Abstract
Neurological disorders are an important global public health problem, but pharmaceutical treatments are limited due to drug access to the central nervous system being restricted by the blood-brain barrier (BBB). Poly(lactic-co-glycolic acid) (PLGA) nanoparticles (NPs) are one of the most promising drug and gene delivery systems for crossing the BBB. While these systems offer great promise, PLGA NPs also have some intrinsic drawbacks and require further engineering for clinical and research applications. Multiple strategies have been developed for using PLGA NPs to deliver compounds across the BBB. We classify these strategies into three categories according to the adaptations made to the PLGA NPs (1) to facilitate travel from the injection site (pre-transcytosis strategies); (2) to enhance passage across the brain endothelial cells (BBB transcytosis strategies) and (3) to achieve targeting of the impaired nervous system cells (post-transcytosis strategies). PLGA NPs modified according to these three strategies are denoted first, second, and third generation NPs, respectively. We believe that fusing these three strategies to engineer multifunctional PLGA NPs is the only way to achieve translational applications.
Keywords: PLGA, nanoparticle, systemic delivery, central nervous system
Introduction
Neurological disorders include more than 600 different diseases, and these disorders account for 12% of total deaths globally [1,2]. The economic costs of these conditions are immense. Neurological disorders contribute to 6.3% of the global burden of disease, and the global economic impact of dementia alone was greater than 600 billion dollars in 2010 [3]. In addition, new drug development for these diseases is generally slow [4]. In 2012, the United States (US) Food and Drug Administration (FDA) approved 39 new drugs, among which only two were approved for neurological disorders. The time required to develop a new neurotropic preparation is nearly 12-16 years, at a cost of 0.8-1.7 billion US dollars [5]. These difficulties in drug development are largely attributable to a shortage of effective delivery systems capable of bringing active compounds across the blood-brain barrier (BBB), an insurmountable obstacle to many promising drugs. Nearly 100% of large-molecule drugs and greater than 98% of small-molecule drugs do not cross the BBB [6].
To overcome the BBB, drug delivery using nanoparticle (NP) carriers has attracted attention as a promising strategy, mainly due to the tunable characteristics of NPs. NPs can be functionalized using specific proteins, peptides, and monoclonal antibodies, and their sub-micron size permits penetration deep into tissues via capillaries, where they can be taken up by the target cells [7].
Among the variety of NPs that can potentially be used as delivery vehicles for treatments for neurological disorders, some of the most promising are those made of poly(lactic-co-glycolic acid) (PLGA). PLGA is a block co-polymer made of polylactic acid (PLA) and polyglycolic acid (PGA) that has been approved by the FDA and European Medicine Agency for human use for many years [8,9]. PLGA has excellent biocompatibility, and upon exposure to the human physical environment, PLGA is hydrolyzed into lactic and glycolic acids, which are naturally-occurring metabolites [10]. PLGA nanoparticles can be easily modified to accommodate a range of functional or targeting options [8]. However, PLGA NPs without modifications also have a number of intrinsic drawbacks. Their negative surface charge makes cellular uptake inefficient. They have a relatively short blood circulation time and they do not pass through the BBB without further modifications [11].
In recent years, a variety of approaches have been developed to deliver PLGA NPs to the brain. In this review, we will first briefly introduce the biology of the BBB and typical approaches for the synthesis of PLGA NPs. Then, we will discuss three approaches that have been developed to engineer PLGA nanoparticles for enhanced drug delivery to the brain: 1) prolonged pre-transcytosis circulation, 2) increased BBB transcytosis, and 3) enhanced post-transcytosis NP-brain interaction.
Blood-brain barrier
The BBB is an active interface between the vascular system and the brain that supports tissue homeostasis, but also presents a major obstacle for drug delivery to the central nervous system (CNS) [12]. The exchange of substances across the BBB between the brain tissue and blood is restricted by both physical (tight junctions) and metabolic (enzymes) barriers [13].
Structure of BBB
The BBB is a complex structure composed of brain endothelial cells (BECs), astrocytes, pericytes (PCs), and the basement membrane (BM). BECs are the majority components and foundation of the BBB. BECs differ from other endothelial cells in their absence of fenestrations, more extensive tight junctions (TJs), and the sparse occurrence of pinocytic vesicular transport in these cells.
Three functional layers exist between the blood and brain: (1) capillary endothelial cells (BECs); (2) a basement membrane completely covering the capillaries, made of type IV collagen, fibronectin, and laminin, and containing embedded pericytes; and (3) astrocyte processes surrounding the basement membrane on the CNS side of the BBB [14]. Each of these layers may contribute to the restriction of solute movement into the brain (Figure 1).
Figure 1.
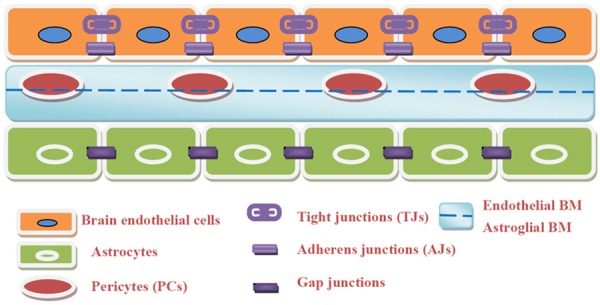
Simplify to a BECs-BM-Astrocytes sandwich-like triple structure of BBB. 1) Brain endothelial cells which connected with TJs and AJs, 2) Basement membranes compose of endothelial BM and astroglial BM with pericytes embedded in them, 3) Astrocyte processes that surround the basement membrane which connected with each other by GJs.
Functions of and routes across the BBB
The BBB controls the movement of electrolytes, xenobiotics, and circulating immune cells between the blood and CNS in order to maintain an optimal milieu for neuronal function [15]. As such, the BBB acts not only as a barrier to cells and solutes, but also as a carrier for selectively transported substances [16].
Potential routes for permeation and transport across the BBB are listed here: (1) transcellular diffusion, in which gaseous molecules and small lipophilic compounds passively diffuse across BECs with or without the assistance of active efflux carriers that may intercept some of these substances and pump them out of the endothelial cells; (2) carrier-mediated influx, which may be passive or secondarily active and serves as the transport mechanism for many essential polar molecules, such as glucose, amino acids, and nucleosides; (3) receptor-mediated transcytosis (RMT), in which macromolecules such as peptides and proteins, are actively transported across the cerebral endothelium; (4) adsorptive-mediated transcytosis (AMT), which appears to be induced non-specifically by certain positively charged macromolecules; and (5) tight junction modulation, in which altered connections between BBB cells increase the permeability of the paracellular aqueous diffusion pathway [17,18] (Figure 2). These mechanisms together account for the barrier properties of the BECs and include physical, active transport, enzymatic, and immunologic barriers to entry into the CNS. Drugs targeting the CNS must be engineered with these barrier functions taken into account.
