Abstract
Background: Keratinocyte growth factor-2 (KGF-2) has been testified to be a multifunctional growth factor, which can stimulate the regeneration and reconstruction of epidermis, corium and mucosa. Its effect on Crohn’s disease has hitherto not been evaluated. Here, we investigated the preventive and therapeutic actions of STEA, a mutant of human KGF-2 with high activity, on trinitrobenzene sulfonic acid (TNBS)-induced rat model of Crohn’s disease. Methods: Rats with TNBS-induced colitis were treated with STEA and clinical scores were evaluated. Body weight, mortality, macroscopic and microscopic damage of the colonic tissue were examined. The levels of inflammatory cytokines in serum were detected by ELISA, the T cell subpopulations and the cell cycle of intestinal epithelial cells were analyzed by flow cytometry. Results: Both preventive and therapeutic administration of STEA significantly ameliorated body weight loss, diarrhea, and intestinal inflammation, reduced the high mortality and histopathologic damage of rats with TNBS-induced colitis. The serum level of inflammatory cytokines, such as TNF-α, IL-1β, IFN-γ and IL-6 were markedly decreased in colitis rats treated with STEA. The CD4+ and CD8+ T lymphocytes in peripheral blood were reduced with STEA administration at early stage of colitis. In addition, STEA treatment could promote the growth of intestinal epithelial cells by increasing the cell proportion in S phase of cell cycle and inhibiting cell apoptosis. Conclusions: Both preventive and therapeutic administration of STEA could ameliorate the colonic damages in rats with TNBS-induced colitis. STEA might be a promising option for the treatment of Crohn’s disease.
Keywords: Mutant of keratinocyte growth factor-2, Crohn’s disease, TNBS, cell proliferation
Introduction
Crohn’s disease (CD) is an idiopathic chronic inflammatory disease of unknown etiology, which easily relapses and affects the whole of the alimentary tract (digestive tube) from mouth to anus. The disease possesses the highest incidence in Europe and North American population, which shows an ascending incidence in Asia population in recent years [1,2]. The pathogenesis of CD is closely related with the intestinal inflammation and damage caused by dysregulation of immune response of intestinal mucosa. It is found that helper T1 (Th1)-like cells, an inflammatory subset of T cells, were selectively activated in intestinal mucosa of CD patients, by which proinflammatory cytokines such as interferon (IFN)-γ, tumor necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6 were persistently secreted in the early stage of the disease [3,4].
Several immunoregulatory agents, such as 5-aminosalicylates, corticosteroids, and McAb against tumor necrosis factor (TNF)-α have mainly been used for the treatment of human CD to control the dysregulated immune response [5]. However, these agents can cause various undesirable side-effects. Besides, some patients with CD are stubborn even to the combined use of these agents. In fact, the intestinal mucosa plays an important barrier role in preventing the penetration of toxic and immunogenic factors. Recent studies have shown that impaired intestinal barrier function permits the penetration of toxic and immunogenic factors, leading to the occurrence and perpetuation of intestinal inflammation. Therefore, keeping the integrity of the intestinal mucosa may also provide an effective approach as a therapeutic strategy for CD.
Keratinocyte growth factor (KGF)-2, also known as fibroblast growth factor (FGF)-10, is initially isolated from rat embryo as a homolog of KGF-1 in 1996 [6]. Human KGF-2 cDNA encoding a 208 aa precursor with a signal sequence (~40Aa) is obtained from the human embryo lung in 1997. KGF-2 can specifically promote the growth, proliferation and differentiation of epithelia cells, and possesses many important biological functions [7-10]. Administration of KGF-2 significantly promotes the re-epithelialization and closure of wounds in an ischaemia-impaired rabbit ear model, which was found to be more effective than the vehicle control at closing the interstices of a human meshed skin graft explanted to athymic nude rats [11,12]. KGF-2 can induce the intestinal mucosa of rat and cynomolgus monkey to reversible incrassation [13,14]. In the dextran sulfate sodium (DSS)-induced murine model of ulcerative colitis, intraperitoneal or subcutaneous injection of KGF-2 could significantly enhance weight recovery, and reduce colonic mucosa damages [15]. The similar results of protection against intestinal injury are achieved in studies on indomethacin-induced jejunal ulceration in rats [16]. A phase 2 trial, conducted to evaluate the safety and efficacy of topical of KGF-2 treatment in the healing of chronic venous ulcer in 94 patients, showed that KGF-2 markedly accelerated wound healing than placebo [17]. Therefore, KGF-2 might be a promising agent for the treatment of mucosal injury in human inflammatory bowel disease.
Animal chronic intestinal inflammation induced by intrarectal administration of trinitrobenzene sulfonic acid (TNBS) resembles many of the clinical, histopathologic, and immune characteristics of CD in humans [18,19]. In our previous studies, we constructed a double-site mutant of KGF-2 (named STEA) by replacing Ser115 and Glu117 with Thr and Ala, and the mutant showed higher bioactivities than that of KGF-2 [20]. Here, we examined the efficacy of intraperitoneal administration of STEA on rat model of TNBS-induced colitis, and found that both preventive and therapeutic administration of STEA significantly alleviated the systemic and local inflammation response involved in colitis rats, accelerated the reepithelialization and restoration of colonic mucosa, and greatly improved histopathologic damage healing of rats with TNBS-induced colitis, which suggests that STEA might be a promising option for the therapy of Crohn’s disease.
Materials and methods
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of Academy of Military Medical Sciences (Permit Number: SCXK-2002-001). All surgery was performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering.
Animals
Male Wistar rats (140±20 g; Animal Center of Academy of Military Medical Sciences, China) were used for the experiments. All rats were housed in specific pathogen-free conditions in the animal facility, fed with standard laboratory chow and tap water, and handled according to institutionally recommended animal care guidelines.
Induction of colitis and treatment protocols
After overnight fasting, rats were anesthetized by intraperitoneal injection of 3% sodium pentobarbital (50 mg/kg). The distal colon was cleaned carefully with a small balloon catheter before the enema. Rats were upside down, and TNBS (100 mg/kg, 5 mg, Sigma, St. Louis, MO) in 40% ethanol was then instilled into the lumen of the colon using the catheter fitted onto a 1 ml syringe (about 8 cm from the anal verge) in 5 seconds. Control rats received 40% ethanol alone. Rats were then kept in an inverted position for 30 seconds and returned to their cages. To investigate the preventive effect of STEA on established colitis, the rats were injected intraperitoneally (IP) with 3 mg/kg STEA once a day for 3 days starting 2 days before initiation of TNBS treatment (day 0), and TNBS group received equal amount of phosphate buffer (PB) via IP injection. The STEA was produced as described previously [20]. Some animals were killed by euthanasia on day 6 and 12. To study the effect of STEA on the recrudescent model of CD, rats were subjected twice infusion of TNBS on day 0 (100 mg/kg) and 18 (10 mg per rat), and were administered IP with 3 mg/kg STEA once a day for 5 days after the second infusion of TNBS. At the same time, control rats received 40% ethanol infusions and were treated with PB. Some animals were killed on day 4, 8, and 12 after the last treatment with TNBS.
