Abstract
A considerable number of studies have demonstrated that cytomegalovirus (CMV) reactivation after allogeneic hematopoietic stem cell transplantation (Allo-HSCT) could enforce graft-versus leukemia (GVL) effect in acute myeloid leukemia (AML) patients. However, the use of antithymocyte globulin (ATG) as part of graft-versus-host disease (GVHD) prophylaxis may dampen this beneficial effect of CMV replication. In this context, we retrospectively analyzed the effect of CMV reactivation on relapse, survival and prognosis in a total of 227 AML patients who received a myeloablative (MA) conditioning regimen at a single research center between January 2010 and April 2013. Of these 227 patients, 110 cases received non-ATG-containing regimens and 117 cases received ATG-containing regimens. CMV reactivation occurred in 45 patients (41%) among non-ATG regimen group and 73 patients (62%) among ATG regimen group (P = 0.001). At a median time to follow-up of 27.5 months, a lower risk of cumulative relapse incidence associated with CMV reactivation was observed in non-ATG group in multivariate analyses (OR 0.28, 95% CI 0.10-0.79; P = 0.016). However, CMV reactivation after transplantation did not significantly decrease the cumulative incidence of relapse in our ATG group (OR 0.28, 95% CI 0.10-0.79; P = 0.016). Collectively, our results demonstrate that in AML patients following sibling HSCT, the CMV-induced beneficial effect on relapse occurs only in the MA regimens containing no ATG, although ATG promotes CMV reactivation.
Keywords: Acute myeloid leukemia, hematopoietic stem cell transplantation, CMV reactivation, relapse, antithymocyte globulin, myeloablative regimen, antileukemic effect
Introduction
Recently, several studies demonstrated that a potential reduction in relapse risk of acute myeloid leukemia (AML) patients could benefit from cytomegalovirus (CMV) reactivation after allogeneic hematopoietic stem cell transplantation (allo-HSCT) [1-5]. This beneficial effect of CMV replication could be affected by the intensity of the conditioning regimen [6], and the use of antithymocyte globulin (ATG) [7] or alemtuzumab [8] as the prophylaxis of graft-versus-host disease (GVHD). The graft-versus leukemia (GVL) effect induced by CMV reactivation may due to a subsequent natural killer (NK) cell or T-cell immune response to CMV reactivation after transplantation [2,9-11]. Based on these results, patients with AML who underwent myeloablative (MA) conditioning regimens might significantly benefit from this GVL effect induced by CMV reactivation [6]. At present, very few studies have reported that the subsequent influence of ATG on this CMV-induced antileukemic effect. Here we retrospectively analyzed the impact of ATG-containing MA regimens on the CMV-induced GVL effect in over 200 AML patients after allo-HSCT.
Material and methods
Study population
A retrospective study was conducted in the First Affiliated Hospital of Soochow University in China, including a serial group of 227 patients with AML undergoing HSCT between January 2010 and April, 2013. The eligibility criteria for all patients with AML was selected as follows: (1) all transplant recipients were diagnosed as de novo AML before HSCT, except for acute promyelocytic leukemia (APL); (2) transplant recipients with a CMV seropositive status pre-transplantation were conformed to the inclusion criteria; (3) peripheral blood stem cells (PBSC), bone marrow (BM) or mix graft (MG) (PBSC plus BM) was obtained as hematopoietic stem cells; (4) patients with regular monitoring of CMV reactivation after transplantation; (5) except for transplant recipients after secondary transplantation; and (6) patients used prophylactic donor lymphocyte infusion after HSCT before disease relapse were also excluded from this study.
One hundred and ten patients received transplantation from HLA-identical sibling HSCT (Sib-HSCT), while 60 cases underwent related HLA-haploidentical HSCT (Haplo-HSCT) and 57 cases underwent unrelated HSCT (URD-HSCT). All patients received a myeloablative (MA) conditioning regimen consisting of busulfan (BU) plus cyclophosphamide (CY), whereas total body irradiation (TBI) plus CY was chosen as a substitute in cases with extramedullary leukemic infiltration pre-transplantation. Prophylaxis of GVHD consisted of a combination of cyclosporine (CSA) and short-term methotrexate (MTX) in all transplant recipients. ATG at a dose of 2.5 mg/kg daily from day -6 to -3 and mycophenolate mofetil (MMF) were performed as were performed as extra GVHD prophylaxis in patients underwent Haplo-HSCT or URD-HSCT. The characteristics of all patients are summarized in Table 1.
Table 1.