Figure 2.
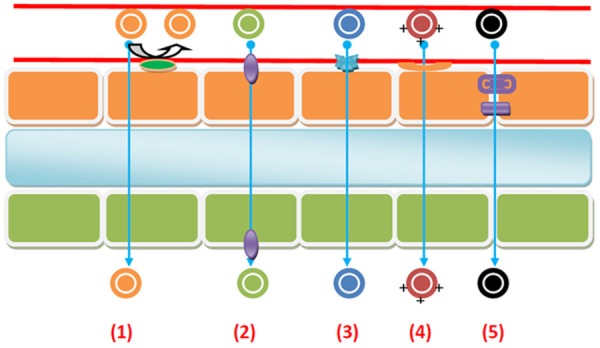
Routes for permeation and transport across the BBB: 1) Passive transcellular diffusion through BBB, while active efflux carriers may pump them out of the endothelial cell. 2) Carrier-mediated influx across BBB. 3) Receptor-mediated transcytosis (RMT) across BBB. 4) Adsorptive-mediated transcytosis (AMT) across the BBB. 5) Tight junction (TJ) modulation across BBB.
PLGA nanoparticles
Many formulation protocols have been developed for PLGA NPs. These can be divided into two classes: bottom-up and top-down techniques. Each has its advantages and disadvantages, but when selecting a formulation protocol, the principal considerations should be the desired chemical characteristics of the active component; the intended interactions with the organic solvents, polymers, and surfactants in use; and the planned application for the NPs.
Salting out methods are suitable for the formation of NPs at higher polymer concentrations, but the demands of these purification processes are a limitation. Nanoprecipitation methods may only be used for low polymer concentrations to maintain a mean NP size of 200 nm. Methods that involve solvent evaporation are more time-consuming and expensive, but these are less sensitive to changes in polymer concentrations. Emulsion evaporation, in particular, can be used for entrapment of hydrophilic (with or without emulsion) or hydrophobic (without emulsion) drugs [19].
The aggregation of PLGA NPs during solvent evaporation processes is a notable problem, regardless of the specific method used. In order to prevent PLGA-NP aggregation, polymer stabilizers are often used to coat NP surfaces, including polyvinyl alcohol (PVA) [20], polyvinylpyrrolidone (PVP), Tween 80 [21] and human serum albumin (HSA). However, these stabilizers are difficult to remove even with thorough washing protocols, and some are toxic to the BBB [22]. In an effort to avoid this problem, Jiang Chang et al. reported that protein adsorption (bovine serum albumin [BSA] or Tf) could be used to coat NPs without the use of detergent and increased particle stability at 37°C. Notably, this process avoided the use of chemical reactants and organic solvents, which may induce partial loss of active protein conformation [23].
Even though PLGA NPs have emerged as potentially promising carriers for CNS therapies, unmodified PLGA NPs present a number of intrinsic drawbacks: negative charge, hydrophobic structure, and non-targeting of the BBB. All of these characteristics adversely impact blood circulation time and the extent to which the NPs will be taken up by the target cells. Therefore, surface functionalization and further engineering of PLGA NPs are needed. Examples of PLGA-NP modifications for enhanced function include attachment of polyethylene glycol (PEG) polymer chains (PEG ylation); polymersome creation; core-shell type hybridization; cell-PLGA hybridization; surface derivatization; bisphosphonate-functionality; lectin-functionality; mannan-decoration; sialic acid-functionality; biotin-functionality; folate-functionality; transferrin-functionality; peptide-functionality; antibody-directed immunonanoparticle creation; nucleotide-functionality; magnetic NP creation; receptor-specific ligand-PLGA conjugation; and many other constructs. Each PLGA-NP formulation system offers distinctive design features [11].
Strategies for engineering PLGA NPs to cross the BBB
Over the last several decades, a variety of approaches have been developed to engineer PLGA NPs with greater efficiency for crossing the BBB and enhanced CNS activity. We classify these approaches into three major categories: (1) approaches for enhancing pre-transcytosis circulation; (2) approaches for enhancing BBB transcytosis; and (3) approaches for enhancing post-transcytosis NP-brain interactions (Table 1, Figure 3).
Table 1.
Strategies for engineering PLGA NPs across the BBB
| Strategies | Purpose | Methods | Corresponding PLGA NPs |
|---|---|---|---|
| Pre-transcytosis | Stabilization in blood circulation and escape reticulo-endothelial system | 1. Size control | The first generation: usually coated with surfactant |
| 2. Hydrophilic modification: PEGylation, human serum albumin (HSA), etc. | |||
| BBB transcytosis | Recognize the BECs and across the BBB | 1. Cell-penetrating peptides: TAT, SynB, etc. | The second generation: usually coated with both surfactant and specific ligands for BECs |
| 2. Receptor-mediated transportation: Polysorbate 80, Poloxamer 188, Transferrin, Leptin Receptor, g7, etc. | |||
| 3. Transporters-mediated transportation: Glutathione | |||
| 4. Adsorption-mediated transportation: Chitosan, Polyethylene imine, Poly-L-lysine, etc. | |||
| Post-transcytosis | Targeting and treating the lesion brain cells | 1. Targeting ligands strategy: Tf-Rs, MMP-2, Pep-1, AS1411(Ap), etc. | The third generation: usually coated with surfactant, specific ligands for BECs and lesion brain cells |
| 2. Environment-response strategy: pH sensitive component, enzyme-mediated activation, etc. |
Figure 3.
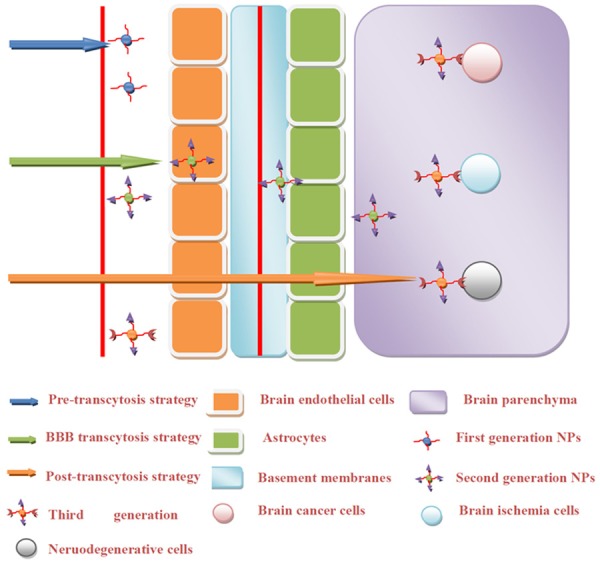
Strategies for engineering PLGA NPs across the BBB. 1) Approaches for enhancing pre-transcytosis circulation, 2) Approaches for enhancing BBB transcytosis, and 3) Approaches for enhancing post-transcytosis NP-brain interaction. Corresponding PLGA NPs are named by the first, second and third generation NPs.
