Abstract
Anthracycline-based chemotherapy is a conventional treatment for breast cancer. However, it can negatively affect host immune function and thereby impair patients’ quality of life. Boosting the host immune system and reducing the adverse effect of chemotherapy are important for effective cancer treatment. Natural killer (NK) cells stimulate immune responses against cancer; autologous immune enhancement therapy with NK cells prolongs patient survival without significant adverse effects. This study investigated the effects of combined treatment with the anthracycline agent epirubicin (EPI) and NK cells on human breast cancer cells. NK cells were obtained by autologous adoptive cell transfer from breast cancer patients and amplified for 14 days in vitro. The cytotoxicity of NK cells against breast cancer cells was higher following EPI (5.0 μg/ml) pretreatment than without EPI pretreatment or application of EPI alone. The expression of NKG2D ligands [unique long 16-binding protein (ULBP) 1, ULBP2, and major histocompatibility complex class I-related chain A] in breast cancer cells was upregulated by pretreatment with EPI, which also increased the secretion of interferon-γ and tumor necrosis factor-α and expression of perforin and granzyme B in NK cells. These results indicate that EPI-NK cell treatment has synergistic cytotoxic effects against breast cancer cells, and suggest that anthracycline-based chemotherapy and NK cell-based immunotherapy can be combined for more effective breast cancer treatment.
Keywords: Natural killer cells, epirubicin, breast cancer
Introduction
Chemotherapy is an important treatment for breast cancer patients. Anthracycline, which targets tumor cell chromosomal DNA, is a widely used and effective drug [1]; however, it also has adverse secondary effects including nausea, vomiting, hematotoxicity, and hair loss [2,3], which can reduce patients’ quality of life. In addition, the lower immune status of breast cancer patients receiving chemotherapy can increase relapse rate [4], since anthracycline-based chemotherapies have been shown to impair immune function [5].
The elimination of tumors requires that the immune system work with standard treatments such as surgery and chemo- and radiotherapies. Autologous immune enhancement therapy (AIET) is an in vitro method used to amplify natural killer (NK) cells and activate T cells, which are then infused into patients with advanced solid tumors [6]. NK cell-based AIET at the Cancer Center of the First Hospital of Jilin University has been effective for treating various types of cancer, including hepatocellular carcinoma [7], lung cancer [8], and gastric carcinoma [9]. Improved prognosis, reflected by a significant downregulation of cancer cell markers, has been accompanied by an increase in the quality of life of patients [10]. NK cells are a critical component of innate immunity against viruses and cancer; cytokine-induced NK activation [11] leads to cytolytic granule-mediated cell apoptosis [12], direct induction of apoptosis by Fas and Fas ligand (FasL) binding [13], and antibody-dependent cell-mediated cytotoxicity [14]. NK cell activity is regulated by interactions between NKG2D-a disulfide-linked homodimeric receptor-and its ligands unique long 16-binding protein (ULBP) and major histocompatibility complex class I-related chain (MIC)A expressed by tumor cells [15]. A prospective cohort study showed that medium and high NK activity was associated with decreased cancer rates, whereas low activity was associated with an increased incidence of cancer [16]. NK activity was also found to be lower in breast cancer patients than in healthy individuals [17]. These data underscore the critical role of NK cells in host defense mechanisms against cancer.
The present study investigated whether combined treatment with anthracycline and NK cell-based AIET is an effective treatment for breast cancer. The anthracycline agent epirubicin (EPI) was used in conjunction with NK cells obtained by autologous adoptive cell transfer from breast cancer patients and expanded for 14 days in vitro, and the cytotoxicity of NK cells was evaluated. We found that pretreatment with EPI enhanced NK cell-induced apoptosis of breast cancer cells. These data suggest that anthracycline-based chemotherapy and NK cell-based AIET can be combined for more effective breast cancer treatment.
Materials and methods
Cell lines and culture
MCF-7, SKBR-3, and MDA-MB-231 breast cancer cells were obtained from the cell bank of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (Hyclone, Logan, UT, USA).
Isolation and expansion of primary NK cells
Peripheral blood mononuclear cells (PBMCs) were obtained from 13 breast cancer patients at the Cancer Center of the First Hospital of Jilin University (Table 1) and were isolated from human peripheral blood by Ficoll gradient centrifugation. To expand NK cells, PBMCs were cultured in AIM-V medium (Invitrogen, Carlsbad, CA, USA) containing 700 U/ml interleukin (IL)-2 (Miltenyi, Cologne, Germany) and 1 ng/ml OK432 (Shandong Lu Ya Pharmaceutical, Jining, China) for 24 h at 37°C in a flask coated with mouse anti-human cluster of differentiation (CD)16 monoclonal antibody (mAb; Beckman Coulter, Marseille, France). This was followed by culture in AIM-V medium containing 700 U/ml IL-2 at 37°C for 2-3 weeks. Expanded NK cells used in this study were cultured for 14 days, and were confirmed as CD3-CD56+ by flow cytometry using anti-CD3 and -CD56 antibodies (BD Pharmingen, San Jose, CA, USA). The purity was ≥ 80%. Written informed consent was obtained from each patient and the experimental protocol was approved by the Institutional Review Committee of Jilin University and the ethics committee of the First Hospital of Jilin University.
Table 1.
