Abstract
Objective: To observe the influence of RNA interference targeting against survivin gene on the biological behaviors of human adenoid cystic cancer (ACC) cells and propose the action mechanism. Method: Specific siRNA (small interfering RNA) was constructed and transfected into ACC-2 cells using liposomes. The expressions of survivin and Caspase-3 in the transfected ACC-2 cells were detected by Western Blot and RT-PCR. Cell apoptosis was detected by transmission electron microscopy, TUNEL method and flow cytometry; ultrastructural changes and cell cycles were observed. Results: Recombinant siRNA interference plasmid specifically targeting against survivin gene was constructed successfully. Survivin protein expression in the transfected ACC-2 cells was downregulated significantly, while Caspase-3 protein and mRNA expressions were upregulated and cell proliferation was inhibited considerably. Conclusion: Recombinant siRNA interference plasmid inhibited survivin mRNA and protein expressions at high efficiency, thereby inhibiting the proliferation of ACC cells.
Keywords: Human adenoid cystic cancer cells, survivin, gene expression, cell apoptosis, proliferation, siRNA, cell cycle
Introduction
Adenoid cystic cancer (ACC) of the salivary gland is a highly malignant and invasive tumor of the oral cavity and the maxillofacial region. A comprehensive approach is preferred for the treatment of ACC, but many problems are still unresolved. Since tumor occurrence and development are associated with the activation of various oncogenes and the deactivation of tumor suppressor genes, the gene therapy targeting at the key genes related to ACC offers hope.
Survivin gene was first discovered and isolated in 1997 by Ambrosini et al. [1] who used effector cell peptidase receptor 1 (EPR-1) cDNA for the hybrid screening in human genome library. As its name infers, Survivin can prolong cell survival. The distribution of survivin gene has high cell selectivity, and the expression is mostly seen in the embryos and most tumor tissues but rarely in fully differentiated normal tissues [2]. Survivin gene is widely expressed in lung cancer, breast cancer, colon cancer, gastric cancer, esophageal cancer and bladder cancer, and also highly expressed in ACC [3]. The high expression of survivin is associated with poor prognosis, high recurrence, drug tolerance and the reduction of apoptosis index [4]. In vitro experiments showed that survivin specifically inhibited the activity of Caspase-3, the downstream effector molecule in apoptosis, thereby inhibiting cell apoptosis [5,6]. At present, survivin is the new diagnostic and treatment target under intensive research due to its tissue-specific distribution and unique role in apoptosis. Conventional chemotherapy and radiotherapy for ACC usually have poor outcomes and cause damage to normal cells near the tumors. RNA interference targeting against survivin gene possesses targeting property, specificity and safety in tumor treatment, which has already shown a good prospect in gastric cancer, pancreatic cancer and esophageal cancer [7-9]. We adopted RNA interference to silence survivin gene, discussed the influence on biological behaviors of ACC cells and proposed an action mechanism.
Materials and methods
Construction and identification of siRNA eukaryotic expression vector targeting survivin
The DNA Oligo synthesized by Life Technologies was subjected to annealing to obtain small fragments and inserted into pGenesil-1 vector digested with BamH I and Hind III restriction enzymes. Thus recombinant plasmid expression siRNA was constructed. It was confirmed by sequencing that siRNA was correctly inserted into the vector. Plasmid Mini Extraction Kit (Sigma, USA) was used to extract the plasmid.
Transfection and clone screening
pGenesil-shRNA-Survivi plasmid enveloped in liposomes was cultured in a CO2 incubator for 15-18 min and placed into the cell culture flask. ACC-2 cell line was provided by Research Room of Cell Biology at West China Center of Medical Sciences of Sichuan University. After cell culture in a 5% CO2 incubator at 37°C for 8-10 h, the medium was replaced by RPMI 1640 containing 10% fetal bovine serum (Gibcol, USA). At 24 h after transfection, clone screening was performed by adding G418 (KeyGen, USA).
Survivin mRNA expression in transfected ACC-2 cells
RNA extraction was performed with the addition of TtriPure Isolation Reagent at room temperature. Reverse transcription was performed using RT-PCR kit, and 8.0 μl of PCR product was subjected to 1.5% agarose gel electrophoresis.
