Abstract
To test polymorphisms rs2200733 (chromosome 4q25) and rs2106261 (ZFHX3) were associated with AF recurrence after catheter ablation in a Chinese Han cohort. A total of 235 AF patients who underwent catheter ablation were recruited consecutively. Two polymorphisms were amplified by polymerase chain reaction and genotyped using high resolution melting analysis. Primary endpoints for AF recurrence were defined as the time to the first recurrence of atrial tachycardia/flutter/fibrillation (AT/AF). AT/AF recurrence was observed in 76 patients (35%). Allelic analysis demonstrated that rs2200733 was strongly associated with AF recurrence after ablation (P = 0.011) and the minor allele T increased the risk for recurrence (OR = 1.715). Diameters of the right atrium as well as the left and right superior pulmonary veins (PVs) were associated with rs2200733 in different genetic models (P = 0.040, 0.047 and 0.028, respectively). No significant association was detected between rs2106261 and AT/AF recurrence after ablation or atrial/PV diameters in any models. On multivariate Cox regression analysis, only rs2200733 was an independent factor of AF recurrence after ablation (HR = 0.532, P = 0.022). In Chinese Han population, rs2200733 but not rs2106261 is associated with AT/AF recurrence after ablation. The patients with genotype TT have larger size of right atrium and superior PVs than those of CC genotype. The findings suggest that rs2200733 may play a key role in regulating proper development and differentiation of atria/PVs.
Keywords: Atrial fibrillation, single nucleotide polymorphism, radiofrequency catheter ablation, pulmonary vein, atrium
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia encountered in the clinical setting and is associated with significant mortality and morbidity worldwide. The prevalence of AF increases substantially with age, ranging from approximately 1% in young adults to nearly 10% in those > 80-years of age [1,2]. A majority of AF patients possess a combination of common and rare genetic variants that impact their predisposition to AF, which clinically manifests in the presence of acquired cardiac or systemic diseases [3].
Genome wide association studies (GWAS) identified rs2200733 and rs2106261 at chromosomes 4q25 and 16q22, respectively, as being associated with AF in individuals of European ancestry [4,5]. These findings were subsequently validated in patients from mainland China [6,7], Hong Kong [4] and Taiwan [8]. On the basis of previous studies, Husser and her colleagues reported that polymorphisms at chromosome 4q25 modulated the risk of AF recurrence after catheter ablation in populations of European ancestry [9]. In another study, risk alleles at the 4q25 loci also predicted impaired clinical response to AF ablation in a White population [10]. Both studies pointed to a potential role for single nucleotide polymorphisms (SNPs) at the 4q25 loci for stratification of AF ablation therapy or pre-interventional management. However, in different races, the minor allele frequency (MAF) of the SNP is different and the correlation between the SNP and the disease is different as well. Thus, we validated the association between rs2200733 and AF recurrence after radiofrequency catheter ablation (RFCA) in non-European/White cohorts-a Chinese Han population. Furthermore, to the best of our knowledge, an association between rs2106261 and AF recurrence after RFCA has not been examined in any patient population.
The aims of our study were to determine: (i) whether the SNPs, rs2200733 and rs2106261, are associated with AF recurrence after ablation in a cohort of patients of Chinese Han origin; (ii) the association between the two SNPs and diameters of the atria/pulmonary veins (PVs); and (iii) the potential independent predictors of AF recurrence after ablation.
Materials and methods
Study subjects
A total of 235 drug-refractory AF patients (mean age 59.41 years, 73.7% male, 55.3% paroxysmal AF) were consecutively enrolled in this study. The Ethics Committee at the First Affiliated Hospital in Dalian Medical University approved all of the protocols used in this study. Written informed consent was obtained from all subjects participating in the study. The study conformed to the principles outlined in the Declaration of Helsinki. At the time of enrollment, all patients were the first time to undergo AF ablation in the Cardiac Pacing and Electrophysiology Department, the First Affiliated Hospital of Dalian Medical University between the period of May, 2010 and December, 2012. Subjects were from northeastern China and self-declared to be of ethnic Chinese Han origin. The criteria used for AF diagnosis was in accordance with the guidelines established by the European Society of Cardiology in 2010 [11], and determined using a standard 12-lead electrocardiogram or Holter recordings, regardless of clinical symptoms. Lone AF (LAF) was defined as AF occurring in the absence of cardiac or systemic disease in patients less than 66 years of age. Paroxysmal AF (PAF) was defined as AF episodes which terminated spontaneously within seven days. Persistent AF (PeAF) was defined as AF episodes lasting greater than seven days and/or requiring termination with pharmacologic or direct current cardioversion. Patients with hyperthyroidism, valvular heart disease, cardiomyopathy, left atrial thrombus, other severe diseases which free survival time less than 1 year, and a positive AF family history were excluded. Patients underwent AF ablation before or lost during the follow up period were also excluded.
Clinical characteristics
The clinical data included age, gender, hypertension, type 2 diabetes mellitus (T2DM), AF type, ablation records, left ventricular ejection fraction (LVEF), atrial diameters measured by echocardiography and PV diameters measured by multi-slice spiral computed tomography (MSCT) (Figure 1). Atrial and PV diameters consisted of averaged data from the supra-inferior and antero-posterior diameters. Hypertension was defined as systolic blood pressure ≥ 140 mmHg and diastolic blood pressure ≥ 90 mmHg. T2DM was diagnosed according to the 2008 American Diabetes Association Standards of Medical Care in Diabetes [12]. We checked for left atrial thrombus in AF patients prior to ablation by transesophageal echocardiography or MSCT.
