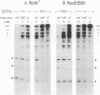
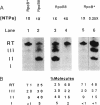
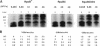Abstract
Rho-dependent terminators constitute one of two major classes of terminators in Escherichia coli. Termination at these sites requires the concerted action of RNA polymerase and rho protein. We present evidence that the efficiency of termination at these sites is governed by kinetic coupling of the rate of transcription of RNA polymerase and the rate of action of rho protein. Termination experiments in vitro indicate that termination efficiency at a rho-dependent terminator is an inverse function of the rate of elongation of RNA polymerase, and each of the mutant phenotypes can be accounted for by the altered rate of elongation of the mutant RNA polymerase. Experiments in vivo show that fast-moving mutant RNA polymerases are termination deficient, while slow-moving mutant RNA polymerases are termination proficient and can suppress the termination deficiency of a slow-acting mutant rho protein. Because of the close coupling of rho action with RNA polymerase, small changes in the elongation rate of RNA polymerase can have very large effects on termination efficiency, providing the cell with a powerful way to modulate termination at rho-dependent terminators.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKWITH J. RESTORATION OF OPERON ACTIVITY BY SUPPRESSORS. Biochim Biophys Acta. 1963 Sep 17;76:162–164. [PubMed] [Google Scholar]
- Baker D., Wuestehube L., Schekman R., Botstein D., Segev N. GTP-binding Ypt1 protein and Ca2+ function independently in a cell-free protein transport reaction. Proc Natl Acad Sci U S A. 1990 Jan;87(1):355–359. doi: 10.1073/pnas.87.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan C. A., Dombroski A. J., Platt T. Transcription termination factor rho is an RNA-DNA helicase. Cell. 1987 Mar 27;48(6):945–952. doi: 10.1016/0092-8674(87)90703-3. [DOI] [PubMed] [Google Scholar]
- Ceruzzi M. A., Bektesh S. L., Richardson J. P. Interaction of rho factor with bacteriophage lambda cro gene transcripts. J Biol Chem. 1985 Aug 5;260(16):9412–9418. [PubMed] [Google Scholar]
- Court D., Brady C., Rosenberg M., Wulff D. L., Behr M., Mahoney M., Izumi S. U. Control of transcription termination: a rho-dependent termination site in bacteriophage lambda. J Mol Biol. 1980 Apr;138(2):231–254. doi: 10.1016/0022-2836(80)90285-5. [DOI] [PubMed] [Google Scholar]
- Das A., Gottesman M. E., Wardwell J., Trisler P., Gottesman S. lambda mutation in the Escherichia coli rho gene that inhibits the N protein activity of phage lambda. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5530–5534. doi: 10.1073/pnas.80.18.5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A., Merril C., Adhya S. Interaction of RNA polymerase and rho in transcription termination: coupled ATPase. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4828–4832. doi: 10.1073/pnas.75.10.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger L. R., Richardson J. P. Procedure for purification of Escherichia coli ribonucleic acid synthesis termination protein rho. Biochemistry. 1981 Mar 17;20(6):1640–1645. doi: 10.1021/bi00509a036. [DOI] [PubMed] [Google Scholar]
- Fisher R. F., Yanofsky C. Mutations of the beta subunit of RNA polymerase alter both transcription pausing and transcription termination in the trp operon leader region in vitro. J Biol Chem. 1983 Jul 10;258(13):8146–8150. [PubMed] [Google Scholar]
- Greenblatt J., McLimont M., Hanly S. Termination of transcription by nusA gene protein of Escherichia coli. Nature. 1981 Jul 16;292(5820):215–220. doi: 10.1038/292215a0. [DOI] [PubMed] [Google Scholar]