Survivin protein expression in transfected ACC-2 cells
Into each cell culture flask 200 μl of lysis buffer containing PMSF was added to extract the proteins. Changes of survivin protein expression were detected by Western Blot, and the protein standard curve was plotted. Protein content was determined by BCA assay. After SDS-PAGE, the proteins were transferred to membrane and sealed with 5% milk. The membrane was washed with TBST three times at room temperature and added with super ECL detection reagent. The results were observed on the gel imager. The molecular weight and optical density of the target bands were analyzed with gel image analysis system.
Detection of cell viability by MTT assay
Cells reaching the logarithmic growth phase were collected and added into the 96-well plate. For each plate, zero adjustment wells, control wells, and plasmid wells were set up, respectively. Three plates were conventionally cultured for 24 h, 48 h and 72, respectively, and 20 μl of MTT was added to further culture cells for 2 h. Then 150 μl of DMSO was added and the cells were incubated on a shaker at low speed for 19 min. OD value was measured at 570 nm with a microplate reader.
Changes of ultrastructure and cell cycle
Cells reaching the logarithmic growth phase were observed under a transmission electron microscope. The apoptotic cells were labeled by TNEUL method. The cells were collected and fixed in 75% ethanol. Changes of cell cycle were detected by using flow cytometry at Children’s Hospital of Chongqing Medical University.
Statistical analysis
Data were expressed as mean ± standard deviation (Mean ± SD), and statistical analysis was performed using SPSS software. t test was performed with P<0.05 indicated significant difference.
Results
Sequencing and identification
Sequencing was performed by Sangon Biotech (Shanghai) Co., Ltd., and the sequences of recombinant clones were obtained. The Survivin-specific siRNA sequences were accurately connected to pGenesil-1 vector and named siSurvivin-1, siSurvivin-2 and siSurvivin-3, respectively.
Identification of ACC-2 cellsafter transfection
The cells were observed under a fluorescence microscope after transient transfection of siSurvivin with cationic liposome DOTAP. The transfected cells emitted green fluorescence as they co-expressed the green fluorescent proteins (Figure 1A).
Figure 1.
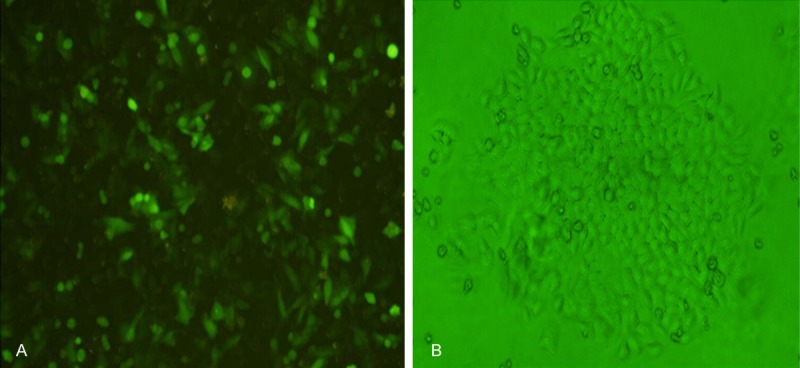
A. Expression of green fluorescent proteins after transfection of ACC-2 cells with pGenesil-shRNA-siSurvivin-2 plasmid. B. Cloning of ACC-2 cells stably transfected with pGenesil-shRNA- siSurvivin-2 plasmid.
Observation of clones under the fluorescence microscope after screening
After screening with G418, the cells were observed under a fluorescence microscope. ACC-2 cells grew in clusters and single clones were formed. Through screening, 2-3 clones of ACC-2 cells stably transfected with plasmid expressingsiSurvivin were obtained (Figure 1B).
Changes of mRNA expression in ACC-2 cells stably transfected with siSurvivin
Quantity One software was used to analyze the gray values of each band of Survivin mRNA and Caspase-3 mRNA in ACC-2 cells. The ratio of gray scale of each type of cells to that of β-actin was calculated. The results are shown in Figure 2. It can be seen from Figure 2A that the survivin mRNA expression in positively transfected cells was lower than that in negatively transfected cells. As seen in Figure 2B, Caspase-3 mRNA expression in positively transfected cells was higher than that in negatively transfected cells.
Figure 2.