Characteristics of NK cell donor breast cancer patients
| Age | Pathological type | ER | PR | HER2 | Ki-67 (%) | Length of illness | Therapy | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Chemotherapy | Radio therapy | Hormonal therapy | Trastuzumab | ||||||||
| 1 | 82 | IDC | - | - | - | +70 | 27 | 3*T+3*FEC | - | - | - |
| 2 | 51 | IDC | + | + | - | +40 | 23 | 1*X | + | Letrozole | - |
| 3 | 42 | ILC | + | + | - | +5 | 52 | 6*CAF | - | Tamoxifen | - |
| 4 | 55 | IDC | - | - | - | < 70 | 11 | 1*TAC+4*AC-T | - | - | - |
| 5 | 48 | IDC | - | - | + | +70 | 14 | 1*AC+5*TCH | - | - | + |
| 6 | 38 | IDC | - | - | + | +60 | 42 | 3*TC+3*NP | + | - | + |
| 7 | 71 | IDC | + | - | - | NA | 57 | 6*TAC | + | Tamoxifen | - |
| 8 | 62 | IDC | + | + | + | +25 | 52 | 3*AC-3*TH | - | Anastrozole | + |
| 9 | 59 | IDC | - | - | + | +60 | 39 | 6*TCH | + | - | + |
| 10 | 46 | IDC | + | + | + | +60 | 15 | 1*TAC+5*TCH | + | Tamoxifen | + |
| 11 | 49 | IDC | - | - | + | +70 | 10 | 4*AC-T | - | - | - |
| 12 | 48 | IDC | + | + | + | +40 | 29 | 6*TAC | + | Anastrozole | - |
| 13 | 44 | IDC | - | - | - | +70 | 27 | 3*T+3*FEC | - | - | - |
A, pirarubicin; C, cyclophosphamide; ER, estrogen receptor; E, epirubicin; F, fluorouracil; H, herceptin; HER2, human epidermal growth factor receptor 2; ILC, invasive lobular carcinoma; IDC, invasive ductal carcinoma; MC, medullary carcinoma; N, navelbine; P, Cisplatinum; PR, progesterone receptor; T, docetaxel/taxol; X, Xeloda. *All patients were female and had undergone surgery.
Cell viability assay
Inhibition of breast cancer cell growth by EPI treatment was assessed with a 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay according to the manufacturer’s instructions. Briefly, MCF-7, SKBR3, and MDA-MB-231 cells were seeded in 96-well plates at a density of 5 × 104/ml and allowed to attach overnight, then treated with EPI at concentrations of 0.625, 1.25, 2.5, 5, 10, and 20 μg/ml for 8, 12, 24, and 48 h at 37°C and 5% CO2. A 20-μl volume of MTT (5 g/l) was added followed by incubation for 4 h. Colored formazan crystals produced by reaction with MTT were dissolved in 150 μl dimethylsulfoxide, and the absorbance was measured at 570 nm using a Model 550 microplate reader (BioTek, Winooski, VT, USA). Inhibition of cell proliferation was calculated with the following formula: % inhibited = [1-(treatment group absorbance/control absorbance)] × 100% [18].
Cytotoxicity assay
The cytotoxicity of EPI and NK cells to breast cancer cells was evaluated with the calcein-acetoxymethyl (AM) ester release assay. Calcein-AM (Dojindo, Shanghai, China) was added to MCF-7, SKBR-3, or MDA-MB-231 cells (106/ml in 1000 μl DMEM) at a final concentration of 1 μM for 30 min at 37°C and 5% CO2. The cells were washed twice with phosphate-buffered saline (PBS) and divided into five groups: no treatment (A), treated with Triton X-100 (B), treated with EPI (C), with added NK cells (D), and pretreated with 5 μg/ml EPI followed by addition of NK cells (E) at a density of 5 × 104/ml. Different concentrations (15 × 104/ml, 50 × 104/ml, and 150 × 104/ml) of expanded NK cells were added for 4 h at 37°C. After centrifugation at 1500 rpm for 3 min, the medium was transferred to another 96-well plate. The released extracellular calcein-AM was visualized and quantified with a microplate reader at excitation/emission wavelengths of 490/515 nm. The cytotoxicity was determined according to the following formula: killing rate (%) = [(C or D or E-A)/(B-A)] × 100% [19].
Enzyme-linked immunosorbent assay (ELISA)
Interferon (IFN)-γ and tumor necrosis factor (TNF)-α secretion by NK cells was measured using commercial ELISA kits (Bioster, Wuhan, China) according to the manufacturer’s instructions. Briefly, MCF-7, SKBR-3, and MDA-MB-231 cells pretreated with 5.0 µg/ml EPI for 12 h were seeded in triplicate in 96-well plates (1 × 104/per well) and co-cultured with the same number of expanded NK cells for an additional 24 h. Expanded NK cells cultured without or with untreated cancer cells served as controls. IFN-γ and TNF-α levels in the supernatant were determined by measuring absorbance at 450 nm on a microplate reader. All samples were prepared in triplicate under the same conditions.
Flow cytometry analysis
NK cell phenotype and ligands expressed by breast cancer cells were determined by flow cytometry using a LSRFortessa system (BD Biosciences, San Diego, CA, USA). Briefly, expanded NK cells were collected, washed, and incubated with mouse mAbs against human CD3, CD56, NKG2D, NKp44, NKp46, CD158a, and Fas (all from BD Pharmingen); and CD158b, CD158b2, CD158e, and FasL (all from Biolegend) for 15 min. Cultured breast cancer cells were washed and incubated with mouse mAbs against ULBP1, ULBP2, and MICA (all from Biolegend) for 15 min.