Transplant recipients’ characteristics classified by CMV reactivation
| Variable | ALL patients | CMV reactivation | P value | |
|---|---|---|---|---|
|
| ||||
| No | Yes | |||
| No. of patients | 227 | 109 | 118 | |
| Male patients | 118 (52%) | 54 (46%) | 64 (54%) | 0.479 |
| Median age, years (range) | 35 (2-63) | 37 (2-59) | 32.5 (3-63) | 0.034 |
| Risk stratification | 0.803 | |||
| Favorable | 28 (12%) | 14 (50%) | 14 (50%) | |
| Intermediate | 128 (56%) | 59 (46%) | 69 (54%) | |
| Adverse | 71 (31%) | 36 (51%) | 35 (49%) | |
| Disease phase at HSCT | 0.931 | |||
| CR1 | 166 (73%) | 80 (48%) | 86 (52%) | |
| ≥ CR2 or active | 61 (27%) | 29 (48%) | 32 (52%) | |
| WBC | 0.923 | |||
| < 100000/MCL | 188 (83%) | 90 (48%) | 98 (52%) | |
| ≥ 100000/MCL | 39 (17%) | 19 (49%) | 20 (51%) | |
| Donor status | 0.004 | |||
| Sib | 110 (49%) | 65 (59%) | 45 (41%) | |
| URD | 57 (25%) | 23 (40%) | 34 (60%) | |
| Haplo | 60 (26%) | 21 (35%) | 39 (65%) | |
| Stem cell source | 0.255 | |||
| BM | 50 (22%) | 20 (40%) | 30 (60%) | |
| PBSC | 125 (55%) | 66 (53%) | 59 (47%) | |
| PBSC+BM | 52 (23%) | 23 (44%) | 29 (56%) | |
| Conditioning regimen | 0.723 | |||
| BU/CY | 211 (93%) | 102 (48%) | 109 (52%) | |
| TBI/CY | 16 (7%) | 7 (44%) | 9 (56%) | |
| ATG regimen | 0.001 | |||
| Yes | 117 (52%) | 44 (38%) | 73 (62%) | |
| No | 110 (48%) | 65 (59%) | 45 (41%) | |
| Acute GVHD | 0.039 | |||
| Grade: 0-1 | 134 (59%) | 72 (54%) | 62 (46%) | |
| Grade: 2-4 | 93 (41%) | 37 (40%) | 56 (60%) | |
| Chronic GVHD | 0.673 | |||
| Present | 122 (54%) | 57 (47%) | 65 (53%) | |
| Absent | 105 (46%) | 52 (50%) | 53 (50%) | |
CMV: cytomegalovirus; HSCT: hematopoietic stem cell transplantation; CR: complete remission; WBC: whole blood count; Sib: HLA-identical sibling; Haplo: related haploidentical; URD: unrelated donor; BM: bone marrow; PBSC: peripheral blood stem cell; BU: busulfan; CY: cyclophosphamide; TBI: total body irradiation; ATG: antithymocyte globulin; GVHD: graft-versus-host disease.
Diagnostic method and standard of CMV reactivation
Quantitative real-time PCR test was chosen as the method for detection of CMV reactivation, and the Diagnostic Kit for Quantification of Human CMV DNA (DaAn Gene co. Ltd of Sun Yat-sen University in Guangzhou, China) was performed as the diagnostic method. CMV reactivation was defined as any two consecutive qPCR tests of blood plasma samples with a CMV viral load of ≥ 100 copies/mL.
Management of CMV reactivation after HSCT
The results of CMV assays in all transplant recipients and donors were negative before transplantation. CMV reactivation evaluation was monitored weekly or biweekly during the first 100 days after transplant, monthly up to 1 year, and on conventional monitoring after the first year. All transplant recipients underwent a standard induction course of ganciclovir (5 mg/kg, twice daily; from day -9 to -2) pre-transplantation, and received acyclovir as prophylactic antiviral therapy from day -1 to 1 year after transplantation. Pre-emptive therapy for CMV reactivation was based on either ganciclovir or foscarnet due to severe cytopenia. After two weeks of induction antiviral therapy, maintenance treatment was followed until two sequential surveillances were negative.
Variable definitions
The risk stratification of AML before first induction therapy was classified both by cytogenetics and molecular abnormality, using the conventional distinction Standard [12]. The assessment of acute GVHD (aGVHD) and chronic GVHD (cGVHD) was performed according to the commonly accepted categories [13,14]. Bone marrow examination was executed twice a month during 100 days post-HSCT and once every 3 months thereafter, or additional reexamination if necessary. Disease relapse was defined using the conventional criteria [15], while appearance of morphologically positive blasts in the cerebrospinal fluid was defined as extramedullary relapse. Non-relapse mortality (NRM) was defined as death from all causes except for relapse. Overall survival (OS) was defined as the time from transplantation to death or to the last observation.