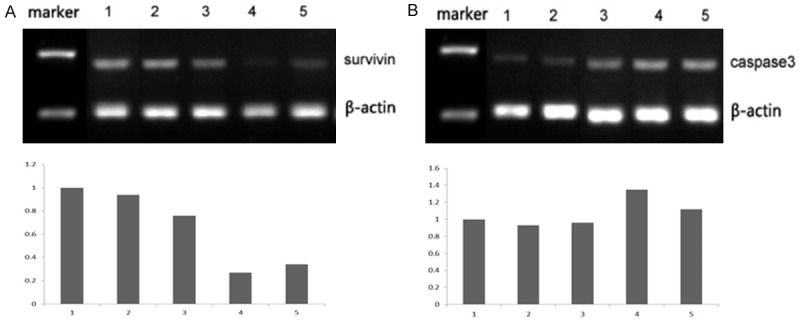
mRNA expression results by RT-PCR in different group. A. Survivin mRNA expression, 1: NT (non-transfection); 2: Negative; 3: siSurvivin-1; 4: siSurvivin-2; 5: siSurvivin-3. B. Caspase-3 mRNA expression, 1: NT (non-transfection); 2: Negative; 3: siSurvivin-1; 4: siSurvivin-2; 5: siSurvivin-3.
Changes of protein expressions
Gray values of each band of survivin and Caspase-3 proteins in ACC-2 cells were analyzed using Quantity One software. The ratio of gray scale of each type of cells to that of β-actin was calculated. Total protein extraction was performed for cells transfected with siSurvivin plasmid. Expressions of survivin and Caspase-3 proteins were detected by Western blot using specific antibodies to survivin and Caspase-3 (Figure 3).
Figure 3.
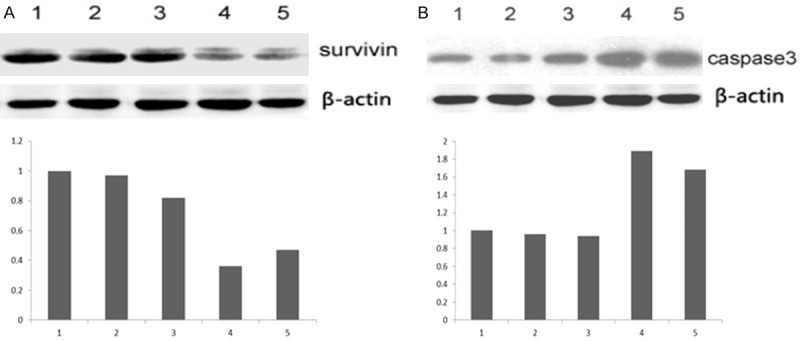
Protein expression in different group. A: Survivin mRNA expression, 1: NT (non-transfection); 2: Negative; 3: siSurvivin-1; 4: siSurvivin-2; 5: siSurvivin-3. B: Caspase-3 mRNA expression, 1: NT (non-transfection); 2: Negative; 3: siSurvivin-1; 4: siSurvivin-2; 5: siSurvivin-3.
It can be seen from Figure 3A that survivin protein expression was obviously inhibited after transfection with β-actin, indicating effective interference on the protein level. However, the effect of interference varied from one plasmid to another, and the best inhibition was achieved with siSurvivin-2. It can be seen from Figure 3B that Caspase-3 expression was greatly upregulated after transfection with siSurvivin. Like the situation with survivin protein, the best interference effect was also achieved with siSurvivin-2.
Detection of cell proliferation activity
As shown by the experimental results, the cell proliferation activity was higher in the non-transfection group and the negative control group. Transfection with siRNA plasmid caused the cell proliferation activity to decrease significantly (Table 1, Figure 4).
Table 1.
OD values of cell Proliferation (Mean ± SD)
| 24 h | 48 h | 72 h | |
|---|---|---|---|
| siSurvivin-2 | 0.213±0.009 | 0.249 ±0.017* | 0.313±0.015* |
| Negative | 0.232±0.017 | 0.326±0.029 | 0.515±0.017 |
| NT | 0.229±0.016 | 0.353±0.016 | 0.536±0.009 |
Compared to NT group;
p<0.05.
Figure 4.