Figure 1.
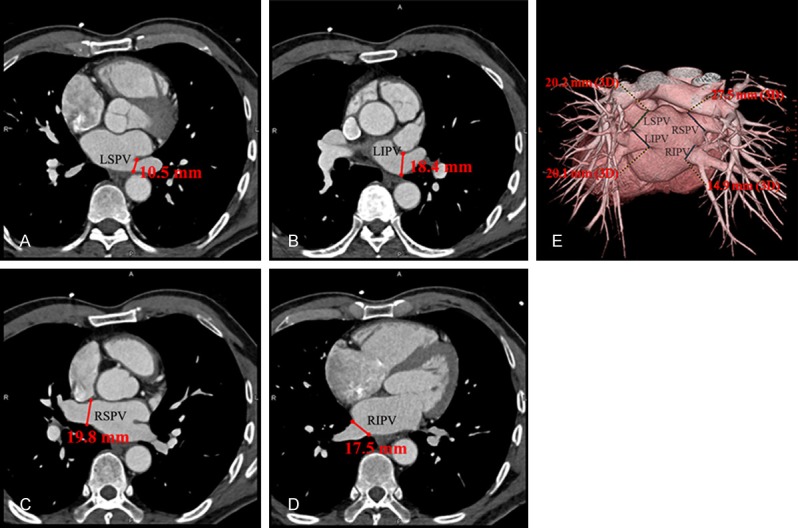
The measuring methods of pulmonary veins diameters by multi-slice spiral computed tomography. A-D. Relatively displayed the antero-posterior diameters of LSPV, LIPV, RSPV and RIPV. E. Displayed the supra-inferior diameters of the four pulmonary veins. LSPV, left superior pulmonary vein; LIPV, left inferior pulmonary vein; RSPV, right superior pulmonary vein; RIPV, right inferior pulmonary vein.
Radiofrequency catheter ablation
There are five experienced physicians to perform RFCA and all of them are blinded to the patients’ genotypes. The details of the electrophysiological study and 3-dimensional mapping have been described in previous studies [13,14]. After femoral venous access was obtained, two multipolar catheters were placed at the coronary sinus and right ventricle apex. Dual trans-septal puncture was performed under fluoroscopic guidance, with delivery of two 8-F long sheaths (SL1 and SR0, St. Jude Medical, St. Paul, MN, USA) into the left atrium (LA). An ablation catheter (NaviStar ThermoCool, Biosense WebsterInc., USA) and a circumferential mapping catheter (LASSO, Biosense Webster Inc., USA) were placed in LA through the two sheaths. In patients with PAF, circumferential pulmonary vein isolation (CPVI) was achieved guided by the CARTO system. In patients with PeAF, ablation was achieved by a step-wise approach. If AF continued after CPVI, the following linear ablations were performed sequentially: roof line of LA, mitral isthmus line, inferior vena cava tricuspid annual isthmus line, and complex fractionated atrial electrograms (CFAE) ablation. The endpoint of ablation included AF termination, establishment of a bi-directional conduction block and voltage reduction or disappearance of CFAE. If AF organized to atrial flutter, entrainment mapping was performed to target and ablate the critical isthmus. The radiofrequency energy was 30-40 W with temperature setting not more than 43°C. If AF was not terminated after the procedure above, electric cardioversion was performed to restore sinus rhythm.
Genotyping
Genomic DNA was isolated from peripheral blood lymphocytes with the TIANamp Blood DNA Kit (TiangenBiotect, Beijing, China) according to the manufacturer’s protocols. The details of rs2200733 and rs2106261 see http://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?rs=2200733 and http://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?rs=2106261, respectively. PCR was performed in a 25 μL final volume containing 1.5 mM Mg2+, 0.2 mM dNTPs, 0.5 μM each primer (Supplementary Table 1), 25 ng of human genomic DNA template, 5 μM SYTO9 green fluorescent intercalating agent, and 0.15 U of Taq DNA polymerase. PCR was performed using the ABI 9700 System (Life Technologies, Grand Island, New York, United States of America) and following the thermal profile: 95°C for 5 min; 40 cycles of 95°C for 10 s, corresponding annealing temperature (59.2°C for rs2200733, 60.3°C for rs2106261) for 10 s, and 72°C for 15 s; and a final cycle of 72°C for 10 min. PCR amplicons were genotyped using high resolution melting analysis (HRM) and the Rotor-Gene 6000 System (Corbett Life Science, Sydney, Australia) using standard protocols with minor modifications [15]. For each HRM run, positive control DNA from samples with known genotypes (TT, CT, and CC for rs2200733; and AA, AG, and GG for rs2106261), and a negative control without DNA template was included. To validate the accuracy of HRM genotyping data, ten samples from each genotype of both SNPs were randomly selected for Sanger sequencing. All sequencing results were consistent with the genotypes as determined by HRM analysis.
Follow-up
After discharge, the patients underwent follow-up (1 day post-catheter ablation as well as 1, 3, 6 and 12 months following the procedure) at our cardiology clinic or with their referring physician. Warfarin was prescribed for 3 months unless contraindicated. Anti-arrhythmic drugs were prescribed for 8 weeks to prevent the early recurrence of AF in patients with PeAF. When patients experienced symptoms consistent with a tachycardia following their ablation, 24-hour Holter monitoring was performed to define the cause of the clinical symptoms. The endpoint for follow-up was AF recurrence which was defined according to the HRS/EHRA/ECAS Consensus Statement recommendations, as any episode of non-sinus atrial tachyarrhythmia, including atrial tachycardia, atrial flutter, or AF (AT/AF) lasting greater than 30 seconds that occurred after the 3-month post-ablation blanking period [16].