Pre-transcytosis circulation
Size control
Size control is a crucial point for approaches intended to enhance pre-transcytosis circulation of NPs for drug delivery. NP size affects the biodistribution profile. NPs with mean diameters of 60-70 nm will be excreted faster, while larger NPs (200 nm or more) may be sequestrated by the liver or spleen. Therefore, NPs with diameters of 70-200 nm are optimal for in vivo applications [24]. Size control of PLGA NPs during synthesis may be achieved by modifying surfactant or polymer concentrations, polymer molecular mass, solvent type, and/or phase ratios. Careful tuning of these parameters has enabled important advances in the manipulation of NP characteristics to allow for improved drug entrapment efficiency [19].
Researchers are continually attempting to decrease the average NPs size with better control [19]. The polymer concentration has the largest effect on size. Higher (w/v) concentrations created larger particles, from between 0.1 and 10% (w/v) [25]. A concentration of 0.1% (w/v) resulted in 80 nm particles whereas 10% (w/v) yielded NPs of about 240 nm. Furthermore, it has been reported that higher molecular weight PLGA polymers make larger particles [26,27]. Konan et al. tested a variety of molecular weights from 12 to 98 kDa at two ratios of poly(lactic) to glycolic acid, namely 50:50 and 75:25. An increase in w/w % resulted in increasing the particle size whereby typically sizes obtained by this method are about 100-160 nm [27]. Also, particle size is affected by stirring speed and time. Stirring speeds were varied between 2000 and 13,500 rpm, and the smallest NPs of 155 nm were obtained at the highest speed. The stirring time for the smallest sized NP was determined to be 15 min. No further decrease in size could be obtained by increasing the stirring time up to 45 min [19,28].
Zhou et al. developed a partial centrifugation technique to get a smaller and desired diameter of PLGA NPs. They subjected the particle solution with low-speed centrifugation (8,000× g for 10 min) first to cause larger particles to pellet and been removed (Figure 4A). Then NPs in the supernatant were collected and washed using high-speed centrifugation (100,000× g for 30 min). SEM showed that NPs isolated using this protocol were 74±18 nm in diameter with a yield of 12±2% (Figure 4B). While the other group using conventional centrifugation techniques were 150±30 nm in diameter with an average yield of 55±5% (Figure 4C). And also the different of the solution can change the size of the particles. They chosen ethyl acetate (EA) as the solvent instead of DCM were 65±16 nm in diameter with yield improved to 44±3% (Figure 4D), and the cryoprotection with trehalose did not alter NPs size (Figure 4E) [29].
Figure 4.
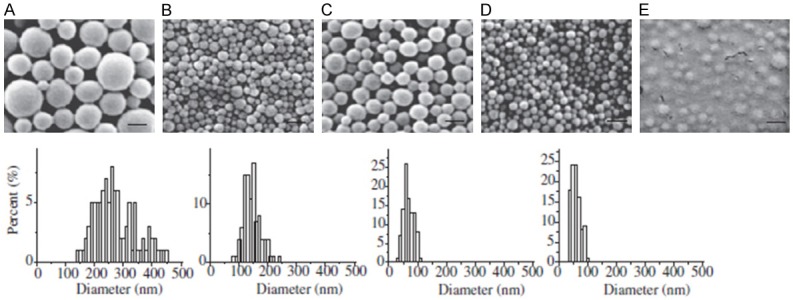
Size and characterization optimizing of PLGA NPs by using partial centrifugation technique. (A) the pellet from the first low-speed centrifugation, (B) the pellet from the high-speed centrifugation of the supernatant from the first centrifugation (small nanoparticles), and (C) nanoparticles synthesized using standard procedures. (D) Morphology and size distribution of small nanoparticles with EA used as solvent. (E) SEM of small nanoparticles cryoprotected with trehalose.
Hydrophilic surface modification
Due to their hydrophobic structure, PLGA NPs experience high rates of opsonization by the reticuloendothelial system (RES). To reduce opsonization and uptake by RES organs, thereby prolonging NP circulation, several strategies have been developed to create a hydrophilic cloud around the NPs. Nanoparticles have been applied to PLGA NPs to provide such hydrophilic coatings, including PEG (polyethylene glycol) [30], human serum albumin (HSA) [31], PEO (polyethylene oxide) [32], poloxamers and poloxamines [33,34], polysorbate 80, TPGS and polysaccharides like dextran [35,36]. Hydrophilic polymers may be applied to the surfaces of NPs by adsorption of surfactants or by utilizing block or branched copolymers [35,36]. Among them, the PEG and the HSA are the most commonly used.
PEGylation
PEG (Polyethylene glycol) is the most used polymers for drug delivery applications and the first PEGylated product is already on the market for over 20 years. In 1990, PEGylated adenosine deaminase became the first approved polymer- protein conjugate in USA [37] and thanks to this, plus the possibility to solve most of other polymers problems in drug delivery, the interest in PEG has grown exponentially [38]. PEGylation technology contributes to (a) increasing the aqueous solubility and stability, (b) reducing intermolecular aggregation, (c) decreasing immunogenicity, and (d) prolonging the systemic circulation time of a compound. PEG is often linked to PLGA to achieve similar beneficial effects [11].
Surface conjugation of human serum albumin (HSA)
Human serum albumin (HSA) is known to be the most abundant native protein in human body which has various advantages including ready availability, biodegradability, and low toxicity and immunogenicity [39]. HSA has received special interest in drug delivery studies due to its ability for passive targeting through enhanced permeation and retention (EPR) effect in tumor tissues [40]. Saeed Manoochehri et al. prepared docetaxel loaded PLGA NPs and surface conjugated with HSA. Result showed that this NP had more cytotoxicity against tumor cell lines and albumin conjugated PLGA NPs may represent a promising drug delivery system in cancer therapy [31]. Wohlfart’s lecithin-containing PLGA/HSA particles (Dox-Lecithin-PLGA/HSA) also reveal a considerable anti-tumour effect and demonstrate that this NP enable delivery of doxorubicin across the BBB in the therapeutically effective concentrations [41].