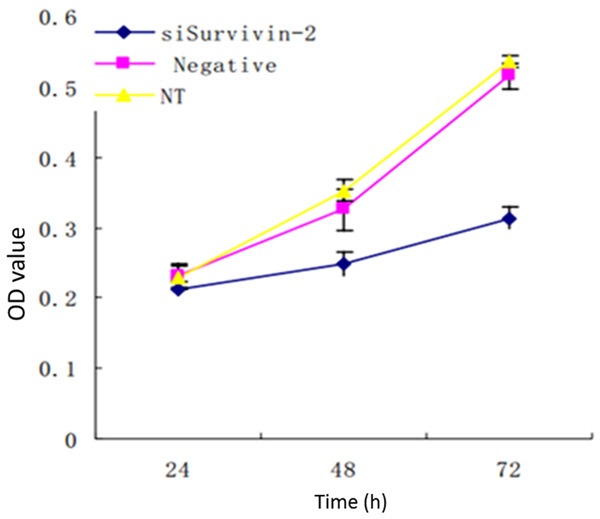
Cell proliferation activity.
Changes of ultrastructure and cell cycle
TEM images are shown in Figure 5. The cells in the non-transfection group showed polymorphism. The cell size varied, and the plasma membrane was covered with abundant microvilli. The cell nuclei were enlarged, and the cell organelles presented with no obvious swelling (Figure 5A). TEM images of the negative control group were similar as above (Figure 5B). For the siSurvivin-2 group, the microvilli-like protuberances became thicker or even shed off. Mitochondrial swelling and cytoplasmic condensation indicated signs of apoptosis (Figure 5C).
Figure 5.
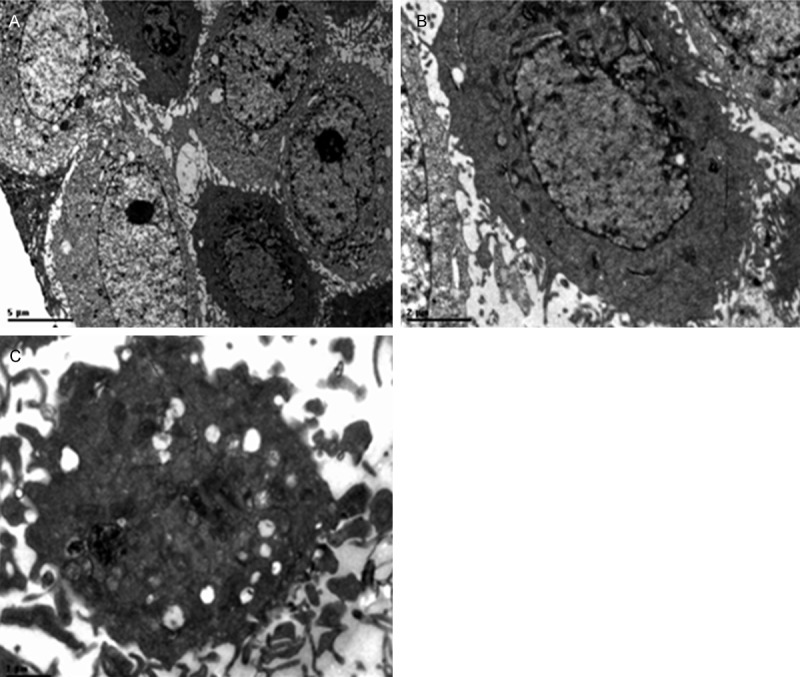
Cell ultrastructure by electron micrographs in different group. A: Non-transfection group; B: Negative control group; C: Transfected with pGenesil-shRNA- siSurvivin-2 plasmid.
TUNEL assay results shown that no change in cell morphology and color in the control group. However in the siSurvivin-2 RNAi group, the majority of cell nuclei were shrunken into a circular and uniform brown staining (Figure 6).
Figure 6.
