Abstract
MiRNAs play crucial roles in abnormal proliferation and invasion of VSMCs. However, the roles and mechanisms of miRNAs in VSMCs are not fully understood. In our study, we demonstrated that PDGF-BB and serum induced proliferation of VSMCs led to the upregulation of miR-541. We also showed that overexpression of miR-541 promoted VSMC proliferation and invasion. In addition, Interferon regulatory factor 7 (IRF7) was found to be a potential target of miR-541 and upregulation of IRF7 could inhibit VSMC proliferation. Restored expression of miR-541 promoted IRF7-inhibited VSMCs proliferation. In conclusion, these findings suggest that inhibitors targeting miR-541 or its specific downstream molecules may be therapeutic strategy for VSMC growth-related diseases.
Keywords: Vascular smooth muscle cells, microRNA, miR-541, IRF7
Introduction
Vascular smooth muscle cells (VSMCs) exhibit quiescent status under normal conditions within adult blood vessels, proliferating at a very low rate [1-3]. However, unlike skeletal and cardiac muscle cells which consist of terminally differentiated cells, VSMCs keep remarkable plasticity and can undergo phenotypic switching under certain circumstances [4]. Proliferation of VSMCs following vascular injury contributes to the pathogenesis of intimal hyperplasia and atherosclerosis [5]. In response to vascular injury, VSMCs can release various growth factors and cytokines, including EGF, leptin, FGF-2, interleukin-1, PDGF, and angiotensin II. and exhibit increased rate of proliferation and migration [7,8]. However, the molecular mechanisms in VSMCs proliferation and its association with atherosclerosis and restenosis are still unclear.
MicroRNAs (MiRNAs) are endogenous, small, noncoding RNAs that control gene expression post-transcriptionally through binding to complementary sequences in their 3’ untranslated regions (3’UTR) [9-11]. MiRNAs are key regulators in almost all cellular functions such as proliferation, differentiation, apoptosis, development, and carcinogenesis [12-15]. Deregulated miRNA is reported to be associated with many diseases such as leukemia, inflammation, cancers, diabetes, and heart diseases [16-20]. Increasing evidences have suggested that miRNAs play crucial roles in proliferation-related diseases, especially in VSMCs proliferation [21,22]. Various miRNAs, such as let-7d, miR-21, miR-490-3p, miR-365, miR-153 and miR-223, are involved in VSMC phenotype and neointima formation [4,8,23-25]. MiR-21, miR-142-5p, and miR-146a were proved to promote VSMC growth through targeting different molecules, such as c-Ski, B cell translocation gene 3 (BTG3), and KLF4, respectively [24,26,27]. However, the function of miR-541 in VSMCs function is still unknown.
In this study, miR-541 increased VSMCs proliferation and invasion via a direct interaction with the 3’UTR of IRF7. In addition, miR-541 was significantly upregulated in proliferating VSMCs induced by PDGF-BB. Inhibitors of miR-541 or its specific downstream molecules may be a potencial therapeutic target for VSMC growth-related diseases.
Materials and methods
Ethics statement
All experimental protocols were approved by the Clinical Research Ethics Committee of the Second Xiangya Hospital.
Cell culture and transfection
The VSMCs were obtained from Cascade Biologics (Portland, OR) and cultured in the recommended conditions supplemented with fetal bovine serum (FBS). All other chemicals and reagents were obtained from Sigma unless otherwise specified. The miR-541 mimics, inhibitors, scramble and control pcDNA-IRF7 and Empty vector were synthesized by GenePharma (Shanghai, China) and then transfected into the VSMCs. All cell transfections were introduced by DharmaFECT1 Reagent (Dharmacon, TX, USA) in accordance with the manufacturer’s protocol.
RNA isolation and quantitative real-time reverse transcription-PCR
Total RNA was extracted from the cells using Trizol reagent (Invitrogen, Calsbad, CA, USA) according manufacturer’s instructions. Relative transcript levels of mRNA or miRNA were detected using the iQ5 Real-Time PCR Detection System (Bio-Rad, California, USA). The real-time PCR reaction was composed of 1x SYBR Green fluorescent dye (Takara, Dalian, China), 1 μl forward primers (10 μM), 1 μl reverse primers (10 μM), 1x qPCR mix, 1 μl cDNA. The sequences of the specific primers are shown in Table S1. GAPDH or U6 was used as an internal control for mRNA or miRNA respectively.
Cell proliferation and cell invasion assay
Cell proliferation was detected using CCK-8 (DOJINDO, Kumamoto, Japan) following to the manufacturer’s instructions. The cells were cultured for 24, 48 and 72 h. The supernatant was removed, and 100 μl of DMEM/F12 medium containing 10 μl of CCK-8 was added to each well for incubating until visual color conversion occurred. The optical density (OD) values were read at 450 nm. Transwell (BD Sciences) coated with matrigel was used to measure cell invasion. Cells were cultured in medium without serum in the upper chamber and medium containing 10% FBS was added in the lower chamber. After 24 hours, cells that were not invaded through medium were removed and the inserts were stained with crystal violet (0.2%), photographed and counted.