BBB transcytosis
As previously mentioned, there are five routes by which cells and molecules may travel across the BBB. Within these routes, interactions of materials with BECs during transit may include eight separate mechanisms: adsorption to BECs, inhibition of efflux systems, opening of tight junctions, endocytosis and transcytosis by the BECs, and others [42,43]. However, not all of these mechanisms are suitable targets for PLGA-NP modifications. Cell-penetrating peptides (CPPs), receptor-mediated endocytosis ligands, and adsorptive-mediated endocytosis ligands are the most prominent candidate biomolecules targeting underlying mechanisms for the transport of drugs across the BBB into the brain [43,44].
Cell-penetrating peptides
CPPs originate from various families and are heterogeneous in size and sequence. They all possess multiple positive charges at physiological pH. CPPs facilitate cellular uptake of various molecular attachments and therefore may be appropriate candidates for facilitating brain delivery of NP-encapsulating peptides [45]. A number of CPPs have already demonstrated improved therapeutic molecule delivery across the BBB for the treatment of CNS diseases [46]. These CPPs include trans-activating transcriptional activator (TAT), Angiopep, penetratin, TP, rabies virus glycoprotein (RVG), prion peptide, and SynB [47].
The TAT peptide is a nonamphipathic arginine-rich CPP derived from a human immunodeficiency virus (HIV)-1 protein which widely used to enhance cellular uptake. Recently, the TAT peptide was modified by adding histidine (H), mTAT, significantly improved gene delivery efficiency, by up to 7000 fold [48]. Zhou et al. conjugated mTAT to PLGA NPs and tested their gene delivery effectiveness when exposed to HEK 293T cells. Gene delivery efficiency peaked with addition of 5 mg avidin/mTAT per 100 mg PLGA, where the luciferase expression was 270 fold greater than that obtained using NPs without mTAT [49].
The SynB peptides are a family of CPPs that show charge-mediated BBB selectivity, with uptake proceeding via a caveolae independent pathway [50]. There have also been recent reports of the use of SynB peptides as brain transport systems for NPs. For example, intravenously injected SynB pegylated gelatin siloxane NP (SynB-PEG-GS) levels in the brain were significantly higher and levels in the liver significantly lower compared to plain NPs [51]. Penetratin, a CPP with a relatively low content of basic amino acids, has been used to functionalize poly(ethylene glycol)-block-poly(lactic acid) (PEG-PLA) NPs, and penetratin enhanced the cellular accumulation [52]. A thermally responsive elastin like polypeptide (ELP) covalently attached to a CPPs and a therapeutic inhibitory peptide has been shown to be able to enhance delivery to rat brain tumors and mediate uptake across the tumor cells’ plasma membranes on intravenous administration [53].
The non-selection of CPPs to all kinds of cells greatly restricts their application as pharmaceutical tools, and hence methods of targeting CPPs are being investigated. The stability of peptide vectors is an important factor regarding their use for in vivo delivery, as the vector must not be metabolically cleaved until it delivers its cargo to the appropriate target [45].
Receptor-mediated transportation
Receptors that are highly expressed on BECs include low-density lipoprotein receptor (LDLR), transferrin receptor (TfR), insulin receptor, insulin-like growth factor receptor, diphtheria toxin receptor, nicotinic acetylcholine receptor (nAChR), and scavenger receptor class B type. These receptors can be targeted with suitable ligands to mediate drug delivery across the BBB [54].
Polysorbate 80 (tween 80) and poloxamer 188
Polysorbate 80 was considered to be a ‘gold standard’ coating surfactant for brain delivery since poly (butyl cyanoacrylate) (PBCA) NPs coated with polysorbate 80 (Tween 80) facilitate the brain delivery of a number of drugs that are unable to cross the BBB in free form [42]. Binding of loperamide and doxorubicin to PBCA NPs coated with polysorbate 80 induced considerable analgesia effects and high anti-tumour effect [55]. Poloxamer 188 (Pluronic F-68) also considerably enhanced the anti-tumour action of doxorubicin loaded to PBCA NPs against intracranial glioblastoma [56], and it appeared to be similarly effective with polysorbate 80. This phenomenon was attributed to these two compounds coating NPs selectively adsorb certain plasma proteins especially the apolipoproteins E and B on the nanoparticle surface. While the apolipoproteins promote receptor-mediated endocytosis of the particles by the BECs, and facilitating these drug-loaded NPs across the BBB [57].
PLGA NPs coated with poloxamer 188 or polysorbate 80 was studied by Svetlana Gelperina for an efficient brain delivery. The result showed all formulations extended the survival times of the tumor-bearing animals when compared to control. And the Dox-PLGA/PVA + P188 was most effective: a long-term remission (>100 days without tumour) was observed in 40% (4/10) of the animals treated with this formulation [55]. Mayank Chaturvedi et al. formulate TIMP-1-loaded PLGA NPs and coating with polysorbate 80 to enhance their BBB penetration. And coming the results of neither Ps80-coated nor uncoated NPs caused significant opening of the BBB, and essentially they were nontoxic. Penetration studies showed that TIMP-1 NPs + Ps80 had 11.21%±1.35% penetration, whereas TIMP-1 alone and TIMP-1 NPs without Ps80 coating did not cross the endothelial monolayer. In vivo studies indicated BBB penetration of intravenously injected TIMP-1 NPs + Ps80 [58].
Transferrin (Tf)-coated and lactoferrin coated NPs
Another possibility is using the transferrin(Tf) since Tf-receptors (Tf-Rs) are overexpressed in BECs and glioma cells [59,60]. Transferrin or anti-transferrin receptor monoclonal antibodies (OX26) coating to the human serum albumin NPs which loading loperamide induced very significant antinociceptive effects in the tail-flick test in ICR (CD-1) mice, while the control group modified with IgG2a antibodies yielded only insignificant marginal effects [61].
Yanna Cui et al. found a noticeable fluorescence signal at the brain tumor site was observed after coumarin-6 (CM)-NPs-Tf injection as compared to CM-NPs injection. In particular, the fluorescence intensities of the circled regions in were 0, 5.9×1010 and 1.3×1011 after injection of blank NPs, CM-NPs and CM-NPs-Tf, respectively [62].
These finding were supported by Chang et al. who evaluated the endocytosis of PLGA NPs coated with Tf in vitro model of the BBB made of a co-culture of brain endothelial cells and astrocytes. PLGA NPs were prepared using a fluorescent dye and coated with Tween 20, BSA and transferrin (Tf). The result revealed that cellular endocytosis of Tf-PLGA NPs was about 20-fold greater than Blank NPs and 2-fold greater than BSA-NPs [23].