Statistical analysis
The chi-square test or Fisher exact test was used to compare categorical variables of patients, while the t test was used for continuous ones. The Gray test [16] was implemented to estimate the cumulative incidence of relapse (CIR) in patients classified by CMV reactivation for statistical difference, comparing the event of relapse with a competing event (NRM). Fine and Gray competing risk regression model [17] was performed as multivariate analysis. The control variables for multivariate analysis were classified as follows: risk stratification (adverse versus intermediate versus favorable), disease phase pre-transplantation (≥ CR2 or active versus CR1), white blood count (WBC) before first induced chemotherapy (≥ 100000/MCL versus < 100000/MCL), degrees of aGVHD (grade 2-4 versus grade 0-1), occurrence of cGVHD (present versus absent), and CMV reactivation (yes versus no).
Kaplan Meier method was used to estimate OS, comparing the two groups by the log-rank test. Cox proportional hazards model and the Wald test were also used in analyzing the risk factors of OS and calculating 95% confidence interval (CI); risk stratification, the disease phase, initial WBC count, the occurrence of aGVHD/cGVHD, and the CMV reactivation status were treated as time-dependent variables. All tests were two-sided and the p values were at the routine 5% significance level. Data were analyzed by R 3.2.1 package cmprsk (The R Foundation for Statistical Computing, Vienna-A; www.R-project.org).
Results
CMV reactivated more frequently in ATG group than that in non-ATG group
Table 1 shows the clinical characteristics of 227 AML patients with or without CMV reactivation after allo-HSCT; with a median age at transplantation of 35 years old (range 2-63). The median follow-up time for all patients was 27.5 months (range 0-65) through April 30th 2015. A total of 118 patients (52%) experienced CMV reactivation after a median time of 54 days (range 5-257), and 92% of which (109/118) developed CMV reactivation during the first 100 days after HSCT. Respectively, the median number copies of CMV-DNA in non-ATG group were 1910 copies/mL (range 190-31000), compared with 1910 copies/mL (range 129-117000) in ATG group (Figure 1, P = 0.718).
Figure 1.
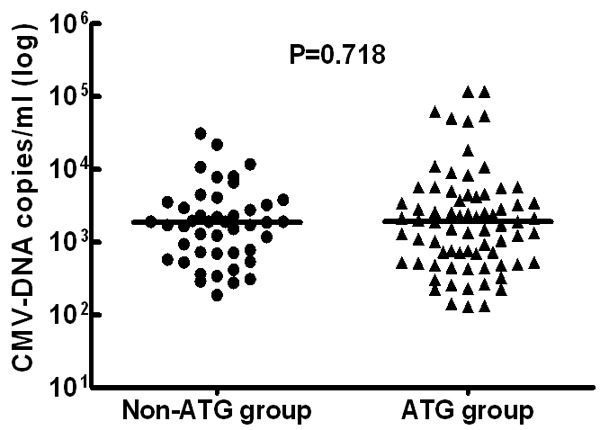
The initial number copies of CMV-DNA in patients without and with use of ATG, and t test was performed to examine the differences between these two groups.
Univariate analysis showed that donor status was the vital determinant of CMV reactivation: 41% in Sib-HSCT, 60% in URD-HSCT, and 65% in Halpo-HSCT (P = 0.004). Furthermore, CMV reactivated more frequently in the ATG group after Haplo- or URD-HSCT. CMV reactivation happened in 45 of 110 (41%) patients among non-ATG regimens group from Sib-HSCT, compared with 73 of 117 (62%) patients in ATG-containing regimens group (P = 0.001). Similarly, median ages and the presence of aGVHD grade ≥ 2 had an important impact on CMV reactivation. In addition, there was also no statistical difference in classification by gender, risk stratification, disease phase, WBC count, stem cell source, conditioning regimen, or the occurrence of cGVHD.
CMV reactivation led to a lower relapse rate in non-ATG group
Among the 227 patients, 58 cases (26%) had relapsed by the end of follow-up period. The median time to relapse was 149 days (range 19 to 892). Respectively, the CIR was 21.1%, 25.7%, and 29.7% in cases without CMV reactivation, compared with 16.1%, 22.0%, and 22.0% for those with CMV reactivation at 1 year, 2 years, and 3 years post-transplantation (Figure 2). Although there is no statistically significant difference (P = 0.237), a trend towards a lower relapse rate could still be seen in patients with CMV reactivation. Meanwhile, 10 patients with CMV reactivation relapsed during the first 100 days after HSCT, of which 3 cases relapsed before CMV replication. When considering the multivariate analyses, no difference was shown in the CIR stratified by CMV reactivation (OR 0.78, 97.5% CI 0.46-1.31; P = 0.340). The occurrence of cGVHD (P < 0.001) and an adverse phase at transplantation (≥ CR2/active) (P < 0.001) were only two prognostic factors of disease relapse (Table 2).
Figure 2.