Evaluation on macroscopic and microscopic damage
All rats were monitored for the appearance of diarrhea, body weight loss, and survival. For evaluation of macroscopic damage, colons were graded for macroscopic lesions (scale 0-10) based on criteria reflecting inflammation (ie, hyperemia, bowel thickening, and extent of ulceration) [21]. The stool score comprised two parts and totaled a maximum score of 4, based on a modification of the system outlined by Elson et al. [22]. Stool consistency was graded as: 0 = firm, 1 = loose, 2 = diarrhea. Blood in the stool was also evaluated on a 0- to 2-point scale: 0 = no blood, 1 = occult blood, 2 = gross rectal bleeding. Stool scores were taken at several points throughout the course of the disease. The length and weight from the ileocecal junction to the anal verge were measured as the colonic length and weight. The distal third of the colon was dissected, fixed in 30% formaldehyde, and stained with H.E. Then the histologic damage was analyzed.
Determination of serum cytokine level by ELISA
Blood samples were collected by cardiac puncture and sera were isolated by centrifugation at 2000 rpm for 10 min. The levels of IL-4, IFN-γ, TNF-α, IL-1β, and IL-6 in sera were measured by rat ELISA kit (ADL Inc, USA) according to the manufacturer’s protocols. Briefly, 100 μl serum was added into each well respectively and incubated for 20 min at 25°C. Then, 100 μl biotin-conjugated antibodies were added and incubated for 20 min at 25°C. Subsequently, 100 μl anti-IgG antibody labeled with HRP was added and incubated for 10 min at 25°C. Each above-mentioned step was followed by washing plate with wash buffer, finally reacted with 100 μl tetramethylbenzidine (TMB) solution for 20 min at 25°C and terminated by addition of 100 μl stop solution. The A450 was measured within 15 min.
Analysis of T cell subpopulations by slow sytometry
The cardiac puncture-collected blood samples were analyzed by fluorescence-activated cell sorting (FACS). Monoclonal antibodies of fluorescein isothiocyanate (FITC)-conjugated anti-rat CD3, phycoerythrin (PE)-conjugated anti-rat CD4, and PE-Cy5-conjugated anti-rat CD8 were purchased from Becton Dickinson (BD Biosciences Pharmingen, San Diego, CA). A two-parameter analysis was performed to determine the percentages of T cells (CD3), CD4 positive T Cells (CD4+) and CD8 positive T cells (CD8+). Briefly, 100 μl whole blood was pre-incubated with antibodies for 30 min at room temperature. Then, 2 ml lysing solution was added and maintained for 15 min at room temperature. After centrifugation at 1500 rpm for 5 min, the cells were washed with PBS and analyzed using a fluorescence cell counter (FACS Calibur Becton Dickinson, USA).
Analysis of cell cycle of intestinal epithelial cells
The whole small intestine of rat was isolated and washed with ice-cold NaCl solution (0.9%) to remove food debris, then rapidly dissected and washed through again to remove any food particles and mucin remained in the intestinal lumen. A 10 cm of intestine proximal to the stomach was discarded and the next 40-45 cm was retained. The intestinal epithelial cells were isolated as described previously [23]. Subsequently, the collected cells were centrifuged and washed twice with 1 mL PBS (pH 7.4). After centrifugation again, the cell pellet was resuspended in 0.3 mL PBS (pH 7.4, containing 10% fetal bovine serum) before adding 0.7 ml ethanol and deposited at -20°C for 30 h. Cells were collected by centrifugation and washed once with 1 ml PBS (pH 7.4). Then, 200 μl (1 mg/ml) RNaseA was added, mixed, and incubated at 37°C for 30 min. Finally, 400 μl of PI (100 μg/ml) solution was added, with an incubation of 20 min, and then cell samples were analyzed using a fluorescence cell counter (FACS Calibur Becton Dickinson, USA).
Statistical analysis
Student t test and the Mann-Whitney U test were used where appropriate for statistical analysis. Survival curves were analyzed by the Kaplan-Meier log-rank test. All values are expressed as mean ± SD. P value < 0.05 was considered statistically significant.
Results
STEA injection protects against TNBS-induced colitis
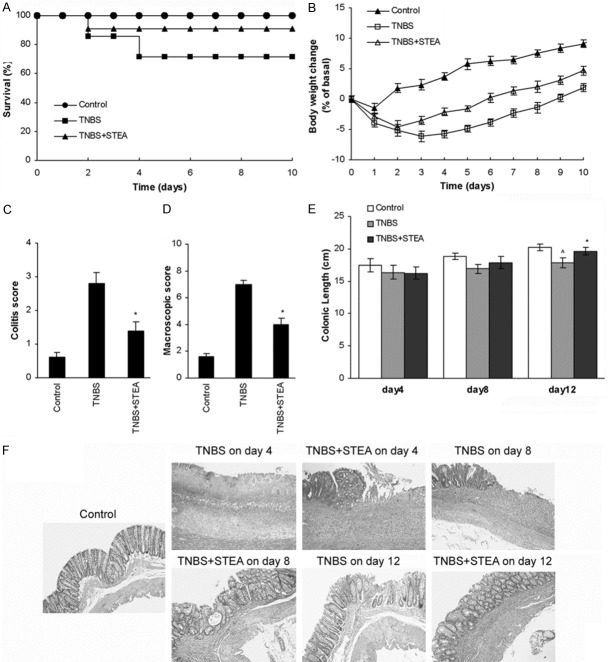
KGF-2 can promote proliferation of epithelial cells and has been manifested possessing perfect effect on epithelial damages as well as (DSS)-induced murine model of ulcerative colitis [11-15]. In our previous studies, we obtained a mutant of KGF-2, named STEA, which showed higher biological activities than that of KGF-2 [20]. Here, we first investigated the potential preventive action of STEA in an experimental model of colitis induced by intrarectal infusion of TNBS, which displays human CD-like clinical, histopathologic, and immunologic features. Rats subjected to intrarectal administration of TNBS in 40% ethanol developed a severe illness characterized by bloody diarrhea, rectal prolapse, and pancolitis accompanied by extensive wasting syndrome, and a profound and sustained weight loss resulting in 53% mortality (Figure 1A-D). Rats pretreated with 3 mg/kg of STEA had an increased survival rate of 82%, rapidly recovered the loss of body weight, and regained a healthy appearance similar to control rats treated with 40% ethanol alone (Figure 1A-D). Macroscopic examination of colons obtained 6 days after colitis induction showed striking hyperemia, inflammation, and necrosis, in contrast, the colons of STEA-pretreated rats showed slight macroscopic inflammation (Figure 1C, 1D). The colonic length in rats with STEA-pretreated was significantly longer than that in rats with TNBS-treated alone on day 12 (P < 0.05) (Figure 1E). Histologic examination of the distal colon of control rats received 40% ethanol showed the characteristic intact surface epithelium, well defined crypt length, and lack of cellular infiltrate in the mucosa and submucosa (Figure 1F). Rats with TNBS-induced colitis showed a remarkable transmural inflammation involving all layers of the bowel wall, adherence to surrounding tissues, loss of the histological structure, focal ulcers with an acute inflammatory cell exudates, and loss of goblet cells on day 6, and a thickening of the colon wall, fibrosis found through the colon and/or the presence of mucosal edema on day 12 (Figure 1F). In contrast, in rats with TNBS-induced colitis pretreated with 3 mg/kg of STEA, a significant reduction of inflammatory activity, crypt regeneration and restoration of colonic mucosa were observed on day 6, though cellular infiltration and edema in the lamina propria and submucosa were observed, and the histologic images were further improved on day 12 (Figure 1F).
