Abstract
Auricular cartilage loss or defect remains a challenge to plastic surgeons, and cartilage regenerative medicine provides a novel method to solve the problem. However, ideal seeding cells seem to be the key point in the development of cartilage regeneration. Although bone marrow-mesenchymal stem cells were considered as the ideal seeding cells in cartilage regeneration, regenerative cartilage differentiated from bone marrow-mesenchymal stem cells still faces some problems. It is reported that many tissues and organs contain a certain number of adult progenitor or stem cells that can replace cells that die or restore tissues and organs after injury. Therefore, we tried to use a fibronectin differential adhesion assay to isolate cartilage stem/progenitor cells from auricular cartilage and perichondrium. Flow cytometric analysis demonstrated the two cell populations expressed mesenchyme stem cell positive surface marker. Meanwhile, the cells differentiate into osteogenic line, chondrogenic line and adipogenic line under different induction conditions. The proliferation of cartilage stem/progenitor cells derived from perichondrium was higher than cartilage stem/progenitor cells derived from auricular cartilage. In addition, there is a difference on osteogenic differentiation, chondrogenic differentiation and adipogenic differentiation between these two cell populations. In conclusion, auricular cartilage and perichondrium both contain cartilage stem/progenitor cells, which may provide an ideal seeding cells for cartilage regeneration.
Keywords: Auricular cartilage, perichondrium, cartilage stem/progenitor cells, fibronectin, differentiation, proliferation
Introduction
Microtia and auricular cartilage defects caused by trauma and tumor are very common, and the current method is reconstruction of auricular cartilage by costal cartilage, which based on the sacrifice of costal cartilage [1-4]. Cartilage regeneration, especially cartilage tissue engineering provides a brand-new strategy for the therapy of auricular cartilage defects and loss [5,6]. Seeding cells, scaffolds, and microenvironment are three elements of tissue engineering, and seeding cells play an important role [7]. Owing to its easy harvest and multipotency, as well as high proliferative potential, bone marrow mesenchymal stem cells (BMSCs) are considered as one of the most promising cell sources in cartilage repair and cartilage regeneration [8-10]. However, more and more reports indicated regenerative cartilages differentiated from BMSCs tend to fibrosis, vascularize or even ossify [11-14]. In addition, some studies also showed stem cells derived from other tissues, such as adipose tissue, synovial membrane, muscle also show chondrogenic potential, but could not maintain stable chondrogenic differentiation permanently [15-17]. Recently, it is reported that cartilage tissue and perichondrium may obtain a huge amount of cartilage stem/progenitor cells (CSPCs), which may be the ideal seeding cells in cartilage regeneration [18-21]. Therefore, in current study, we try to isolate stem cells from auricular cartilage tissue and perichondrium in a pig model by a fibronectin differential adhesion assay, and evaluate the stemness of the two cell populations. The Q-PCR assay and CCK-8 assay studies were performed to compare differentiation ability and cell proliferation of the two cells population. In addition, we also compare the cartilage stem/progenitor cells with the bone marrow-mesenchymal stem cells on proliferation and differentiation.
Materials and methods
All experimental protocols involving animal tissues and cells was approved by the Ethics Committee of Shanghai Jiao Tong University School of Medicine.
Cartilage stem/progenitor cells in auricular cartilage tissue and perichondrium of newborn pigs (Chang Feng hybrid piglets provided by Shanghai Chuansha Breeding Factory, n = 6) were harvested via a differential adhesion to fibronectin in vitro as described previously. The obtained pig ears were seperated into cartilage tissue and perichondrium. The specimen were excised and minced into 1 mm2 pieces, then washed in sterile chloromycetin and PBS thrice each. The tissues were digested in high-glucose DMEM containing 0.2% collagenase NB4 (Serva, Heidelberg, Germany) in a 37°C shaking water bath for 8 h. The suspension was then filtered through a 200 μm filter to remove undigested particles. After isolation, cells (4000 ml-1) were seeded onto 100 mm plastic Petri dishes (10 μg/ml fibronectin treated overnight) at 37°C for 20 minutes in low-glucose DMEM. After 20 minutes, media and non-adherent cells were removed and low-glucose DMEM containing 10% FBS was added into dishes. The adherent cells were cultured for 14 days until cells reached confluence. Cells were then digested with 0.25% trypsin plus 0.02% EDTA (Invitrogen) and subcultured in a new plate at a density of 2.0 × 104 cells/cm2.
Swine bone marrow was obtained from the posterior superior iliac crest of newborn pigs. Dulbecco’s modified Eagle’s medium (DMEM) containing low glucose (Invitrogen, Carlsbad, Calif., USA) supplemented with 10% fetal bovine serum (FBS) was added to the aspirate (1:1) and loaded over Percoll (Sigma, St. Louis, Mo., USA) for density gradient centrifugation. Mononucleated cells were harvested from the interface after centrifugation at 3,000 rpm for 10 min followed by two washes with phosphate-buffered saline (PBS). Cells were resuspended in low-glucose DMEM supplemented with 10% FBS, plated into 100-mm culture dishes at a density of 2.0 × 105 cells/cm2 and incubated at 37°C in an atmosphere of 5% CO2 in air. Nonadherent cells were removed by medium change after 24 h. Adherent cells were cultured for 7-10 days until cells reached over 80% confluence. Cells were then digested with 0.25% trypsin plus 0.02% EDTA (Invitrogen) and subcultured in a 100-mm culture dish at a density of 2.5 × 104 cells/cm2. BMSCs at passage 2 were used for tissue engineering.