Dual luciferase assays
Cells were co-transfected with the reporter construct (0.4 μg) of Luciferase Reporter Vectors control vector (0.2 μg), and miR-541 or scramble. After 24 h of post-transfection, cells were harvested and assayed with Dual Luciferase Assay (Promega, WI, USA) following the manufacturer’s protocol. Firefly luciferase value was normalized to Renilla luciferase activity values, and the ratio of Firefly/Renilla was reported.
Western blotting analysis
Western blot analysis was carried out using standard protocols [30]. Proteins were separated by 12% SDS-PAGE. Subsequently, proteins were transferred to PVDF membranes (Amersham, Buckinghamshire, UK), which were blocked with non-fat dried milk (5%) for 2 h. Then they were incubated overnight with anti-PCNA antibody or anti-IRF7 antibody (Abcam, England) at 1:2000 dilutions; anti-GAPDH antibody (Proteintech, Chicago, USA) at 1:50,000 dilution., The membranes were washed with TBST and then incubated with goat anti-rabbit antibody (1:5000 dilutions, zsgb-bio, Beijing, China) for 2 h. Protein levels of GAPDH were used as loading controls.
Statistical analysis
Statistics were analyzed using SPSS 17.0. Data are presented as the mean ± standard deviation. Either an analysis of variance (ANOVA) or Student’s t-test was used for statistical analysis. α=0.05 (two-side) was set as the statistical significance level. Each experiment was performed at least three times.
Results
MiR-541 was upregulated by PDGF-BB and FBS in VSMCs
The CCK8 assay showed that PDGF-BB (20 ng/ml) enhanced VSMCs proliferation (Figure 1A) and induced miR-541 expression (Figure 1B). We also confirmed that FBS could induce the VSMCs proliferation (Figure 1C) and miR-541 expression (Figure 1D).
Figure 1.
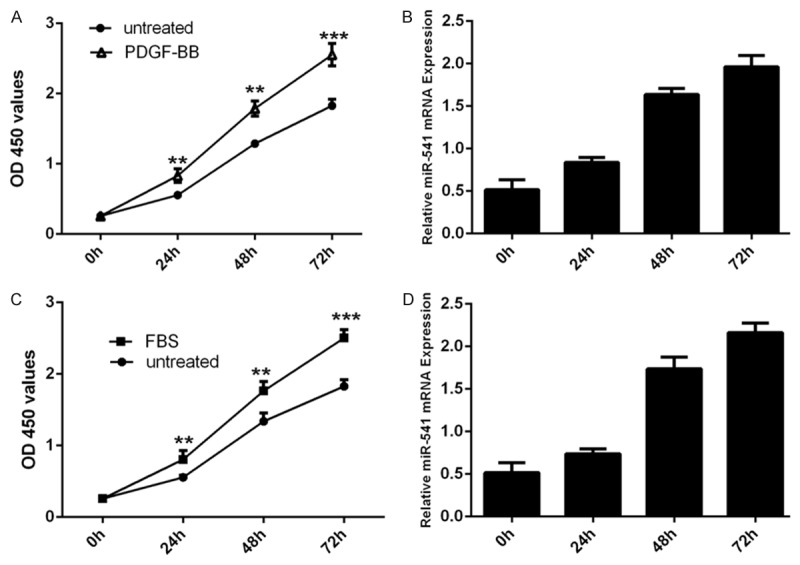
miR-541 was up-regulated by PDGF-BB and FBS in VSMCs. A. The CCK8 proliferation assay has shown that PDGF-BB can induce the VSMCs proliferation. B. qRT-PCR analysis was performed to the levels of miR-541 in VSMCs after PDGF-BB treatment. PDGF-BB can enhance the expression of miR-541. U6 snRNA was used as internal control. C. The CCK8 proliferation assay has shown that (fetal bovine serum) FBS can induce the VSMCs proliferation. D. qRT-PCR was performed to the levels of miR-541 in VSMCs after PBS treatment. FBS can enhance the expression of miR-541. U6 snRNA was used as internal control. *p<0.05, and **p<0.01, *** p<0.001.
Overexpression of miR-541 promoted the proliferation of VSMCs
MiR-541 expression was increased in VSMCs transfected with miR-541 mimics (Figure 2A), and decreased in VSMCs transfected with miR-541 inhibitor (Figure 2B). The CCK-8 proliferation assay showed that the growth rate of VSMCs was enhanced in cells transfected with miR-541 mimics compared with cells transfected with scramble mimics or untreated (Figure 2C). Meanwhile, miR-541 inhibitor inhibited the VSMCs proliferation (Figure 2D). The proliferative effect of miR-541 was further confirmed by Western blot and qRT-PCR of PCNA, a well known cell proliferation marker. As shown in Figure 2E and 2F, there was an increase in the mRNA and protein of PCNA in the group transfected with miR-541 mimics compared with the scrambled group or untreated group.
Figure 2.