- Guarente L. P., Beckwith J. Mutant RNA polymerase of Escherichia coli terminates transcription in strains making defective rho factor. Proc Natl Acad Sci U S A. 1978 Jan;75(1):294–297. doi: 10.1073/pnas.75.1.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L. Restoration of termination by RNA polymerase mutations is rho allele-specific. J Mol Biol. 1979 Apr 5;129(2):295–304. doi: 10.1016/0022-2836(79)90283-3. [DOI] [PubMed] [Google Scholar]
- Hager D. A., Jin D. J., Burgess R. R. Use of Mono Q high-resolution ion-exchange chromatography to obtain highly pure and active Escherichia coli RNA polymerase. Biochemistry. 1990 Aug 28;29(34):7890–7894. doi: 10.1021/bi00486a016. [DOI] [PubMed] [Google Scholar]
- Jin D. J., Gross C. A. Characterization of the pleiotropic phenotypes of rifampin-resistant rpoB mutants of Escherichia coli. J Bacteriol. 1989 Sep;171(9):5229–5231. doi: 10.1128/jb.171.9.5229-5231.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D. J., Gross C. A. Mapping and sequencing of mutations in the Escherichia coli rpoB gene that lead to rifampicin resistance. J Mol Biol. 1988 Jul 5;202(1):45–58. doi: 10.1016/0022-2836(88)90517-7. [DOI] [PubMed] [Google Scholar]
- Jin D. J., Gross C. A. RpoB8, a rifampicin-resistant termination-proficient RNA polymerase, has an increased Km for purine nucleotides during transcription elongation. J Biol Chem. 1991 Aug 5;266(22):14478–14485. [PubMed] [Google Scholar]
- Jin D. J., Gross C. A. Three rpoBC mutations that suppress the termination defects of rho mutants also affect the functions of nusA mutants. Mol Gen Genet. 1989 Apr;216(2-3):269–275. doi: 10.1007/BF00334365. [DOI] [PubMed] [Google Scholar]
- Jin D. J., Walter W. A., Gross C. A. Characterization of the termination phenotypes of rifampicin-resistant mutants. J Mol Biol. 1988 Jul 20;202(2):245–253. doi: 10.1016/0022-2836(88)90455-x. [DOI] [PubMed] [Google Scholar]
- Kepes A. Transcription and translation in the lactose operon of Escherichia coli studied by in vivo kinetics. Prog Biophys Mol Biol. 1969;19(1):199–236. doi: 10.1016/0079-6107(69)90006-6. [DOI] [PubMed] [Google Scholar]
- Kingston R. E., Nierman W. C., Chamberlin M. J. A direct effect of guanosine tetraphosphate on pausing of Escherichia coli RNA polymerase during RNA chain elongation. J Biol Chem. 1981 Mar 25;256(6):2787–2797. [PubMed] [Google Scholar]
- Kuchta R. D., Benkovic P., Benkovic S. J. Kinetic mechanism whereby DNA polymerase I (Klenow) replicates DNA with high fidelity. Biochemistry. 1988 Sep 6;27(18):6716–6725. doi: 10.1021/bi00418a012. [DOI] [PubMed] [Google Scholar]
- Landick R., Yanofsky C. Stability of an RNA secondary structure affects in vitro transcription pausing in the trp operon leader region. J Biol Chem. 1984 Sep 25;259(18):11550–11555. [PubMed] [Google Scholar]
- Lau L. F., Roberts J. W., Wu R. RNA polymerase pausing and transcript release at the lambda tR1 terminator in vitro. J Biol Chem. 1983 Aug 10;258(15):9391–9397. [PubMed] [Google Scholar]
- Lau L. F., Roberts J. W., Wu R. Transcription terminates at lambda tR1 in three clusters. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6171–6175. doi: 10.1073/pnas.79.20.6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery-Goldhammer C., Richardson J. P. An RNA-dependent nucleoside triphosphate phosphohydrolase (ATPase) associated with rho termination factor. Proc Natl Acad Sci U S A. 1974 May;71(5):2003–2007. doi: 10.1073/pnas.71.5.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan E. A. Antitermination mechanisms in rRNA operons of Escherichia coli. J Bacteriol. 1986 Oct;168(1):1–5. doi: 10.1128/jb.168.1.1-5.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan W. D., Bear D. G., von Hippel P. H. Rho-dependent termination of transcription. I. Identification and characterization of termination sites for transcription from the bacteriophage lambda PR promoter. J Biol Chem. 1983 Aug 10;258(15):9553–9564. [PubMed] [Google Scholar]
