Abstract
Both POEMS syndrome and IgG4 related diseases are uncommon and clinical manifestations have been multifarious and nonspecific. There is still no literature describing the relationships between these two diseases. This study describes a case of a 33-year-old woman with POEMS syndrome, who had high serum IgG4 concentration and monoclonal IgG4 positive plasmacyte tissue infiltration. This is the first available report of the IgG4 subtype of POEMS syndrome. It is found that the best treatment is to find a novel therapy that targets the plasma cells directly.
Keywords: POEMS syndrome, IgG4 related diseases
Introduction
POEMS syndrome is the acronym of an uncommon paraneoplastic syndrome, due to plasma cell dysplasia characterized by polyneuropathy, organomegaly, endocrinopathy, monoclonal protein and skin changes. Other important traits are sclerotic bone lesions, Castleman’s disease, papilloedema, peripheral oedema, clubbing, ascites, pleural effusions, thrombocytosis, fatigue and increased levels of vascular endothelial growth factor [1-7]. IgG4-related disease is a newly recognized fibroinflammatory condition characterized by tumefactive lesions, a dense lymphoplasmacytic infiltrate rich in IgG4-positive plasma cells, storiform fibrosis, and, often but not always, elevated serum IgG4 concentrations [8-12]. Both of the diseases are related to plasma cell abnormality. Currently there are no reports regarding the relationships between two diseases. In this study, an interesting case of POEMS syndrome-IgG4 subtype was present.
Case report
A 33 year-old female patient was hospitalized in March 2014 with two years of anasarca. From April 2012, she started to suffer from eyelids and lower limbs edema without obvious causes. Meantime, her daily urine volume decreased. Later, chest distress and abdominal distension appeared gradually. After an abdominal ultrasound showing pyoperitoneum, she was asked to take diuretics. The diuretics were helpful in eliminating the edema. However anasarca symptoms frequently recurred. Her abdominal distension symptoms aggravated and she asked to be hospitalized.
She was diagnosed with hyperthyroidism in 2011. She started to take thyroxin tablets regularly because of hypothyroidism after the 131I treatment. There was nothing remarkable about her family history. Physically, she had non-specific but obvious hyperpigmentation and there were changes similar to the thickening of skin. She had abdominal distention and her spleen was palpable above her umbilicus. Shifting dullness was positive. Clubbing and edema were presented in her extremities.
Blood tests showed her white blood cell count was 3.42×109/L, with a hemoglobin concentration of 112 g/L, and a platelet count of 203×109/L. Blood albumin was 28.3 g/L, while globulin was 46.2 g/L. Her liver zymogram, renal function, electrolytes and routine urine examination were normal. The thyroid functions were also measured, from which we discovered that her thyroid-stimulating hormone (TSH) and free thyroxine (T4) level were within the normal ranges, but her free triiodothyronine (T3) level was only 1.50 pg/mL.
The patient complained that she kept suffering from chest distress and abdominal distension and ultrasonic cardiogram (UCG) and abdominal CT scan examinations were received. The UCG revealed that she had left and right atrial dilatation, pulmonary arterial hypertension and cardiac effusion. The abdominal CT scan showed that she had bilateral pleural effusion, pyoperitoneum and pelvic effusion. Lymph nodes located in the porta hepatis, retroperitoneum, mesenteric, pelvic cavity and inguen were noticeably enlarged and increased. One low-density shadow, which might be a neoplastic lesion, was found in her left hepatic lobe. This was a markedly enlarged spleen. There were many bone dense shadows in her thoracic vertebra, lumbar vertebra and pelvis, which were suspiciously similar to tumor metastatic foci.
In addition, the patient also took a series of laboratory examinations such as serum and urine with immunofixation electrophoresis. The results of the tests were as follows: there was IgG-κ monoclonal immunoglobulin in both her blood and urine samples, the measurement of which was 32.9%. IgA-λ monoclonal immunoglobulin could also be observed in her serum. A quantitative analysis of the immunoglobulins in her blood showed that she has hyper-IgG of 24.7 g/L and hypo-C3 of 0.69 g/L. All of her autoantibodies were negative, including the anti-nuclear antibody, extractable nuclear antigens, anti-neutrophils cytoplasm antibodies, antiphospholipid antibody and lupus anticoagulants. Tumor Markers, such as AFP, CEA, NSE, CA125, CA199, CA153, were also negative.