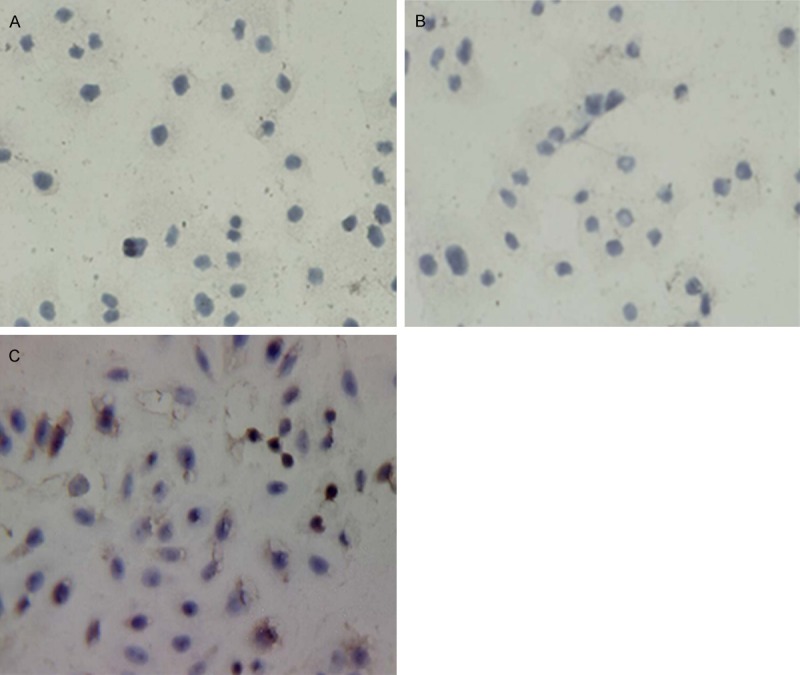
TUNEL detection results in different group. A: Non-transfection group; B: Negative control group; C: Transfected with pGenesil-shRNA- siSurvivin-2 plasmid.
The detection of cell cycle is shown in Figure 7. After stable transfection, the proportion of cells arrested in G1 phase increased obviously, while that of cells in S phase and G2/M phase decreased. This tendency coincided with the reduced cell proliferation.
Figure 7.
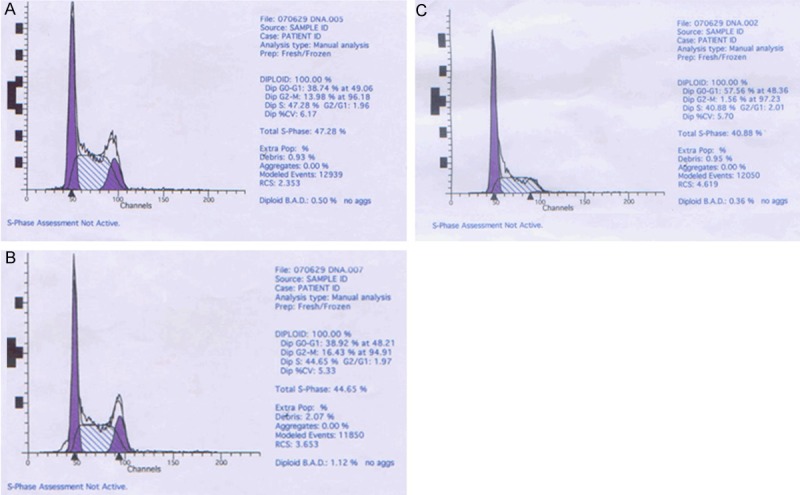
Cell cycle analysis results in different group. A: Non-transfection group; B: Negative control group; C: Transfected with pGenesil-shRNA- siSurvivin-2 plasmid.
Discussion
RNA interference (RNAi) is a biological process that achieves gene silencing through transfection with specific sequences in various species. Also an intracellular immune defense mechanism, RNAi prevents the genome from the damage of double-stranded RNA (dsRNA), thereby maintaining the relative stability of the genome. RNAi can be applied as a reverse genetics technique and occupies an important place in the studies of functional genes in the post-genomics era [10]. Gene silencing induced by dsRNA was first discovered in 1995 when Guo and Kemphues [11] blocked the expression of parl gene in Caenorhabditis elegans by using antisense RNA technique. They found that antisense RNA inhibited parl gene expression, while positive-sense RNA had similar inhibitory effect. However, the two effects were mutually independent from each other and involved different mechanisms. RNAi that can be induced by dsRNA has high specificity and acts as a gene regulatory mechanism on the post-transcriptional level [13]. Vectors expressing siRNA are mainly RNA polymerase III promoters, including human U6 promoter, H1 promoter, 7SK promoter or mouse U6 promoter [14]. Some researchers introduced RNA polymerase III promoters into shRNA expression vectors. The pGenesil-1 vector used in the present study is an optimized siRNA expression vector.
We performed molecular cloning to construct recombinant siRNA expression plasmid targeting 3 loci of the open reading frame of survivin gene. Sequence alignment showed that there were no abnormalities or wrong bases. The clone of ACC-2 cells with silenced Survivin gene was selected. Detections showed that survivin mRNA and protein expressions were effectively inhibited by transfection with siSurvivin. The degree of gene silencing varied at different target loci in terms of mRNA and protein expressions. siRNA expressions plasmid targeting No. 2 locus had the highest inhibition rate on the survivin gene.