To assess the levels of the intracellular granule granzyme B (BD Pharmingen) and of perforin (BD Pharmingen) in NK cells, the Golgi transport inhibitors Golgi Stop and Golgi Plug (both from BD Biosciences) were added to the experimental system for 12 h. Cells were washed and incubated with antibodies against CD3 and CD56 for 15 min; the membranes were ruptured by treatment with 10% BD Cytofix/Cytoperm, followed by incubation with granzyme B and perforin at 20°C for 30 min. Cells were then washed with wash buffer (10% BD Perm/Wash). Data were analyzed using FlowJo v.9.6.4 software (Tree Star, Ashland, OR, USA).
Statistical analysis
Statistical analyses were carried out with Prism 6 (GraphPad, La Jolla, CA, USA) and SPSS v.17.0 (SPSS Inc., Chicago, IL, USA) software. Data are expressed as mean ± SEM. The Student’s t test was used to assess differences between groups. P < 0.05 was considered as statistically significant.
Results
Expanded human NK cells are cytotoxic to breast cancer cells
We investigated whether combining chemotherapy and AIET can improve the treatment of breast cancer. NK cell-based AIET is widely used as cancer immunotherapy in our hospital; we obtained CD3-CD56+ NK cells from breast cancer patients, and evaluated the expression of NK cell receptors by flow cytometry (Figure 1A). The activating receptors NKG2D, NKp44, and NKp46 were highly expressed whereas the inhibitory receptors CD158a, CD158b, CD158b2, and CD158e were detected at low levels. Expanded NK cells were also cytotoxic to MCF-7, SKBR-3, and MDA-MB-231 breast cancer cells (Figure 1B), with cytotoxicity increased at higher E:T ratios. These results indicate that the expanded NK cells were activated.
Figure 1.
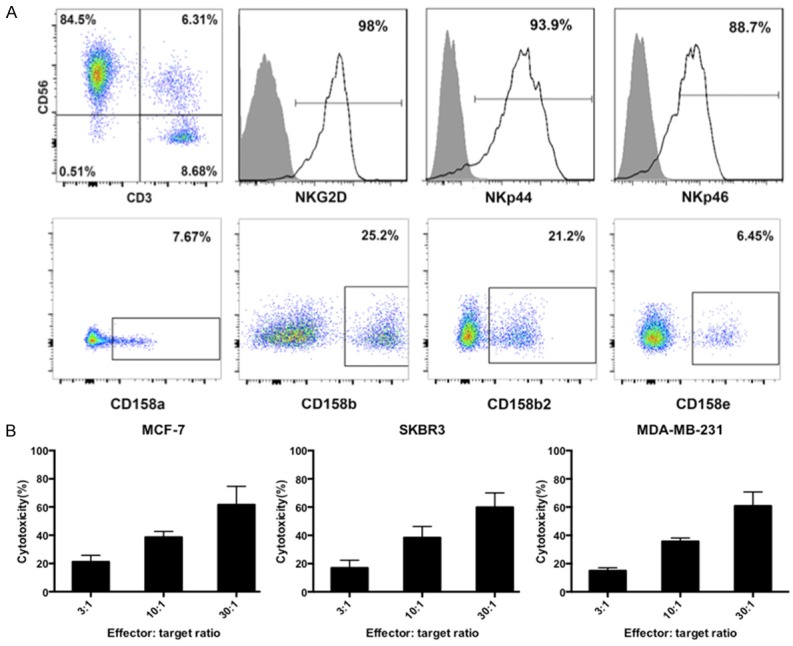
Phenotype of expanded human NK cells. A. PBMCs from breast cancer patients were cultured for 14 days in AIM-V medium the presence of IL-2. Expanded NK cells were induced by IL-2, OK432, and anti-human CD16 antibody. Expression of activating and inhibitory NK cell receptors were detected by flow cytometry. B. The cytotoxicity of expanded NK cells against MCF-7, SKBR-3, and MDA-MB-231 and breast cancer cells at different effector: target ratios (3:1, 10:1, and 30:1) was assessed at 24 h. Experiments were performed in triplicate; data are expressed as mean ± SEM.
EPI inhibits breast cancer cell proliferation
The effects of EPI on breast cancer cells and expanded NK cells were investigated by cell proliferation and cytotoxicity assays. MCF-7, SKBR-3, and MDA-MB-231 cells and NK cells were treated with various concentrations of EPI. Proliferation was inhibited in all three breast cancer cell lines (Figure 2A) and in NK cells (Figure 2B) in a dose- and time-dependent manner by EPI treatment, suggesting that directly combining EPI with NK cells is not an effective strategy for inducing cytotoxicity in breast cancer cells. However, when breast cancer cells were pretreated with EPI followed by expanded NK cells, the cytotoxic effect was potentiated as compared to either treatment alone (Figure 2C). Thus, sequential treatment with EPI followed by expanded NK cells leads to increased cytotoxicity in breast cancer cells.
Figure 2.
