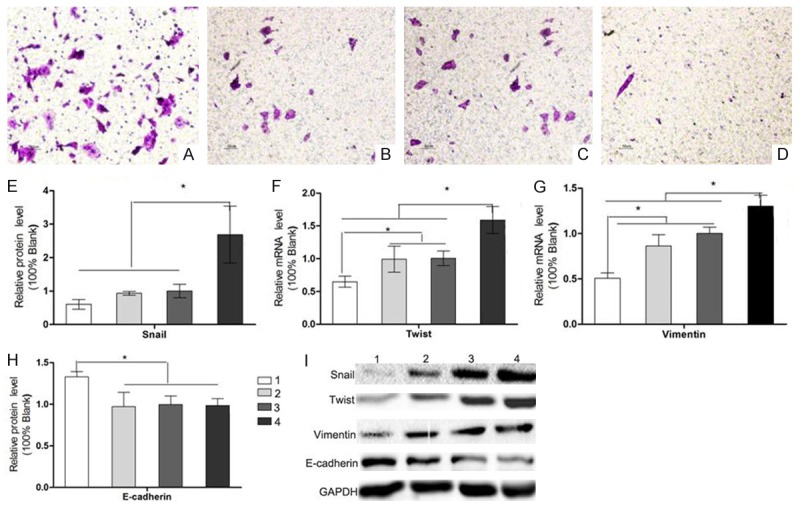
RSPO2 promotes the invasive ability of HCT116 spheroid cells through EMT procedure. Transwell invasion assay of rRSPO2 40ng/ml (A), blank (B), negative control (C) and LGR5-small interfering RNA (siRNA) group (D). Cells that invaded through the Matrigel-coated filter were fixed, stained and counted (×100). (E) Relative snail levels of HCT116 spheroid cells in rRSPO2 40 ng/ml, blank, negative control (NC) and LGR5-small interfering RNA (siRNA) group; (F) Relative twist levels of HCT116 spheroid cells in rRSPO2 40 ng/ml, blank, negative control (NC) and LGR5-small interfering RNA (siRNA) group; (G) Relative vimentin levels of HCT116 spheroid cells in rRSPO2 40 ng/ml, blank, negative control (NC) and LGR5-small interfering RNA (siRNA) group; (H) Relative E-cadherin levels of HCT116 spheroid cells in rRSPO2 40 ng/ml, blank, negative control (NC) and LGR5-small interfering RNA (siRNA) group. (I) WB result. *P<0.05.
Discussion
In last decades, cancer stem cells (CSCs) became a promising approach to explain the recurrence and metastasis of cancer. This theory proposed that tumor was heterogeneous, containing subpopulations of cells with stemness characteristics: permanent differentiation and proliferative capacities, strong tumorigenicity, high invasive and migrated abilities. From then the CSCs were proved and distinguished in multiple solid tumors except the colon cancer stem cells (CCSCs) since the specific molecular marker of CCSCs remain unclear. CD133, cd44, EpCAM, ALDH, CD24 and LGR5 were all expected markers for CCSCs. Due to the marker uncertainty of CCSCs, Spheroid colon cancer cells derived from SFM became a substitute method to enrich CCSCs, we previously successfully obtained HCT116 stem-like spheroid cells from SFM culture and confirmed their stemness both in vitro and in vivo. According to our experience, HCT116 was an accessible spheroid cells rather than other colon cancer lines, in this study we continued applying the HCT116 spheroid cells as the CCSCs model.
RSPO2 was pivotal in various embryo development such as midfacial, limb, and lung morphogenesis in vertebrates [20], and also were linked to tumorigenesis such as pancreatic and mammary cancer with or without the activation of Wnt signaling [9,10]. Starr first identified RSPO2 was a candidate colorectal oncogene in mice [21] and then Kaneda [11] also found RSPO2 was upregulated in H1299 colon cancer cell lines, but recently Kazuya Shinmura examined 75 primary CRCs in the Japanese population found RSPO2 was downregulated in most CRCs except RSPO fusion transcripts [22], Wu also pointed out that RSPO2 expression was lower or undetectable in in human CRC cells and primary tumours compared with their matched adjacent normal mucosa [12]. To ignore the RSPO2 expression issue, our results showed that RSPO2 expression had a significant increase in HCT116 spheroid cells, given RSPOs expressed and played a role in ISCs, we believed that RSPO2 also had effects on CCSCs.