Two major types of genes, namely, oncogenes and tumor suppressor genes (or antioncogenes), are involved in tumor occurrence. In normal conditions, the two types of genes interact with each other, maintaining the normal growth, differentiation and apoptosis of cells. Once the oncogenes are activated or antioncogenes are deactivated, excess proliferation, differentiation and abnormal apoptosis of tumor cells take place, which may lead to tumors. We observed the ACC-2 cells at 24 h, 48 h and 72 h after transfection. Compared with the control group, the cells with survivin gene silenced had a more mild proliferation curve, indicating that tumor cell proliferation was effectively inhibited by the silencing of survivin gene. Microstructures, organelles and morphological changes of the transfected ACC-2 cells were observed by transmission electron microscopy. Early morphological changes during apoptosis include nuclear chromatin condensation and concentration of chromatin near the nuclear membrane. Then cytoplasmic condensation and plasma membrane blebbing occur, and the cell nuclei are disintegrated into fragments which are wrapped in cell membranes. Many vesicles and apoptotic bodies with intact membrane structures are formed in the cytoplasm [15]. These changes are different from those during cell necrosis which is usually accompanied by cell swelling, cell membrane rupture and cell disintegration. Our experiment showed that the cells underwent the above early morphological changes during apoptosis after stable transfection. Ben-Izhak O et al. [16] compared TUNEL assay for pathological sections from 66 cases with salivary gland cancers with detections of p53 and Ki67 expressions. They found that TUNEL assay was effective for pathological classification and diagnosis of tumor metastasis. In our experiment, no morphological changes were detected by TUNEL assay in the non-transfection group and the negative control group, and the cells were not stained. In RNAi group, some cells were stained uniformly with brownish yellow. Thus after the silencing of survivin gene, more apoptotic ACC cells were induced compared with the model group, Caspase-3 normally exists in the cytoplasm as pro-enzyme (32 KD) and is activated in early stage of apoptosis. The activated Caspase-3 consists of two large subunits (17 KD) and two small subunits (12 KD), and the two subunits constitute the active form of Caspase-3. The cytoplasmic and nuclear substrate can be lysed by the activated Caspase-3, leading to cell apoptosis [17-19]. Caspase-3 is the most important terminal digestive enzyme in cell apoptosis and the key component in CTL-mediated cell death. Survivin blocks cell apoptosis induced by various factors by inhibiting the activity of effectors Caspase-3 and Caspase-7 [20-23]. Some researchers found that the upregulation of survivin gene was significantly correlated with the reduction of Caspase-3 in liver cancer [24] and tongue squamous cell carcinoma [25]. The Caspase-3 mRNA and protein expressions in the tranfection group were obviously upregulated compared with the non-transfection group and the negative control. It is apparent that the activation of Caspase-3 by the transfection of siRNA targeting Survivin is the major cause of apoptosis of ACC cells. Thus survivin is a target gene for the treatment of ACC.
The cycle of duplication and division is known as the cell cycle (CC). Each CC consists of four phases successively: G1 interval, S phase (the phase of DNA synthesis), G2 interval, and M phase (the phase of mitosis). Cells not engaged in any of these phases are in Go phase (resting phase). CC is regulated by the check points [26-27]. Survivin gene not only inhibits cell apoptosis, but also promotes mitosis [28,29]. During cell division, survivin specifically acts in G2/M phase [30], promoting tumor cell proliferation and spread, which explains the periodicity of survivin gene expression. After silencing of survivin gene by RNAi, the proportion of cells arrested in G1 phase increased obviously, while that of cells in S phase and G2/M phase decreased, which was consistent with the mild proliferation curve in MTT assay. This also coincided with other in vitro experiments [31]. The downregulation of survivin gene inhibited the proliferation of ACC cells by inhibiting mitosis. As fewer cells were in G2/M phase, the cells could not complete mitosis, and therefore the cell proliferation was inhibited.
We explored the strategy of silencing of survivin gene to inhibit the proliferation of ACC cells and probable mechanism. The findings shed new light into the development of RNAi-based drugs targeting ACC-related genes, thereby promoting the treatment of ACC.
Acknowledgements
This work was supported by the Clinical Innovation Fund Of Southwest Hospital, Third Military Medical University. No SWH2013LC16.