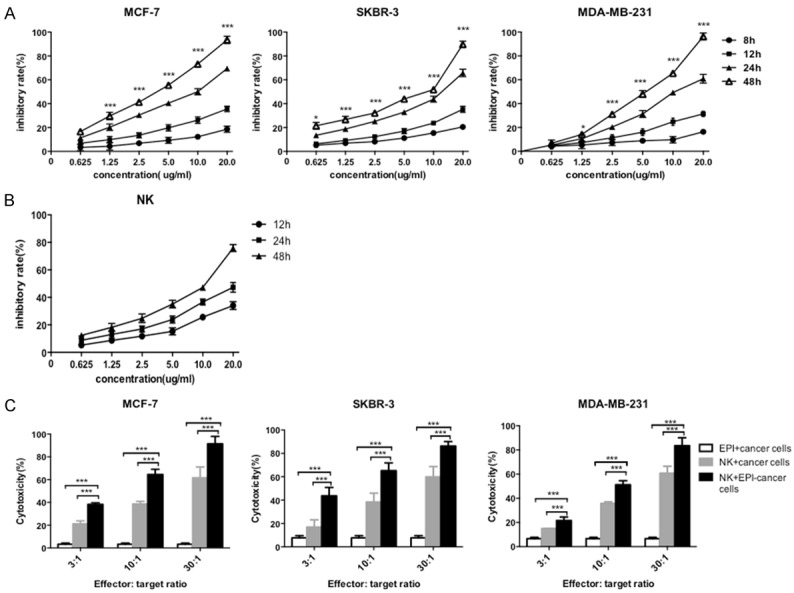
EPI pretreatment enhances the cytotoxicity of NK cells to human breast cancer cells. (A, B) MCF-7, SKBR-3, and MDA-MB-231 breast cancer cells (A) or expanded human NK cells (B) were cultured with indicated concentrations of EPI at for 8 to 48 h. Cell viability was assessed with the MTT assay. (C) Breast cancer cells were pretreated with EPI for 12 h, followed by addition of NK cells for 24 h; cells treated with EPI or NK cells only served as controls. Cytotoxicity was assessed with calcein-AM. Experiments were performed in triplicate; data are expressed as mean ± SEM. *P < 0.05, ***P < 0.01.
EPI induces NKG2D ligand expression in breast cancer cells
NKG2D, an activating receptor of NK cells, plays an essential role in immune responses, and NKG2D ligands and downstream signaling are important for anti-tumorigenic activity. NKG2D ligands are expressed by many types of cancer cell; we therefore evaluated the expression of the NKG2D ligands ULBP1, ULBP2, and MICA in breast cancer cells after EPI pretreatment by flow cytometry. The application of EPI induced the upregulation of all three NKG2D ligands in MCF-7 (Figure 3A), SKBR-3 (Figure 3B), and MDA-MB-231 (Figure 3C) cells (P < 0.01). We did not observe any differences in the expression of the activating NK cell receptors NKG2D, NKp44, or NKp46 owing to their high basal expression levels (data not shown). These results suggest that inducing the upregulation of NKG2D ligands is a mechanism by which EPI enhances the cytotoxicity of NK cells against breast cancer cells.
Figure 3.
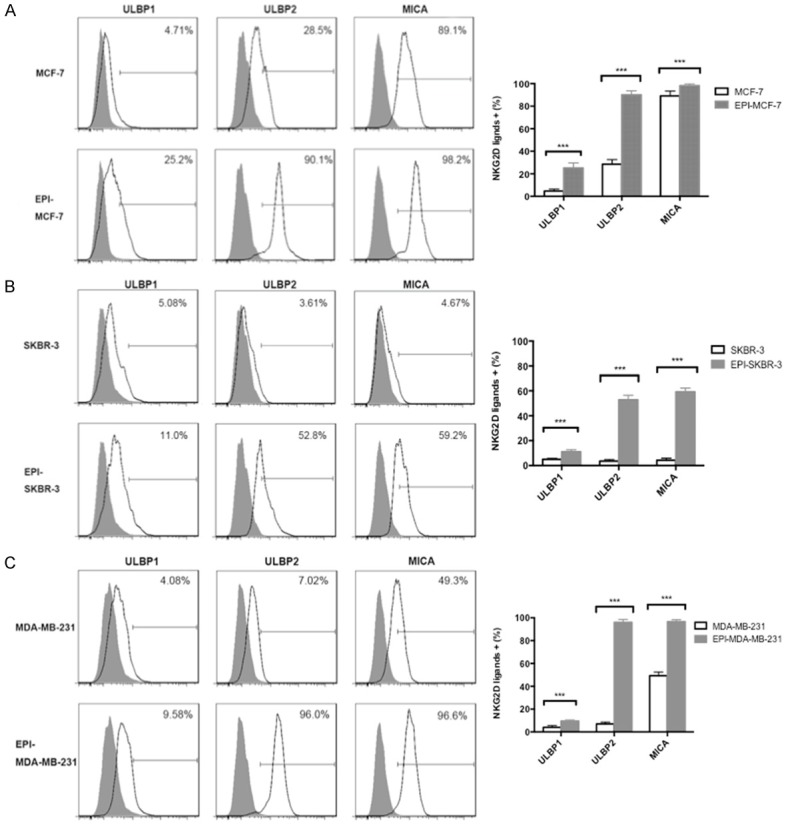
Upregulation of NKG2D ligand expression in breast cancer cells by EPI pretreatment. (A-C) MCF-7 (A), SKBR-3 (B), and MDA-MB-231 (C) breast cancer cells were pretreated with EPI for 12 h, washed, and cultured for 24 h. Untreated cells were used as a control. ULBP1, ULBP2, and MICA expression was detected by flow cytometry. Experiments were performed in triplicate; data are expressed as mean ± SEM. ***P < 0.01.