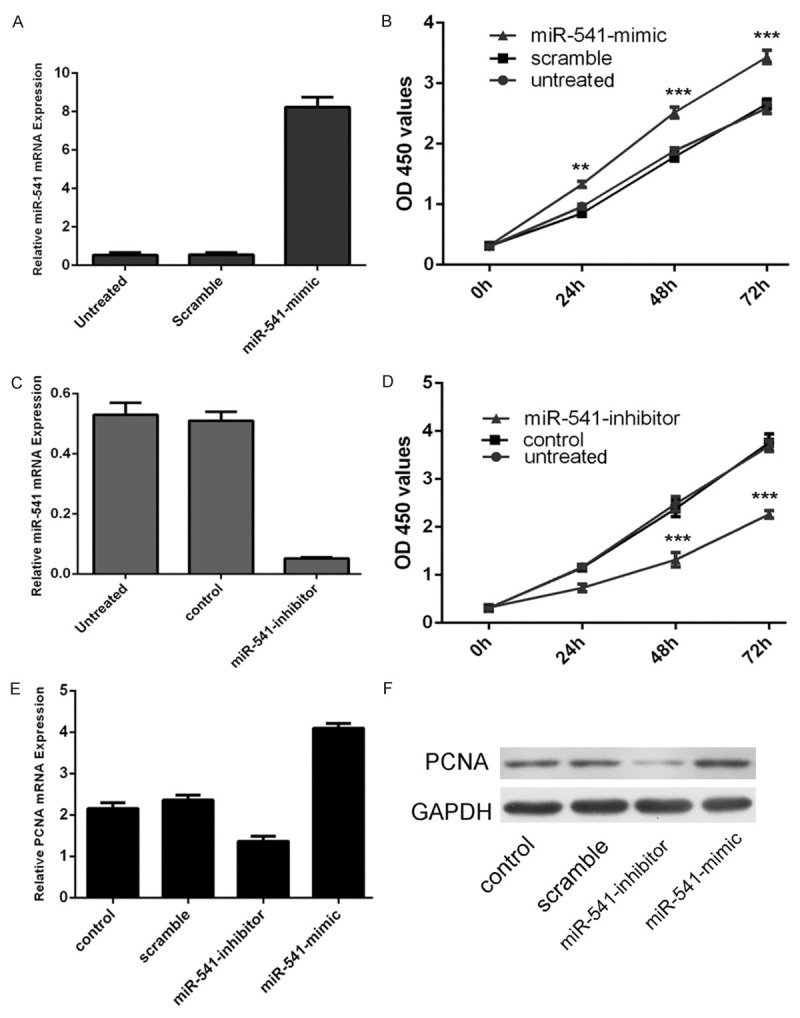
Overexpression of miR-541 promoted the proliferation of VSMCs. A. qRT-PCR analysis of miR-541 in VSMCs upon transfection of miR-541 mimics. The expression of miR-541 in VSMCs transfected with miR-541 mimics was up-regulated. U6 snRNA was used as internal control. B. The CCK8 proliferation assay has shown that miR-541 overexpression can induce the VSMCs proliferation. C. qRT-PCR analysis of miR-541 in VSMCs upon transfection of miR-541 inhibitor. The expression of miR-541 in VSMCs transfected with miR-541 inhibitors was down-regulated. U6 snRNA was used as internal control. D. The CCK8 proliferation assay has shown that miR-541 downregulation can inhibit the VSMCs proliferation. E. qRT-PCR analysis of PCNA in VSMCs upon transfection of miR-541 mimics, inhibitor, scramble or control. F. Western blot analysis of PCNA in VSMCs upon transfection of miR-541 mimics, inhibitors, or scramble or control. *p<0.05, and **p<0.01, *** p<0.001.
Overexpression of miR-541 promoted the invasion of VSMCs
The invasiveness was dramatically increased in cells transfected with miR-541 mimics compared with the scramble group and control group cells. Meanwhile, the invasiveness was decreased transfected in cells transfected with miR-541 inhibitor (Figure 3A and 3B).
Figure 3.
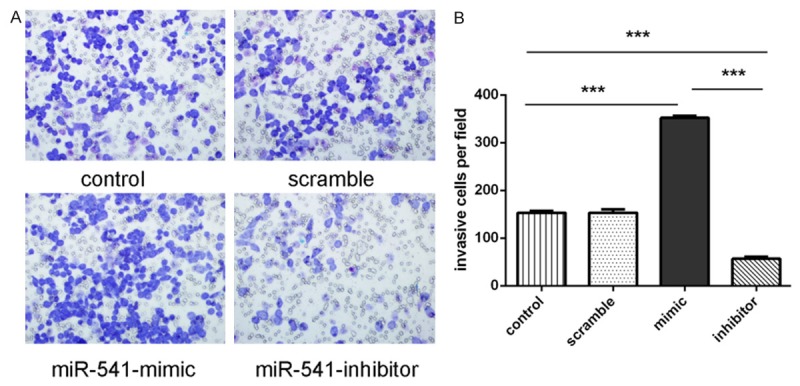
Overexpression of miR-541 promoted the invasion of VSMCs. A. Invasion analysis of VSMCs after treatment with miR-541 mimics, inhibitors or scramble or control. B. The relative ratio of invasive cells per field is shown, *p<0.05, **p<0.01, and ***p<0.001.