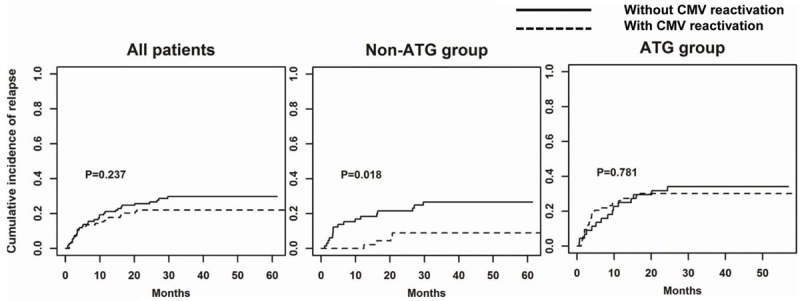
Cumulative incidence of relapse classified by CMV reactivation in all patients, Non-ATG group, and ATG group. The Gray test was performed to examine the differences between patients with and without CMV reactivation.
Table 2.
Multivariate analysis of cumulative incidence of relapse and OS
| CIR | OS | |||
|---|---|---|---|---|
|
|
||||
| P value+ | OR+ (95% CI) | P value* | OR* (95% CI) | |
| All patients | ||||
| Risk stratification | ||||
| Favorable | 1.0┼ | 1.0┼ | ||
| Intermediate | 0.620 | 1.36 (0.40-4.62) | 0.182 | 1.67 (0.79-3.53) |
| Adverse | 0.091 | 2.85 (0.85-9.61) | 0.025 | 2.41 (1.12-5.18) |
| Disease phase: ≥ CR2 or active vs CR1 | < 0.001 | 4.32 (2.53-7.40) | < 0.001 | 4.00 (2.66-6.03) |
| WBC: ≥ 100000/MCL vs < 100000/MCL | 0.940 | 0.97 (0.48-1.97) | 0.710 | 0.90 (0.53-1.55) |
| Acute GVHD: Grade: 2-4 vs 0-1 | 0.950 | 1.02 (0.60-1.73) | 0.002 | 1.86 (1.25-2.77) |
| Chronic GVHD: Present vs Absent | < 0.001 | 0.21 (0.12-0.39) | 0.002 | 0.53 (0.35-0.79) |
| CMV: yes vs no | 0.340 | 0.78 (0.46-1.31) | 0.012 | 0.60 (0.40-0.89) |
| Patients in non-ATG group | ||||
| Risk stratification | ||||
| Favorable | 1.0┼ | 1.0┼ | ||
| Intermediate | 0.420 | 2.43 (0.28-20.84) | 0.121 | 2.36 (0.80-6.95) |
| Adverse | 0.054 | 8.07 (0.97-67.47) | 0.037 | 3.34 (1.07-10.37) |
| Disease phase: ≥ CR2 or active vs CR1 | 0.046 | 2.62 (1.02-6.78) | < 0.001 | 4.34 (2.08-9.05) |
| WBC: ≥ 100000/MCL vs < 100000/MCL | 0.810 | 1.16 (0.34-3.99) | 0.380 | 0.62 (0.22-1.80) |
| Acute GVHD: Grade: 2-4 vs 0-1 | 0.840 | 0.90 (0.32-2.53) | 0.037 | 1.99 (1.04-3.81) |
| Chronic GVHD: Present vs Absent | 0.008 | 0.27 (0.10-0.71) | 0.325 | 0.72 (0.38-1.38) |
| CMV: yes vs no | 0.016 | 0.28 (0.10-0.79) | 0.005 | 0.37 (0.18-0.74) |
| Patients in ATG group | ||||
| Risk stratification | ||||
| Favorable | 1.0┼ | 1.0┼ | ||
| Intermediate | 0.780 | 0.82 (0.20-3.39) | 0.923 | 1.05 (0.36-3.05) |
| Adverse | 0.740 | 1.28 (0.31-5.29) | 0.428 | 1.54 (0.53-4.47) |
| Disease phase: ≥ CR2 or active vs CR1 | < 0.001 | 5.26 (2.64-10.47) | <0.001 | 3.53 (2.08-5.98) |
| WBC: ≥ 100000/MCL vs < 100000/MCL | 0.690 | 0.82 (0.31-2.17) | 0.836 | 1.07 (0.56-2.05) |
| Acute GVHD: Grade: 2-4 vs 0-1 | 0.970 | 1.01 (0.52-1.97) | 0.033 | 1.76 (1.05-2.96) |
| Chronic GVHD: Present vs Absent | < 0.001 | 0.19 (0.08-0.41) | 0.003 | 0.45 (0.26-0.76) |
| CMV: yes vs no | 0.840 | 1.07 (0.55-2.07) | 0.220 | 0.72 (0.42-1.22) |
CIR: cumulative incidence of relapse; OS: overall survival;
Reference category.
Multivariate Fine and Gray competing risk regression model.