LGR5 belongs to the GPCR family and was a well-accepted intestinal stem cell marker [23], Previous studies have shown that LGR5 was overexpressed in colon cancer tissue and more likely been the marker for CCSCs [23], as the specific ligand of LGR5, RSPO2 up-regulated the LGR5 expression of CCSCs. Flow cytometry detected LGR5+ cells increased after the exogenous recombinant RSPO2 added or SFM cultured. Since we previously confirmed that SFM culture enriched the LGR5+ CCSCs, LGR5 was also associated the maintenance with the stemness of spheroid cells, then we used qRT-PCR, WB and IF revealed that cytoplasmic LGR5 expression also increased with the RSPO2 added, indicated that RSPO2 might also have the same effect on enriching and maintaining CCSCs through up-regulating LGR5 expression. Besides, we found the LGR5+ had a significant raise in adherent cells than spheroid cells possibly because spheroid cells already had a higher percentage of LGR5+ CCSCs and higher RSPO2 expression, and the adherent cells had little LGR5+ CCSCs and lower RSPO2 expression which were more susceptible to exogenous RSPO2. For further understanding the effect of RSPO2 on the LGR5 expression of CCSCs, we continued down-regulated the internal RSPO2 expression of HCT116 spheroid cells and found a parallel down-regulation of LGR5 expression. The above results gave a clue on that RSPO2 in favor of enriching CCSCs by regulating the LGR5 expression of cancer cells.
To further evaluate the potential role for Rspo2 in CCSCs, we investigated typically stem properties of spheroid cells in vitro: sphere formation, proliferation, migration and invasion. As expected the spheres were increased when the RSPO2 concentration in SFM culture system was elevated by recombinant RSPO2 and reduced by si-RSPO2, which unforeseen was that RSPO2 has little effect on the proliferation of spheroid cells. From the growth curve the recombinant RSPO2 even attenuated the growth of spheroid cells insignificantly. Same in the spheres formation assay, we found that RSPO2 only elevated the spheres numbers without the total cell numbers variation. This results seemed contradictory to the RSPO2’s function of promote the cell proliferation through activating the Wnt/beta-catenin pathway. Recently Changjie Wu’s report that RSPO2 might suppress the growth of CRC cells by negatively regulating Wnt/β-catenin signaling through an LGR5-dependent feedback mechanism probably explained our findings, but in Kazuya Shinmura’ article he found an increased proliferation of DLD-1 cells after RSPO2 expression up-regulated which was contrary to Changjie Wu’s increased proliferation of DLD-1 cells after RSPO2 expression down-regulated. So the RSPO2’s role in the proliferation of CRC cells and CCSCs still need further comprehensive studies.
Migration and invasion are another important properties of CCSCs, Klauzinska [10] found that RSPO2 had regulates invasiveness of mammary epithelial cells but had little effect on proliferation of mammary epithelial cells, this was also happened to CCSCs in our studies, the invasiveness of CCSCs was up -regulated by recombinant RSPO2 or down-regulated by si-RSPO2, meanwhile, the epithelial-mesenchymal transition (EMT), which associated with maintenance of stemness in HCT116 spheroid cells according our previous study [18], also was regulated by RSPO2 in CCSCS. Given that RSPO2 promoted the spheres formation of CCSCs without cell numbers increased, we suggested that the RSPO2 might contributed to the spheres formation and the invasion of CCSCs through enhancing EMT procedure.
In our study we tried changing the RSPO2 concentration of culture environment of CCSCs by adding exogenous recombinant RSPO2 or silencing the intrinsic RSPO2 expression, then we disclosed that RSPO2 regulated part of stemness properties of CCSCs such as the sphere formation and the invasion but had little effect on the proliferation of CCSCs, the EMT might associated with the RSPO2’ role in CCSCs. However, there still lots of confuse between CCSCs and RSPO2’ waiting for us to discover, Wnt signaling might be the key to this and that is our future study.