FACS analysis
To assess characteristics of cells, auricular cartilage stem/progenitor cells (CSPCs) and perichondrium stem/progenitor cells (PSPCs) at passage 1, passage 2, and passage 3 were used for FACS analysis. 1 × 106 cells were washed in PBS and incubated for 1 hour at 4°C with fluorescence-conjugated mouse anti-human monoclonal antibodies (CD29, CD34, CD44, CD45, and CD90). Cells were centrifuged at 2000 × g, supernatants removed and cells washed thrice in PBS. Finally, labelled cells were re-suspended in 1 ml PBS and subjected to analyze with Beckman Coulter FC 500.
Colony forming efficiency
To study the colony forming efficiency of two cell populations, cells were seeded at a density of 100 cells/plate (100 mm plastic Petri dishes) and were cultured in DMEM containing 10% FBS at an incubator with 5% CO2 and 95% humidity at 37°C for 2 weeks. The number of colonies was quantified after 2 weeks by staining with toluidine blue [22].
Cell proliferation measurement
The cell proliferation rate was assessed with a cell counting kit (CCK)-based colorimetric assay (CCK-8; Dojindo China Co., Ltd.). BMSCs, CSPCs and PSPCs resuspended in 100 μL DMEM containing 10% FBS were seeded at a density of 1000 cells/well in 96-well plates (BD Pharmingen) and cultured for 1 day, 3 days, 5 days, and 7 days. Before every test, 10 μL of CCK-8 solution was added into each well and incubated for 4 hours, then the absorbance of the supernatant was measured spectrophotometrically at 450 nm.
Cell differentiation
CSPCs and PSPCs were seeded at a density of 5000 cells/well in a 12-well culture plate, and cultured in different induction mediums to evaluate osteogenic, adipogenic, and chondrogenic differentiation.
Cells were induced in osteogenic induction medium (DMEM containing 10% FBS, 0.01 μM 1,25-dihydroxyvitamin D3, 50 μM ascorbate-2-phosphate, 10 mM β-glycerophosphate, 1% antibiotic/antimycotic, all reagent were purchased from Sigma company, USA) for 16 days, the culture medium was replaced every 2 days. To test osteogenesis, cells in a 12-well plate were washed by PBS, and fixed with 4% Paraformaldehyde (PFA) for 30 min at room temperature, incubated with 1% Alizarin Red S solution for 10 min at room temperature using a Alizarin Red Stain Kit (Sigma-Aldrich Co., St Louis, MO, USA).
Cells were induced in adipogenic induction medium (DMEM containing 10% FBS, 0.5 mM isobutyl-methylxanthine, 1 μM dexamethasone, 10 μM insulin, 200 μM indomethacin, 1% antibiotic/antimycotic, all reagent were purchased from Sigma company, USA) for 14 days, the culture medium was replaced every 2 days. To test adipogenesis, cells in a 12-well plate were fixed with 4% paraformaldehyde (PFA) for 30 min at room temperature, and stained with oil red O solution for 10 min at room temperature using an oil Red O Stain Kit (Sigma-Aldrich Co., St Louis, MO, USA).
For chondrogenesis, cells were seeded at a density of 5000 cells/well in a 12-well culture plate and cultured using high glucose DMEM containing 10% FBS, 10 ng/ml transforming growth factor-1, 10-7 M dexamethasone, and 40 ng/ml insulin-like growth factor-1. After two week of induction, the cells were stained with toluidine blue.
Gene expression
After osteogenic, adipogenic, and chondrogenic induction, total RNA in different groups (BMSCs as control group) was extracted from each specimen and cDNA was obtained by reverse transcription (RT) according to previously described methods. The mRNA expression of osteogenic markers (osteocalcin, runx2, and collagen I), adipogenic markers (adiponectin, adipocyte fatty acid binding protein [AP2] and fatty acid synthase [FAS]), as well as chondrogenic genes (aggrecan, collagen II, and sox-9, as well as elastin) were used to evaluate the osteogenic differentiation, adipogenic differentiation, and chondrogenic differentiation respectively.
The primers used in this study are shown in Table 1.
Table 1.