MiR-541 targeted IRF7 in VSMCs
The alignment of miR-541 and the 3’-UTR of IRF7 was shown of Figure 4A. Forced expression of miR-541 remarkably reduced luciferase activity of reporter gene with wild type, but not mutant IRF7 3’UTR, indicating that miR-541 directly targeted IRF7 3’UTR (Figure 4B). Overexpression of miR-541 could reduce the mRNA and protein expression of IRF7 (Figure 4C and 4D). Meanwhile, inhibition of miR-541 could promote the mRNA and protein expression of IRF7 (Figure 4E and 4F).
Figure 4.
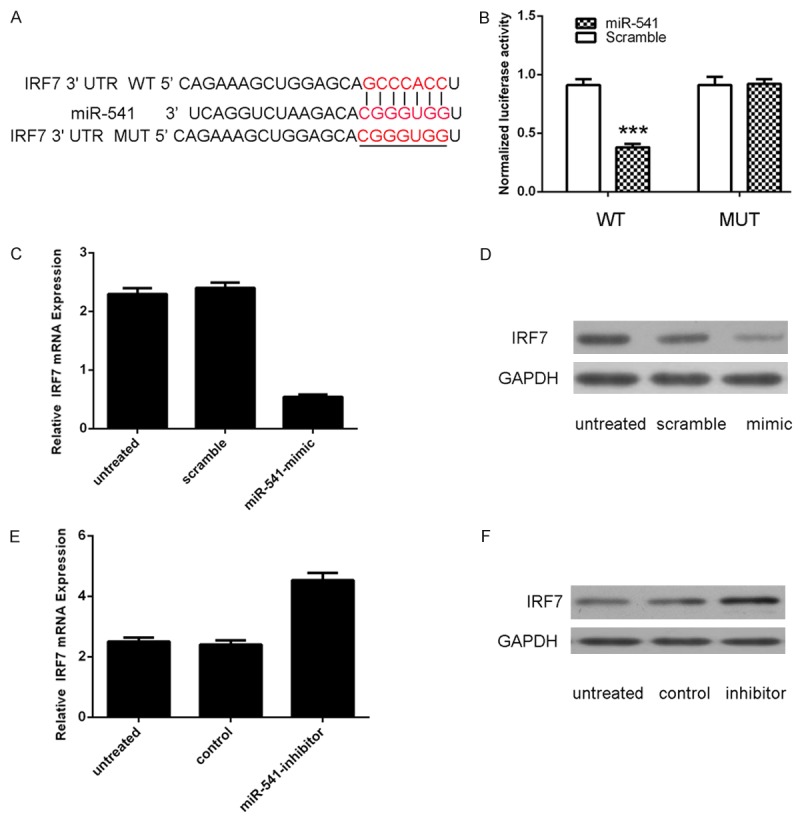
miR-541 targets IRF7 in VSMCs. A. Schematic representation of IRF7 3’UTR showing the putative miR-541 target site. B. Relative luciferase activity of the indicated IRF7 reporter construct in VSMCs is shown. Firefly luciferase values were normalized to Renilla luciferase activity and plotted as relative luciferase activity. C. RT-PCR analysis was performed to examine the effects of miR-541 mimic on IRF7 expression in VSMCs. Ectopic expression of miR-541 significantly decreased IRF7 transcripts. GAPDH was used as internal control. D. Western blotting was performed to examine the effect of miR-541 mimic on the expression of IRF7. GAPDH was also detected as a loading control. E. Inhibition of miR-541 promoted the protein expression of IRF7. F. RT-PCR analysis was performed to examine the effects of miR-541 inhibitor on IRF7 expression in VSMCs. ***p<0.001.
Overexpression of IRF7 inhibited VSMCs proliferation
PDGF-BB (20 ng/ml) or FBS could inhibit IRF7 expression (Figure 5A and 5B). Western blot data showed that pcDNA-IRF7 enhanced the IRF7 protein expression (Figure 5C). Overexpression of IRF7 inhibited the VSMCs proliferation (Figure 5D). As shown in Figure 5E and 5F, there was a decrease in the mRNA and protein of PCNA in the group transfected with pcDNA-IRF7 compared with the control group or untreated group.
Figure 5.