Multivariate Cox proportional hazards model.
Based on conditioning regimens with or without ATG, we divided all patients into 2 groups. In the subgroup analysis, differences in relapse in conjunction with CMV reactivation status were evaluated in non-ATG and ATG groups. In non-ATG cohort from Sib-HSCT, 21 of 110 cases (19.1%) relapsed at a median time of 297 days (range 29-892) after transplantation. Respectively, the CIR was 0%, 8.9%, and 8.9% in cases with CMV reactivation, compared with 18.5%, 21.5%, and 26.7% for those without CMV reactivation at 1 year, 2 years, and 3 years post-transplantation (Figure 2). After controlling for other variables in our multivariate analyses in the non-ATG group (Table 2), the occurrence of CMV reactivation was still listed as a favorable independent factor correlating with decreased relapse incidence (OR 0.28, 97.5% CI 0.10-0.79; P = 0.016). Applying the same analysis to all patients, disease phase (≥ CR2/active) (P = 0.046) and cGVHD (P = 0.008) were other two crucial prognostic factors predictive of disease relapse.
In the ATG group, 37 of 117 (31.6%) patients relapsed after a median time of 120 days (range 19-731) post-HSCT. Other than these findings in our non-ATG group, both the univariate and multivariate analyses showed that the effect of CMV reactivation on disease relapse was statistically meaningless in the ATG group. The CIR was 26.0%, 30.1%, and 30.1% in cases with CMV reactivation, compared with 25.0%, 31.8%, and 34.1% for those without CMV reactivation at 1 year, 2 years, and 3 years after HSCT (Figure 2). Consistent with our analysis of the group containing all patients we found that within the non-ATG group, cGVHD (P < 0.001) and disease phase (≥ CR2/active) (P < 0.001) greatly influenced the cumulative incidence of relapse in multivariate analyses (Table 2).
CMV reactivation with a better OS but not affecting on non-relapse mortality in non-ATG group
At the end of the follow-up period, 125 cases are still alive and 102 died [(51 died of disease progression and 51 due to non-relapse mortality (NRM)]. The estimate of OS rate in all patients was 70.9%, 59.9%, and 56.3% at 1 year, 2 years, and 3 years, respectively.
In NRM rate analysis with regard to CMV reactivation status, no statistical significance was observed in groups containing all patients, the non-ATG group, or the ATG group (Figure 3). However, a higher estimated OS rate with CMV replication was observed in the non-ATG group but not in all patients or the ATG group (Figure 4). Respectively, the estimated OS rate stratified by CMV reactivation was 77.8% and 56.0% at 3 years after transplantation in the non-ATG group (P = 0.050). Multivariate analysis (Table 2) illuminated that CMV reactivation was still a good prognostic factor for better OS in the non-ATG group (OR 0.37, 95% CI 0.18-0.74; P = 0.005). In the ATG group, by contrast, the analysis of OS had no significant relationship with CMV reactivation. Both in the group containing all patients or subgroups thereof, OS was greatly worsened in patients who developed aGVHD grade ≥ 2, and who underwent transplantation at an adverse phase of disease (CR ≥ 2 or active). In contrast with the non-ATG group, the presence of cGVHD rather than CMV reactivation was a good prognostic factor for better OS in the ATG group (OR 0.45, 95% CI 0.26-0.76; P = 0.003).
Figure 3.
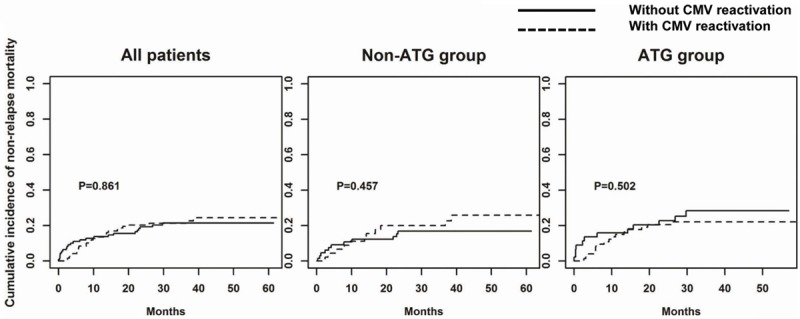
Cumulative incidence of non-relapse mortality (NRM) classified by CMV reactivation in all patients, Non-ATG group, and ATG group. The Gray test was performed to examine the differences between patients with and without CMV reactivation.
Figure 4.