Acknowledgements
This study was supported by Science and Technology program of Guangdong Province China (2013B021800078, and 2014A020211009). Special Project on the Integration of Industry, Education and Research of Guang-zhou, China (2060404).
Disclosure of conflict of interest
None.
References
- 1.Yoon JK, Lee JS. Cellular signaling and biological functions of R-spondins. Cell Signal. 2012;24:369–377. doi: 10.1016/j.cellsig.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phesse TJ, Sansom OJ. Responding to Rspondin: slit2 potentiates intestinal regeneration. Cell Stem Cell. 2013;13:512–514. doi: 10.1016/j.stem.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Kim KA, Zhao J, Andarmani S, Kakitani M, Oshima T, Binnerts ME, Abo A, Tomizuka K, Funk WD. R-Spondin proteins: a novel link to beta-catenin activation. Cell Cycle. 2006;5:23–26. doi: 10.4161/cc.5.1.2305. [DOI] [PubMed] [Google Scholar]
- 4.MacDonald BT, Tamai K, He X. Wnt/betacatenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao J, de Vera J, Narushima S, Beck EX, Palencia S, Shinkawa P, Kim KA, Liu Y, Levy MD, Berg DJ, Abo A, Funk WD. R-spondin1, a novel intestinotrophic mitogen, ameliorates experimental colitis in mice. Gastroenterology. 2007;132:1331–1343. doi: 10.1053/j.gastro.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Bhanja P, Saha S, Kabarriti R, Liu L, Roy-Chowdhury N, Roy-Chowdhury J, Sellers RS, Alfieri AA, Guha C. Protective role of R-spondin1, an intestinal stem cell growth factor, against radiation-induced gastrointestinal syndrome in mice. PLoS One. 2009;4:e8014. doi: 10.1371/journal.pone.0008014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ootani A, Li X, Sangiorgi E, Ho QT, Ueno H, Toda S, Sugihara H, Fujimoto K, Weissman IL, Capecchi MR, Kuo CJ. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat Med. 2009;15:701–706. doi: 10.1038/nm.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohkawara B, Glinka A, Niehrs C. Rspo3 binds syndecan 4 and induces Wnt/PCP signaling via clathrin-mediated endocytosis to promote morphogenesis. Dev Cell. 2011;20:303–314. doi: 10.1016/j.devcel.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Ilmer M, Boiles AR, Regel I, Yokoi K, Michalski CW, Wistuba II, Rodriguez J, Alt E, Vykoukal J. RSPO2 Enhances Canonical Wnt Signaling to Confer Stemness-Associated Traits to Susceptible Pancreatic Cancer Cells. Cancer Res. 2015;75:1883–1896. doi: 10.1158/0008-5472.CAN-14-1327. [DOI] [PubMed] [Google Scholar]
- 10.Klauzinska M, Baljinnyam B, Raafat A, Rodriguez-Canales J, Strizzi L, Greer YE, Rubin JS, Callahan R. Rspo2/Int7 regulates invasiveness and tumorigenic properties of mammary epithelial cells. J Cell Physiol. 2012;227:1960–1971. doi: 10.1002/jcp.22924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaneda H, Arao T, Tanaka K, Tamura D, Aomatsu K, Kudo K, Sakai K, De Velasco MA, Matsumoto K, Fujita Y, Yamada Y, Tsurutani J, Okamoto I, Nakagawa K, Nishio K. FOXQ1 is overexpressed in colorectal cancer and enhances tumorigenicity and tumor growth. Cancer Res. 2010;70:2053–2063. doi: 10.1158/0008-5472.CAN-09-2161. [DOI] [PubMed] [Google Scholar]