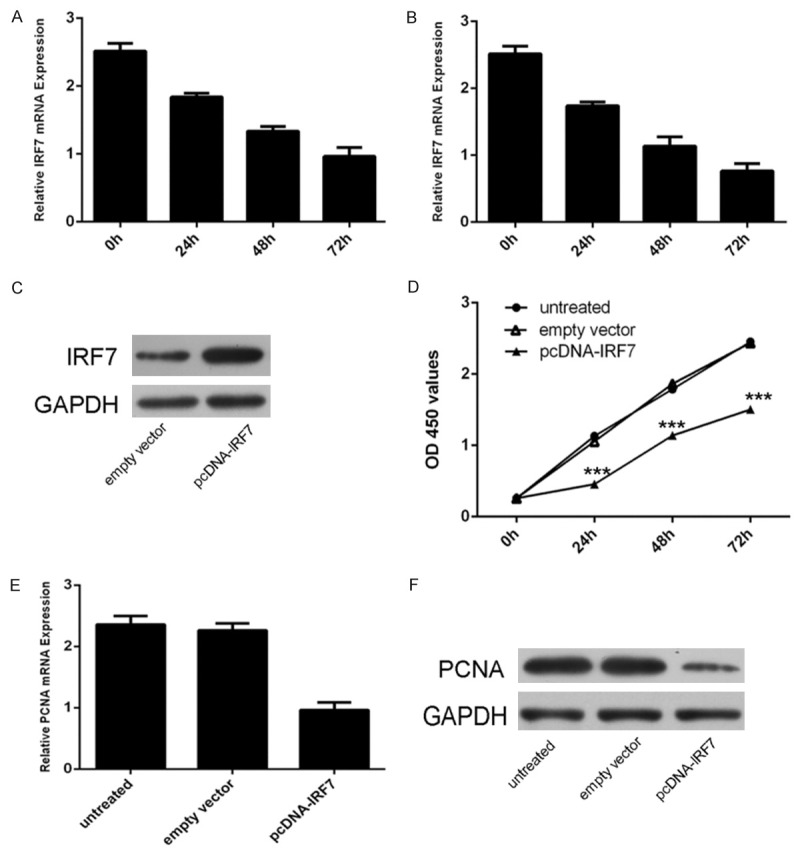
Overexpression of IRF7 can inhibit VSMCs proliferation. A. qRT-PCR analysis was performed to the levels of IRF7 in VSMCs after PDGF-BB treatment. PDGF-BB can inhibit the expression of IRF7. GAPDH was used as internal control. B. qRT-PCR analysis was performed to the levels of IRF7 in VSMCs after FBS treatment. FBS can inhibit the expression of IRF7. GAPDH was used as internal control. C. Western blotting was performed to examine the effects of pcDNApcDNA-IRF7 on the expression of IRF7. GAPDH was also detected as a loading control. D. The CCK8 proliferation assay has shown that IRF7 can inhibit the VSMCs proliferation. E. IRF7 overexpression depressed the PCNA mRNA expression. GAPDH was used as internal control. F. IRF7 overexpression inhibited the PCNA protein expression. GAPDH was also detected as a loading control. *p<0.05, ** p<0.01, and ***p<0.001.
Restoration of miR-541 promoted IRF7-inhibited VSMCs proliferation
When miR-541 mimic and pcDNA-IRF7 were cotransfected into VSMCs, miR-541 expression significantly enhanced the IRF7-inhibited VSMCs proliferation (Figure 6B and 6C). Moreover, overexpression of miR-541 enhanced the IRF7-inhibited the expression of PCNA in the VSMCs (Figure 6C and 6D).
Figure 6.
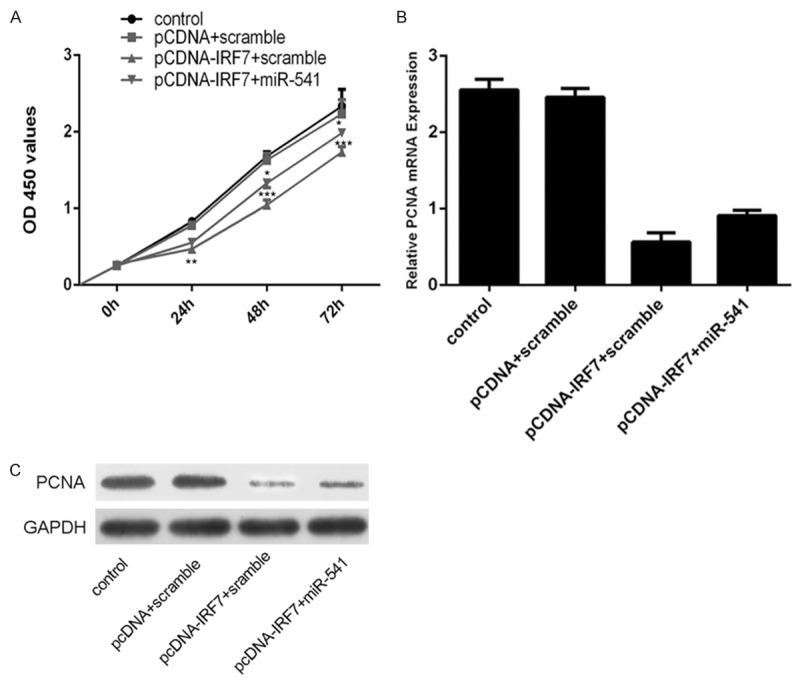
Restoration of miR-541 promoted IRF7-inhibited VSMCs proliferation. A. The cell growth in VSMCs co-transfected with either miR-541 mimic or scramble and pcDNA-IRF7 or pcDNAempty vector using CCK-8 proliferation assay. B. The mRNA expression of PCNA in VSMCs co-transfected with either miR-541 mimic or scramble and pcDNA-IRF7 or pcDNA empty vector using qRT-PCR analysis. GAPDH was used as internal control. C. The protein expression of PCNA in VSMCs co-transfected with either miR-541 mimic or scramble and pcDNA-IRF7 or pcDNA empty vector using western blot analysis. GAPDH was also detected as a loading control. *p<0.05, ** p<0.01, and ***p<0.001.