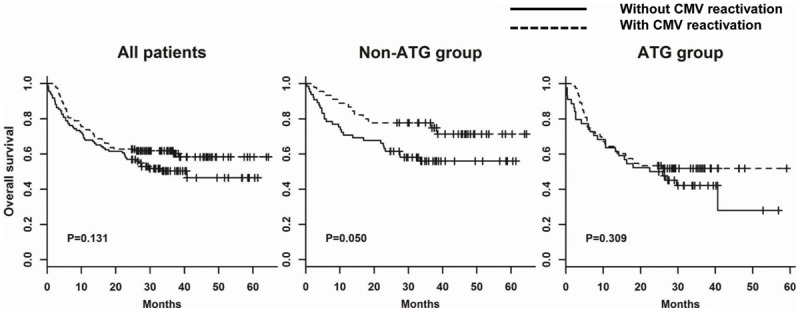
Overall survival (OS) classified by CMV reactivation in all patients, Non-ATG group, and ATG group. A log-rank test was performed to examine the differences between patients with and without CMV reactivation.
Discussion
In recent years, several studies have confirmed the antileukemic effect induced by CMV reactivation in AML patients after allo-HSCT [3-6,18]. However, few systematic and control analysis was performed to evaluate the impact of ATG on this CMV-induced effect. Lately Busca A et al. showed that this CMV-induced profitable effect could be dampened by the use of ATG in vivo [7]. Even so, this moderate effect of ATG in that study seems to be ambiguous currently. Hence, our retrospective study sought to analyze whether the use of ATG regimens might abolish this antileukemic effect. Accordingly, a total of 227 patients with AML who had undergone HSCT were divided into two groups based on whether ATG was used as part of their MA conditioning regimen.
Our study demonstrated a trend towards a lower tendency of relapse incidence associated with CMV reactivation after transplantation in all patients; however, these differences did not achieve statistical significance. By contrast, subgroup analysis showed that CMV reactivation resulted in decreasing cumulative incidence of relapse and a better OS rate in the non-ATG regimen group but not in the ATG group. Similarly, relapse incidence was affected by both the presentation of cGVHD and disease phase at transplantation in all patients, the non-ATG group and the ATG group, while both aGVHD grade ≥ 2 and disease phase (≥ CR2/active) were two poor prognostic factors affecting the final outcome. In the assessment of NRM affected by CMV replication, all analyses in all patient groups showed no statistical significance. Aside from our findings regarding cumulative incidence of relapse and OS analysis in the non-ATG group, our other findings were consistent with those in previous studies.
On the basis of previous studies [19], CMV seropositivity and mismatched donors were the two foremost risk factors of CMV replication. Moreover, severe immunosuppression included ATG after transplantation resulted in more frequent CMV reactivation [20,21]. In our data, ATG-group from haplo- or URD-HSCT had a higher incidence of CMV reactivation (73/110), compared with 45 of 110 patients among non-ATG group from sib-HSCT (62% versus 41%, P = 0.001). Despite this higher incidence of CMV replication in ATG group, all patients from this group have not benefited from the CMV-induced GVL effect yet. Among the non-ATG group, the beneficial effects induced by CMV replication were more apparent, both in univariate and multivariate analyses. However, the mechanism behind antileukemic effects induced by CMV infection after allo-HSCT is not clarified currently. Foley et al. reported that the NKG2C+CD57+ NK cells expanded by CMV replication enhanced the antileukemic effect in vitro [10]. CMV replication after HSCT could raise the expression of leucocyte fixation antigen-3 on AML blasts, resulting in increased NK cell-mediated antileukemic effects [2,9]. Furthermore, Knight A et al. [22] has demonstrated that Vδ2NEG γδT cells participate in the immune response to clear CMV, and these expanded γδT cells have displayed cytolytic activity against tumor cells in vitro [23]. Meanwhile, γδT cells induced by CMV replication that can cross-recognize residual leukemic blasts have been reported recently [11]. When the latent CMV in leukemic blasts becomes reactivated, T cells specific for CMV derived from the donor could also be cytotoxic to these leukemic blasts [2]. All these findings may make the CMV-induced antileukemic effect more persuasive. According to several studies about the effect of ATG on immune reconstruction [21,24], ATG can greatly delay the reconstitution of CD4+ T cells. Nevertheless, ATG seemed to have no obvious immunosuppressive effects on NK cells. And no explicit data could be used to evaluate the impact of ATG on the reconstitution of NK cells, especially the NKG2C+CD57+ NK cells. In this context, we had great difficulty in demarcating the role of ATG in our study. In contrast to the previous study of Manjappa S et al., a few patients (2 out of 206) used ATG as part of the MA conditioning regimen, so it was not surprising that both all patients and cohorts underwent MA regimens in that study have significantly decreased relapse incidence associated with CMV reactivation [6].