Lalani J et al. compare brain targeting efficiency of tramadol-loaded PLGA NPs surface modified with transferrin (Tf) and lactoferrin (Lf). Tf and Lf anchored NPs exhibit enhanced uptake with 2.38 and 3.85 folds higher targeting respectively in the brain when compared with unconjugated NPs. The brain targeting observed for Lf anchored PLGA NPs (Lf-TMD-PLGA-NP) was 1.62 folds that of Tf anchored PLGA NPs (Tf-TMD-PLGA-NP) [63]. Hence, all the study revealed Tf and Lf as promising and effective targeting ligands that could facilitate transport of PLGA NPs across BBB and improve drug accumulation in the brain.
Leptin receptor
Leptin can bind to the leptin receptor in the choroid plexus and BECs, where it is taken up into the brain parenchyma. Tosi et al. conjugated G21, a leptin12-32 fragment, on the surface of PLGA NPs and found this NPs across the BBB on intravenous injection, with 0.16% of the injected dose of nanoparticles reaching the brain after 2 h [64].
Others
Peptides such as g7 (NH2-Gly-L-Phe-D-Thr-Gly-L-Phe-L-Leu-L-Ser(O-β-D-Glucose)-COOH, g7) are similar to opioid peptides and can be employed to deliver model drugs into the central nervous system. G7 bound to PLGA NPs also enabled the delivery of loperamide and yielded prolonged antinociceptive reactions [65]. The reason for this behaviour is not clear; since it is known that the parent opioid peptides crosses the BBB by adsorbption-mediated endocytosis, due to their amphypathic character and helical conformation. It is possible to hypothesize a similar mechanism for the BBB crossing of these drug carriers [66].
Pep TGN (TGNYKALHPHNG), a 12-amino-acid-peptide, was employed a drug delivery system targeting to the brain and which was displayed by bacteriophage clone 12-2 and finally selected by rounds of in vivo screening. The green fluorescence could be observed much stronger for Pep TGN- CM-PEG-PLGA NPs than that unmodified NP, which suggesting that the Pep TGN conjugation NPs resulted in higher cellular uptake when incubated with bEnd.3 cells. Enhanced brain accumulation efficiency together with lower accumulation in liver and spleen was observed in the nude mice intravenously injected with Pep TGN conjugated NPs compared with those injected with plain NPs, showing powerful brain selectivity of Pep TGN [67].
These results indicate that different receptors in the BECs could be employed for the delivery of drugs across the BBB. It is possible that any ligand for which a receptor exists on the BECs may be used for this purpose. However, there is no idea which one ligand is the best and whether any other ligand exist. Maybe the phage display and screening skill for new ligands is a promising stratagem. On the other hand, since most of these receptors are ubiquitously expressed, there is the danger of non-specific adverse effects resulting in potential limitations of this approach.
Transporters-mediated transportation
In addition to receptors, active transport systems crossing the BBB can also be used for brain-targeted delivery. These include amino acid, hexose, and monocarboxylate transporters [68]. In particular, the transporter for glutathione is highly expressed at the BBB. G-Technology is a glutathione-conjugated liposome delivery system that was developed for the transport of molecules across the BBB, and several drugs have been successfully delivered to the brain using this system. One of these, glutathione PEGylated liposomal doxorubicin has undergone phase I/II clinical evaluation and may become the first targeted nanomedicine approved in the world. This well-defined system offers favorable pharmacokinetics and safety for the CNS delivery of bioactive ligands [54].
Glutathione is an endogenous tripeptide thiol that helps protect cells from reactive oxygen species (ROS). The use of glutathione-coated NPs takes advantage of the large number of glutathione transporters present at the BBB. As an example of this type of system, Werner Geldenhuys et al. used doxorubicin-loaded PLGA-COOH (DL-lactide-co-glycolide) coated with a glutathione-PEG conjugate (PEG-GSH) to target drug delivery to the brain and demonstrated NP permeation in an in vitro BBB model. These authors reported that 5% GSH-PEG-coated NPs achieved the greatest permeability across their system, which included BECs and a porous support membrane. In this study, 1 mM GSH-PEG-coated NPs demonstrated significantly greater permeation efficiency than a free drug solution over 48 hours and at all time periods investigated [69].
Adsorption-mediated transportation
Ultrastructural studies show that both the luminal and abluminal surface of BECs presents an overall negative charge, they will repel anionic molecules and help positively charged delivery systems interact with the BBB through adsorption-mediated endocytosis [70]. Cationic surfactant coating is believed to render positive charge to nanoparticles, therefore improving their interaction with cells and tissues. Another reason for cationic coating with surfactants would be significant reduction in size, leading to ‘stealth’ properties and prolonged circulation time [71]. However, poor selectivity is the predominant problem of adsorptive-mediated targeting.
Chitosan
Chitosan is a natural cationic polymer which has been endowed with positive charges from the protonated amines. Jaruszewski KM et al. have demonstrated that the surface adsorption of chitosan improves the stability of PLGA NPs during lyophilization; lowers their propensity to aggregate in aqueous solutions; protects the antibodies conjugated to the nanoparticle surface; and enhances their cellular uptake. Moreover, due to structural similarity with sugars that are widely used as cryoprotectants, chitosan also serves as a cryoprotectant capable of preserving the integrity of PLGA NPs as well as the proteins conjugated to the nanovehicle surface during lyophilization [72].
Polyethylene imine
Polyethylene imine (PEI), a commercially available cationic polyamine, is one of the most successful and widely studied cationic polymers. It is widely regarded as a gold standard for the comparison of transfection efficiencies among non-viral vectors[71]. Jayeeta et al. introduce PEI to STAT3 siRNA PLGA NPs and find that PEI dramatically changed the surface charge from negative to positive charge. It facilitated adherence of the NPs to the negatively charged cellular membranes [73]. Jayeeta have also noticed that release of siRNA from PEI-PLGA complex was faster, possibly due to compatible size of the NPs and possibly for higher electrostatic interaction between PEI pre-complex and PLGA [74].
However, PEI is associated with toxicity issues which limits its application. Conjugation of hydrophilic polymer poly(ethylene glycol) (PEG) was proposed as a solution to this problem, while it also shield the positive charge of the NPs and prolonging circulation time [71].
Poly-L-lysine
Poly-L-lysine (PLL) is obtained through polymerization of N-carboxy-anhydride of lysine. Jeremy et al. use DCC as the coupling agent to conjugate PLL to PLGA, and they find that the incorporation of PLGA-PLL reduced the overall negative surface charge in particles. The surface charge became positive for blank particles in which half or more PLGA-PLL/PLGA was incorporated. The utility of these particles as a vehicle for transfection was evaluated using COS cells. Transfection efficacy for all particles was determined by quantifying the expression of luciferase encoded on the plasmid. Levels of luciferase expression were statistically greater than blank particles at higher weight percentages of PLGA-PLL/PLGA (≥10%) particles [75].