Co-culture of expanded NK cells and EPI-pretreated breast cancer cells increases IFN-γ and TNF-α secretion by NK cells
We investigated whether EPI pretreatment can stimulate the release of cytokines by NK cells. IFN-γ and TNF-α levels in the supernatant of expanded NK cells without or with co-cultured, EPI-pretreated or untreated breast cancer cells were measured by ELISA. IFN-γ and TNF-α secretion was higher in the supernatant of NK cells co-cultured with EPI-pretreated breast cancer cells (Figure 4), suggesting that EPI pretreatment caused increased cytokine secretion by NK cells, thereby potentiating their cytotoxic effects.
Figure 4.
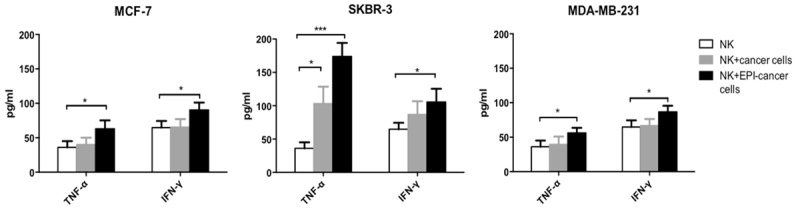
EPI pretreatment increases IFN-γ and TNF-α secretion by NK cells. MCF-7, SKBR-3, and MDA-MB-231 breast cancer cells were pretreated with EPI for 12 h before adding expanded human NK cells for 24 h. IFN-γ and TNF-α levels in the supernatant were detected by ELISA. The supernatant of NK cells without or with untreated cancer cells served as controls. Experiments were performed in triplicate; data are expressed as mean ± SEM. *P < 0.05, ***P < 0.01.
Granzyme B and perforin expression is upregulated in NK cells in the presence of EPI-pretreated breast cancer cells
To further examine how EPI influences the cytotoxicity of NK cells to breast cancer cells, we evaluated the two main mechanisms of NK cell-mediated cytotoxicity-i.e., the granzyme B/perforin and Fas/FasL pathways. Both granzyme B and perforin were produced at high levels (> 85%) by expanded NK cells, as determined by flow cytometry. Expression was higher in NK cells co-cultured with EPI-pretreated MCF-7 (Figure 5A), SKBR-3 (Figure 5B), and MDA-MB-231 (Figure 5C) cells as compared to those cultured alone, indicating that the granzyme B/perforin pathway is activated in NK cells in the presence of EPI-pretreated breast cancer cells.
Figure 5.
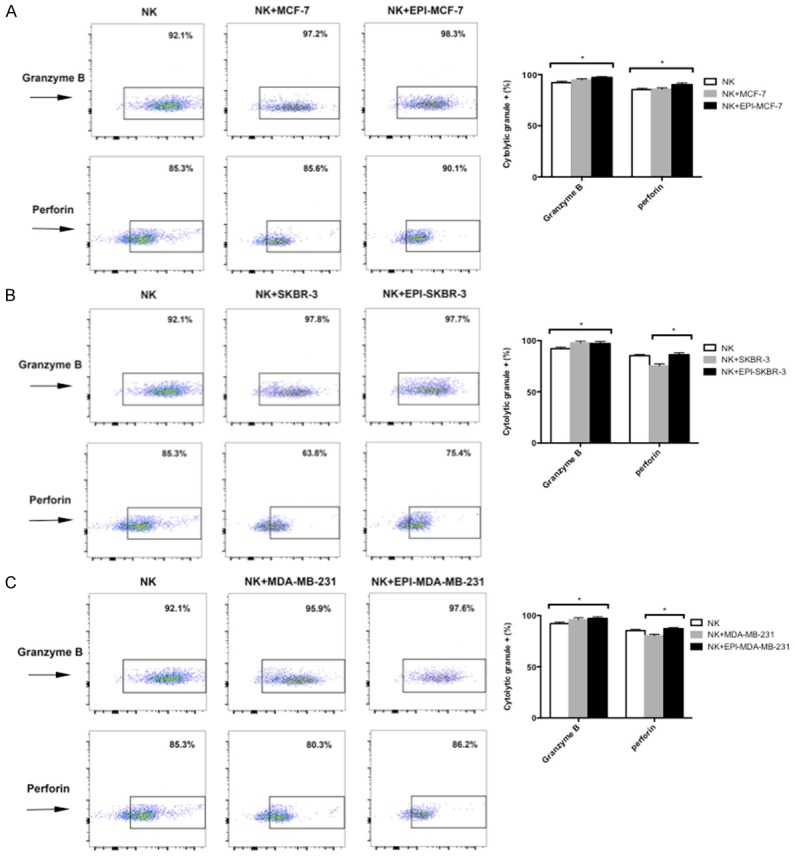
EPI pretreatment increases granzyme B and perforin expression in NK cells. (A-C) MCF-7 (A), SKBR-3 (B), and MDA-MB-231 (C) breast cancer cells were pretreated with EPI for 12 h before adding expanded NK cells for 12 h. Granzyme B and perforin expression was detected by flow cytometry. NK cells cultured without or with untreated cancer cells were used as controls. Experiments were performed in triplicate; data are expressed as mean ± SEM. *P < 0.05.