- Morgan W. D., Bear D. G., von Hippel P. H. Rho-dependent termination of transcription. II. Kinetics of mRNA elongation during transcription from the bacteriophage lambda PR promoter. J Biol Chem. 1983 Aug 10;258(15):9565–9574. [PubMed] [Google Scholar]
- Morgan W. D., Bear D. G., von Hippel P. H. Specificity of release by Escherichia coli transcription termination factor rho of nascent mRNA transcripts initiated at the lambda PR. J Biol Chem. 1984 Jul 10;259(13):8664–8671. [PubMed] [Google Scholar]
- Mori H., Imai M., Shigesada K. Mutant rho factors with increased transcription termination activities. II. Identification and functional dissection of amino acid changes. J Mol Biol. 1989 Nov 5;210(1):39–49. doi: 10.1016/0022-2836(89)90289-1. [DOI] [PubMed] [Google Scholar]
- Platt T. Transcription termination and the regulation of gene expression. Annu Rev Biochem. 1986;55:339–372. doi: 10.1146/annurev.bi.55.070186.002011. [DOI] [PubMed] [Google Scholar]
- Richardson J. P., Carey J. L., 3rd rho Factors from polarity suppressor mutants with defects in their RNA interactions. J Biol Chem. 1982 May 25;257(10):5767–5771. [PubMed] [Google Scholar]
- Richardson J. P. Rho-dependent transcription termination. Biochim Biophys Acta. 1990 Apr 6;1048(2-3):127–138. doi: 10.1016/0167-4781(90)90048-7. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D., Shimatake H., Brady C., Wulff D. L. The relationship between function and DNA sequence in an intercistronic regulatory region in phage lambda. Nature. 1978 Mar 30;272(5652):414–423. doi: 10.1038/272414a0. [DOI] [PubMed] [Google Scholar]
- Schleif R., Hess W., Finkelstein S., Ellis D. Induction kinetics of the L-arabinose operon of Escherichia coli. J Bacteriol. 1973 Jul;115(1):9–14. doi: 10.1128/jb.115.1.9-14.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. C., Chamberlin M. J. Binding of rho factor to Escherichia coli RNA polymerase mediated by nusA protein. J Biol Chem. 1984 Dec 25;259(24):15000–15002. [PubMed] [Google Scholar]
- Stroynowski I., Kuroda M., Yanofsky C. Transcription termination in vitro at the tryptophan operon attenuator is controlled by secondary structures in the leader transcript. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2206–2210. doi: 10.1073/pnas.80.8.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurushita N., Shigesada K., Imai M. Mutant rho factors with increased transcription termination activities. I. Functional correlations of the primary and secondary polynucleotide binding sites with the efficiency and site-selectivity of rho-dependent termination. J Mol Biol. 1989 Nov 5;210(1):23–37. doi: 10.1016/0022-2836(89)90288-x. [DOI] [PubMed] [Google Scholar]
- Yager T. D., von Hippel P. H. A thermodynamic analysis of RNA transcript elongation and termination in Escherichia coli. Biochemistry. 1991 Jan 29;30(4):1097–1118. doi: 10.1021/bi00218a032. [DOI] [PubMed] [Google Scholar]
- Yang X. J., Roberts J. W. Gene Q antiterminator proteins of Escherichia coli phages 82 and lambda suppress pausing by RNA polymerase at a rho-dependent terminator and at other sites. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5301–5305. doi: 10.1073/pnas.86.14.5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C. Transcription attenuation. J Biol Chem. 1988 Jan 15;263(2):609–612. [PubMed] [Google Scholar]
- von Hippel P. H., Yager T. D. Transcript elongation and termination are competitive kinetic processes. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2307–2311. doi: 10.1073/pnas.88.6.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]