Primer sequences for RT-PCR
| Gene | Primer | Product length |
|---|---|---|
| Osteocalcin | Forward Primer 5’-TACCCAGATCCTCTGGAGCC-3’ | 109 bp |
| Reverse Primer 5’-TGCCATAGAAGCGCCGATAG-3’ | ||
| RunX2 | Forward Primer 5’-CCAGCAGCACTCCATACCTC-3’ | 166 bp |
| Reverse Primer 5’-ACGCCATCGTTCTGGTTAGG-3’ | ||
| COL1A2 | Forward Primer 5’-GGTTTCGGCAAAGTTGGAGG-3’ | 218 bp |
| Reverse Primer 5’-GCCCTTTCTTGCAGTTGCC-3’ | ||
| Adiponectin | Forward Primer 5’-AGTCCTGAAGAAGGCACATCC-3’ | 238 bp |
| Reverse Primer 5’-TGTGTAACCTTGGGCGTTCA-3’ | ||
| AP2 | Forward Primer 5’-TGCTACCAGGAAAGTGGCTG-3’ | 215 bp |
| Reverse Primer 5’-CATCCCACTTCTGCACCTGT-3’ | ||
| FAS | Forward Primer 5’-CATGGAGGAGGTGGTGATCG-3’ | 303 bp |
| Reverse Primer 5’-CGAAGGGAAGCAGGGTTGAT-3’ | ||
| Aggrecan | Forward Primer 5’-CTCACGGTGAAACCCGTCTT-3’ | 146 bp |
| Reverse Primer 5’-TCGGGAAAAGCCCAGGGT-3’ | ||
| COL2A1 | Forward Primer 5’-CGAGACAGGTGCTGCAAGTC-3’ | 141 bp |
| Reverse Primer 5’-TGATCACCTGGTTTCCCACC-3’ | ||
| Sox-9 | Forward Primer 5’-CTCAGCAAGACTCTGGGCAA-3’ | 200 bp |
| Reverse Primer 5’-TTGGGAGAGATGTGCGTCTG-3’ | ||
| Elastin | Forward Primer 5’-GTTGGACCCTTTGGAGGTCA-3’ | 126 bp |
| Reverse Primer 5’-TCCAGGCCCAAAGCCGTAG-3’ | ||
| GAPDH | Forward Primer 5’-CCTCAACGACCACTTCGTCA-3’ | 237 bp |
| Reverse Primer 5’-GGGTCTGGGATGGAAACTGG-3’ |
Statistical analysis
Statistical evaluations were performed using a Student’s t test. A p value less than 0.05 was considered statistically significant.
Results
Culture of cartilage stem/progenitor cells from cartilage tissue and perichondrium
After 2 weeks of primary culture, a single cell formed a spherical colony of cells. CSPCs were polygon, while PSPCs showed fibroblastic morphology (Figure 1).
Figure 1.
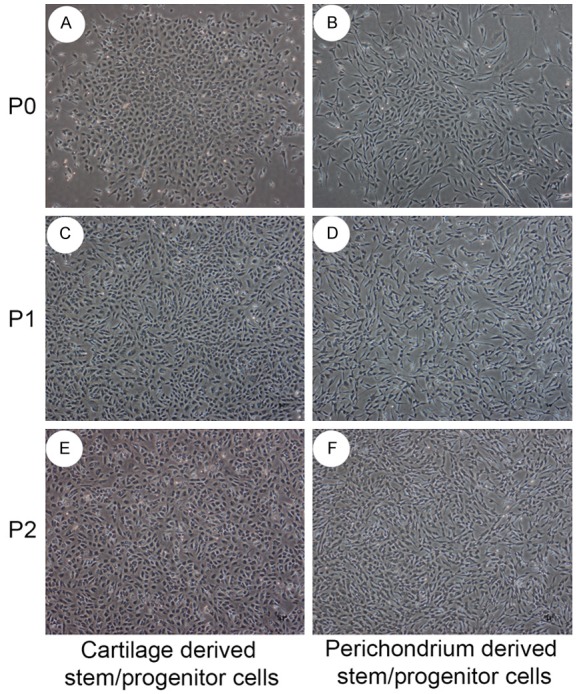
Cytomorphology of cartilage derived stem/progenitor cells and perichondrium derived stem/progenitor cells. Stem/progenitor cells in primary culture could proliferate and will form colonies. Auricular cartilage derived stem/progenitor cells in primary culture were small rounded and polygonal, and polygonal and spindle shaped in passage 1 culture and passage 2 culture. Perichondrium derived stem/progenitor cells were generally elongated and spindle shaped, as fibroblast-like.
Flow cytometry analysis
To identify the cell population isolated from two different tissues, flow cytometry was performed to characterize the cell-surface marker profile. The positive markers for MSCs such as CD29, CD44, and CD90, the negative markers for MSCs such as CD34 and CD45 were analyzed. High expressions of positive markers were observed in the cell population (CD29, 81.9±7.8% and CD44, 47.8±4.1%, as well as CD90, 86.8±9.1% for CSPCs, while CD29, 76.9±7.9% and CD44, 53.6±5.5%, as well as CD90, 82.9±8.9% for PSPCs, and almost no expressions of negative markers, indicating the cell population may be a stem cells population. In addition, CSPCs and PSPCs at passage 1, passage 2, and passage 3 showed the high expression of the positive markers (CD29, CD44 and CD 90), indicating they retained their markers over time (Figure 2).
Figure 2.