Polymethacrylates
Polymethacrylates are cationic polymers with vinyl-base and they also possess the ability to form polyplexes on condensation of polynucleotide in the nanometer range. Commercially available polymethacrylate Eudragit E100 was combined with FDA-approved polymers PLGA/PLA to give nanoparticles of a cationic nature using cationic surfactant cetyltrimethylammonium bromide (CTAB) with significantly high transfection efficiency [76,77].
Poly(b-amino ester)
Poly(b-amino ester) (PBAE) is a new class of cationic polymers of biodegradable and non-toxic nature. PBAE polyplexes showed transfection efficiencies similar to PEI and low cytotoxicity in vitro. PBAE in combination with PLGA was used for DNA vaccination and PBAE/PLGA NPs succeeded in significant reduction of tumor size in mice. PBAE/PLGA particles were also able to delay the release of plasmid DNA for several days [78,79].
Other cationic material including DODAB, cetrimide, protamine and DC-chol (3b-[N-(dimetyhylaminoethane carbamoyl)] cholesterol), coating PLGA NPs have been evaluated for delivery of plasmids in pulmonary epithelial. A prolonged and high level of gene expression was observed for positively charged coated PLGA NPs. These NPs were also found to be less toxic than PEI NPs while highest cellular uptake was observed with DC-chol coating [80]. PLGA NPs coated with cationic surfactant DMAB were studied for oral delivery of paclitaxel which showed an equivalent anticancer efficacy to intravenously administration, while it obtained with 50% lower dose of paclitaxel encapsulated in this NPs [81].
Post-transcytosis NP-brain interaction
Once PLGA NPs cross the BBB into the CNS, targeted delivery systems are needed so that NPs reach and enter the sites of disease. In this review, we will focus on adaptations targeting brain tumors, but the principles of this discussion may be generalized to other clinical targets.
Targeting ligands strategy
Brain tumor cells overexpress several receptors, including epidermal growth factor receptor, matrix metalloproteinase-2 (MMP-2), integrins, interleukin 13 receptor, nucleolin, TfRs, and low-density lipoprotein receptor [82-84]. Previous studies of brain tumor-targeted drug delivery studies have generally focused on these receptors.
TfRs and low-density lipoprotein receptor-related protein (LRP) are highly expressed on both glioma cells and BECs, making it possible for only one ligand to both facilitate passage across the BBB and also to target tumor cells. Coating PLGA NPs with transferrin (Tf) has also been found to greatly prolong blood half-life when coated NPs are intravenously injected in rat and mouse models. In a study using F98 cells, the uptake of Tf-NP by F98 cells increased both in vitro and in vivo, with excellent penetration of Tf-NPs into brain tumors. These data support Tf-NPs as a promising nanomedicine construct for the delivery of antineoplastic drugs to glioma cells at early and late tumor stages [85].
Matrix metalloproteinase-2 (MMP-2), an essential proteinase regulating brain tumor invasion and angiogenesis, was used in PLGA NPs delivery system as one of the therapeutic target. A RNAi gene which specifically suppress MMP-2 was designed and co-capsulated with paclitaxel into PLGA NPs to achieve a sustained release of both agents. The vivo in BALB/c nude mice showed the dual delivery system was able to impose significant tumor regression compared with single delivery system [86].
Pep-1, one specific ligand of interleukin 13 receptor α2 (IL-13Rα2) which over-expressed on glioma cells and primary glioblastoma cells, was identified to exhibit excellent capacity of crossing the blood tumor barrier (BTB) and homing to giloma. Pep-1 was functionalized to the surface of PEG-PLGA NPs and it exhibited a significantly enhanced cellular association in rat C6 glioma cells and improved penetration in 3D avascular C6 glioma spheroids compared with non-targeting NPs. In vivo, Pep-NP could facilitate the distribution of the coumarin-6 about 2.21 times higher than non-targeting NPs in glioma region [87].
Recently, several technologies were developed to screen for peptides or aptamers that possess high binding efficiency and high specificity. Phage display and SELEX screens are examples. AS1411 (Ap), a DNA aptamer specifically binding to nucleolin which was highly expressed in the plasma membrane of both cancer cells and endothelial cells in angiogenic blood vessels, was discovered by SELEX [88]. AS1411 modified PLGA NPs (AsNPs) as the targeting ligand displayed approximately 2-fold higher uptake by and localization in brain tumor cells compared with unmodified NPs. In vivo, paclitaxel-loaded AsNP effectively slowed tumor growth and prolonged the median survival time of brain tumor-bearing mice, which was significantly better than the values obtained using unmodified NPs [89].
Environment-response strategy
A large variety of NPs responding to physical stimuli (temperature, electrical, electrochemical, light, magnetic, and ultrasonic), chemical stimuli (pH, ionic, and redox), or biological stimuli (enzymes, glucose, and inflammation) have been synthesized and developed as effective delivery systems. Stimulus-responsive NPs are termed as ‘smart’, ‘intelligent’, or ‘environmentally sensitive’, and have greater potential than traditional delivery systems [90,91].
Among the different types of stimuli, the major releasing mechanisms are pH-sensitive release in brain cancer therapy. The tumor microenvironment is slightly acidic with a pH of about 6.5-7.2 while the endosomes and lysosomes in cells have a pH around 4.5-5.5 [92]. To overcome lysosomal degradation and achieve NPs and/or loaded proteins release into cytosol, Resham Chhabra et al. propose the formulation of hybrid NPs by adding 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) as pH sensitive component in the formulation of PLGA NPs. Their experiments show that NPs are able to transiently destabilize the integrity of lysosomes in which they are taken up, speeding their escape and favoring cytoplasmatic localization [93]. Another example is a multifunctional NPs system that consists of TAT-PEG-PLGA and a pH-sensitive diblock copolymer, poly(L-cystine bisamide-g-sulfadiazine)-b-PEG (PCBS-b-PEG). At an acidic tumor site, the NPs are subject to a change in the charge of sulfonamide, which in turn destroys the physical association between sulfonamide and TAT. This deshielding leads to TAT-mediated translocation of the TAT-PEG-PLGA micelles into tumor cells. On the other hand, the detached PCBS-b-PEG is degraded by glutathione [11].
Enzyme-mediated activation is also an attractive mechanism for therapeutic activation of NPs. Gu et al. developed a activatable cell penetrating peptides (ACPP) strategy by linked LMWP with an MMP-2/9-cleavable peptide (PLGLAG), which formed a activatable LWMP (ALMWP). This ALWMP was conjugated to NPs, allowing for delivery of paclitaxel. In mice bearing intracranial C6 glioma tumors, ALWMP NPs carrying paclitaxel led to significantly longer survival times when compared to Taxol (p < 0.01) as well as LMWP-modified NPs (p < 0.05) [94].