Figure 1.
Pretreatment with STEA protects against colitis. Colitis was induced by intracolonic administration of TNBS (100 mg/kg). Rats were treated intraperitoneally with 3 mg/kg STEA once a day for 3 days starting 2 days before TNBS treatment (day 0). Control rats received 40% ethanol or PB alone. Clinical evolution was monitored by survival (A) and body weight changes (B). Colitis score (C) and macroscopic damage score (D) were determined at 6 days after TNBS administration. Colonic length (E) was measured from the ileocecal junction to the anal verge. (F) Representative histologic findings in rats with TNBS-induced colitis pretreated with STEA. Sections of the distal colon were collected at necropsy and stained with H&E (original magnification, ×100). Data are expressed as means ± SD, n = 14 rats/group in (A) and (B), n = 5 rats/group in (C) to (E). *p < 0.05 vs TNBS-treated rats; ^p < 0.05 vs control rats.
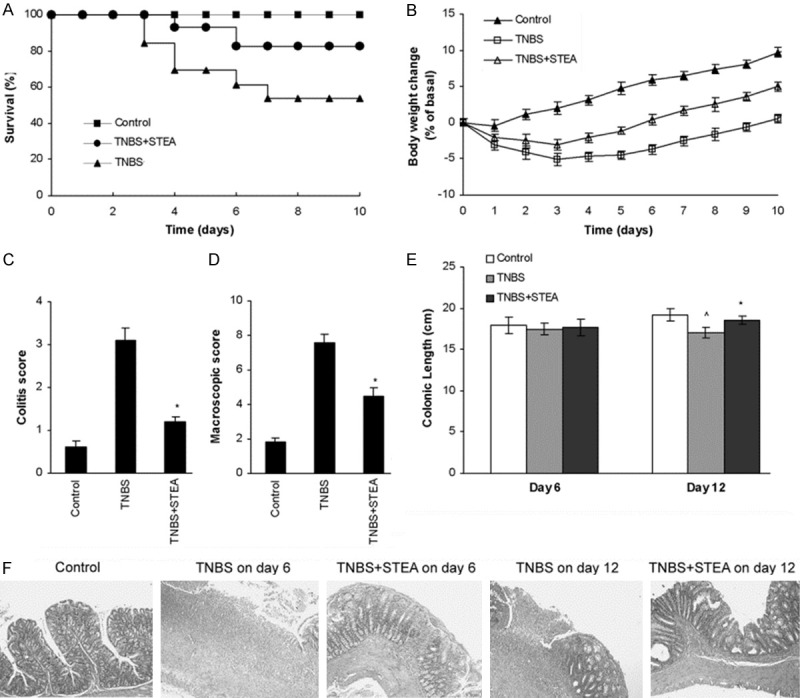
About the therapeutic action of STEA on the recrudescent model of CD, rats subjected to infusion of TNBS on days 0 (100 mg/kg) and 18 (10 mg per rat), developed a severe illness characterized by bloody diarrhea and pancolitis, and a profound weight loss resulting in 35% mortality (Figure 2A-D). Rats treated with 3.0 mg/kg of STEA displayed an increased survival rate of 91%, rapidly recovered from the loss of body weight, and regained a healthy appearance similar to control rats treated with 40% ethanol (Figure 2A-D). Macroscopic examination of colons obtained at day 4 after colitis induction showed striking hyperemia and inflammation, in contrast, the colons of STEA-pretreated rats showed obviously reduced macroscopic inflammation (Figure 2C, 2D). At necropsy, the colonic length was longer than that in rats with TNBS-induced colitis on day 12 (P < 0.05) (Figure 2E). The histologic assay of the colonic tissues from rats with TNBS-induced colitis showed severe inflammation, characterized by mucosal necrosis and transmural inflammation on day 4, and the similar severe damage status were still remained on day 8, suggesting the slow reepithelialization and restoration of colonic mucosa (Figure 2F). In contrast, crypt regeneration, reepithelialization and restoration of colonic mucosa were relatively rapid, though there were severe damages of colonic mucosa observed in rats with TNBS-induced colitis treated with 3.0 mg/kg of STEA on day 4, and the histologic images were greatly improved on day 8, and nearly recovered to normal structure on day 12 (Figure 2F).
Figure 2.
Treatment with STEA protects against colitis. The recrudescent colitis of rat was induced by twice infusion of TNBS on day 0 (100 mg/kg) and day 18 (10 mg per rat), and rats were administered IP with 3 mg/kg STEA once a day for 5 days after the second infusion of TNBS. Simultaneously, control rats received 40% ethanol infusions and were treated with PB. Clinical evolution was monitored by survival (A) and body weight changes (B). Colitis score (C) and macroscopic damage score (D) were determined at 4 days after TNBS administration. Colonic length (E) was measured from the ileocecal junction to the anal verge at necropsy. (F) Representative histologic findings in rats with TNBS-induced colitis treated with STEA. Sections of the distal colon were collected at necropsy and stained with H&E (original magnification, ×100). Data are expressed as means ± SD, n = 14 rats/group in (A) and (B), n = 5 rats/group in (C) to (E). *p < 0.05 vs TNBS-treated rats; ^p < 0.05 vs control rats.