Discussion
MiRNAs are a class of small RNAs that regulate gene expression. Increasing research suggests that deregulated miRNAs play important roles in abnormal VSMC proliferation and migration [7,31]. Abnormal proliferation of VSMCs is associated with various diseases, such as restenosis and atherosclerosis [21]. In our study, the expression of miR-541 was increased in the PDGF-BB and FBS induced VSMCs. Moreover, overexpression of miR-541 promoted the VSMCs proliferation and invasion by directly targeting IRF7. Furthermore, forced of IRF7 expression could inhibit VSMCs proliferation. Restoration of miR-541 promoted IRF7-inhibited VSMCs proliferation.
Recent study has shown that miR-541 is downregulated in cardiomyocytes treated by angiotensin II (Ang-II) and overexpression of miR-541 decreases the hypertrophic phenotype upon Ang-II treatment in cellular models [32]. MiR-541 is proved to be the target of microphthalmia-associated transcription factor (MITF), which can negatively regulate the expression of miR-541. Another study reported that Her2 and Erk1/2 might be the targets of miR-541 in breast cancer cells [33]. Here, our results show that the expression of miR-541 is upregulated in the VSMCs induced by PDGF-BB and FBS. Moreover, enforced expression of miR-541 promotes the VSMCs proliferation and invasion. The proliferation of VSMCs is an important event in the pathogenesis of vascular diseases. It is characterized by intimal thickening that is commonly seen in restenosis after vein grafting, atherosclerosis, vascular rejection, and coronary intervention. However, the roles of miR-541 in the proliferation of VSMCs remain unknown.
IRF7 is a protein first isolated as a transcriptional factor binding to the Epstein-Barr virus BamHI Q promoter. It is predominantly expressed in lymphoid cells and participates in innate and adaptive immunity [34,35]. IRF7 is an IFN-inducible transcription factor necessary for IFN-stimulated gene (ISG) induction by IFNs [36]. When activated by phosphorylation, IRF7 translocates from the cytoplasm to the nucleus to induce target gene expression by binding to the promoter regulatory elements [37]. IRF7 is not expressed in cells normally, but it can be induced by various cytokines, such as IFN-a, the STAT pathway, double-stranded RNA, or viral infection [38]. Huang et al. reported that IRF7 expression was decreased in response to carotid injury and that IRF7 inhibited VSMC proliferation and neointima formation [39]. Although IRFs displayed opposite changes in their expression in different studies, they led to a similar effect on VSMC proliferation in response to vascular injury [39]. IRF7 can target PCNA and lead to a similar effect on VSMC proliferation [39]. IRF7 plays important roles in neointima formation after wire injury through different mechanisms, indicating that the IRFs are crucial regulators in the neointima formation [39]. In our study, expression levels of IRF7 were significantly inhibited in miR-541-overexpressed cells. We also demonstrated that overexpression of IRF7 inhibited the VSMC proliferation. Moreover, restoration of miR-541 promoted IRF7-inhibited VSMCs proliferation.
In conclusion, our study identified miR-541 as an activator of VSMC proliferation by regulating IRF7. MiR-541 expression was upregulated in proliferating VSMCs induced by PDGF-BB. In addition, miR-541 promoted VSMC proliferation and invasion by inhibiting IRF7, suggesting that miR-541 inhibitor can be potential therapeutic target for VSMC growth-related diseases.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Yu X, Li Z. MicroRNAs regulate vascular smooth muscle cell functions in atherosclerosis (review) Int J Mol Med. 2014;34:923–933. doi: 10.3892/ijmm.2014.1853. [DOI] [PubMed] [Google Scholar]
- 2.Cheng Y, Liu X, Yang J, Lin Y, Xu DZ, Lu Q, Deitch EA, Huo Y, Delphin ES, Zhang C. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res. 2009;105:158–166. doi: 10.1161/CIRCRESAHA.109.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang YS, Wang HY, Liao YC, Tsai PC, Chen KC, Cheng HY, Lin RT, Juo SH. MicroRNA-195 regulates vascular smooth muscle cell phenotype and prevents neointimal formation. Cardiovasc Res. 2012;95:517–526. doi: 10.1093/cvr/cvs223. [DOI] [PubMed] [Google Scholar]
- 4.Kim MH, Ham O, Lee SY, Choi E, Lee CY, Park JH, Lee J, Seo HH, Seung M, Min PK, Hwang KC. MicroRNA-365 Inhibits the Proliferation of Vascular Smooth Muscle Cells by Targeting Cyclin D1. J Cell Biochem. 2014;115:1752–61. doi: 10.1002/jcb.24841. [DOI] [PubMed] [Google Scholar]