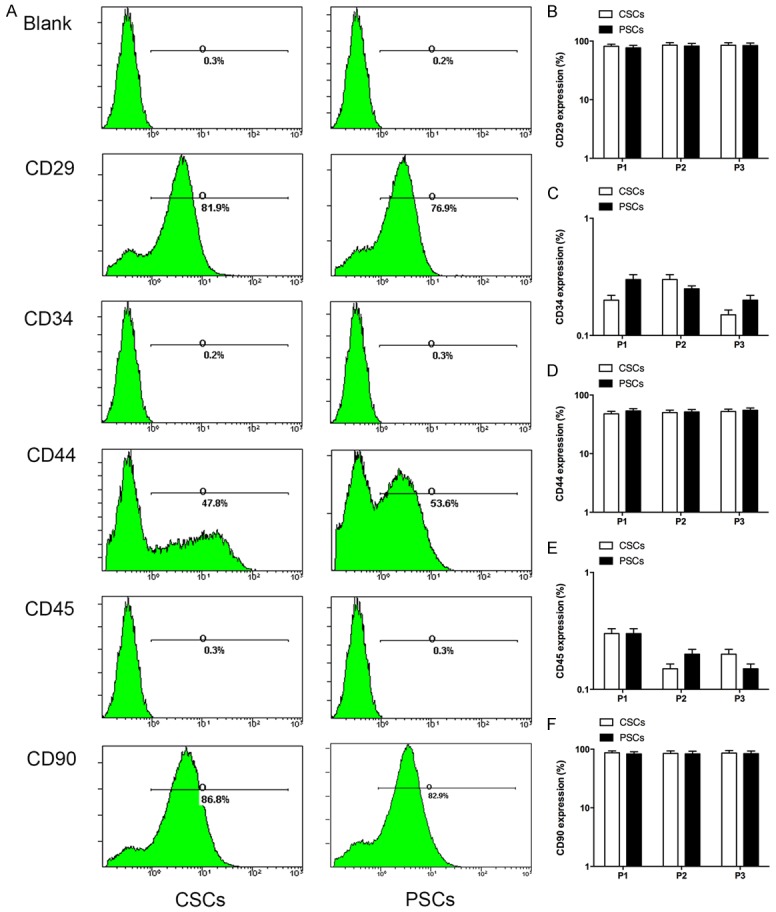
The expression of cell surface markers. The two kinds of stem/progenitor cells showed high expression levels of bone marrow mesenchyme stem cell positive surface markers (CD29, CD44 and CD90), while almost no expressions of mesenchyme stem cell negative surface markers (CD34 and CD45). The surface marker at passage 1, passage 2, and passage 3 showed a high expression, indicating they could retain their markers over time.
Clonogenicity assay
The clonogenicity was analyzed to assess the proliferation potency of single cells. Cells were maintained in monolayer cultures in DMEM medium containing 10% FBS. After seeding 100 cells on a plastic dish for 2 weeks, colonies were stained. CSPCs and PSPCs generated 94±8 and 96±7 colonies respectively. These findings indicated that most of cells could form colonies (Figure 3).
Figure 3.
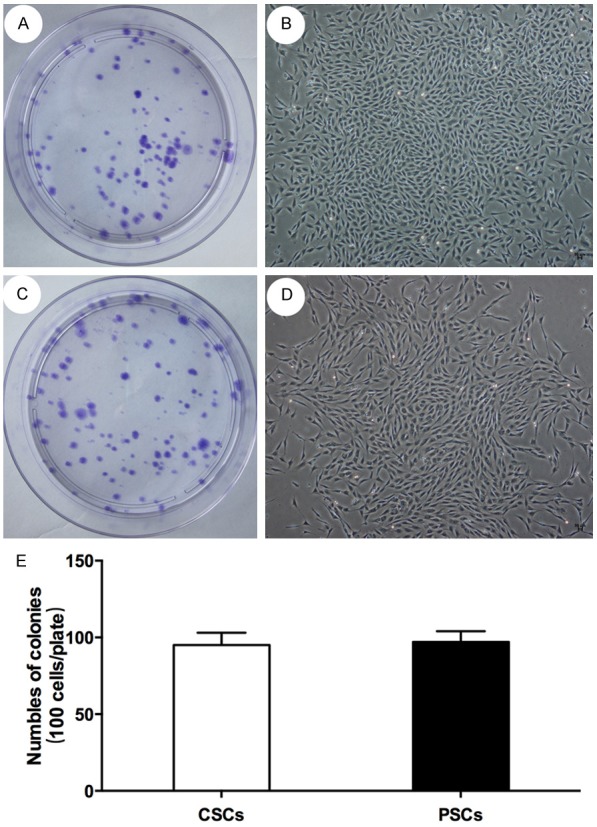
Colony formation assay. Auricular cartilage derived stem/progenitor cells and perichondrium cartilage derived stem/progenitor cells could gererate almost 95 colonies respectively in every 100 cells, indicating that most of cells could form colonies.
Cell proliferation
To test the cell proliferating capability, the proliferative rates were analyzed by using CCK-8 assay. There was a significant difference on proliferation between CSPCs and PSPCs (p<0.05), indicating PSPCs showed higher proliferative ability than CSPCs (Figure 7).
Figure 7.
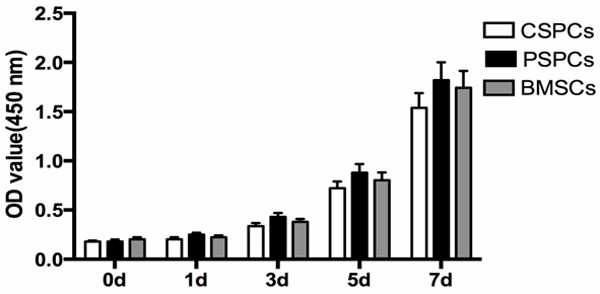
Cell proliferation. A significant difference on proliferation between perichondium derived stem/progenitor cells and cartilage derived stem cells was observed (p< 0.05), perichondium derived stem/progenitor cells are more proliferative.