Statistical analysis
Pearson’s chi-squared (χ2) and unpaired student’s t-tests were performed with the SPSS version 22.0 software (IBM Incorporated, Armonk, New York, United States of America) for categorical traits (gender, hypertension, T2DM, PAF, PeAF, LAF) and continuous traits (age, LVEF, and left atrial diameter (LAD)), respectively. For allelic association analysis, 2×2 contingency tables assessed by Pearson’s chi-squared (χ2) test were used to compare differences in the frequencies of the rs2200733 and rs2106261 minor alleles between the recurrence and non-recurrence groups. Odds ratios (ORs) and corresponding 95% confidential intervals (95% CI) were also calculated. Genotypic association analysis under three genetic models (dominant, recessive and additive) was performed using 2×3 contingency tables assessed by Pearson’s chi-squared (χ2) test. Multiple logistic regression analysis was used to adjust covariates such as gender, age, hypertension, T2DM, AF type. Survival analysis was performed to test the effect of the risk allele, rs2200733, on AF recurrence-free time using the Kaplan-Meier test. For genotypic associations between rs2200733/rs2106261 and atrial/PV diameters, a nonparametric test (the Kruskal-Wallis H test for the additive model or the Mann-Whitney U test for the recessive/dominant models) was used. Univariate and multivariate analysis were generated using the Cox proportion regression to identify potential risk factors independently associated with recurrence after RFCA (gender, age, LAF, rs2200733, rs2106261, hypertension, T2DM, LSPV, LIPV, RSPV, RIPV, LAD, RAD). The former only included one variate with “ENTER” method in every single analysis and the latter was calculated by “BACKWARD: LR” method. Two-tailed P < 0.05 was accepted as statistically significant.
Results
Baseline characteristics
The final study cohort consisted of 217 patients. Based on the follow-up results, the study subjects were divided into recurrence and non-recurrence groups. AF recurrence was observed in 76 patients (35%). Age, gender, LAD, LVEF, hypertension, T2DM, AF type were distributed similarly between the two groups (Table 1). However, a greater number of subjects with LAF were observed in the non-recurrence compared to the recurrence group (P = 0.014).
Table 1.
Baseline clinical characteristics of atrial fibrillation subjects
| Characteristics | Total Subjects | Recurrence Group | Non-recurrence Group | P-value |
|---|---|---|---|---|
| Number | 217 | 76 | 141 | N/A |
| Age (M ± SD, y) | 59.41±10.90 | 60.66±9.68 | 58.74±1.48 | 0.350 |
| Male/Female | 160/57 | 53/23 | 107/34 | 0.326 |
| LAD (M ± SD, mm) | 38.90±5.18 | 38.05±5.29 | 38.82±4.53 | 0.269 |
| LVEF (M ± SD, %) | 56.96±4.78 | 57.98±2.73 | 57.50±3.40 | 0.137 |
| Hypertension (1/0) | 84/133 | 35/41 | 49/92 | 0.107 |
| T2DM (1/0) | 26/191 | 10/66 | 16/125 | 0.669 |
| Paroxysmal AF | 123 | 39 | 81 | 0.395 |
| Persistent AF | 94 | 37 | 60 | |
| Lone AF | 90 | 23 | 67 | 0.014 |
AF, atrial fibrillation; LAD, left atrial diameter; LVEF, left ventricular ejection fraction; M ± SD, mean ± standard deviation; T2DM, type 2 diabetes mellitus.
Allelic association between rs2200733/rs2106261 and AF recurrence after RFCA
The genotype TT/CT/CC distributions of rs2200733 were 19/26/31 and 16/52/73 in recurrence group and non-recurrence group, respectively; the genotype AA/AG/GG distributions of rs2106261 were 14/38/24 and 30/61/50, respectively. Both the SNPs were found to be in Hardy-Weinberg equilibrium in the non-recurrence group (P > 0.05). Analyses were performed to test the hypothesis that the SNPs were related to AF recurrence after ablation (Table 2). Overall, the minor allele T of rs2200733 exhibited a significant allelic association with increased risk of AF recurrence after RFCA (P = 0.010, OR = 1.714). The association remained significant with an OR of 1.832 (P-adj = 0.011) after adjusting for age, gender, LAD, LVEF, hypertension, T2DM, AF type. However, the minor allele A of rs2106261 was not associated with AF recurrence after RFCA (P = 0.685, OR = 1.106). After adjusting covariates, rs2106261 also failed to correlate with AF recurrence after ablation (P-adj = 0.659, OR = 0.992).
Table 2.
Allelic and genotypic analysis of rs2200733/rs2106261 with AF recurrence after RFCA
| Models | P-value | OR (95% CI) | P-adj | Exp(B) (95% CI) |
|---|---|---|---|---|
| rs2200733 | ||||
| Allelic | 0.010 | 1.714 (1.137-2.585) | 0.011 | 1.832 (1.150-2.919) |
| Additive | 0.029 | N/A | 0.019 | 1.694 (1.092-2.627) |
| Dominant | 0.154 | 0.309 (0.146-0.654) | 0.218 | 1.499 (0.787-2.854) |
| Recessive | 0.009 | 2.604 (1.249-5.432) | 0.003 | 3.524 (1.516-8.913) |
| rs2106261 | ||||
| Allelic | 0.685 | 1.106 (0.744-1.645) | 0.659 | 0.992 (0.957-1.028) |
| Additive | 0.242 | N/A | 0.436 | 0.851 (0.567-1.277) |
| Dominant | 0.666 | 1.563 (0.890-2.740) | 0.742 | 0.800 (0.212-3.019) |
| Recessive | 0.176 | 1.580 (0.813-3.071) | 0.119 | 3.599 (0.720-17.989) |
CI, confidence interval; Exp(B), adjusted OR; OR, odds ratio; P-adj, adjusted P value.