Generally speaking in our study, patients not using ATG as a part of GVHD prophylaxis could definitely benefit from the enhanced GVL effect induced by CMV reactivation. CMV reactivation foreboded a lower cumulative incidence of relapse and a better OS in the non-ATG group, while no statistical significance was presented in the ATG group. Thus, the use of ATG in the MA conditioning regimen abrogates this beneficial effect. In addition, the disease phase pre-transplantation has been observed as the consistent significant determinant factor for the final outcome of the group containing all patients. In this study, 43 of 117 (37%) patients with pre-transplant ATG regimen underwent HSCT in an adverse disease phase (≥ CR2/active), compared with 18/110 (16%) of non-ATG group patients (P = 0.001). Further studies are necessary to confirm whether this more adverse disease phase might obscure the CMV-induced beneficial anti-leukemic effects in ATG group patients.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 81370626; 81273266; 81100390), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), National Clinical Key Specialty Development Project and National Public Health Grand Research Foundation (No. 201202017), the Network Hospital of Clinical Medicine Science and Technology Project, Jiangsu Science and Technology Department (No. BL2012005), Jiangsu “333” talent project (BRA2015497).
Disclosure of conflict of interest
None.
References
- 1.Lönnqvist B, Ringdèn O, Ljungman P, Wahren B, Gahrton G. Reduced risk of recurrent leukaemia in bone marrow transplant recipients after cytomegalovirus infection. Br J Haematol. 1986;63:671–679. doi: 10.1111/j.1365-2141.1986.tb07551.x. [DOI] [PubMed] [Google Scholar]
- 2.Barrett AJ. CMV: when bad viruses turn good. Blood. 2011;118:1193–1194. doi: 10.1182/blood-2011-06-354340. [DOI] [PubMed] [Google Scholar]
- 3.Elmaagacli AH, Steckel NK, Koldehoff M, Hegerfeldt Y, Trenschel R, Ditschkowski M, Elmaagacli AH, Steckel NK, Koldehoff M, Hegerfeldt Y, Trenschel R, Ditschkowski M, Christoph S, Gromke T, Kordelas L, Ottinger HD, Ross RS, Horn PA, Schnittger S, Beelen DW. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood. 2011;118:1402–1412. doi: 10.1182/blood-2010-08-304121. [DOI] [PubMed] [Google Scholar]
- 4.Ito S, Pophali P, Co W, Koklanaris EK, Superata J, Fahle GA, Childs R, Battiwalla M, Barrett AJ. CMV reactivation is associated with a lower incidence of relapse after allo-SCT for CML. Bone Marrow Transplant. 2013;48:1–4. doi: 10.1038/bmt.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green ML, Leisenring WM, Xie H, Walter RB, Mielcarek M, Sandmaier BM, Riddell SR, Boeckh M. CMV reactivation after allogeneic HCT and relapse risk: evidence for early protection in acute myeloid leukemia. Blood. 2013;122:1316–1324. doi: 10.1182/blood-2013-02-487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manjappa S, Bhamidipati PK, Stokerl-Goldstein KE, DiPersio JF, Uy GL, Westervelt P, Liu J, Schroeder MA, Vij R, Abboud CN, Fehniger TA, Cashen AF, Pusic I, Jacoby M, Meera SJ, Romee R. Protective effect of cytomegalovirus reactivation on relapse after allogeneic hematopoietic cell transplantation in acute myeloid leukemia patients is influenced by conditioning regimen. Biol Blood Marrow Transplant. 2014;20:46–52. doi: 10.1016/j.bbmt.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busca A, Passera R, Pini M, Zallio F, Dellacasa C, Audisio E, Giaccone L, Maffini E, Costa C, Cavallo R, Bruno B. The use of ATG abrogates the antileukemic effect of cytomegalovirus reactivation in patients with acute myeloid leukemia receiving grafts from unrelated donors. Am J Hematol. 2015;90:E117–21. doi: 10.1002/ajh.23998. [DOI] [PubMed] [Google Scholar]
- 8.Thomson KJ, Mackinnon S, Peggs KS. CMVspecific cellular therapy for acute myeloid leukemia? Blood. 2012;119:1088–1090. doi: 10.1182/blood-2011-10-383943. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher JM, Prentice HG, Grundy JE. Natural killer cell lysis of cytomegalovirus (CMV)-infected cells correlates with virally induced changes in cell surface lymphocyte functionassociated antigen-3 (LFA-3) expression and not with the CMV-induced down-regulation of cell surface class I HLA. J Immunol. 1998;161:2365–2374. [PubMed] [Google Scholar]