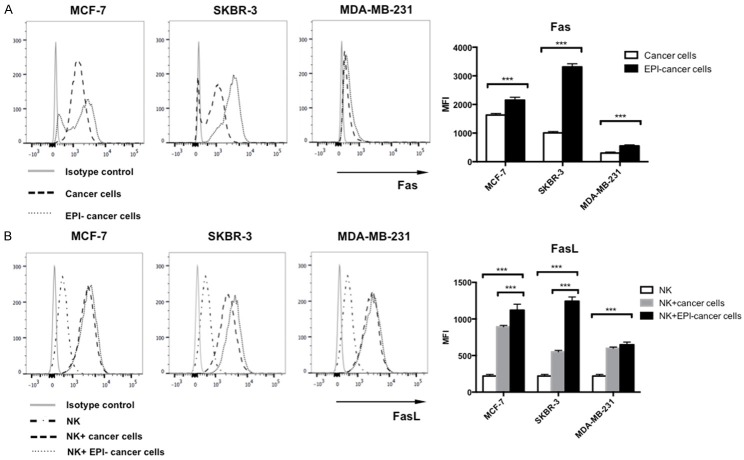
EPI pretreatment induces the upregulation of Fas receptor expression in breast cancer cells and FasL expression in NK cells
The apoptosis-inducing effects of NK cells depend on binding of FasL to the Fas receptor. We therefore examined the expression of Fas and FasL in NK and breast cancer cells, respectively, by flow cytometry. EPI pretreatment increased Fas expression in MCF-7, SKBR-3, and MDA-MB-231 cells (Figure 6A). NK cells co-cultured with EPI-pretreated breast cancer cells had higher FasL expression as compared to those cultured alone (Figure 6B). These results indicate that EPI pretreatment induces the upregulation of both Fas and FasL expression.
Figure 6.
EPI pretreatment induces upregulation of Fas receptor on breast cancer cells and FasL on expanded NK cells. A. MCF-7, SKBR-3, and MDA-MB-231 breast cancer cells were pretreated with EPI for 12 h, washed, and cultured for 24 h. Untreated cells served as a control. Fas receptor expression by cancer cells was detected by flow cytometry. B. MCF-7, SKBR-3, and MDA-MB-231 cells were pretreated with EPI for 12 h before adding expanded NK cells for 24 h. FasL expression by NK cells was detected by flow cytometry. Mean fluorescence intensity is shown. Experiments were performed in triplicate; data are expressed as means ± SEM. ***P < 0.01.
Discussion
A side effect of anthracycline-based chemotherapeutic agents is impairment of immune function [5], which can lead to tumor relapse and metastasis. NK cells recognize stressed cells in the absence of antibodies and the major histocompatibility complex (MHC) and play an essential role in immune surveillance by inducing tumor cell death [20]. In this study, we treated human breast cancer cells (Table 2) with the anthracycline-based agent EPI followed by NK cell-based AIET, which significantly enhanced cytotoxicity to breast cancer cells via a mechanism involving the upregulation of NKG2D ligand and FasL expression in NK cells and of Fas receptor in breast cancer cells, as well as increases in IFN-γ and TNF-α secretion and granzyme B and perforin expression by NK cells.
Table 2.
Molecular properties of human breast cancer cell lines used in this study
| Cell line | ER/PR | HER2 | Ki-67% | Molecular subtype |
|---|---|---|---|---|
| MCF-7 | + | - | < 14% | Luminal A |
| SKBR-3 | - | + | - | HER2-positive |
| MDA-MB-231 | - | - | - | Basal-like |
ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PR, progesterone receptor.
NK cell-based AIET has been widely used to treat breast cancer patients who have received anthracycline-based chemotherapy [8], and has been clinically effective [21]. In this study, we demonstrated that the cytotoxicity of EPI to breast cancer cells was dose- and time-dependent. Due to its potency, the concentration of EPI we used in subsequent experiments (5.0 µg/ml for 12 h) was lower than the IC50. Since EPI was also toxic to NK cells, we pretreated breast cancer cells with EPI and washed the cells thoroughly before adding NK cells to the culture. The cytotoxic effects on breast cancer cells was evaluated with the calcein-AM release assay, which is comparable to the standard 51Cr release assay [22]. The three breast cancer cell lines examined showed variable sensitivity to EPI and NK cells. Furthermore, the cytotoxicity of NK cells increased as a function of E:T ratio, indicating that in clinical situations, an appropriate amount of NK cells can enhance their therapeutic effects while remaining within patients’ limits of tolerance. Large-scale ex vivo NK cell transfusion has been shown to decrease tumor size and prolong patient survival without adverse effects [21,23]. The fact that sequential EPI and NK cell treatment was more toxic to breast cancer cells than either one by itself, and produced an effect that was greater than the sum of EPI and NK cell treatment alone, suggests that there are other mechanisms by which EPI potentiates the cytotoxicity of NK cells.