The bone marrow cytomorphologic examination revealed that plasmacytes accounted for 2.5% of all nucleated cells. The examination further revealed that immature plasma cells were occasionally observed and binucleated plasma cells could be clearly seen. The bone marrow histologic examination showed that plasma cells had scattered and the big patchy hyperplasia was partially naïve. CD138(+), κ(+), λ(-), CD38(+), MPO(-), CD5(-), CD20(-), CD10(-), CD23(-), CD45(-) were found from Marrow immunohistochemical staining, which suggests monoclonal plasma cell proliferation. Based on these findings, a diagnosis of a plasma cell disease, such as POEMS Syndrome or multiple myeloma, was considered.
The patient experienced a lassitude of the extremities. The Electromyogram (EMG) revealed multiple peripheral nerve damage involving the bilateral median nerve, cubital nerve and peroneal nerve. The patient then received further examinations. The Fundus cope turned out to be a choked disc. Sex hormone examinations were abnormal and a decreased corticosteroid level was discovered. A positron emission tomography-computed tomography (PET-CT) revealed several enlarged and increased lymph nodes, including: the bilateral neck, mediastina, bilateral hilus of lung, cardio-diaphragmatic angle, bilateral armpit, hepatic portal region, liver stomach gap, spleen stomach gap, root of mesentery, peritoneum, retroperitoneum, bilateral pelvic cavity and inguen. The metabolism of some lymph nodes increased slightly. Her salivary glands experienced a slight increase in metabolism as well. It was found that she had hepatosplenomegaly and her spleen metabolism was higher than her liver. Interstitial changes were found in her double lung. There were signs of pulmonary arterial hypertension, cardiac enlargement and pericardial effusion, pyoperitoneum and pelvic effusion. Extensive osteosclerotic lesions were also found. Yet the low-density shadow found in her left hepatic lobe by the abdominal CT scan did not have an increased metabolism (Figures 1, 2 and 3).
Figure 1.
Positron emission tomography-computed tomography (PET-CT) images of this patient (Part I). The lymph nodes of her neck (A), armpit (B), mediastina (C) were enlarged and increased. Their metabolism slightly increased. Her double lung had interstitial changes (D, E). Cardiac enlargement and pericardial effusion could be observed (F).
Figure 2.
PET-CT images of this patient (Part II). Her lymph nodes of liver stomach gap (A), spleen stomach gap (B), hepatic portal region (C), root of mesentery (D), pelvic cavity (E), inguen (F) were enlarged and increased. Their metabolism slightly increased.
Figure 3.
PET-CT images of this patient (Part III). She had hepatosplenomegaly (A). The low-density shadow found in her left hepatic lobe didn’t have increased metabolism (B). She had extensive osteosclerotic lesions as well (C).
According to the patient’s medical history and examination results, she was diagnosed with POEMS syndrome because of the multisystem disorder characterized by polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes. Before she was diagnosed with POEMS syndrome, however, considering she could have some systemic disease, her blood IgG4 level was detected during the testing process. It was found remarkably that her blood IgG4 level was 4.7 g/L (normal < 1.35 g/L) and this result was reexamined and confirmed.
After reviewing previous study, no similar cases have been reported. Considering this lack of available experience, a bold hypothesis regarding a particular subtype of POEMS syndrome involving IgG4 was put forward. In order to validate this hypothesis, this patient’s bone marrow immunohistochemical staining including IgG and IgG4 were further examined. The staining results showed that almost all of the CD138(+) plasma cells were also IgG(+) and most of the IgG(+) plasma cells were also IgG4(+) (Figure 4). This finding indicates that pathogenic plasma cells mainly secrete IgG4 monoclonal immunoglobulin which confirmed the prior hypothesis.
Figure 4.
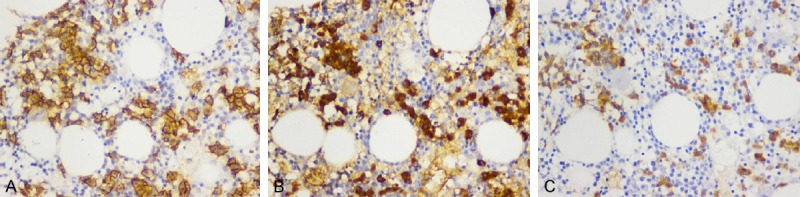
Bone marrow immunohistochemical staining. A: CD138 immunohistochemical staining, B: IgG immunohistochemical staining, C: IgG4 immunohistochemical staining. In this patient’s bone marrow tissue, positive cells (yellow cells) were clearly shown. Almost all of CD138(+) plasma cells were also IgG(+) and most of IgG(+) plasma cells were also IgG4(+).