- 5.Li P, Liu Y, Yi B, Wang G, You X, Zhao X, Summer R, Qin Y, Sun J. MicroRNA-638 is highly expressed in human vascular smooth muscle cells and inhibits PDGF-BB-induced cell proliferation and migration through targeting orphan nuclear receptor NOR1. Cardiovasc Res. 2013;99:185–193. doi: 10.1093/cvr/cvt082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan MC, Hilyard AC, Wu C, Davis BN, Hill NS, Lal A, Lieberman J, Lagna G, Hata A. Molecular basis for antagonism between PDGF and the TGFbeta family of signalling pathways by control of miR-24 expression. EMBO J. 2010;29:559–573. doi: 10.1038/emboj.2009.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Wang Y, Wang X, Eisner GM, Asico LD, Jose PA, Zeng C. Insulin promotes vascular smooth muscle cell proliferation via microRNA-208-mediated downregulation of p21. J Hypertens. 2011;29:1560–1568. doi: 10.1097/HJH.0b013e328348ef8e. [DOI] [PubMed] [Google Scholar]
- 8.Song L, Duan P, Guo P, Li D, Li S, Xu Y, Zhou Q. Downregulation of miR-223 and miR-153 mediates mechanical stretch-stimulated proliferation of venous smooth muscle cells via activation of the insulin-like growth factor-1 receptor. Arch Biochem Biophys. 2012;528:204–211. doi: 10.1016/j.abb.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 9.Li M, Yu M, Liu C, Zhu H, He X, Peng S, Hua J. miR-34c works downstream of p53 leading to dairy goat male germline stem-cell (mGSCs) apoptosis. Cell Prolif. 2013;46:223–231. doi: 10.1111/cpr.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JE, Hong EJ, Nam HY, Kim JW, Han BG, Jeon JP. MicroRNA signatures associated with immortalization of EBV-transformed lymphoblastoid cell lines and their clinical traits. Cell Prolif. 2011;44:59–66. doi: 10.1111/j.1365-2184.2010.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohdaira H, Sekiguchi M, Miyata K, Yoshida K. MicroRNA-494 suppresses cell proliferation and induces senescence in A549 lung cancer cells. Cell Prolif. 2012;45:32–38. doi: 10.1111/j.1365-2184.2011.00798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, You T, Jing J. MiR-125b inhibits cell biological progression of Ewing’s sarcoma by suppressing the PI3K/Akt signalling pathway. Cell Prolif. 2014;47:152–160. doi: 10.1111/cpr.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mun J, Tam C, Chan G, Kim JH, Evans D, Fleiszig S. MicroRNA-762 is upregulated in human corneal epithelial cells in response to tear fluid and Pseudomonas aeruginosa antigens and negatively regulates the expression of host defense genes encoding RNase7 and ST2. PLoS One. 2013;8:e57850. doi: 10.1371/journal.pone.0057850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li R, Yuan W, Mei W, Yang K, Chen Z. MicroRNA 520d-3p inhibits gastric cancer cell proliferation, migration, and invasion by downregulating EphA2 expression. Mol Cell Biochem. 2014;396:295–305. doi: 10.1007/s11010-014-2164-6. [DOI] [PubMed] [Google Scholar]
- 15.Cesana M, Cacchiarelli D, Legnini I, Santini T, Sthandier O, Chinappi M, Tramontano A, Bozzoni I. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147:358–369. doi: 10.1016/j.cell.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishioka C, Ikezoe T, Yang J, Nobumoto A, Tsuda M, Yokoyama A. Downregulation of miR-217 correlates with resistance of ph leukemia cells to ABL tyrosine kinase inhibitors. Cancer Sci. 2014;105:297–307. doi: 10.1111/cas.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kong D, Piao YS, Yamashita S, Oshima H, Oguma K, Fushida S, Fujimura T, Minamoto T, Seno H, Yamada Y, Satou K, Ushijima T, Ishikawa TO, Oshima M. Inflammation-induced repression of tumor suppressor miR-7 in gastric tumor cells. Oncogene. 2012;31:3949–3960. doi: 10.1038/onc.2011.558. [DOI] [PubMed] [Google Scholar]
- 18.Weber DG, Johnen G, Bryk O, Jockel KH, Bruning T. Identification of miRNA-103 in the cellular fraction of human peripheral blood as a potential biomarker for malignant mesothelioma--a pilot study. PLoS One. 2012;7:e30221. doi: 10.1371/journal.pone.0030221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong X, Chung AC, Chen HY, Dong Y, Meng XM, Li R, Yang W, Hou FF, Lan HY. miR-21 is a key therapeutic target for renal injury in a mouse model of type 2 diabetes. Diabetologia. 2013;56:663–674. doi: 10.1007/s00125-012-2804-x. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Yan CH, Li Y, Xu K, Tian XX, Peng CF, Tao J, Sun MY, Han YL. MicroRNA-31 controls phenotypic modulation of human vascular smooth muscle cells by regulating its target gene cellular repressor of E1A-stimulated genes. Exp Cell Res. 2013;319:1165–1175. doi: 10.1016/j.yexcr.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Kim S, Kang H. miR-15b induced by platelet-derived growth factor signaling is required for vascular smooth muscle cell proliferation. BMB Rep. 2013;46:550–554. doi: 10.5483/BMBRep.2013.46.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao S, Wassler M, Zhang L, Li Y, Wang J, Zhang Y, Shelat H, Williams J, Geng YJ. MicroRNA-133a regulates insulin-like growth factor-1 receptor expression and vascular smooth muscle cell proliferation in murine atherosclerosis. Atherosclerosis. 2014;232:171–179. doi: 10.1016/j.atherosclerosis.