STEA administration reduces inflammatory responses in rats with TNBS-induced colitis
The TNBS-induced murine model of CD by rectal elution is associated with Th1 cell mediated response, therefore, the inflammatory cytokines released by Th1 cells, such as IFN-γ, TNF-α, IL-1β and IL-6 play important roles in the pathogenesis and persistent inflammatory responses of colitis [3,4,24]. IL-4 is an important anti-inflammatory cytokine and decreased mucosal IL-4 is found in IBD patients [25]. Here, we evaluated the effect of STEA injection on the serum level of TNF-α, IFN-γ, IL-1β, IL-6, and IL-4 in STEA-treated and untreated rats with TNBS induced colitis. In the protective action study of STEA, the serum IL-1β was significantly increased in rats with TNBS-induced colitis compared with control rats (P < 0.05) on day 6, and the difference became more remarkable on day 12. However, no significant difference was found in serum IL-1β between the STEA-pretreated rats and control rats, though IL-1β in STEA-pretreated rats was slightly increased on day 6 (Figure 3A). The serum levels of TNF-α, IL-6 and IFN-γ in rats with TNBS-induced colitis were also significantly up-regulated on day 6. Pretreatment with 3 mg/kg STEA obviously reduced these inflammatory cytokines levels in the blood serum, especially on day 12 (Figure 3A). No obvious differences were found in serum IL-4 among the three studied groups (Figure 3A). Similar results were observed in the study to the therapeutic effect of STEA on the recrudescent model of CD. The serum levels of TNF-α, IL-6 and IFN-γ were increased in rats with TNBS-induced colitis, but decreased by STEA pretreatment, and significant decline was observed on day 8 and 12 (Figure 3B).
Figure 3.
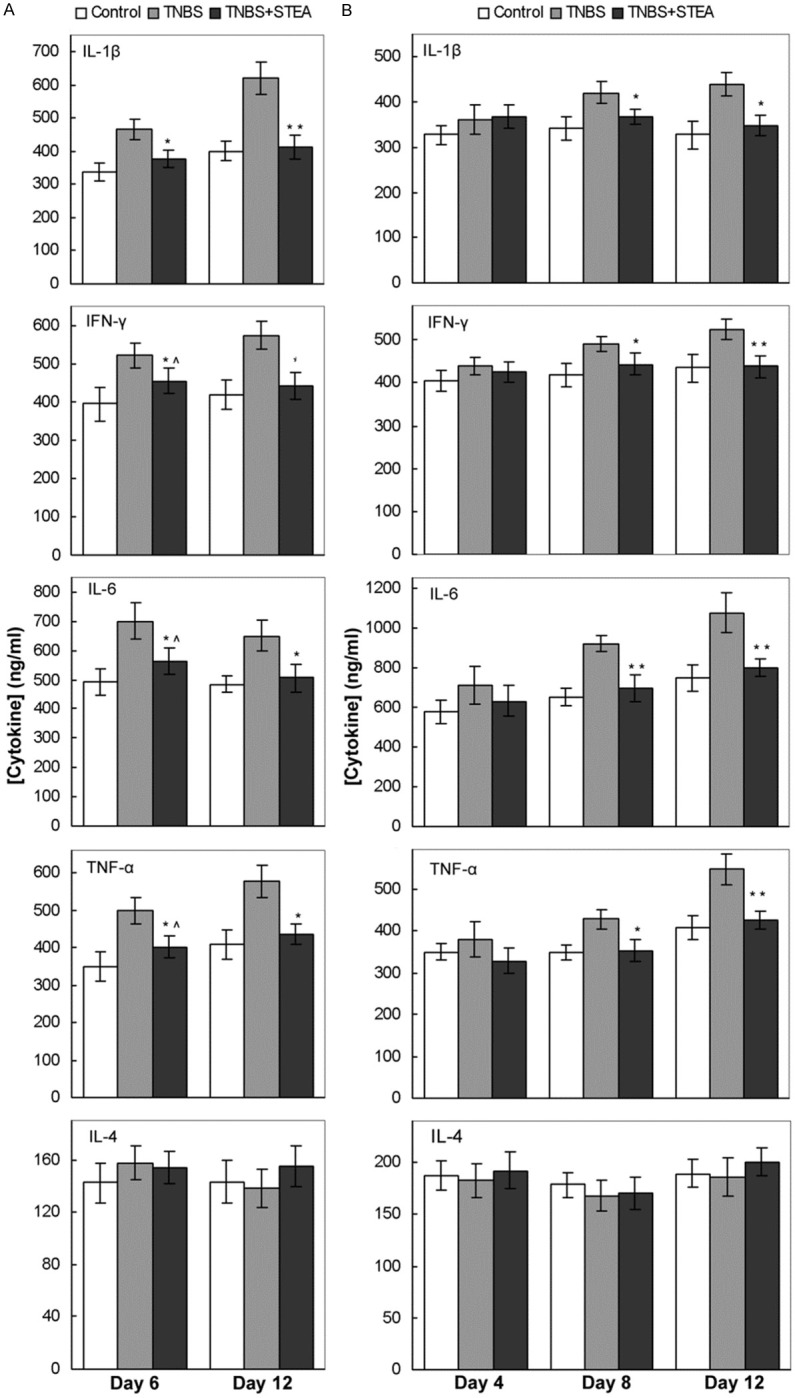
Effect of STEA administration on the serum level of inflammatory cytokines. The preventive (A) and therapeutic (B) effects of STEA administration on the serum levels of inflammatory cytokines in rats with TNBS-induced colitis were investigated. Colitis induced by TNBS infusion and administration of STEA were performed as described in the experiment methods. Sera were collected at the indicated times, and cytokines levels were measured by ELISA. Results represent mean ± SE . **p < 0.01 and *p < 0.05 vs TNBS-treated rats; ^p < 0.05 vs control rats.