- 10.Foley B, Cooley S, Verneris MR, Pitt M, Curtsinger J, Luo X, Lopez-Verges S, Lanier LL, Weisdorf D, Miller JS. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2012;119:2665–2674. doi: 10.1182/blood-2011-10-386995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scheper W, van Dorp S, Kersting S, Pietersma F, Lindemans C, Hol S, Heijhuurs S, Sebestyen Z, Gründer C, Marcu-Malina V, Marchant A, Donner C, Plachter B, Vermijlen D, van Baarle D, Kuball J. γδT cells elicited by CMV reactivation after allo-SCT cross-recognize CMV and leukemia. Leukemia. 2013;27:1328–1338. doi: 10.1038/leu.2012.374. [DOI] [PubMed] [Google Scholar]
- 12.O’Donnell MR, Abboud CN, Altman J, Appelbaum FR, Arber DA, Attar E, Borate U, Coutre SE, Damon LE, Goorha S, Lancet J, Maness LJ, Marcucci G, Millenson MM, Moore JO, Ravandi F, Shami PJ, Smith BD, Stone RM, Strickland SA, Tallman MS, Wang ES, Naganuma M, Gregory KM. Acute myeloid leukemia. J Natl Compr Canc Netw. 2012;10:984–1021. doi: 10.6004/jnccn.2012.0103. [DOI] [PubMed] [Google Scholar]
- 13.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED. 1994 consensus conference on acute gvhd grading. Bone Marrow Transplant. 1995;15:825. [PubMed] [Google Scholar]
- 14.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, Palmer J, Weisdorf D, Treister NS, Cheng GS, Kerr H, Stratton P, Duarte RF, McDonald GB, Inamoto Y, Vigorito A, Arai S, Datiles MB, Jacobsohn D, Heller T, Kitko CL, Mitchell SA, Martin PJ, Shulman H, Wu RS, Cutler CS, Vogelsang GB, Lee SJ, Pavletic SZ, Flowers ME. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versushost disease. I. The 2014 Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant. 2015;21:389–401. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA, Lo-Coco F, Willemze R, Biondi A, Hiddemann W, Larson RA, Löwenberg B, Sanz MA, Head DR, Ohno R, Bloomfield CD International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J. Clin. Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 16.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 17.Fine JP, Gray RJ. A proportional hazards model for the sub-distribution of a competing risk. J Am Statist Assoc. 1999;94:496–509. [Google Scholar]
- 18.Jang JE, Kim SJ, Cheong JW, Hyun SY, Kim YD, Kim YR, Kim JS, Min YH. Early CMV replication and subsequent chronic GVHD have a significant anti-leukemic effect after allogeneic HSCT in acute myeloid leukemia. Ann Hematol. 2015;94:275–282. doi: 10.1007/s00277-014-2190-1. [DOI] [PubMed] [Google Scholar]
- 19.Boeckh M, Ljungman P. How we treat cytomegalovirus in hematopoietic cell transplant recipients. Blood. 2009;113:5711–5719. doi: 10.1182/blood-2008-10-143560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chevallier P, Hebia-Fellah I, Planche L, Guillaume T, Bressolette-Bodin C, Coste-Burel M, Rialland F, Mohty M, Imbert-Marcille BM. Human herpes virus 6 infection is a hallmark of cord blood transplant in adults and may participate to delayed engraftment: a comparison with matched unrelated donors as stem cell source. Bone Marrow Transplant. 2010;45:1204–1211. doi: 10.1038/bmt.2009.326. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt-Hieber M, Schwarck S, Stroux A, Ganepola S, Reinke P, Thiel E, Uharek L, Blau IW. Immune reconstitution and cytomegalovirus infection after allogeneic stem cell transplantation: The important impact of in vivo T cell depletion. Int J Hematol. 2010;91:877–885. doi: 10.1007/s12185-010-0597-6. [DOI] [PubMed] [Google Scholar]
- 22.Knight A, Madrigal AJ, Grace S, Sivakumaran J, Kottaridis P, Mackinnon S, Travers PJ, Lowdell MW. The role of Vdelta2-negative gammadelta T cells during cytomegalovirus reactivation in recipients of allogeneic stem cell transplantation. Blood. 2010;116:2164–2172. doi: 10.1182/blood-2010-01-255166. [DOI] [PubMed] [Google Scholar]
- 23.Scotet E, Martinez LO, Grant E, Barbaras R, Jenö P, Guiraud M, Monsarrat B, Saulquin X, Maillet S, Estève JP, Lopez F, Perret B, Collet X, Bonneville M, Champagne E. Tumor recognition following Vgamma9Vdelta2 T cell receptor interactions with a surface F1-ATPaserelated structure and apolipoprotein A-I. Immunity. 2005;22:71–80. doi: 10.1016/j.immuni.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 24.Servais S, Menten-Dedoyart C, Beguin Y, Seidel L, Gothot A, Daulne C, Willems E, Delens L, Humblet-Baron S, Hannon M, Baron F. Impact of Pre-Transplant Anti-T Cell Globulin (ATG) on Immune Recovery after Myeloablative Allogeneic Peripheral Blood Stem Cell Transplantation. PLoS One. 2015;10:e0130026. doi: 10.1371/journal.pone.0130026. [DOI] [PMC free article] [PubMed] [Google Scholar]