Conclusions and future directions
In most cases, a single strategy does not achieve both goals of crossing the BBB and targeting treatment for CNS disease. In order to provide successful therapies for the full range of neurological disorders, fusion methods using different strategies are needed. Such targeted therapies require the formulation and construction of multifunctional engineered PLGA NPs.
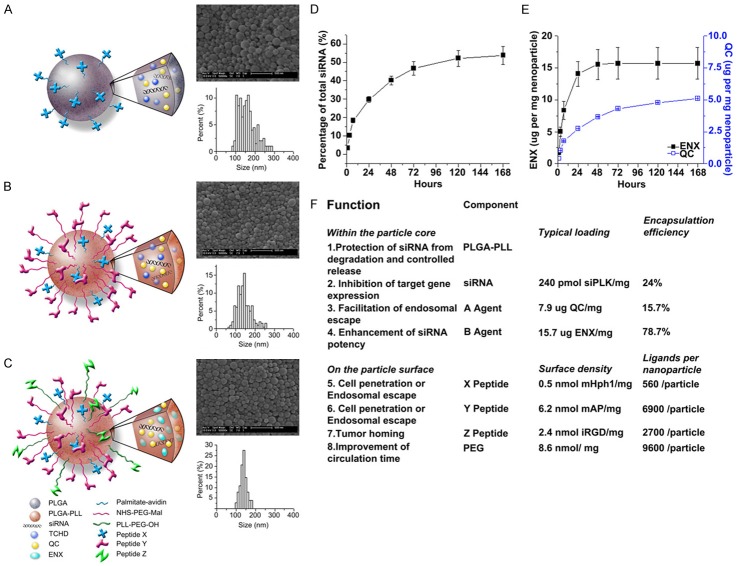
Examples of this next generation of PLGA NPs are those described by Hanjie Wang et al. This group has constructed multifunctional, magnetic PLGA/MPLs which are mainly self-assembled from two parts: (1) hydrophobic PLGA cores for loading drugs and magnetic nanocrystals and (2) polymeric lipid shells anchored with functional molecules, such as PEG chains, TAT peptides, and RGD peptides that can provide additional functionality, including helping the vectors condense genes, prolonging circulation time, enhancing BBB penetration, and targeting delivery to neoplastic tissue [95]. A second example of multifunctional PLGA NPs is the work of Zhou et al., further improving multifunctional PLGA NPs by synthesizing particles with three levels of complexity (Figure 5A-C). Zhou et al. report the formulation of PLGA NPs with surface modification by two peptides (X and Y) for the purposes of cell penetration or endosomal escape and by Z peptides for the function of tumor uptake. These modifications provided access across the BBB and tissue targeting for encapsulated nucleic acids (siRNA) and drugs (quinacrine and enoxacin) that were then slowly released at their target sites (Figure 5D, 5E). Potential modifications to these NPs included polymer matrix selection for stabilization or controlled release, the introduction of gene knockdown capabilities, enhanced cell penetration or endosomal escape properties, tumor targeting options, and other choices [49] (Figure 5F).
Figure 5.
Characterization of eight separate functions PLGA NPs. (A-C): schematics, morphology e as captured by SEM, and size distribution of multifunctional nanoparticles conjugated with a single peptide X (A), two peptides X and Y (B) and three peptides X, Y, and Z (C). (D) Controlled release of siRNA from nanoparticles loaded with siRNA against PLK1. (E) Controlled release of QC and ENX from nanoparticles loaded with siRNA against PLK1. (F) Components of a functional nanoparticle loaded with siRNA against PLK1 and their individual functions.
For pre-transcytosis strategies to increase BBB permeation, NPs are usually coated with surfactants to prolong their time in circulation, and these approaches are designated as first generation modifications. The addition of specific ligands to NPs that are intended to enhance transcytosis passage across the BBB are characteristic of second generation approaches. The ultimate NPs for translational applications are engineered multifunctional PLGA NPs that combine first and second generation capabilities and post-transcytosis activity against CNS diseases. We designate these CNS-targeted therapies capable of crossing the BBB as third generation. The evolution of third generation engineered PLGA NPs offers great promise for the delivery of bioactive substances to the CNS to treat neurological disorders (Figure 3).
Acknowledgements
This work is supported by Nature Science Foundation of Hubei Province of China (2015CFB182), Hubei Province health and family planning scientific research project (WJ2015MB092) and the foundation of China Scholarship Council.
Disclosure of conflict of interest
Here we claim that none of the material in our manuscript (Systemic delivery to central nervous system by Engineered PLGA nanoparticles) has been published or is under consideration for publication elsewhere, and no potential conflicts of interest were disclosed. And this manuscript is in accordance with the authorship statement of ethical standards for manuscripts submitted to American Journal of Translation Research.