Effects of STEA on T cell subpopulations in peripheral blood
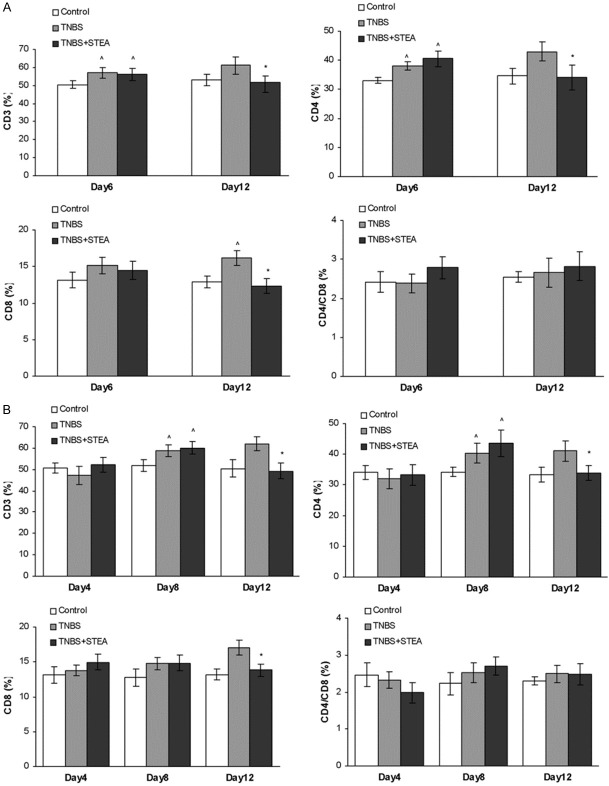
Damage to the epithelial gut mucosa in IBD has been linked to the elevated levels of effecter immune cells such as activated CD4+ and CD8+ cytotoxic T cells [26,27]. In this study, we investigated the ratio of T cell subpopulations in the peripheral blood from rats with TNBS-induced colitis using flow cytometry. As shown in Figure 4, CD3+ and CD4+ T lymphocytes in peripheral blood were markedly increased after TNBS infusion at observed times, and CD8+ T lymphocytes was slightly elevated on day 6 and significantly increased on day 12 (Figure 4A). Pretreatment with STEA significantly reduced CD3+, CD4+, and CD8+ T lymphocytes in peripheral blood on day 12 (Figure 4A). However, no significant differences were found on the CD4/CD8 ratio among the studied groups during our observation, though the number of T cell subpopulations was changed with TNBS infusion. In the therapeutic effect study of STEA, obvious increase was observed in the number of CD3+, CD4+, and CD8+ T cells on day 8 and 12 after TNBS infusion (Figure 4B). Administration with 3 mg/kg STEA significantly reduced CD3+, CD4+, and CD8+ T lymphocytes in peripheral blood on day 12 (Figure 4B). Also, no significant differences have been found on the CD4/CD8 ratio among the studied groups during our observation.
Figure 4.
Effects of STEA treatment on T Cell subpopulations. The preventive (A) and therapeutic (B) effects of STEA administration on T cell subpopulations from rats with TNBS-induced colitis were investigated. Colitis induced by TNBS infusion and administration of STEA were performed as described in the experiment methods. Blood samples were collected by cardiac puncture. The T-cell numbers were determined by FACS. Results are means ± SD. *p < 0.05 vs TNBS-treated rats; ^p < 0.05 vs control rats.
Effects of STEA pretreatment on the cell cycle of intestinal epithelial cells
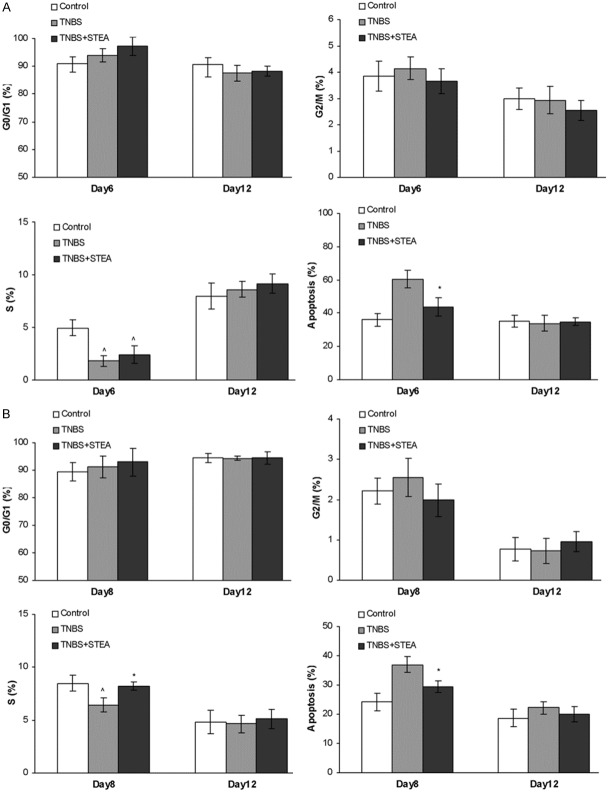
The small intestine proximal to the colon was often suffered from infusion of TNBS, so we investigated the cell cycle and apoptosis of epithelial cells from intestine adjacent to colon. The whole small intestine of rat was removed and intestinal epithelial cells were isolated, and then analyzed by flow cytometry. It seems that the proportion of intestinal epithelial cells in G0/G1 phase had slightly raised in rats treated with TNBS on day 6, and such trend was enhanced after pretreating with STEA, however, no significant differences were found among the studied groups (Figure 5A). The proportion of intestinal epithelial cells in G2/M phase was nearly unaffected by treatment with TNBS or TNBS and STEA (Figure 5A). The intestinal epithelial cells in S phase were significantly reduced in rats treated with TNBS on day 6, and pretreated with 3 mg/kg of STEA slightly increased the number of cells in S phase. On day 12, the intestinal epithelial cells in S phase recovered to normal level in rats treated with TNBS or TNBS and STEA (Figure 5A). In the apoptosis assay of intestinal epithelial cells, the proportion of cells in apoptosis was significantly increased on day 6 in rats with TNBS infusion alone, but not in rats pretreated with 3 mg/kg of STEA. In the further observation, no obvious differences were found among the studied groups (Figure 5A). Similar results were observed in the therapeutic effect of STEA on the recrudescent model of CD (Figure 5B). Treatment with 3 mg/kg of STEA significantly elevated the intestinal epithelial cells in S phase and reduced the cells in apoptosis on day 8 (Figure 5B).
Figure 5.
Effects of STEA treatment on the cell cycle of intestinal epithelial cells. The preventive (A) and therapeutic (B) effects of STEA administration on the cell cycle of intestinal epithelial cells from rats with TNBS-induced colitis were investigated. Colitis induction, administration of STEA, and isolation of the intestinal epithelial cells were performed as described in the experiment methods. The cell cycle was analyzed using a fluorescence cell counter. Results are means ± SD. *p < 0.05 vs TNBS-treated rats; ^p < 0.05 vs control rats.