Osteogenic, adipogenic, and chondrogenic differentiation
To test the pluripotency, osteogenic, adipogenic and chondrogenic differentiation studies were performed (Figure 4).
Figure 4.
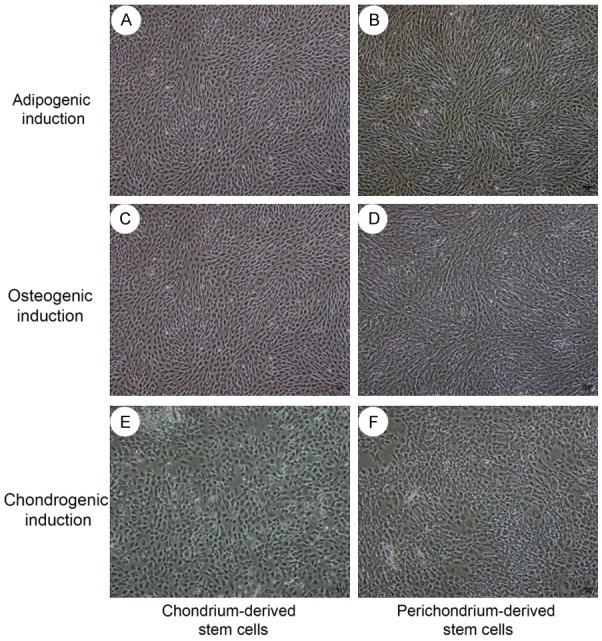
Cytomorphological change under osteocytic, adipocytic, and chondrogenic differentiation. After 3 days of osteocytic, adipocytic, and chondrogenic induction, the two cell populations showed special cytomorphologic change. Especially in chondrogenic induction, the cells changed into polygonal.
In osteogenic induction medium, all the two groups formed aggregates or nodules that were strongly positive for Alizarin Red after 16 days stimulation (Figure 5A, 5B).
Figure 5.
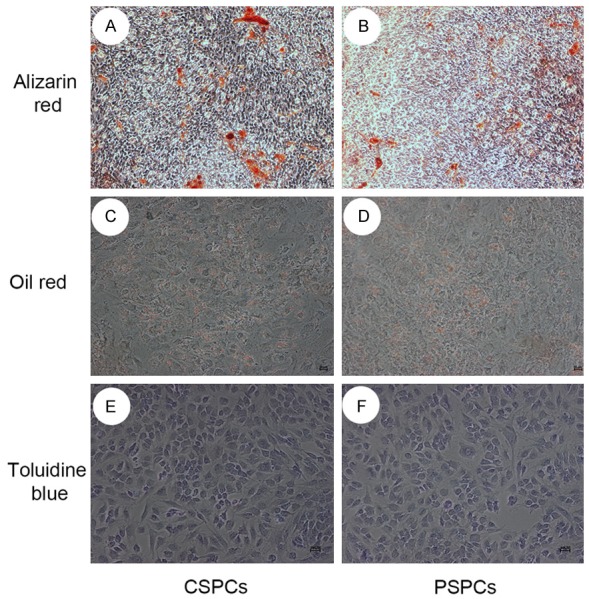
Osteocytic, adipocytic, and chondrogenic differentiation. Cells in osteogenic induction medium for 16 days could form nodules that were strongly positive for Alizarin Red. Lipid-rich vacuoles stained with oil red O were observed in cells after 2 weeks induction in adipocytic differentiation. After 2 week of chondrogenic induction in vitro, most cells in two groups were positive with toluidine blue.
For adipogenic differentiation, lipid-rich vacuoles were observed in cells with increased induction time, and lipid-rich vacuoles in induction group were stained with oil red O (Figure 5C, 5D).
After 2 weeks of culture in vitro, most cells were positive with toluidine blue, indicating CSPCs and PSPCs could differentiate into chondrocytes (Figure 5E, 5F).
Gene expression
The expression of chondrogenic genes (aggrecan, collagen II, and sox-9, as well as elastin) and osteogenic markers (osteocalcin, runx2, and collagen I) in CSPCs is higher than those in PSPCs after induction, indicating CSPCs is superior to PSPCs in chondrogenic differentiation and osteogenic differentiation. The expression of chondrogenic genes in CSPCs and PSPCs is higher than those in BMSCs after induction. In addition, the expression of collagen II and sox-9 in CSPCs and PSPCs without chondrogenic induction was observed (data not shown), indicating CSPCs and PSPCs are superior to BMSCs in chondrogenic differentiation (Figure 6B, 6C).
Figure 6.