Genotypic association between rs2200733/rs2106261 and AF recurrence after RFCA
Genotypic associations between the two SNPs and AF recurrence after RFCA were analyzed under the additive, dominant and recessive genetic models (Table 2). For rs2200733, a significant association was found between the T allele and an increased risk of AF recurrence after RFCA under the additive and recessive models both before and after adjusting for covariates in the study groups (additive model: P = 0.029, P-adj = 0.019; recessive model: P = 0.009, P-adj = 0.003). Survival analysis of subjects with rs2200733 and AF recurrence after ablation under the recessive model demonstrated that the TT genotype had shorter AF recurrence-free survival time compared to the CT+CC genotypes for patients undergoing ablation (10.14 months versus 11.44 months, Figure 2). For rs2106261, no association with AF recurrence after RFCA was revealed by any of the three models, even after adjusting for covariates (P-adj = 0.436, 0.742 and 0.119 for additive, dominant and recessive models, respectively).
Figure 2.
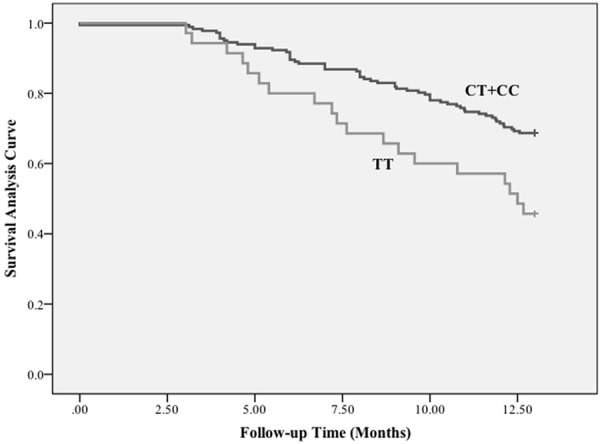
Survival analysis of rs2200733 and AF recurrence after RFCA. Kaplan-Meier curve of rs2200733 TT genotype versus CT+CC genotypes demonstrates shorter AT/AF recurrence-free survival time for patients undergoing ablation.
Genotypic association between rs2200733/rs2106261 and atrial/PV diameters
After excluding the patients with abnormal amount of PVs (5 patients displayed three PVs and 18 patients displayed five PVs), we documented the diameters of four PVs in a total of 194 patients. The relationship between diameters of the atria/PVs and rs2200733 was examined under the three genotypic models (Table 3). For rs2200733, (i) the right atrial diameter (RAD) with the risk allele T group was larger than the CC group (38.7±6.3/38.5±5.3 mm versus 37.0±5.2 mm, P = 0.040) under the dominant model; (ii) the diameter of the left superior PV (LSPV) with genotype TT was larger than CT+CC genotypes under the recessive model (21.1±2.8 mm versus 19.3±4.7/19.8±3.6 mm, P = 0.047); (iii) the diameter of right superior PV (RSPV) was associated with rs2200733 genotypes under the three genotypic models (P = 0.024, 0.028 and 0.017, respectively) and (iv) the diameter of RSPV in the TT group was larger than that of the CC group (22.2±4.2 mm versus 19.7±3.8 mm). No significant associations were revealed for the LAD and the inferior PVs in any of the three models. For rs2106261, no association was detected by any of the three models for any diameter (whether atria and PVs, Supplementary Table 2).
Table 3.
Association between rs2200733 and diameters of atria and pulmonary veins
| Clinical Parameters | Genotypes (M ± SD, mm) | Inherent Models (P-value) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| TT | CT | CC | Additive | Recessive | Dominant | |
| LA | 37.8±5.6 | 38.1±5.5 | 36.9±5.3 | 0.407 | 0.847 | 0.199 |
| RA | 38.7±6.3 | 38.5±5.3 | 37.0±5.2 | 0.128 | 0.272 | 0.040 |
| LSPV | 21.1±2.8 | 19.3±4.7 | 19.8±3.6 | 0.136 | 0.047 | 0.525 |
| LIPV | 16.7±2.1 | 16.6±2.7 | 16.8±3.3 | 0.938 | 0.946 | 0.770 |
| RSPV | 22.2±4.2 | 20.75±3.9 | 19.7±3.8 | 0.024 | 0.028 | 0.017 |
| RIPV | 18.6±2.7 | 17.9±2.4 | 17.8±3.3 | 0.426 | 0.192 | 0.627 |
LA, left atrium; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; M ± SD, mean ± standard deviation; RA, right atrium; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein.
Univariate and multivariate analysis of potential risk factors in association with AF recurrence after RFCA
The risk factors included gender, age, rs2200733 (under a recessive model), rs2106261 (under a recessive model), LAF, hypertension, T2DM, LSPV, LIPV, RSPV, RIPV, LAD and RAD (Table 4). For univariate analysis, age, rs2200733 and LAF were risk factors of AF recurrence after RFCA (P = 0.029, 0.005 and 0.045, respectively); after multivariate analysis using proportional Cox regression, only rs2200733 was the independent factor of AF recurrence after ablation (HR = 0.532, P = 0.022) which consistent with previous results.