- 12.Wu C, Qiu S, Lu L, Zou J, Li WF, Wang O, Zhao H, Wang H, Tang J, Chen L, Xu T, Sun Z, Liao W, Luo G, Lu X. RSPO2-LGR5 signaling has tumour-suppressive activity in colorectal cancer. Nat Commun. 2014;5:3149. doi: 10.1038/ncomms4149. [DOI] [PubMed] [Google Scholar]
- 13.Seshagiri S, Stawiski EW, Durinck S, Modrusan Z, Storm EE, Conboy CB, Chaudhuri S, Guan Y, Janakiraman V, Jaiswal BS, Guillory J, Ha C, Dijkgraaf GJ, Stinson J, Gnad F, Huntley MA, Degenhardt JD, Haverty PM, Bourgon R, Wang W, Koeppen H, Gentleman R, Starr TK, Zhang Z, Largaespada DA, Wu TD, de Sauvage FJ. Recurrent R-spondin fusions in colon cancer. Nature. 2012;488:660–664. doi: 10.1038/nature11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shinmura K, Kahyo T, Kato H, Igarashi H, Matsuura S, Nakamura S, Kurachi K, Nakamura T, Ogawa H, Funai K, Tanahashi M, Niwa H, Sugimura H. RSPO fusion transcripts in colorectal cancer in Japanese population. Mol Biol Rep. 2014;41:5375–5384. doi: 10.1007/s11033-014-3409-x. [DOI] [PubMed] [Google Scholar]
- 15.Ricci-Vitiani L, Fabrizi E, Palio E, De Maria R. Colon cancer stem cells. J Mol Med (Berl) 2009;87:1097–1104. doi: 10.1007/s00109-009-0518-4. [DOI] [PubMed] [Google Scholar]
- 16.Fujii M, Sato T. Culturing intestinal stem cells: applications for colorectal cancer research. Front Genet. 2014;5:169. doi: 10.3389/fgene.2014.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X, Wei B, Han X, Zheng Z, Huang J, Liu J, Huang Y, Wei H. LGR5 is required for the maintenance of spheroid-derived colon cancer stem cells. Int J Mol Med. 2014;34:35–42. doi: 10.3892/ijmm.2014.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han XY, Wei B, Fang JF, Zhang S, Zhang FC, Zhang HB, Lan TY, Lu HQ, Wei HB. Epithelial-mesenchymal transition associates with maintenance of stemness in spheroid-derived stem-like colon cancer cells. PLoS One. 2013;8:e73341. doi: 10.1371/journal.pone.0073341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carmon KS, Gong X, Lin Q, Thomas A, Liu Q. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc Natl Acad Sci U S A. 2011;108:11452–11457. doi: 10.1073/pnas.1106083108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamada W, Nagao K, Horikoshi K, Fujikura A, Ikeda E, Inagaki Y, Kakitani M, Tomizuka K, Miyazaki H, Suda T, Takubo K. Craniofacial malformation in R-spondin2 knockout mice. Biochem Biophys Res Commun. 2009;381:453–458. doi: 10.1016/j.bbrc.2009.02.066. [DOI] [PubMed] [Google Scholar]
- 21.Starr TK, Allaei R, Silverstein KA, Staggs RA, Sarver AL, Bergemann TL, Gupta M, O’Sullivan MG, Matise I, Dupuy AJ, Collier LS, Powers S, Oberg AL, Asmann YW, Thibodeau SN, Tessarollo L, Copeland NG, Jenkins NA, Cormier RT, Largaespada DA. A transposon-based genetic screen in mice identifies genes altered in colorectal cancer. Science. 2009;323:1747–1750. doi: 10.1126/science.1163040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi S, Yamada-Okabe H, Suzuki M, Natori O, Kato A, Matsubara K, Jau Chen Y, Yamazaki M, Funahashi S, Yoshida K, Hashimoto E, Watanabe Y, Mutoh H, Ashihara M, Kato C, Watanabe T, Yoshikubo T, Tamaoki N, Ochiya T, Kuroda M, Levine AJ, Yamazaki T. LGR5-positive colon cancer stem cells interconvert with drug-resistant LGR5-negative cells and are capable of tumor reconstitution. Stem Cells. 2012;30:2631–2644. doi: 10.1002/stem.1257. [DOI] [PubMed] [Google Scholar]