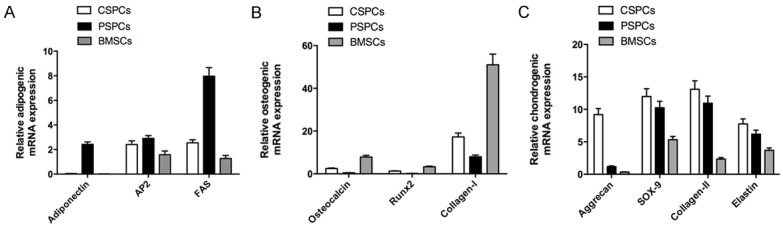
Q-PCR. The osteogenic markers (osteocalcin, runx2, and collagen I), and chondrogenic genes (aggrecan, collagen II, and sox-9, as well as elastin) in cartilage derived stem/progenitor cells after treatment is higher than those in perichondrium derived stem/progenitor cells (p<0.05). The expression of chondrogenic genes in cartilage derived stem/progenitor cells after treatment is higher than those in bone mesenchymal stem cells (p<0.05). However, the mRNA expression of adipogenic markers (adiponectin, adipocyte fatty acid binding protein [AP2] and fatty acid synthase [FAS]) in cartilage derived stem/progenitor cells after treatment is lower than those in perichondrium derived stem/progenitor cells (p<0.05).
While the expression of adipogenic markers (adiponectin, adipocyte fatty acid binding protein [AP2] and fatty acid synthase [FAS]) in PSPCs is higher than those in CSPCs after induction, indicating PSPCs is superior to CSPCs in adipogenic differentiation (Figure 6A).
Discussion
Stem or progenitor cells play an important role in the restore and recovery of damaged tissues or organs [23-25]. Stem cells based tissue engineering or regenerative medicine provide a promising therapy for the repair of cartilage defects [26-28]. For the past ten years, stem cells showed their magic effects in the treatment of diseases, especially, the application of BMSCs in clinical experiments [29,30]. BMSCs were widely used in cartilage tissue engineering and cartilage regeneration, owing to their easy accessibility, and high proliferation rate, as well as multipotency. The in vivo stable ectopic chondrogenesis by mesenchymal stem cells remains a problem [12]. Cui et al. found that low-intensity ultrasound induced ectopic chondrogenesis of MSCs in subcutis became ossified eventually at 6 weeks post-implantation [16]. Pelttari et al. reported that cartilage-like micromass formed by human MSCs in vitro was apt to calcify with vascular invasion after being implanted subcutaneously into SCID mice [11]. De Bari also et al. found that in vitro chondrogenically induced synovial membrane derived MSCs failed to form stable ectopic cartilage after being implanted intramuscularly or subcutaneously into nude mice, although these cells exhibited their chondrogenic phenotypes before implantation [17]. These results suggest that in chondrogenic differentiated MSCs may not necessarily render fully differentiated and thus they tend to lose induced phenotypes in long term, indicating that MSCs from these origins are not ideal seeding cells in cartilage tissue engineering or cartilage regeneration. To look for a novel seeding cells plays a vital role in the cartilage, and it is reported that many tissues and organs contain a certain number of adult stem cells [31]. Therefore, in current study, we tried to find stem cells in auricular cartilage and perichondrium.
Auricular cartilage is a specialized, gristly connective tissue, wrapped closely by perichondrium, which is a dense membrane that is composed of fibrous connective tissue. Takeshi Togo et al. reported the perichondrium contained tissue progenitor stem cells, which could differentiate into adipocytes as well as osteocytes in differentiation induction medium, and for cartilage reconstruction in vivo, the composites with perichondrocytes generated the same weight of cartilaginous tissue as those with chondrocytes, significantly bigger than rabbit bone marrow mesenchymal stem cells in the implant study [22]. In addition, Shinji Kobayashi et also found that human auricular perichondrium harbors a unique cell population, termed as cartilage stem/progenitor stem cells (CSPCs) [21]. In our current study cartilage and perichondrium were separated in the experiment and stems cells were isolated by a fibronectin differential adhesion assay, which was used by Gary P. Dowthwaite to isolate stem cells in the articular cartilage [19]. The difference between our study and previous studies on isolation of stem or progenitor cells in the perichondrium is that we used the fibronectin differential adhesion assay, so we can obtain much more stem cells than other methods. In addition, we also isolated stem or progenitor cells from auricular cartilage. The results of the flow cytometry showed these two kind of cell populations expressed mesenchyme stem cell positive surface marker highly, such as CD29, and CD44, as well as CD90, with little expression of CD34 and CD45. The colony forming assay also showed the high colony forming efficiency of the two cell populations.
Meanwhile, we tried to compared the difference on the proliferation and differentiation between two kinds of stems, and explore which kind of stem cell was more suitable in the cartilage regeneration. In current study, we found that the perichondrium derived stem or progenitor cells showed higher proliferation than the cartilage derived stem or progenitor cells, which was similar with the results of Takeshi [22]. In Takeshi experiment, they found perichondrocytes proliferated more rapidly than chondrocytes from the beginning of the primary culture. In addition, Shinji also demonstrated perichondrocytes morphologically resembled fibroblasts and maintained this appearance after long-term culture, and perichondrocytes contain highly expandable clones and proliferate 13% faster than chondrocytes [21]. We speculated that cartilage derived stem or progenitor cells and perichondrium derived stem cells were derived from different origins: perichondrium derived stem cells were derived from connective tissue, which contains higher proliferative cell populations, such as fibroblasts. We also found that perichondrium derived stem cells morphologically fibroblast-like, while cartilage derived stem cells are polygonal. For cell differentiation, cartilage derived stem cells were more superior in the chondrogenic and osteogenic differentiation than perichondrium derived stem cells, while perichondrium derived stem cells were more superior in the adipogenic differentiation than cartilage derived stem cells. This may be because cartilage derived stem cells derived from cartilage, they were more apt to chondrogenic and osteogenic differentiation. Especially in chondrogenic differentiation, cartilage derived stem cells and perichondrium derived stem cells experienced morphological changes toward chondrocytes-like after 3 days chondrogenic induction, which were superior to BMSCs (data not shown). In addition, the expression of chondrogenic genes in CSPCs and PSPCs is higher than those in BMSCs after induction, indicating they are more suitable in cartilage regeneration comparing with BMSCs.