At last, the patient accepted one adjusted MPT regimen treatment (28 days/each cycle), including melphalan 4 mg/(m2·d), prednisone 25 mg/d, for 7 days every month and thalidomide continuously, 28 days for one cycle. She couldn’t tolerate 100 mg/d thalidomide due to the neurotoxicity. Therefore she took 75 mg of thalidomide every night instead. After 4 courses of MPT, her symptoms involving anasarca, chest distress, abdominal distension and lassitude of the extremities all relieved.
In November 2014, the patient was re-hospitalized because of fever, swelling and aching gums after 8 courses of MPT. Blood tests showed her white blood cell count was 1.15×109/L, neutrophil count was only 0.29×109/L, with a hemoglobin concentration of 88 g/L, and platelet count of 113×109/L. The level of free triiodothyronine (T3) increased to 1.59 pg/mL and blood IgG4 level decreased to 1.5 g/L. Immunofixation electrophoresis still found IgG-κ monoclonal immunoglobulin, but the percentage was reduced to 25.3% (Figure 5). Only mild IgA-λ monoclonal immunoglobulin could be shown. There was no monoclonal immunoglobulin in her urine (Figure 5). The bone marrow cytomorphologic examination revealed that there was a maturation arrest of neutrophils. However, plasmacytes accounted for only 0.5% of the nucleated cells and all of the plasmacytes were mature and didn’t show paramorphia. The abdominal CT scan showed that she had no bilateral pleural effusion, pyoperitoneum or pelvic effusion. Her enlarged spleen decreased in size. Only a small amount of retroperitoneum lymph nodes could be observed. There was minimal alteration to the low-density shadow found in her left hepatic lobe, which was also true of the bone dense shadows in her vertebras. Her fever is likely due to her gum infection. After accepting the antibiotics and granulocyte colony stimulating factor (G-CSF) treatment, the patient recovered quickly and was discharged.
Figure 5.

Serum and urine with immunefixation electrophoresis. A: There was IgG-κ monoclonal immunoglobulin in her serum and its percentage was 32.9%. And IgA-λ monoclonal immunoglobulin also could be seen (first visit). B: There was IgG-κ monoclonal immunoglobulin in her urine (first visit). C: After 8 courses MPT treatment, there was still IgG-κ monoclonal immunoglobulin in her serum but its percentage was reduced to 25.3%. Only mild IgA-λ monoclonal immunoglobulin could be shown. D: After 8 courses MPT treatment, there was no monoclonal immunoglobulin in her urine. (ELP: electrophoresis, G: IgG, A: IgA, M: IgM, K: kappa light chain, L: Lamda light chain, Kf: free kappa light chain, Lf: free lamda light chain).
Discussion
There are some similarities between POEMS syndrome and IgG4 related diseases. They are both uncommon resulting in multifarious and nonspecific clinical manifestations, which can make it difficult for physicians to distinguish one from the other in some special cases. For example, both of them can lead to systemic damage and organomegaly (Liver, spleen, lymph nodes are more common). However, to date, there are no reports that patients with IgG4 related diseases appeared polyneuropathy. Meanwhile, polyneuropathy is one of two mandatory criteria of POEMS syndrome. Accordingly, physicians should consider higher possibility of POEMS syndrome in intractable cases once polyneuropathy is observed. In this case, the patient experienced multiple peripheral nerve damage confirmed by EMG. However, this alone is not enough and final diagnosis will still depend on the evidence monoclonal plasma cell proliferation.