Discussion
In recent years, some FGFs such as aFGF, bFGF, and KGF-1, have been tested as potent therapeutic agents in a variety of experimental models of human [5,28,29]. KGF-2, a homolog of KGF-1, also a member of the FGF family, has the special ability to stimulate the growth, proliferation and differentiation of epithelium. Studies have showed that KGF-2 is effective in accelerating healing of epithelial damage in some tissues, such as skin and oral mucosa [30,31]. In a DSS-induced murine model of ulcerative colitis, KGF-2 could significantly enhance weight recovery and reduce colonic mucosa damages [15,32]. The mutant of KGF-2 (named STEA) with higher biological activities in vitro than that of KGF-2 was previously constructed by our lab [20], but its role in vivo was not known. In this study, we evaluated the preventive and therapeutic effect of STEA administration on rats with TNBS-induced colitis.
Intraperitoneal injection of 3 mg/kg STEA once a day for 3 days at day 2 before initiation of TNBS treatment could significantly ameliorate the clinical and histopathologic severity of the wasting disease, abrogate body weight loss, diarrhea, and intestinal inflammation, and reduce the high mortality caused by this syndrome. In the recrudescent model of CD, therapeutic administration of 3 mg/kg STEA once a day for 5 days could improve the damage healing of colon mucosa, reduce intestinal inflammation, and promote the restoration of colonic mucosa.
TNBS-induced model of colonic inflammation by intrarectal elution displays pathologic, clinical and immunological similarities to human CD [19]. The TNBS, as a hapten, is supposed to depend on the haptenization of autologous proteins and presentation of MHC class II-fitting peptides to CD4+ T cells by antigen presenting cells, ultimately leading to specific CD4+ T cell recognition, T cell expansion, and T cell cytokine responses. On the contrary, cytokines can stimulate the rapid division, proliferation of a large number of T cells and formation into sensitized lymphocyte, and induce the expression of adhesion molecules and degranulation of mast cells, which results in the enhanced and persistent inflammatory responses of CD. Therefore, the enhanced T cell response and greatly released inflammatory cytokines play important roles in the pathogenesis and persistent inflammatory responses of CD [33,34]. In fact, damage to the epithelial gut mucosa in IBD has been linked to elevated levels of immune effector cells such as activated CD4+ and CD8+ cytotoxic T cells, and CD4+ T cells are the predominant cell type that infiltrates the intestine in IBD [26,27]. In this study, increased CD4+ T cells and CD8+ cytotoxic T cells are identified in peripheral blood from rats with TNBS-induced colitis, in accordance, severe infiltration of inflammatory cells in the histologic investigation of colonic mucosa were discovered (Figure 1). Pretreated with STEA significantly reduced CD4+ and CD8+ T lymphocytes in peripheral blood on day 12 but not on day 6, which was consistent with the histologic findings, infiltration of inflammatory cells were observed in colonic mucosa from rats with colitis and pretreated with STEA on day 6 (Figure 1). The cytokine pattern of T cells in the murine TNBS-induced colitis is consistent with that found in CD patients, which is characterized by enhanced production of TNF-α and IFN-γ. TNF-α exerts its proinflammatory effects through increased production of IL-1β and IL-6 as well as initiation of cytotoxic, apoptotic, and acute-phase responses [3,4,24]. Here, we investigated levels of these proinflammatory cytokines in blood serum from rats with TNBS-induced colitis. After treatment with TNBS, the serum levels of IL-1β, IL-6, TNF-α, and IFN-γ from TNBS treated rats were prominently increased on day 6 and even higher on day 12, however, significant decreases were observed in colitis rats with protective and therapeutic admistration of 3 mg/kg STEA. The result was consistent with changes in CD4+ and CD8+ T lymphocytes in peripheral blood. These results suggested that the administration of STEA could alleviate T cell-mediated immune responses including inflammation response involved in rats with TNBS-induced colitis.
IL-4 is an important anti-inflammatory cytokine in Th2 T cell mediated response, which has immunoregulatory activity and may play a central role in gut immunology. IL-4 level and IL-4 mRNA were found to be reduced in IBD, which may cause defective immunosuppressive and anti-inflammatory mechanisms and may contribute to the pathogenic cascade leading to inflammation [25]. In this study, we also investigated the IL-4 level in peripheral blood. However, we found that neither TNBS- nor STEA-treatment would significantly affect the level of IL-4 in peripheral blood. Therefore, the effect of STEA ameliorating the TNBS-induced colitis was not mediated by driving the Th2 cell response to greatly release the anti-inflammatory cytokines.
In previous study of efficacy of KGF-2 in DSS-induced murine colitis, the exact mechanism of KGF-2 ameliorating clinical and histopathologic damage of colonic mucosa hasn’t been clarified [15]. In our study, the fact that STEA administration reduced both the number of T cell subpopulations and the production of multiple inflammatory cytokines, including TNF-α, IL-6, IL-1β, and IFN-γ, could partially explain the low inflammatory infiltrates in the colonic mucosa of rats with STEA-treated colitis. The decrease in inflammatory mediators could be the consequence of a diminished infiltration of inflammatory cells in the colonic mucosa in the STEA-treated rats. In fact, histologic analysis revealed that reepithelialization and restoration of colonic mucosa were relatively rapid by STEA administration, and the colonic length of STEA-treated group was significantly longer than that of TNBS group. In addition, many studies have identified that KGF-2 enhances in vivo proliferation of gastrointestinal tissue [14-16]. Thus, our presumption is that STEA administration could ameliorate the pathologic and clinical severity of TNBS-induced colitis mainly by stimulating epithelial cell proliferation and growth, and hasten wound closure to enhance the mucosal barrier function by restoring injured epithelial structures but not by exerting a direct anti-inflammatory effect on T cells. As shown in Figure 1, by maintaining or rapidly reestablishing the integrity of the epithelial mucosa, STEA helped reinforce the barrier function of that tissue, thereby effectively reducing the degree of inflammatory infiltrate. It has been difficult to verify intestinal cell proliferation in vivo, because the background level of colonic proliferation is so high, even in the TNBS-treated group. Our presumption is partly supported by the study on the cell cycle of intestinal epithelial cells. The proportion of intestinal epithelial cells in S phase was significantly reduced at early stage of TNBS-induced colitis. While, STEA protective and therapeutic administration could increase the proportion of intestinal epithelial cells in S phase, which suggested the promoting effect of STEA on mitosis of intestinal epithelial cells. We also investigated apoptosis of the intestinal epithelial cells. As shown in Figure 5, the cells in apoptosis was increased at early stage of TNBS-induced colitis, but was markedly reduced after treatment with STEA, which suggested that STEA might have some positive effect on promoting survival of intestinal epithelial cells. By histological analysis, more crypt/goblet cells were observed in colonic mucosa from rats treated with STEA, and similar results have been reported, in which KGF-2 is identified as a survival factor for maintaining the stem cell population in developing incisor and crypt cells in intestinal mucosa [14,35]. In addition, KGF-2 administration stimulated goblet cell hyperplasia, presumably resulting in enhanced mucin secretion, which could provide additional mucosal protection [14,15]. Therefore, STEA might have some effect on enhancing the survival of intestinal crypt cells, causing an increase in the protective capacity of the mucosal lining of the intestine.