The apoptosis of tumor cells by NK cells is mediated by receptors such as NKG2D [24], which recognizes MICA and MICB as well as ULBP family members [25]. We found that EPI pretreatment increased the levels of ULBP1, ULBP2, and MICA expression in breast cancer cells, but not of NKG2D, NKp44, and NKp46 in NK cells, suggesting that the NK cell-based AIET could be performed between two courses of chemotherapy, which would prevent the escape of tumor cells from the effects of EPI.
Cytokines play crucial roles in the activation of NK cells, which function in immune surveillance by secreting IFN-γ and TNF-α [26]. The former activates macrophages for phagocytosis and cell lysis, while the latter stimulates the induction of apoptosis by NK cells [11,27]. We found that IFN-γ and TNF-α secretion was increased by EPI pretreatment alone, which may explain the synergistic effects of this combination therapy.
Perforin and granzymes are present in their cytoplasm of NK cells. Upon release, perforin forms pores in the plasma membrane of the target cell, creating an aqueous channel through which granzymes and associated molecules can enter and induce either apoptosis or osmotic cell lysis [27]. Our results demonstrate that EPI followed by NK cell treatment increased the levels of granzyme B relative to controls, suggesting another mechanism for the cytotoxicity induced by this combination therapy.
Fas/FasL signaling regulates apoptosis in cancer cells and functions as a critical component of host immune surveillance [28]. Fas has been shown to be essential for NK cell-induced apoptosis [29]. In this study, we found that Fas expression was upregulated in breast cancer cells pretreated with EPI, while FasL levels were increased in NK cells co-cultured with EPI-treated breast cancer cells, providing evidence that EPI enhances breast cancer cell apoptosis induced by NK cells via activation of Fas/FasL signaling.
In summary, the combination of EPI followed by NK cell was more toxic to breast cancer cells than either of these treatments alone, indicating that NK cell-based AIET between two periods of EPI-based chemotherapy can be an effective treatment for breast cancer.
Acknowledgements
This work was supported by the Scientific and Technological Developing Plan of Jilin Province (nos. 20140414014GH, 120204YY01012460, and 140204YY010315227) and the Platform Construction Project of Development and Reform Commission of Jilin Province (no. 2014N147).
Disclosure of conflict of interest
None.
References
- 1.Taatjes DJ, Fenick DJ, Koch TH. Nuclear targeting and nuclear retention of anthracyclineformaldehyde conjugates implicates DNA covalent bonding in the cytotoxic mechanism of anthracyclines. Chem Res Toxicol. 1999;12:588–596. doi: 10.1021/tx990008q. [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 3.Kornblith AB, Lan L, Archer L, Partridge A, Kimmick G, Hudis C, Winer E, Casey R, Bennett S, Cohen HJ, Muss HB. Quality of life of older patients with early-stage breast cancer receiving adjuvant chemotherapy: a companion study to cancer and leukemia group B 49907. J. Clin. Oncol. 2011;29:1022–1028. doi: 10.1200/JCO.2010.29.9859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira JP, Chompret A, Joulin V, Clavel-Chapelon F, Bourhis J, Andre F, Delaloge S, Tursz T, Kroemer G, Zitvogel L. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 5.Mozaffari F, Lindemalm C, Choudhury A, Granstam-Bjorneklett H, Helander I, Lekander M, Mikaelsson E, Nilsson B, Ojutkangas ML, Osterborg A, Bergkvist L, Mellstedt H. NK cell and T-cell functions in patients with breast cancer: effects of surgery and adjuvant chemo- and radiotherapy. Br J Cancer. 2007;97:105–111. doi: 10.1038/sj.bjc.6603840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manjunath SR, Ramanan G, Dedeepiya VD, Terunuma H, Deng X, Baskar S, Senthilkumar R, Thamaraikannan P, Srinivasan T, Preethy S, Abraham SJ. Autologous immune enhancement therapy in recurrent ovarian cancer with metastases: a case report. Case Rep Oncol. 2012;5:114–118. doi: 10.1159/000337319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui J, Wang N, Zhao H, Jin H, Wang G, Niu C, Terunuma H, He H, Li W. Combination of radiofrequency ablation and sequential cellular immunotherapy improves progression-free survival for patients with hepatocellular carcinoma. Int J Cancer. 2014;134:342–351. doi: 10.1002/ijc.28372. [DOI] [PubMed] [Google Scholar]
- 8.Ding X, Cao H, Chen X, Jin H, Liu Z, Wang G, Cai L, Li D, Niu C, Tian H, Yang L, Zhao Y, Li W, Cui J. Cellular immunotherapy as maintenance therapy prolongs the survival of the patients with small cell lung cancer. J Transl Med. 2015;13:158. doi: 10.1186/s12967-015-0514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui J, Li L, Wang C, Jin H, Yao C, Wang Y, Li D, Tian H, Niu C, Wang G, Han W, Xu J, Chen J, Li W. Combined cellular immunotherapy and chemotherapy improves clinical outcome in patients with gastric carcinoma. Cytotherapy. 2015;17:979–988. doi: 10.1016/j.jcyt.2015.03.605. [DOI] [PubMed] [Google Scholar]