In conclusion, we isolated cell populations from auricular cartilage and perichondrium, and confirmed their stem cells properties by expression of stem cell surface marker, and colony forming assay, as well as multiple differentiation potential. In addition, we compare the proliferation and differentiation ability among CSPCs, PSPCs and BMSCs. The current results indicated progenitor/stem cells derived from cartilage and perichondrium may be a new source of seeding cell in cartilage tissue or cartilage regeneration.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (81272128, 81471878) and National 863 program (SS2014AA20705).
Disclosure of conflict of interest
The authors report no conflict of interest.
References
- 1.Wilkes GH, Wong J, Guilfoyle R. Microtia reconstruction. Plast Reconstr Surg. 2014;134:464e–479e. doi: 10.1097/PRS.0000000000000526. [DOI] [PubMed] [Google Scholar]
- 2.Cabin JA, Bassiri-Tehrani M, Sclafani AP, Romo T 3rd. Microtia Reconstruction: Autologous Rib and Alloplast Techniques. Facial Plast Surg Clin North Am. 2014;22:623–638. doi: 10.1016/j.fsc.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Kasrai L, Snyder-Warwick AK, Fisher DM. Single-stage autologous ear reconstruction for microtia. Plast Reconstr Surg. 2014;133:652–662. doi: 10.1097/PRS.0000000000000063. [DOI] [PubMed] [Google Scholar]
- 4.Nomura H. Chest wall deformity at the site of costal cartilage harvesting. Adv Otorhinolaryngol. 2014;75:110–113. doi: 10.1159/000350976. [DOI] [PubMed] [Google Scholar]
- 5.Fujihara Y, Takato T, Hoshi K. Macrophageinducing FasL on chondrocytes forms immune privilege in cartilage tissue engineering, enhancing in vivo regeneration. Stem Cells. 2014;32:1208–1219. doi: 10.1002/stem.1636. [DOI] [PubMed] [Google Scholar]
- 6.Viti F, Scaglione S, Orro A, Milanesi L. Guidelines for managing data and processes in bone and cartilage tissue engineering. BMC Bioinformatics. 2014;15(Suppl 1):S14. doi: 10.1186/1471-2105-15-S1-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leor J, Amsalem Y, Cohen S. Cells, scaffolds, and molecules for myocardial tissue engineering. Pharmacol Ther. 2005;105:151–163. doi: 10.1016/j.pharmthera.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Bara JJ, McCarthy HE, Humphrey E, Johnson WE, Roberts S. Bone marrow-derived mesenchymal stem cells become antiangiogenic when chondrogenically or osteogenically differentiated: implications for bone and cartilage tissue engineering. Tissue Eng Part A. 2014;20:147–159. doi: 10.1089/ten.tea.2013.0196. [DOI] [PubMed] [Google Scholar]
- 9.Brady K, Dickinson SC, Guillot PV, Polak J, Blom AW, Kafienah W, Hollander AP. Human fetal and adult bone marrow-derived mesenchymal stem cells use different signaling pathways for the initiation of chondrogenesis. Stem Cells Dev. 2014;23:541–554. doi: 10.1089/scd.2013.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Izal I, Aranda P, Sanz-Ramos P, Ripalda P, Mora G, Granero-Molto F, Deplaine H, Gomez-Ribelles JL, Ferrer GG, Acosta V, Ochoa I, Garcia-Aznar JM, Andreu EJ, Monleon-Pradas M, Doblare M, Prosper F. Culture of human bone marrow-derived mesenchymal stem cells on of poly (L-lactic acid) scaffolds: potential application for the tissue engineering of cartilage. Knee Surg Sports Traumatol Arthrosc. 2013;21:1737–1750. doi: 10.1007/s00167-012-2148-6. [DOI] [PubMed] [Google Scholar]
- 11.Pelttari K, Winter A, Steck E, Goetzke K, Hennig T, Ochs BG, Aigner T, Richter W. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54:3254–3266. doi: 10.1002/art.22136. [DOI] [PubMed] [Google Scholar]
- 12.Liu K, Zhou GD, Liu W, Zhang WJ, Cui L, Liu X, Liu TY, Cao Y. The dependence of in vivo stable ectopic chondrogenesis by human mesenchymal stem cells on chondrogenic differentiation in vitro. Biomaterials. 2008;29:2183–2192. doi: 10.1016/j.biomaterials.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 13.Xue K, Qi L, Zhou G, Liu K. A two-step method of constructing mature cartilage using bone marrow-derived mesenchymal stem cells. Cells Tissues Organs. 2013;197:484–495. doi: 10.1159/000347238. [DOI] [PubMed] [Google Scholar]
- 14.Xue K, Zhu Y, Zhang Y, Chiang C, Zhou G, Liu K. Xenogeneic chondrocytes promote stable subcutaneous chondrogenesis of bone marrow-derived stromal cells. Int J Mol Med. 2012;29:146–152. doi: 10.3892/ijmm.2011.830. [DOI] [PubMed] [Google Scholar]