In summary, our study indicates that both the preventive and therapeutic administrations of STEA are effective to ameliorate the colonic damages induced by the hapten reagent TNBS, and STEA may be a promising option for the treatment of CD. Of course, the combination of STEA with an immunoregulatory compound, in which different disease targets are simultaneously attacked, may be a good strategy to treatment of CD.
Acknowledgements
The authors would like to thank Professor QingLiang Luo, HaiXiao Huang, GuoLiang Xiong, Ling Xie, and Shuang Xing for kindly assistance and suggestions on animal treatment. In addition, we gratefully acknowledge GuoShan Cao for providing antibodies of FITC anti-rat CD4 and antibodies of PE anti-rat CD3. This work was financially supported by National Natural Science Foundation of China (No. 30672403), Preclinical Program of Beijing Municipal Science and Technology Commission (Z141100000514007) and National Key New Drug Creation and Development Program (2011ZXJ09101B-10B, 2009ZX09301-002/05/14). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hilmi I, Tan YM, Goh KL. Crohn’s disease in adults: observations in a multiracial Asian population. World J Gastroenterol. 2006;12:1435–1438. doi: 10.3748/wjg.v12.i9.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oh SH, Kim KM. Current issues of pediatric inflammatory bowel disease in Korea. Korean J Pediatr. 2014;57:465–471. doi: 10.3345/kjp.2014.57.11.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boismenu R, Chen Y. Insights from mouse models of colitis. J Leukoc Biol. 2000;67:267–278. doi: 10.1002/jlb.67.3.267. [DOI] [PubMed] [Google Scholar]
- 4.Kaistha A, Levine J. Inflammatory bowel disease: the classic gastrointestinal autoimmune disease. Curr Probl Pediatr Adolesc Health Care. 2014;44:328–334. doi: 10.1016/j.cppeds.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Dotan I, Ron Y, Yanai H, Becker S, Fishman S, Yahav L, Ben Yehoyada M, Mould DR. Patient factors that increase infliximab clearance and shorten half-life in inflammatory bowel disease: a population pharmacokinetic study. Inflamm Bowel Dis. 2014;20:2247–2259. doi: 10.1097/MIB.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 6.Yamasaki M, Miyake A, Tagashira S, Itoh N. Structure and expression of the rat mRNA encoding a novel member of the fibroblast growth factor family. J Biol Chem. 1996;271:15918–15921. doi: 10.1074/jbc.271.27.15918. [DOI] [PubMed] [Google Scholar]
- 7.Emoto H, Tagashira S, Mattei MG, Yamasaki M, Hashimoto G, Katsumata T, Negoro T, Nakatsuka M, Birnbaum D, Coulier F, Itoh N. Structure and expression of human fibroblast growth factor-10. J Biol Chem. 1997;272:23191–23194. doi: 10.1074/jbc.272.37.23191. [DOI] [PubMed] [Google Scholar]
- 8.Ramasamy SK, Mailleux AA, Gupte VV, Mata F, Sala FG, Veltmaat JM, Del Moral PM, De Langhe S, Parsa S, Kelly LK, Kelly R, Shia W, Keshet E, Minoo P, Warburton D, Bellusci S. Fgf10 dosage is critical for the amplification of epithelial cell progenitors and for the formation of multiple mesenchymal lineages during lung development. Dev Biol. 2007;307:237–247. doi: 10.1016/j.ydbio.2007.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El Agha E, Al Alam D, Carraro G, MacKenzie B, Goth K, De Langhe SP, Voswinckel R, Hajihosseini MK, Rehan VK, Bellusci S. Characterization of a novel fibroblast growth factor 10 (Fgf10) knock-in mouse line to target mesenchymal progenitors during embryonic development. PLoS One. 2012;7:e38452. doi: 10.1371/journal.pone.0038452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watanabe Y, Zaffran S, Kuroiwa A, Higuchi H, Ogura T, Harvey RP, Kelly RG, Buckingham M. Fibroblast growth factor 10 gene regulation in the second heart field by Tbx1, Nkx2-5, and Islet1 reveals a genetic switch for down-regulation in the myocardium. Proc Natl Acad Sci U S A. 2012;109:18273–18280. doi: 10.1073/pnas.1215360109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Zhou X, Ma J, Tian H, Jiao Y, Zhang R, Huang Z, Xiao J, Zhao B, Qian H, Li X. Effects of keratinocyte growth factor-2 on corneal epithelial wound healing in a rabbit model of carbon dioxide laser injury. Biol Pharm Bull. 2010;33:971–976. doi: 10.1248/bpb.33.971. [DOI] [PubMed] [Google Scholar]
- 12.Smith PD, Polo M, Soler PM, McClintock JS, Maggi SP, Kim YJ, Ko F, Robson CM. Efficacy of growth factors in the accelerated closure of interstices in explanted meshed human skin grafts. J Burn Care Rehabil. 2000;21:5–9. doi: 10.1097/00004630-200021010-00003. [DOI] [PubMed] [Google Scholar]
- 13.Sung C, Parry TJ, Riccobene TA, Mahoney A, Roschke V, Murray J, Gu ML, Glenn JK, Caputo F, Farman C, Odenheimer DJ. Pharmacologic and pharmacokinetic profile of repifermin (KGF-2) in monkeys and comparative pharmacokinetics in humans. AAPS PharmSci. 2002;4:E8. doi: 10.1208/ps040208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han DS, Li F, Holt L, Connolly K, Hubert M, Miceli R, Okoye Z, Santiago G, Windle K, Wong E, Sartor RB. Keratinocyte growth factor-2 (FGF-10) promotes healing of experimental small intestinal ulceration in rats. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1011–1022. doi: 10.1152/ajpgi.2000.279.5.G1011. [DOI] [PubMed] [Google Scholar]
- 15.Miceli R, Hubert M, Santiago G, Yao DL, Coleman TA, Huddleston KA, Connolly K. Efficacy of keratinocyte growth factor-2 in dextran sulfate sodium-induced murine colitis. J Pharmacol Exp Ther. 1999;290:464–471. [PubMed] [Google Scholar]