According to the revised POEMS syndrome criteria, two mandatory criteria (polyneuropathy and monoclonal plasma cell proliferative disorder) including one major criterion (sclerotic bone lesion, Castleman disease or vascular endothelial growth factor [VEGF] elevation) and one minor criterion (organomegaly, edema, endocrinopathy, skin changes, papilledema or thrombocytosis) are required to confirm the diagnosis [1,3]. FDG PET/CT is useful in detecting and selecting bone lesions and lymph nodes in patients with suspected POEMS syndrome [13]. Though FDG PET/CT also can find multiple lymph node enlargements/organomegaly in IgG4 related disease, their metabolism levels are usually normal. It is worth to mention that bone lesions have not been reported in IgG4 related disease cases. In the case of this paper, this POEMS patient had extensive osteosclerotic lesions observed by PET/CT.
A comprehensive diagnostic criteria of IgG4 related disease has been published, however, the key histopathological features are: non-clonotypic lymphoplasmacytic infiltrate, storiform fibrosis, tumefactive lesions, increased IgG4/IgG staining of plasma cells and obliterative phlebitis [14-19]. In this case, the patient had high serum IgG4 level and IgG4 plasmacyte tissue infiltration, while her plasmacytes were monoclonal, an observation which was confirmed by bone marrow immunohistochemical staining as well as immunofixation electrophoresis. As a result, this female patient was diagnosed with POEMS syndrome but not an IgG4 related disease.
The reason why this patient was considered to have suffered from a special subtype of POEMS syndrome is because she indeed had high serum IgG4 concentration as well as monoclonal IgG4 plasmacyte tissue infiltration, which had never been reported before in patients suffering from POEMS syndrome. Therefore it is not clear whether IgG4-positive plasma cell tissue infiltration and elevated serum IgG4 concentrations are origins or outcomes of IgG4 related diseases. This case indeed helped solving this confusion. It is reasonable to speculate that these phenomena are not the origins but the outcomes in the course of illnesses since these phenomena could also be observed in other diseases such as POEMS syndrome and multicentric Castleman disease [20,21].
IgG4 related disease is a benign course. Glucocorticoid and immunosuppressive agents are usually available to treat it. However, due to an underlying plasma cell neoplasm, POEMS syndrome is a paraneoplastic syndrome. During the treatment, corticosteroids are palliative while alkylators are the main form of treatment, which can be used in low dose conventional therapy as well as a high dose with stem cell transplantation [22]. Lenalidomide shows promise with controllable toxicity [23]. Thalidomide and bortezomib also take effect. But they should be weighed carefully regarding their potential benefit and risk since they may aggravate the peripheral neuropathy [24]. The benefit of anti-VEGF antibodies is controversial [25,26]. For this patient, we tried to use an MPT regimen for treatment. From the present results, this regimen appears effective as it can clearly alleviate symptoms. Her serum IgG4 concentration could significantly decrease and her monoclonal immunoglobulin level could also be reduced, at least to some degree. However, the side effects associated with this regimen, including possible infection, would hinder the sustained treatment.
In conclusion, as a new subtype of POEMS syndrome, POEMS syndrome-IgG4 subtype is still not well investigated. Following up with further research and collecting more cases are both important. It is also advised that every suspected POEMS patients should be tested for their serum IgG4 concentration to promptly recognize any abnormalities. In addition, the best choice of treatment this disease is to find a novel therapy to target the plasma cells directly.
Consent
Written informed consent was obtained from the patient for publication of this case study and any accompanying images related. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Acknowledgements
This study was supported by grants from China scholarship council (No. 201306165030) and the National Natural Science Foundation of China (81300387 and 81301053).
Disclosure of conflict of interest
None.