Table 4.
Univariate and multivariate analysis of AF recurrence after RFCA
| Variates | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| B | P value | HR (95% CI) | B | P value | HR (95% CI) | |
| Gender | -0.204 | 0.420 | 0.815 (0.496-1.340) | |||
| Age | 0.024 | 0.029 | 1.024 (1.002-1.047) | 0.017 | 0.155 | 1.017 (0.994-1.041) |
| rs2200733 | -0.742 | 0.005 | 0.476 (0.283-0.801) | -0.630 | 0.022 | 0.532 (0.310-0.913) |
| rs2106261 | 0.094 | 0.750 | 1.099 (0.615-1.965) | |||
| LAF | 0.504 | 0.045 | 1.651 (1.010-2.698) | 0.397 | 0.153 | 1.488 (0.862-2.568) |
| LSPV | 0.050 | 0.111 | 1.052 (0.988-1.119) | |||
| LIPV | 0.040 | 0.375 | 1.041 (0.953-1.138) | |||
| RSPV | 0.043 | 0.184 | 1.044 (0.980-1.111) | |||
| RIPV | 0.011 | 0.774 | 1.011 (0.939-1.088) | |||
| LAD | -0.001 | 0.964 | 0.999 (0.953-1.047) | |||
| RAD | 0.012 | 0.611 | 1.012 (0.968-1.058) | |||
| Hypertension | -0.296 | 0.201 | 0.744 (0.472-1.171) | |||
| T2DM | -0.110 | 0.748 | 0.896 (0.470-1.743) | |||
AF, atrial fibrillation; HR, hazard ratio; CI, confidence interval; LAF, lone atrial fibrillation; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein; LAD, left atrial diameter; RAD, right atrial diameter; T2DM, type 2 diabetes mellitus.
Discussion
Main findings
We found that rs2200733 but not rs2106261 was associated with AF recurrence after RFCA in the Chinese Han population, and the minor allele T of rs2200733 increased risk of AF recurrence after ablation. We also demonstrated that rs2200733 was positively associated with RAD, LSPV and RSPV diameters, with diameters of the TT group being larger than those of the CC group. To our knowledge, this is the first demonstration of a lack of association between rs2106261 and AF recurrence after RFCA.
The relationship between rs2200733 and AF recurrence after ablation as well as diameters of atria/PVs
In our study, we found the minor allele T of rs2200733 increased the risk of AF recurrence after ablation, which was consistent with previous studies [9,10]. The exact mechanisms by which SNPs at chromosome 4q25 modulate the susceptibility of patients for AF and AF relapse after catheter ablation remain unknown. However, recent studies have highlighted the importance of nearby gene--PITX2, which play a critical role in atrial development, and differentiation, proliferation and expansion of pulmonary myocardial cells [17,18]. Considering these findings together with the data of the current study, it is now tempting to speculate that chromosome 4q25 variant carriers may differ in their pulmonary vein phenotype (i.e., amount, diameter, extension and arrhythmogenicity of pulmonary vein myocardium) and consequently on AF mechanisms, which will impact on catheter ablation of the pulmonary veins [9]. Therefore, we compared the diameters of PVs in the three genotypes of rs2200733 and found that patients with the risk allele T had larger diameters of superior PVs, which was proved the part of our hypothesis. Saito et al. and Tagawa et al. reported that the myocardial sleeves of the superior PVs were longer and wider than their inferior counterparts [19,20]. These observations were consistent with previous results that the ostia or internal structures of the superior PVs were more common ectopic loci driving AF than the inferior PVs [21]. Considering these results together with our data, it was validated that rs2200733 may be involved in the structural development of the superior PVs. However, this was in contrast with observations made by Marek et al. that reported enlargement of the left inferior PV in patients with the T allele [22]. The result needs to be proved by further studies.
We also observed that patients with the risk allele T of rs2200733 had larger RA. On the one hand, it was consistent with the fact that 4q25 polymorphisms increased the risk for atrial flutter, which is confined to the right atrium [4,23]. On the other hand, it was contrary to most studies, in that the majority of AF excited loci originated from ectopic beats in the LA [24,25]. However, some other studies have indicated that RA loci may indeed act as AF drivers. For instance, dominant frequency mapping studies in patients with PeAF uncovered dominant frequency max sites in the RA of 16% of patients [26] with clear reports of AF termination during exclusive or dominant ablation in the RA, although these cases are uncommon [27,28]. Hocini et al. demonstrated that the RA is driving AF in approximately 20% of PeAF cases and that pre-procedural as well as procedural independent predictors indicate the need for RA ablation, including the duration of AF and the RA size [29].
In summary, (i) RFCA restricted to the pulmonary veins may be sufficient in patients with the wildtype and heterozygous, (ii) other ablation strategies (strengthening superior PVs and RA ablation) or more aggressive post-ablation management may be warranted in TT patients in whom superior PVs and right atrial areas may be more important for AF induction and sustenance.
The relationship between rs2106261 and AF recurrence after ablation as well as diameters of atria/PVs
We observed no association between rs2106261 and AF recurrence after RFCA as well as atrial/PV diameters. The rs2106261 polymorphism is an intronic variant located at chromosome 16q22 in the ZFHX3, which was found to be a regulatory factor for STAT3-mediated signal transduction. Tsai et al. suggested that activation of the angiotensin II/Rac1/STAT signaling transduction pathway may contribute to the structural and inflammatory changes associated with AF [30]. Since rs2106261 does not appear to participate in the development of the PVs and atria, it may not be associated with AF recurrence after ablation and the dimensions of the atria/PVs.