- 15.Hennig T, Lorenz H, Thiel A, Goetzke K, Dickhut A, Geiger F, Richter W. Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGFbeta receptor and BMP profile and is overcome by BMP-6. J Cell Physiol. 2007;211:682–691. doi: 10.1002/jcp.20977. [DOI] [PubMed] [Google Scholar]
- 16.Cui JH, Park SR, Park K, Choi BH, Min BH. Preconditioning of mesenchymal stem cells with low-intensity ultrasound for cartilage formation in vivo. Tissue Eng. 2007;13:351–360. doi: 10.1089/ten.2006.0080. [DOI] [PubMed] [Google Scholar]
- 17.De Bari C, Dell’Accio F, Luyten FP. Failure of in vitro-differentiated mesenchymal stem cells from the synovial membrane to form ectopic stable cartilage in vivo. Arthritis Rheum. 2004;50:142–150. doi: 10.1002/art.11450. [DOI] [PubMed] [Google Scholar]
- 18.Derks M, Sturm T, Haverich A, Hilfiker A. Isolation and chondrogenic differentiation of porcine perichondrial progenitor cells for the purpose of cartilage tissue engineering. Cells Tissues Organs. 2013;198:179–189. doi: 10.1159/000354897. [DOI] [PubMed] [Google Scholar]
- 19.Dowthwaite GP, Bishop JC, Redman SN, Khan IM, Rooney P, Evans DJ, Haughton L, Bayram Z, Boyer S, Thomson B, Wolfe MS, Archer CW. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004;117:889–897. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- 20.Henriksson H, Thornemo M, Karlsson C, Hagg O, Junevik K, Lindahl A, Brisby H. Identification of cell proliferation zones, progenitor cells and a potential stem cell niche in the intervertebral disc region: a study in four species. Spine (Phila Pa 1976) 2009;34:2278–2287. doi: 10.1097/BRS.0b013e3181a95ad2. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi S, Takebe T, Inui M, Iwai S, Kan H, Zheng YW, Maegawa J, Taniguchi H. Reconstruction of human elastic cartilage by a CD44+ CD90+ stem cell in the ear perichondrium. Proc Natl Acad Sci U S A. 2011;108:14479–14484. doi: 10.1073/pnas.1109767108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Togo T, Utani A, Naitoh M, Ohta M, Tsuji Y, Morikawa N, Nakamura M, Suzuki S. Identification of cartilage progenitor cells in the adult ear perichondrium: utilization for cartilage reconstruction. Lab Invest. 2006;86:445–457. doi: 10.1038/labinvest.3700409. [DOI] [PubMed] [Google Scholar]
- 23.Cashman TJ, Gouon-Evans V, Costa KD. Mesenchymal stem cells for cardiac therapy: practical challenges and potential mechanisms. Stem Cell Rev. 2013;9:254–265. doi: 10.1007/s12015-012-9375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kode JA, Mukherjee S, Joglekar MV, Hardikar AA. Mesenchymal stem cells: immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy. 2009;11:377–391. doi: 10.1080/14653240903080367. [DOI] [PubMed] [Google Scholar]
- 25.Shi Y, Hu G, Su J, Li W, Chen Q, Shou P, Xu C, Chen X, Huang Y, Zhu Z, Huang X, Han X, Xie N, Ren G. Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell Res. 2010;20:510–518. doi: 10.1038/cr.2010.44. [DOI] [PubMed] [Google Scholar]
- 26.Cancedda R, Dozin B, Giannoni P, Quarto R. Tissue engineering and cell therapy of cartilage and bone. Matrix Biol. 2003;22:81–91. doi: 10.1016/s0945-053x(03)00012-x. [DOI] [PubMed] [Google Scholar]
- 27.Guilak F, Awad HA, Fermor B, Leddy HA, Gimble JM. Adipose-derived adult stem cells for cartilage tissue engineering. Biorheology. 2004;41:389–399. [PubMed] [Google Scholar]
- 28.Tuan RS, Boland G, Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther. 2003;5:32–45. doi: 10.1186/ar614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Derubeis AR, Cancedda R. Bone marrow stromal cells (BMSCs) in bone engineering: limitations and recent advances. Ann Biomed Eng. 2004;32:160–165. doi: 10.1023/b:abme.0000007800.89194.95. [DOI] [PubMed] [Google Scholar]
- 30.Martin-Rendon E, Brunskill SJ, Hyde CJ, Stanworth SJ, Mathur A, Watt SM. Autologous bone marrow stem cells to treat acute myocardial infarction: a systematic review. Eur Heart J. 2008;29:1807–1818. doi: 10.1093/eurheartj/ehn220. [DOI] [PubMed] [Google Scholar]
- 31.Geetha VS, Kameswaran L. Effect of prostaglandins E2 and F2 alpha on tissue mast cells. Indian J Med Res. 1979;70:83–87. [PubMed] [Google Scholar]