Disclosure of conflict of interest
None.
References
- 1.Adida C, Crotty PL, McGrath J, Berrebi D, Diebold J, Altieri DC. Developmentally regulated expression of the novel cancer anti-apoptosis gene survivin in human and mouse differentiation. Am J Pathol. 1998;152:43–9. [PMC free article] [PubMed] [Google Scholar]
- 2.Altieri DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene. 2003;22:8581–8589. doi: 10.1038/sj.onc.1207113. [DOI] [PubMed] [Google Scholar]
- 3.Zhang B, Mu HB, Xu XG, Liu W, Hu NR. Role of Survivin gene on the apoptosis of adenoid cystic carcinoma-2 cells induced by arsenic trioxide. Hua Xi Kou Qiang Yi Xue Za Zhi. 2010;28:246–249. [PubMed] [Google Scholar]
- 4.Engels K, Knauer SK, Metzler D, Simf C, Struschka O, Bier C, Mann W, Kovács AF, Stauber RH. Dynamic intracellular Survivin in oral squamous cell carcinoma: underlying molecular mechanism and potential as an early prognostic marker. J Pathol. 2007;211:532–540. doi: 10.1002/path.2134. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Xu Z, Zhang M. Downregulation of survivin expression and elevation of caspase-3 activity involved in pitavastatin-induced HepG2 cell apoptosis. Oncol Rep. 2007;18:383–387. [PubMed] [Google Scholar]
- 6.Wang ZM, Zhao ZG, Guan XM, Liu F, Zhang LP. Expression of Survivin mRNA and Caspase-3 mRNA and their correlation in tongue squamous cell carcinoma. Shanghai Kou Qiang Yi Xue. 2007;16:582–586. [PubMed] [Google Scholar]
- 7.Miao GY, Lu QM, Zhang XL. Downregulation of Survivin by RNAi inhibits growth of human gastric carcinoma cells. World J Gastroenterol. 2007;13:1170–1174. doi: 10.3748/wjg.v13.i8.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan HT, Xue XH, Dai ZJ, Wang XJ, Li A, Qin ZY. Downregulation of Survivin expression by small interfering RNA induces pancreatic cancer cell apoptosis and enhances its radiosensitivity. World J Gastroenterol. 2006;12:2901–2907. doi: 10.3748/wjg.v12.i18.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Zhu H, Quan L, Zhou C, Bai J, Zhang G, Zhan Q, Xu N. Downregulation of Survivin by RNAi Inhibits the Growth of Esophageal Carcinoma Cells. Cancer Biol Ther. 2005;4:974–8. doi: 10.4161/cbt.4.9.1914. [DOI] [PubMed] [Google Scholar]
- 10.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double - stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–11. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 11.Guo S, Kemphues KJ. par-1, a gene required for establishing polarity in C. elegans embryos, encodes a putative Ser/Thr kinase that isasymmetrically distributed. Cell. 1995;81:611–620. doi: 10.1016/0092-8674(95)90082-9. [DOI] [PubMed] [Google Scholar]
- 12.Vu-Dac N, Gervois P, Jakel H, Nowak M, Bauge E, Dehondt H, Staels B, Pennacchio LA, Rubin EM, Fruchart-Najib J, Fruchart JC. Apolipoprotein A5, a crucialdeterminant of plasma t riglyceride levels, is highly responsive toperoxisome proliferator2activated receptor alpha activators. J Biol Chem. 2003;278:17982–17985. doi: 10.1074/jbc.M212191200. [DOI] [PubMed] [Google Scholar]
- 13.van der Vliet HN, Sammels MG, Leegwater AC, Levels JH, Reitsma PH, Boers W, Chamuleau RA. Apoli-poprotein AV: a novel apolipoprotein associated with an earlyphase of liver regeneration. J Biol Chem. 2001;276:44512–20. doi: 10.1074/jbc.M106888200. [DOI] [PubMed] [Google Scholar]
- 14.O’Brien PJ, Alborn WE, Sloan JH, Ulmer M, Boodhoo A, Knierman MD, Schultze AE, Konrad RJ. The novel apolipoprotein A5 is present in human serum, is associated with VLDL, HDL, and chylomicrons, and circulates at very low concent rations compared with other apolipoproteins. Clin Chem. 2005;51:351–359. doi: 10.1373/clinchem.2004.040824. [DOI] [PubMed] [Google Scholar]