Correlation of diameters of the atria/PVs and AF recurrence after RFCA
In multivariate analysis, we found that only rs2200733 was an independent factor for AF recurrence after ablation. Atrial structural and electrical remodeling are key factors dictating the initiation and maintenance of AF. An enlarged atrial size modulates the substrate for AF in part by promoting the existence of multiple fibrillatory rotors. Previous studies have reported that left atrial enlargement was an independent risk factor for AF recurrence after ablation [31,32]. Our study, which does not support this observation, suggests the potential importance of electrical rather than structural remodeling in the propensity for AF recurrence after ablation.
Although previous result demonstrated diameters of superior PVs of patients with rs2200733 TT genotype were larger than CT+CC genotypes, we found diameters of PVs were not the predictors of AF recurrence after ablation. The association between diameters of PVs and AF or AF recurrence after cardioversion has been controversial thus far. Wei et al. reported that the enlargement of all superior PVs is an independent risk factor for postoperative recurrence of AF [33]; Tsao et al. also showed that the superior PVs were larger in paroxysmal/chronic AF than control group, but was similar after correction for other variables [31]. Another study has indicated that the diameter of PVs had no impact on the outcome of catheter ablation [34]. It needs to be validated by further studies.
Limitations
There are three limitations to our study. First, our findings are generated from a relatively small number of individuals, which should be validated in larger cohorts of Chinese Han patients. Second, the diameter of the atria and PVs were measured using echocardiography and MSCT. Such measurements are subject to artifacts. Third, the study was limited to observation of 2 AF-associated loci. Other genes may also modulate the development and structure of atria/PVs, and consequently the response to AF therapies.
Conclusions
The polymorphism rs2200733 at chromosome 4q25, but not rs2106261 at 16q22, is associated with AF recurrence after RFCA, potentially by influencing the size of the RA and superior PVs. The present findings suggest that rs2200733 may play a key role in regulating proper development and differentiation of atria/PVs and guide the selection of ablation strategies in AF patients.
Acknowledgements
We thank Prof. Xin Tu and Prof. Qing Wang for their technical support with polymorphisms genotyping. This work was supported by grants from the Specialized Research Fund for the Doctoral Program of Higher Education (No. 20092105110003), Dalian Science and Technology Project (No. 2011503391), and the National Basic Research Program of China (No. 201381270274).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillatiorn analysis and implications. Arch Intern Med. 1995;55:469–473. [PubMed] [Google Scholar]
- 2.Ryder KM, Benjamin EJ. Epidemiology and significance of atrial fibrillation. Am J Cardiol. 1999;84:131R–138R. doi: 10.1016/s0002-9149(99)00713-4. [DOI] [PubMed] [Google Scholar]
- 3.Ritchie MD, Rowan S, Kucera G, Stubblefield T, Blair M, Carter S, Roden DM, Darbar D. Chromosome 4q25 variants are genetic modifiers of rare ion channel mutations associated with familial atrial fibrillation. J Am Coll Cardiol. 2012;60:1173–1181. doi: 10.1016/j.jacc.2012.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gudbjartsson DF, Arnar DO, Helgadottir A, Gretarsdottir S, Holm H, Sigurdsson A, Jonasdottir A, Baker A, Thorleifsson G, Kristjansson K, Palsson A, Blondal T, Sulem P, Backman VM, Hardarson GA, Palsdottir E, Helgason A, Sigurjonsdottir R, Sverrisson JT, Kostulas K, Ng MC, Baum L, So WY, Wong KS, Chan JC, Furie KL, Greenberg SM, Sale M, Kelly P, MacRae CA, Smith EE, Rosand J, Hillert J, Ma RC, Ellinor PT, Thorgeirsson G, Gulcher JR, Kong A, Thorsteinsdottir U, Stefansson K. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature. 2007;448:353–357. doi: 10.1038/nature06007. [DOI] [PubMed] [Google Scholar]
- 5.Gudbjartsson DF, Holm H, Gretarsdottir S, Thorleifsson G, Walters GB, Thorgeirsson G, Gulcher J, Mathiesen EB, Njølstad I, Nyrnes A, Wilsgaard T, Hald EM, Hveem K, Stoltenberg C, Kucera G, Stubblefield T, Carter S, Roden D, Ng MC, Baum L, So WY, Wong KS, Chan JC, Gieger C, Wichmann HE, Gschwendtner A, Dichgans M, Kuhlenbäumer G, Berger K, Ringelstein EB, Bevan S, Markus HS, Kostulas K, Hillert J, Sveinbjörnsdóttir S, Valdimarsson EM, Løchen ML, Ma RC, Darbar D, Kong A, Arnar DO, Thorsteinsdottir U, Stefansson K. A sequence variant in ZFHX3 on 16q22 associates with atrial fibrillation and ischemic stroke. Nat Genet. 2009;41:876–878. doi: 10.1038/ng.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi L, Li C, Wang C, Xia Y, Wu G, Wang F, Xu C, Wang P, Li X, Wang D, Xiong X, Bai Y, Liu M, Liu J, Ren X, Gao L, Wang B, Zeng Q, Yang B, Ma X, Yang Y, Tu X, Wang QK. Assessment of association of rs2200733 on chromosome 4q25 with atrial fibrillation and ischemic stroke in a Chinese Han population. Hum Genet. 2009;126:843–849. doi: 10.1007/s00439-009-0737-3. [DOI] [PubMed] [Google Scholar]