- 16.Zeeh JM, Procaccino F, Hoffmann P, Aukerman SL, McRoberts JA, Soltani S, Pierce GF, Lakshmanan J, Lacey D, Eysselein VE. Keratinocyte growth factor ameliorates mucosal injury in an experimental model of colitis in rats. Gastroenterology. 1996;110:1077–1083. doi: 10.1053/gast.1996.v110.pm8612996. [DOI] [PubMed] [Google Scholar]
- 17.Robson MC, Phillips TJ, Falanga V, Odenheimer DJ, Parish LC, Jensen JL, Steed DL. Randomized trial of topically applied repifermin (recombinant human keratinocyte growth factor-2) to accelerate wound healing in venous ulcers. Wound Repair Regen. 2001;9:347–352. doi: 10.1046/j.1524-475x.2001.00347.x. [DOI] [PubMed] [Google Scholar]
- 18.Motavallian-Naeini A, Andalib S, Rabbani M, Mahzouni P, Afsharipour M, Minaiyan M. Validation and optimization of experimental colitis induction in rats using 2, 4, 6-trinitrobenzene sulfonic acid. Res Pharm Sci. 2012;7:159–169. [PMC free article] [PubMed] [Google Scholar]
- 19.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Cai X, Zou M, Xu T, Liu S, Wang Y, Wang J, Xu D. Construction and characterization of a high activity mutant of human keratinocyte growth factor-2. Biotechnol Lett. 2009;31:797–802. doi: 10.1007/s10529-009-9948-x. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez-Rey E, Chorny A, Delgado M. Therapeutic action of ghrelin in a mouse model of colitis. Gastroenterology. 2006;130:1707–1720. doi: 10.1053/j.gastro.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 22.Elson CO, Cong Y, McCracken VJ, Dimmitt RA, Lorenz RG, Weaver CT. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–276. doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 23.Higashiguchi T, Hasselgren PO, Wagner K, Fischer JE. Effect of glutamine on protein synthesis in isolated intestinal epithelial cells. JPEN J Parenter Enteral Nutr. 1993;17:307–314. doi: 10.1177/0148607193017004307. [DOI] [PubMed] [Google Scholar]
- 24.Begue B, Wajant H, Bambou JC, Dubuquoy L, Siegmund D, Beaulieu JF, Canioni D, Berrebi D, Brousse N, Desreumaux P, Schmitz J, Lentze MJ, Goulet O, Cerf-Bensussan N, Ruemmele FM. Implication of TNF-related apoptosis-inducing ligand in inflammatory intestinal epithelial lesions. Gastroenterology. 2006;130:1962–1974. doi: 10.1053/j.gastro.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez-Munoz F, Dominguez-Lopez A, Yamamoto-Furusho JK. Role of cytokines in inflammatory bowel disease. World J Gastroenterol. 2008;14:4280–4288. doi: 10.3748/wjg.14.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller S, Lory J, Corazza N, Griffiths GM, Z’Graggen K, Mazzucchelli L, Kappeler A, Mueller C. Activated CD4+ and CD8+ cytotoxic cells are present in increased numbers in the intestinal mucosa from patients with active inflammatory bowel disease. Am J Pathol. 1998;152:261–268. [PMC free article] [PubMed] [Google Scholar]
- 27.Brimnes J, Allez M, Dotan I, Shao L, Nakazawa A, Mayer L. Defects in CD8+ regulatory T cells in the lamina propria of patients with inflammatory bowel disease. J Immunol. 2005;174:5814–5822. doi: 10.4049/jimmunol.174.9.5814. [DOI] [PubMed] [Google Scholar]
- 28.Matsuura M, Okazaki K, Nishio A, Nakase H, Tamaki H, Uchida K, Nishi T, Asada M, Kawasaki K, Fukui T, Yoshizawa H, Ohashi S, Inoue S, Kawanami C, Hiai H, Tabata Y, Chiba T. Therapeutic effects of rectal administration of basic fibroblast growth factor on experimental murine colitis. Gastroenterology. 2005;128:975–986. doi: 10.1053/j.gastro.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Ma B, Cheng DS, Xia ZF, Ben DF, Lu W, Cao ZF, Wang Q, He J, Chai JK, Shen CA, Sun YH, Zhang GA, Hu XH. Randomized, multicenter, double-blind, and placebo-controlled trial using topical recombinant human acidic fibroblast growth factor for deep partial-thickness burns and skin graft donor site. Wound Repair Regen. 2007;15:795–799. doi: 10.1111/j.1524-475X.2007.00307.x. [DOI] [PubMed] [Google Scholar]
- 30.Jimenez PA, Rampy MA. Keratinocyte growth factor-2 accelerates wound healing in incisional wounds. J Surg Res. 1999;81:238–242. doi: 10.1006/jsre.1998.5501. [DOI] [PubMed] [Google Scholar]
- 31.Radek KA, Taylor KR, Gallo RL. FGF-10 and specific structural elements of dermatan sulfate size and sulfation promote maximal keratinocyte migration and cellular proliferation. Wound Repair Regen. 2009;17:118–126. doi: 10.1111/j.1524-475X.2008.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sandborn WJ, Sands BE, Wolf DC, Valentine JF, Safdi M, Katz S, Isaacs KL, Wruble LD, Katz J, Present DH, Loftus EV Jr, Graeme-Cook F, Odenheimer DJ, Hanauer SB. Repifermin (keratinocyte growth factor-2) for the treatment of active ulcerative colitis: a randomized, double-blind, placebo-controlled, dose-escalation trial. Aliment Pharmacol Ther. 2003;17:1355–1364. doi: 10.1046/j.1365-2036.2003.01589.x. [DOI] [PubMed] [Google Scholar]
- 33.Yao Y, Han W, Liang J, Ji J, Wang J, Cantor H, Lu L. Glatiramer acetate ameliorates inflammatory bowel disease in mice through the induction of Qa-1-restricted CD8(+) regulatory cells. Eur J Immunol. 2013;43:125–136. doi: 10.1002/eji.201242758. [DOI] [PubMed] [Google Scholar]
- 34.Neurath MF, Finotto S, Glimcher LH. The role of Th1/Th2 polarization in mucosal immunity. Nat Med. 2002;8:567–573. doi: 10.1038/nm0602-567. [DOI] [PubMed] [Google Scholar]
- 35.Sahara S, O’Leary DD. Fgf10 regulates transition period of cortical stem cell differentiation to radial glia controlling generation of neurons and basal progenitors. Neuron. 2009;63:48–62. doi: 10.1016/j.neuron.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]