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu ML, Wang JF, Wang GK, You XH, Zhao XX, Jing Q, Qin YW. Vascular smooth muscle cell proliferation is influenced by let-7d microRNA and its interaction with KRAS. Circ J. 2011;75:703–709. doi: 10.1253/circj.cj-10-0393. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Zhao L, He X, Yang T, Yang K. MiR-21 inhibits c-Ski signaling to promote the proliferation of rat vascular smooth muscle cells. Cell Signal. 2014;26:724–729. doi: 10.1016/j.cellsig.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 25.Sun Y, Chen D, Cao L, Zhang R, Zhou J, Chen H, Li Y, Li M, Cao J, Wang Z. MiR-490-3p modulates the proliferation of vascular smooth muscle cells induced by ox-LDL through targeting PAPP-A. Cardiovasc Res. 2013;100:272–279. doi: 10.1093/cvr/cvt172. [DOI] [PubMed] [Google Scholar]
- 26.Kee HJ, Park S, Kwon JS, Choe N, Ahn Y, Kook H, Jeong MH. B cell translocation gene, a direct target of miR-142-5p, inhibits vascular smooth muscle cell proliferation by down-regulating cell cycle progression. FEBS Lett. 2013;587:2385–2392. doi: 10.1016/j.febslet.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Sun SG, Zheng B, Han M, Fang XM, Li HX, Miao SB, Su M, Han Y, Shi HJ, Wen JK. miR-146a and Kruppel-like factor 4 form a feedback loop to participate in vascular smooth muscle cell proliferation. EMBO Rep. 2011;12:56–62. doi: 10.1038/embor.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X, Cheng Y, Zhang S, Lin Y, Yang J, Zhang C. A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res. 2009;104:476–487. doi: 10.1161/CIRCRESAHA.108.185363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wen P, Cao H, Fang L, Ye H, Zhou Y, Jiang L, Su W, Xu H, He W, Dai C, Yang J. miR-125b/Ets1 axis regulates transdifferentiation and calcification of vascular smooth muscle cells in a high-phosphate environment. Exp Cell Res. 2014;322:302–312. doi: 10.1016/j.yexcr.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 30.Li Z, Yu X, Wang Y, Shen J, Wu WK, Liang J, Feng F. By downregulating TIAM1 expression, microRNA-329 suppresses gastric cancer invasion and growth. Oncotarget. 2015;6:17559–69. doi: 10.18632/oncotarget.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stein JJ, Iwuchukwu C, Maier KG, Gahtan V. Thrombospondin-1-induced vascular smooth muscle cell migration and proliferation are functionally dependent on microRNA-21. Surgery. 2014;155:228–233. doi: 10.1016/j.surg.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Liu F, Li N, Long B, Fan YY, Liu CY, Zhou QY, Murtaza I, Wang K, Li PF. Cardiac hypertrophy is negatively regulated by miR-541. Cell Death Dis. 2014;5:e1171. doi: 10.1038/cddis.2014.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leivonen SK, Sahlberg KK, Makela R, Due EU, Kallioniemi O, Borresen-Dale AL, Perala M. High-throughput screens identify microRNAs essential for HER2 positive breast cancer cell growth. Mol Oncol. 2014;8:93–104. doi: 10.1016/j.molonc.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edwards CL, Best SE, Gun SY, Claser C, James KR, de Oca MM, Sebina I, de Labastida Rivera F, Amante FH, Hertzog PJ, Engwerda CR, Renia L, Haque A. Spatio-temporal requirements for IRF7 in mediating type I IFN-dependent susceptibility to blood-stage Plasmodium infection. Eur J Immunol. 2015;45:130–141. doi: 10.1002/eji.201444824. [DOI] [PubMed] [Google Scholar]
- 35.Zhou P, Cowled C, Mansell A, Monaghan P, Green D, Wu L, Shi Z, Wang LF, Baker ML. IRF7 in the Australian black flying fox, Pteropus alecto: evidence for a unique expression pattern and functional conservation. PLoS One. 2014;9:e103875. doi: 10.1371/journal.pone.0103875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Love AC, Schwartz I, Petzke MM. Borrelia burgdorferi RNA induces type I and III interferons via Toll-like receptor 7 and contributes to production of NF-kappaB-dependent cytokines. Infect Immun. 2014;82:2405–2416. doi: 10.1128/IAI.01617-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim N, Kukkonen S, Martinez-Viedma Mdel P, Gupta S, Aldovini A. Tat engagement of p38 MAP kinase and IRF7 pathways leads to activation of interferon-stimulated genes in antigen-presenting cells. Blood. 2013;121:4090–4100. doi: 10.1182/blood-2012-10-461566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ning S, Huye LE, Pagano JS. Regulation of the transcriptional activity of the IRF7 promoter by a pathway independent of interferon signaling. J Biol Chem. 2005;280:12262–12270. doi: 10.1074/jbc.M404260200. [DOI] [PubMed] [Google Scholar]
- 39.Huang L, Zhang SM, Zhang P, Zhang XJ, Zhu LH, Chen K, Gao L, Zhang Y, Kong XJ, Tian S, Zhang XD, Li H. Interferon regulatory factor 7 protects against vascular smooth muscle cell proliferation and neointima formation. J Am Heart Assoc. 2014;3:e001309. doi: 10.1161/JAHA.114.001309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