References
- 1.Dispenzieri A. POEMS syndrome: 2014 Update on diagnosis, risk-stratification,and management. Am J Hematol. 2014;89:213–216. doi: 10.1002/ajh.23644. [DOI] [PubMed] [Google Scholar]
- 2.Li J, Zhou DB, Huang Z, Jiao L, Duan MH, Zhang W, Zhao YQ, Shen T. Clinical characteristics and long-term outcome of patients with POEMS syndrome in China. Ann Hematol. 2011;90:819–826. doi: 10.1007/s00277-010-1149-0. [DOI] [PubMed] [Google Scholar]
- 3.Dispenzieri A. How I treat POEMS syndrome. Blood. 2012;119:5650–5658. doi: 10.1182/blood-2012-03-378992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi XF, Hu SD, Li JM, Luo XF, Long ZB, Zhu Y, Xi XD. Multimodal imaging and clinical characteristics of bone lesions in POEMS syndrome. Int J Clin Exp Med. 2015;8:7467–7476. [PMC free article] [PubMed] [Google Scholar]
- 5.Dispenzieri A. POEMS syndrome. Blood. 2007;21:285–299. doi: 10.1016/j.blre.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Latov N. Diagnosis and treatment of chronic acquired demyelinating polyneuropathie. Nat Rev Neurol. 2014;10:435–436. doi: 10.1038/nrneurol.2014.117. [DOI] [PubMed] [Google Scholar]
- 7.Dispenzieri A, Kyle RA, Lacy MQ, Rajkumar SV, Therneau TM, Larson DR, Greipp PR, Witzig TE, Basu R, Suarez GA, Fonseca R, Lust JA, Gertz MA. POEMS syndrome: definitions and longterm outcome. Blood. 2003;101:2496–2506. doi: 10.1182/blood-2002-07-2299. [DOI] [PubMed] [Google Scholar]
- 8.Masaki Y, Shimizu H, Sato Nakamura T, Nakamura T, Nakajima A, Iwao Kawanami H, Miki M, Sakai T, Kawanami T, Fujita Y, Tanaka M, Fukushima T. IgG4-related disease: diagnostic methods and therapeutic strategies in Japan. J Clin Exp Hematop. 2014;54:95–101. doi: 10.3960/jslrt.54.95. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto M, Hashimoto M, Takahashi H, Shinomura Y. IgG4 disease. J Neuroophthalmol. 2014;34:393–399. doi: 10.1097/WNO.0000000000000172. [DOI] [PubMed] [Google Scholar]
- 10.Nakazawa T, Naitoh I, Hayashi K, Miyabe K, Simizu S, Joh T. Diagnosis of IgG4-related sclerosing cholangitis. World J Gastroenterol. 2013;19:7661–7670. doi: 10.3748/wjg.v19.i43.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L, Lewis JT, Abraham SC, Smyrk TC, Leung S, Chari ST, Poterucha JJ, Rosen CB, Lohse CM, Katzmann JA, Wu TT. IgG4+ plasma cell infiltrates in liver explants with primary sclerosing cholangitis. Am J Surg Pathol. 2010;34:88–94. doi: 10.1097/PAS.0b013e3181c6c09a. [DOI] [PubMed] [Google Scholar]
- 12.Stone JH, Khosroshahi A, Deshpande V, Chan JK, Heathcote JG, Aalberse R, Azumi A, Bloch DB, Brugge WR, Carruthers MN, Cheuk W, Cornell L, Castillo CF, Ferry JA, Forcione D, Klöppel G, Hamilos DL, Kamisawa T, Kasashima S, Kawa S, Kawano M, Masaki Y, Notohara K, Okazaki K, Ryu JK, Saeki T, Sahani D, Sato Y, Smyrk T, Stone JR, Takahira M, Umehara H, Webster G, Yamamoto M, Yi E, Yoshino T, Zamboni G, Zen Y, Chari S. Recommendations for the nomenclature of IgG4-related disease and its individual organ system manifestations. Arthritis Rheum. 2012;64:3061–3067. doi: 10.1002/art.34593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alberti MA, Martinez-Yelamos S, Fernandez A, Vidaller A, Narváez JA, Cano LM, Gamez C, Martinez-Matos JA. 18F-FDG PET/CT in the evaluation of POMES syndrome. Eur J Radiol. 2010;76:180–182. doi: 10.1016/j.ejrad.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Masaki Y, Kurose N, Umehara H. IgG4-related disease: a novel lymphoproliferative disorder discovered and established in Japan in the 21st century. J Clin Exp Hematop. 2011;51:13–20. doi: 10.3960/jslrt.51.13. [DOI] [PubMed] [Google Scholar]