- 10.Subramani B, Pullai CR, Krishnan K, Sugadan SD, Deng X, Hiroshi T, Ratnavelu K. Efficacy of activated and expanded natural killer cells and T lymphocytes for colorectal cancer patients. Biomed Rep. 2014;2:505–508. doi: 10.3892/br.2014.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gras Navarro A, Bjorklund AT, Chekenya M. Therapeutic potential and challenges of natural killer cells in treatment of solid tumors. Front Immunol. 2015;6:202. doi: 10.3389/fimmu.2015.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawaguchi Y, Kono K, Mizukami Y, Mimura K, Fujii H. Mechanisms of escape from trastuzumab-mediated ADCC in esophageal squamous cell carcinoma: relation to susceptibility to perforin-granzyme. Anticancer Res. 2009;29:2137–2146. [PubMed] [Google Scholar]
- 13.Oshimi Y, Oda S, Honda Y, Nagata S, Miyazaki S. Involvement of Fas ligand and Fasmediated pathway in the cytotoxicity of human natural killer cells. J Immunol. 1996;157:2909–2915. [PubMed] [Google Scholar]
- 14.Arnould L, Gelly M, Penault-Llorca F, Benoit L, Bonnetain F, Migeon C, Cabaret V, Fermeaux V, Bertheau P, Garnier J, Jeannin JF, Coudert B. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br J Cancer. 2006;94:259–267. doi: 10.1038/sj.bjc.6602930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terunuma H, Deng X, Dewan Z, Fujimoto S, Yamamoto N. Potential role of NK cells in the induction of immune responses: implications for NK cell-based immunotherapy for cancers and viral infections. Int Rev Immunol. 2008;27:93–110. doi: 10.1080/08830180801911743. [DOI] [PubMed] [Google Scholar]
- 16.Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356:1795–1799. doi: 10.1016/S0140-6736(00)03231-1. [DOI] [PubMed] [Google Scholar]
- 17.Dewan MZ, Takada M, Terunuma H, Deng X, Ahmed S, Yamamoto N, Toi M. Natural killer activity of peripheral-blood mononuclear cells in breast cancer patients. Biomed Pharmacother. 2009;63:703–706. doi: 10.1016/j.biopha.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Tang Y, Zhang X, Qi F, Chen M, Li Y, Liu L, He W, Li Z, Zu X. Afatinib inhibits proliferation and invasion and promotes apoptosis of the T24 bladder cancer cell line. Exp Ther Med. 2015;9:1851–1856. doi: 10.3892/etm.2015.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rautio J, Humphreys JE, Webster LO, Balakrishnan A, Keogh JP, Kunta JR, Serabjit-Singh CJ, Polli JW. In vitro p-glycoprotein inhibition assays for assessment of clinical drug interaction potential of new drug candidates: a recommendation for probe substrates. Drug Metab Dispos. 2006;34:786–792. doi: 10.1124/dmd.105.008615. [DOI] [PubMed] [Google Scholar]
- 20.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terunuma H, Deng X, Nishino N, Watanabe K. NK cell-based autologous immune enhancement therapy (AIET) for cancer. J Stem Cells Regen Med. 2013;9:9–13. doi: 10.46582/jsrm.0901003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Q, Wang N, Wang Y, Wang GY, Piao XX. [Protective effect of ligustilide against glutamate-induced apoptosis in PC12 cells] . Yao Xue Xue Bao. 2015;50:162–168. [PubMed] [Google Scholar]
- 23.Dedeepiya V, Terunuma H, Manjunath S, Senthilkumar R, Thamaraikannan P, Srinivasan T, HelenReena C, Preethy S, Abraham S. Autologous Immune Enhancement Therapy for cancer using NK cells and CTLs without feeder layers; our six year experience in India. J Stem Cells Regen Med. 2011;7:95. [PubMed] [Google Scholar]
- 24.Mandelboim O, Lieberman N, Lev M, Paul L, Arnon TI, Bushkin Y, Davis DM, Strominger JL, Yewdell JW, Porgador A. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409:1055–1060. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 25.Pittari G, Filippini P, Gentilcore G, Grivel JC, Rutella S. Revving up Natural Killer Cells and Cytokine-Induced Killer Cells Against Hematological Malignancies. Front Immunol. 2015;6:230. doi: 10.3389/fimmu.2015.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDowell KA, Hank JA, DeSantes KB, Capitini CM, Otto M, Sondel PM. NK cell-based immunotherapies in Pediatric Oncology. J Pediatr Hematol Oncol. 2015;37:79–93. doi: 10.1097/MPH.0000000000000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iannello A, Debbeche O, Samarani S, Ahmad A. Antiviral NK cell responses in HIV infection: I. NK cell receptor genes as determinants of HIV resistance and progression to AIDS. J Leukoc Biol. 2008;84:1–26. doi: 10.1189/jlb.0907650. [DOI] [PubMed] [Google Scholar]
- 28.Yang D, Torres CM, Bardhan K, Zimmerman M, McGaha TL, Liu K. Decitabine and vorinostat cooperate to sensitize colon carcinoma cells to Fas ligand-induced apoptosis in vitro and tumor suppression in vivo. J Immunol. 2012;188:4441–4449. doi: 10.4049/jimmunol.1103035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thoren FB, Riise RE, Ousback J, Della Chiesa M, Alsterholm M, Marcenaro E, Pesce S, Prato C, Cantoni C, Bylund J, Moretta L, Moretta A. Human NK Cells induce neutrophil apoptosis via an NKp46- and Fas-dependent mechanism. J Immunol. 2012;188:1668–1674. doi: 10.4049/jimmunol.1102002. [DOI] [PubMed] [Google Scholar]