- 15.Hacker G. The morphology of apoptosis. Cell Tissue Res. 2000;301:5–17. doi: 10.1007/s004410000193. [DOI] [PubMed] [Google Scholar]
- 16.Ben-Izhak O, Laster Z, Araidy S, Nagler RM. TUNEL - an efficient prognosis predictor of salivary malignancies. Br J Cancer. 2007;96:1101–1106. doi: 10.1038/sj.bjc.6603655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srinivasula SM, Fernandes-Alnemri T, Zangrilli J, Robertson N, Armstrong RC, Wang L, Trapani JA, Tomaselli KJ, Litwack G, Alnemri ES. The Ced-3/interleukin 1beta converting enzymelike homolog Mch6 and the lamin-cleaving enzyme Mch2alpha are substrates for the apoptotic mediator CPP32. J Biol Chem. 1996;271:27099–27106. doi: 10.1074/jbc.271.43.27099. [DOI] [PubMed] [Google Scholar]
- 18.Chai J, Wu Q, Shiozaki E, Srinivasula SM, Alnemri ES, Shi Y. Crystal structure of a procaspase-7 zymogen: mechanisms of activation and substratebinding. Cell. 2001;107:399–407. doi: 10.1016/s0092-8674(01)00544-x. [DOI] [PubMed] [Google Scholar]
- 19.Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, Pedersen IM, Ricci JE, Edris WA, Sutherlin DP, Green DR, Salvesen GS. A unified model for apical caspase activation. Mol Cell. 2003;11:529–541. doi: 10.1016/s1097-2765(03)00051-0. [DOI] [PubMed] [Google Scholar]
- 20.Ambrosini G, Adida C, Altieri DC. A novel antiapoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 21.Tamm I, Wang Y, Sausville E, Scudiero DA, Vigna N, Oltersdorf T, Reed JC. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas(CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998;58:5315–5320. [PubMed] [Google Scholar]
- 22.Dohi T, Beltrami E, Wall NR, Plescia J, Altieri DC. Mitochondrial survivin inhibits apoptosis and promotes tumorigenesis. J Clin Invest. 2004;114:1117–1127. doi: 10.1172/JCI22222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shin S, Sung BJ, Cho YS, Kim HJ, Ha NC, Hwang JI, Chung CW, Jung YK, Oh BH. An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7. Biochemistry. 2001;40:1117–23. doi: 10.1021/bi001603q. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Xu Z, Zhang M. Downregulation of Survivin expression and elevation of Caspase-3 activity involved in pitavastatin-induced HepG 2 cell apoptosis. Oncol Rep. 2007;18:383–387. [PubMed] [Google Scholar]
- 25.Wang ZM, Zhao ZG, Guan XM, Liu F, Zhang LP. Expression of Survivin mRNA and Caspase-3 mRNA and their correlation in tongue squamous cell carcinoma. Shanghai Kou Qiang Yi Xue. 2007;16:582–586. [PubMed] [Google Scholar]
- 26.Hartwell LH, Ted A. WeinertChekpoints: Controls that ensure the order of cell cycle events. Science. 1989;246:629–633. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- 27.Chiou SK, Jones MK, Tarnawski AS. Survivin-an antiapoptosis protein: its biological roles and implications for cancer and beyond. Med Sci Monit. 2003;9:P125–129. [PubMed] [Google Scholar]
- 28.Altieri DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene. 2003;22:8581–8589. doi: 10.1038/sj.onc.1207113. [DOI] [PubMed] [Google Scholar]
- 29.Altieri DC. Molecular circuits of apoptosis regulation and cell division control: the survivin paradigm. J Cell Biochem. 2004;92:656–663. doi: 10.1002/jcb.20140. [DOI] [PubMed] [Google Scholar]
- 30.Kayaselcuk F, Nursal TZ, Polat A. Expression of Survivin, bcl22, P53 and bax in breast carcinoma and ductal intraepithelial neoplasia (DIN21a) J Exp Clin Cancer Res. 2004;23:105–112. [PubMed] [Google Scholar]
- 31.Miao GY, Lu QM, Zhang XL. Downregulation of survivin by RNAi inhibits growth of human gastric carcinoma cells. World J Gastroenterol. 2007;13:1170–1174. doi: 10.3748/wjg.v13.i8.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]