- 7.Li C, Wang F, Yang YZ, Fu F, Xu C, Shi L, Li S, Xia Y, Wu G, Cheng X, Liu H, Wang C, Wang P, Hao J, Ke Y, Zhao Y, Liu M, Zhang R, Gao L, Yu B, Zeng Q, Liao Y, Yang B, Tu X, Wang QK. Significant association of SNP rs2106261 in the ZFHX3 gene with atrial fibrillation in a Chinese Han GeneID population. Hum Genet. 2011;129:239–246. doi: 10.1007/s00439-010-0912-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee KT, Yeh HY, Tung CP, Chu CS, Cheng KH, Tsai WC, Lu YH, Chang JG, Sheu SH, Lai WT. Association of rs2200733 but not rs10033464 on 4q25 with atrial fibrillation based on the recessive model in a Taiwanese population. Cardiology. 2010;116:151–156. doi: 10.1159/000318172. [DOI] [PubMed] [Google Scholar]
- 9.Husser D, Adams V, Piorkowski C, Hindricks G, Bollmann A. Chromosome 4q25 variants and atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol. 2010;55:747–753. doi: 10.1016/j.jacc.2009.11.041. [DOI] [PubMed] [Google Scholar]
- 10.Shoemaker MB, Muhammad R, Parvez B, Parvez B, White BW, Streur M, Song Y, Stubblefield T, Kucera G, Blair M, Rytlewski J, Parvathaneni S, Nagarakanti R, Saavedra P, Ellis CR, Patrick Whalen S, Roden DM, Darbar RD. Common atrial fibrillation risk alleles at 4q25 predict recurrence after catheter-based atrial fibrillation ablation. Heart Rhythm. 2013;10:394–400. doi: 10.1016/j.hrthm.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery. Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohloser SH, Kolh P, Le Heuzey JY, Ponikowski P, Rutten FH. Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 12.America Diabetes Association. American Diabetes Association Standards of medical care in diabetes-2008. Diabetes Care. 2008;31:S12–S54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 13.Pappone C, Oreto G, Rosanio S, Vicedomini G, Tocchi M, Gugliotta F, Salvati A, Dicandia C, Calabrò MP, Mazzone P, Ficarra E, Di Gioia C, Gulletta S, Nardi S, Santinelli V, Benussi S, Alfieri O. Atrial electroanatomic remodeling after circumferential radiofrequency pulmonary vein ablation: efficacy of an anatomic approach in a large cohort of patients with atrial fibrillation. Circulation. 2001;104:2539–2544. doi: 10.1161/hc4601.098517. [DOI] [PubMed] [Google Scholar]
- 14.Ouyang F, Bänsch D, Ernst S, Schaumann A, Hachiya H, Chen M, Chun J, Falk P, Khanedani A, Antz M, Kuck KH. Complete isolation of left atrium surrounding the pulmonary veins: new insights from the double- Lasso technique in paroxysmal atrial fibrillation. Circulation. 2004;110:2090–2096. doi: 10.1161/01.CIR.0000144459.37455.EE. [DOI] [PubMed] [Google Scholar]
- 15.Xu C, Wang F, Wang B, Li X, Li C, Wang D, Xiong X, Wang P, Lu Q, Wang X, Yang Q, Yin D, Huang Y, Ji L, Wang N, Chen S, Cheng X, Liao Y, Ma X, Su D, Chen G, Xia H, Shi L, Tu X, Wang QK. Minor allele C of chromosome 1p32 single nucleotide polymorphism rs11206510 confers risk of ischemic stroke in the Chinese Han population. Stroke. 2010;41:1587–1592. doi: 10.1161/STROKEAHA.110.583096. [DOI] [PubMed] [Google Scholar]
- 16.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ Jr, Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D Heart Rhythm Society Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2012;9:632–696. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 17.Logan M, Pagan-Westphal SM, Smith DM, Paganessi L, Tabin CJ. The transcription factor Pitx2 mediates situs-specific morphogenesis in response to left-right asymmetric signals. Cell. 1998;94:307–317. doi: 10.1016/s0092-8674(00)81474-9. [DOI] [PubMed] [Google Scholar]
- 18.Mommersteeg MT, Brown NA, Prall OW, de Gier-de Vries C, Harvey RP, Moorman AF, Christoffels VM. Pitx2c and Nkx2-5 are required for the formation and identity of the pulmonary myocardium. Circ Res. 2007;101:902–909. doi: 10.1161/CIRCRESAHA.107.161182. [DOI] [PubMed] [Google Scholar]
- 19.Saito T, Waki K, Becker AE. Left atrial myo cardial extension onto pulmonary veins in humans: anatomic observations relevant for atrial arrhythmias. J Cardiovasc Electrophysiol. 2000;11:888–894. doi: 10.1111/j.1540-8167.2000.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 20.Tagawa M, Higuchi K, Chinushi M, Washizuka T, Ushiki T, Ishihara N, Aizawa Y. Myocardium extending from the left atrium onto the pulmonary veins. Pacing Clin Electrophysiol. 2001;24:1459–1463. doi: 10.1046/j.1460-9592.2001.01459.x. [DOI] [PubMed] [Google Scholar]