- 15.Grimm KE, Barry TS, Chizhevsky V, Hii A, Weiss LM, Siddiqi IN, Brynes RK, O’Malley DP. Histopathological findings in 29 lymph node biopsies with increased IgG4 plasma cells. Mod Pathol. 2012;25:480–491. doi: 10.1038/modpathol.2011.177. [DOI] [PubMed] [Google Scholar]
- 16.Masaki Y, Dong L, Kurose N, Kitagawa K, Morikawa Y, Yamamoto M, Takahashi H, Shinomura Y, Imai K, Saeki T, Azumi A, Nakada S, Sugiyama E, Matsui S, Origuchi T, Nishiyama S, Nishimori I, Nojima T, Yamada K, Kawano M, Zen Y, Kaneko M, Miyazaki K, Tsubota K, Eguchi K, Tomoda K, Sawaki T, Kawanami T, Tanaka M, Fukushima T, Sugai S, Umehara H. Proposal for a new clinical entity, IgG4-positive multiorgan lymphoproliferative syndrome: analysis of 64 cases of IgG4-related disorders. Ann Rheum Dis. 2009;68:1310–1315. doi: 10.1136/ard.2008.089169. [DOI] [PubMed] [Google Scholar]
- 17.Kawamura E, Hisano S, Nakashima H, Takeshita M, Saito T. Immunohistological analysis for immunological response and mechanism of interstitial fibrosis in IgG4 related kidney disease. Mod Rheumatol. 2015;25:571–578. doi: 10.3109/14397595.2014.1001474. [DOI] [PubMed] [Google Scholar]
- 18.Fox RI, Fox CM. IgG4 levels and plasmablasts as a marker for IgG4-related disease (IgG4-RD) Ann Rheum Dis. 2015;74:1–3. doi: 10.1136/annrheumdis-2014-205476. [DOI] [PubMed] [Google Scholar]
- 19.Wallace ZS, Stone JH. An update on IgG4-related disease. Curr Opin Rheumatol. 2015;27:83–90. doi: 10.1097/BOR.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 20.Sato Y, Kojima M, Takata K, Morito T, Mizobuchi K, Tanaka T, Inoue D, Shiomi H, Iwao H, Yoshino T. Multicentric Castleman’s disease with abundant IgG4-positive cells: a clinical and pathological analysis of six cases. J Clin Pathol. 2010;63:1084–1089. doi: 10.1136/jcp.2010.082958. [DOI] [PubMed] [Google Scholar]
- 21.Izumi Y, Takeshita H, Moriwaki Y, Hisatomi K, Matsuda M, Yamashita N, Kawahara C, Shigemitsu Y, Iwanaga N, Kawakami A, Kurohama H, Niino D, Ito M, Migita K. Multicentric Castleman disease mimicking IgG4-related disease: A case report. Mod Rheumatol. 2014;22:1–4. doi: 10.3109/14397595.2014.985356. [DOI] [PubMed] [Google Scholar]
- 22.Jang IY, Yoon DH, Kim S, Lee K, Kim KK, Lim YM, Min WK, Suh C. Advanced POEMS syndrome treated with high-dose melphalan followed by autologous blood stem cell transplantation: a single-center experience. Blood Res. 2014;49:42–48. doi: 10.5045/br.2014.49.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lestang E, Caristan A, Néel A, Graveleau J, Duhamel E, Masseau A, Connault J, Maisonneuve H, Le Gouill S, Blin N, Touzeau C, Moreau P, Hamidou M. Lenalidomide as frontline therapy in polyneuropathy, organomegaly, endocrinopathy, monoclonal protein and skin changes syndrome: a retrospective case series of eight patients. Leuk Lymphoma. 2015;56:1895–1896. doi: 10.3109/10428194.2014.974595. [DOI] [PubMed] [Google Scholar]
- 24.Kawano Y, Nakama T, Hata H, Kimura E, Maruyoshi N, Uchino M, Mitsuya H. Sueeessful treatment with rituximab and thalidomide of POEMS syndrome associated with Waldenstrom maeroglobulinemia. J Neurol Sci. 2010;297:101–104. doi: 10.1016/j.jns.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 25.Briani C, Torre CD, Lessi F, Cavallaro T, Scarlato M, Ferrari S, Campagnolo M, Lucchetta M, Cabrini I, Morbin M, Lauria G, Adami F, Manfredi AA. Pentraxin-3 and VEGF in POEMS syndrome: A 2-year longitudinal study. J Neuroimmunol. 2014;277:189–192. doi: 10.1016/j.jneuroim.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 26.Searlato M, Previtali SC, Carpo M, Pareyson D, Briani C, Del Bo R, Nobile-Orazio E, Quattrini A, Comi GP. Polyneuropathy in POEMS syndrome: role of angiogenic factors in the pathogenesis. Brain. 2005;128:1911–1920. doi: 10.1093/brain/awh519. [DOI] [PubMed] [Google Scholar]