- 21.Pappone C, Rosanio S, Oreto G, Tocchi M, Gugliotta F, Vicedomini G, Salvati A, Dicandia C, Mazzone P, Santinelli V, Gulletta S, Chierchia S. Circumferential radiofrquency ablation of pulmonary vein ostia: A new anatomic approach for curing atrial fibrillation. Circulation. 2000;102:2619–2628. doi: 10.1161/01.cir.102.21.2619. [DOI] [PubMed] [Google Scholar]
- 22.Marek K, Maria F, Edward K, Lodzinski P, Piatkowska A, Broda G, Ploski R, Opolski G. Association between Variants on Chromosome 4q25, 16q22 and 1q21 and Atrial Fibrillation in the Polish Population. PLoS One. 2011;6:e21790–e21794. doi: 10.1371/journal.pone.0021790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viviani Anselmi C, Novelli V, Roncarati R, Malovini A, Bellazzi R, Bronzini R, Marchese G, Condorelli G, Montenero AS, Puca AA. Association of rs2200733 at 4q25 with atrial flutter/fibrillation diseases in an Italian population. Heart. 2008;94:1394–1396. doi: 10.1136/hrt.2008.148544. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh MH, Chen SA, Tai CT, Tsai CF, Prakash VS, Yu WC, Liu CC, Ding YA, Chang MS. Double multielectrode mapping catheters facilitate radiofrequency catheter ablation of focal atrial fibrillation originating from pulmonary veins. J Cardiovasc Electrophysiol. 1999;10:136–144. doi: 10.1111/j.1540-8167.1999.tb00654.x. [DOI] [PubMed] [Google Scholar]
- 25.Hwang C, Karagueuzian HS, Chen PS. Idiopathic paroxysmal atrial fibrillation induced by a focal discharge mechanism in the left superior pulmonary vein: possible roles of the ligament of Marshall. J Cardiovasc Electrophysiol. 1999;10:636–648. doi: 10.1111/j.1540-8167.1999.tb00240.x. [DOI] [PubMed] [Google Scholar]
- 26.Atienza F, Almendral J, Jalife J, Zlochiver S, Ploutz-Snyder R, Torrecilla EG, Arenal A, Kalifa J, Fernández-Avilés F, Berenfeld O. Real-time dominant frequency mapping and ablation of dominant frequency sites in atrial fibrillation with left-to-right frequency gradients predicts long-term maintenance of sinus rhythm. Heart Rhythm. 2009;6:33–40. doi: 10.1016/j.hrthm.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calo L, Lamberti F, Loricchio ML, Castro A, Shpun S, Boggi A, Pandozi C, Santini M. Longterm follow-up of right atrial ablation in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2004;15:37–43. doi: 10.1046/j.1540-8167.2004.03264.x. [DOI] [PubMed] [Google Scholar]
- 28.Calo L, Lamberti F, Loricchio ML, De Ruvo E, Colivicchi F, Bianconi L, Pandozi C, Santini M. Left atrial ablation versus biatrial ablation for persistent and permanent atrial fibrillation: a prospective and randomized study. J Am Coll Cardiol. 2006;47:2504–2512. doi: 10.1016/j.jacc.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 29.Hocini M, Nault I, Wright M, Veenhuyzen G, Narayan SM, Jaïs P, Lim KT, Knecht S, Matsuo S, Forclaz A, Miyazaki S, Jadidi A, O’Neill MD, Sacher F, Clémenty J, Haïssaguerre M. Disparate evolution of right and left atrial rate during ablation of long-lasting persistent atrial fibrillation. J Am Coll Cardiol. 2010;55:1007–1016. doi: 10.1016/j.jacc.2009.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsai C, Lin J, Lai L, Lin CS, Huang SK. Membrane translocation of small GTPase Rac1 and activation of STAT1 and STAT3 in pacing-induced sustained atrial fibrillation. Heart Rhythm. 2008;5:1285–1293. doi: 10.1016/j.hrthm.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 31.Tsao HM, Yu WC, Cheng HC, Wu MH, Tai CT, Lin WS, Ding YA, Chang MS, Chen SA. Pulmonary vein dilation in patients with atrial fibrillation: detection by magnetic resonance imaging. J Cardiovasc Electrophysiol. 2001;12:809–813. doi: 10.1046/j.1540-8167.2001.00809.x. [DOI] [PubMed] [Google Scholar]
- 32.Berruezo A, Tamborero D, Mont L, Benito B, Tolosana JM, Sitges M, Vidal B, Arriagada G, Méndez F, Matiello M, Molina I, Brugada J. Preprocedural predictors of atrial fibrillation recurrence after circumferential pulmonary vein ablation. Eur Heart J. 2007;28:836–841. doi: 10.1093/eurheartj/ehm027. [DOI] [PubMed] [Google Scholar]
- 33.Wei W, Ge JB, Zhu WQ. Roles of left atrial diameter and anatomic structure of pulmonary veins for the prediction of postoperative recurrence after radiofrequency catheter ablation of atrial fibrillation. Chin J Cardiac Arrhyth. 2012;16:206–210. [Google Scholar]
- 34.den Uijl DW, Tops LF, Delgado V, Schuijf JD, Kroft LJ, de Roos A, Boersma E, Trines SA, Zeppenfeld K, Schalij MJ, Bax JJ. Effect of pulmonary vein anatomy and left atrial dimensions on outcome of circumferential radiofrequency catheter ablation for atrial fibrillation. Am J Cardiol. 2011;107:243–249. doi: 10.1016/j.amjcard.2010.08.069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