References
- 1.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Honjo K, Black SE, Verhoeff NP. Alzheimer’s disease, cerebrovascular disease, and the beta-amyloid cascade. Can J Neurol Sci. 2012;39:712–728. doi: 10.1017/s0317167100015547. [DOI] [PubMed] [Google Scholar]
- 3.Abbott A. Dementia: a problem for our age. Nature. 2011;475:S2–4. doi: 10.1038/475S2a. [DOI] [PubMed] [Google Scholar]
- 4.Hwang SR, Kim K. Nano-enabled delivery systems across the blood-brain barrier. Arch Pharm Res. 2014;37:24–30. doi: 10.1007/s12272-013-0272-6. [DOI] [PubMed] [Google Scholar]
- 5.Matschay A, Nowakowska E, Hertmanowska H, Kus K, Czubak A. Cost analysis of therapy for patients with multiple sclerosis (MS) in Poland. Pharmacol Rep. 2008;60:632–644. [PubMed] [Google Scholar]
- 6.Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2:3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vinogradov SV, Bronich TK, Kabanov AV. Nanosized cationic hydrogels for drug delivery: preparation, properties and interactions with cells. Adv Drug Deliv Rev. 2002;54:135–147. doi: 10.1016/s0169-409x(01)00245-9. [DOI] [PubMed] [Google Scholar]
- 8.Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Preat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161:505–522. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 9.Shive MS, Anderson JM. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv Rev. 1997;28:5–24. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 10.Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces. 2010;75:1–18. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Sah H, Thoma LA, Desu HR, Sah E, Wood GC. Concepts and practices used to develop functional PLGA-based nanoparticulate systems. Int J Nanomedicine. 2013;8:747–765. doi: 10.2147/IJN.S40579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wohlfart S, Gelperina S, Kreuter J. Transport of drugs across the blood-brain barrier by nanoparticles. J Control Release. 2012;161:264–273. doi: 10.1016/j.jconrel.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Brasnjevic I, Steinbusch HW, Schmitz C, Martinez-Martinez P European NanoBioPharmaceutics Research Initiative. Delivery of peptide and protein drugs over the blood-brain barrier. Prog Neurobiol. 2009;87:212–251. doi: 10.1016/j.pneurobio.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Hawkins RA, O’Kane RL, Simpson IA, Vina JR. Structure of the blood-brain barrier and its role in the transport of amino acids. J Nutr. 2006;136:218S–226S. doi: 10.1093/jn/136.1.218S. [DOI] [PubMed] [Google Scholar]
- 15.Alvarez JI, Cayrol R, Prat A. Disruption of central nervous system barriers in multiple sclerosis. Biochim Biophys Acta. 2011;1812:252–264. doi: 10.1016/j.bbadis.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 16.Wilhelm I, Krizbai IA. In Vitro Models of the Blood-Brain Barrier for the Study of Drug Delivery to the Brain. Mol Pharm. 2014;11:1949–63. doi: 10.1021/mp500046f. [DOI] [PubMed] [Google Scholar]
- 17.Abbott NJ. Blood-brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis. 2013;36:437–449. doi: 10.1007/s10545-013-9608-0. [DOI] [PubMed] [Google Scholar]
- 18.Bicker J, Alves G, Fortuna A, Falcao A. Blood-brain barrier models and their relevance for a successful development of CNS drug delivery systems: A review. Eur J Pharm Biopharm. 2014;87:409–32. doi: 10.1016/j.ejpb.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Astete CE, Sabliov CM. Synthesis and characterization of PLGA nanoparticles. J Biomater Sci Polym Ed. 2006;17:247–289. doi: 10.1163/156856206775997322. [DOI] [PubMed] [Google Scholar]
- 20.Abdelwahed W, Degobert G, Fessi H. A pilot study of freeze drying of poly(epsilon-caprolactone) nanocapsules stabilized by poly(vinyl alcohol): formulation and process optimization. Int J Pharm. 2006;309:178–188. doi: 10.1016/j.ijpharm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Alyautdin RN, Petrov VE, Langer K, Berthold A, Kharkevich DA, Kreuter J. Delivery of loperamide across the blood-brain barrier with polysorbate 80-coated polybutylcyanoacrylate nanoparticles. Pharm Res. 1997;14:325–328. doi: 10.1023/a:1012098005098. [DOI] [PubMed] [Google Scholar]
- 22.Olivier JC, Fenart L, Chauvet R, Pariat C, Cecchelli R, Couet W. Indirect evidence that drug brain targeting using polysorbate 80-coated polybutylcyanoacrylate nanoparticles is related to toxicity. Pharm Res. 1999;16:1836–1842. doi: 10.1023/a:1018947208597. [DOI] [PubMed] [Google Scholar]
- 23.Chang J, Jallouli Y, Kroubi M, Yuan XB, Feng W, Kang CS, Pu PY, Betbeder D. Characterization of endocytosis of transferrin-coated PLGA nanoparticles by the blood-brain barrier. Int J Pharm. 2009;379:285–292. doi: 10.1016/j.ijpharm.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 24.Owens DE 3rd, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Prakobvaitayakit M, Nimmannit U. Optimization of polylactic-co-glycolic acid nanoparticles containing itraconazole using 2(3) factorial design. AAPS PharmSciTech. 2003;4:E71. doi: 10.1208/pt040471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoo HS, Oh JE, Lee KH, Park TG. Biodegradable nanoparticles containing doxorubicin-PLGA conjugate for sustained release. Pharm Res. 1999;16:1114–1118. doi: 10.1023/a:1018908421434. [DOI] [PubMed] [Google Scholar]
- 27.Konan YN, Gurny R, Allemann E. Pr-e paration and characterization of sterile and freeze-dried sub-200 nm nanoparticles. Int J Pharm. 2002;233:239–252. doi: 10.1016/s0378-5173(01)00944-9. [DOI] [PubMed] [Google Scholar]
- 28.Mohamed F, van der Walle CF. Engineering biodegradable polyester particles with specific drug targeting and drug release properties. J Pharm Sci. 2008;97:71–87. doi: 10.1002/jps.21082. [DOI] [PubMed] [Google Scholar]
- 29.Zhou J, Patel TR, Sirianni RW, Strohbehn G, Zheng MQ, Duong N, Schafbauer T, Huttner AJ, Huang Y, Carson RE, Zhang Y, Sullivan DJ Jr, Piepmeier JM, Saltzman WM. Highly penetrative, drug-loaded nanocarriers improve treatment of glioblastoma. Proc Natl Acad Sci U S A. 2013;110:11751–11756. doi: 10.1073/pnas.1304504110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang N, Du G, Wang N, Liu C, Hang H, Liang W. Improving penetration in tumors with nanoassemblies of phospholipids and doxorubicin. J Natl Cancer Inst. 2007;99:1004–1015. doi: 10.1093/jnci/djm027. [DOI] [PubMed] [Google Scholar]
- 31.Manoocheheri S, Darvishi B, Kamalinia G, Amini M, Fallah M, Ostad SN, Atyabi F, Dinarvand R. Surface modification of PLGA nanoparticles via human serum albumin conjugation for controlled delivery of docetaxel. Daru. 2013;21:58. doi: 10.1186/2008-2231-21-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release. 2001;70:1–20. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 33.Moghimi SM, Hunter AC. Poloxamers and poloxamines in nanoparticle engineering and experimental medicine. Trends Biotechnol. 2000;18:412–420. doi: 10.1016/s0167-7799(00)01485-2. [DOI] [PubMed] [Google Scholar]
- 34.Mozafari MR, Pardakhty A, Azarmi S, Jazayeri JA, Nokhodchi A, Omri A. Role of nanocarrier systems in cancer nanotherapy. J Liposome Res. 2009;19:310–321. doi: 10.3109/08982100902913204. [DOI] [PubMed] [Google Scholar]
- 35.Sadat Tabatabaei Mirakabad F, Nejati-Koshki K, Akbarzadeh A, Yamchi MR, Milani M, Zarghami N, Zeighamian V, Rahimzadeh A, Alimohammadi S, Hanifehpour Y, Joo SW. PLGA-based nanoparticles as cancer drug delivery systems. Asian Pac J Cancer Prev. 2014;15:517–535. doi: 10.7314/apjcp.2014.15.2.517. [DOI] [PubMed] [Google Scholar]
- 36.Mahapatro A, Singh DK. Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J Nanobiotechnology. 2011;9:55. doi: 10.1186/1477-3155-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenwald RB, Choe YH, McGuire J, Conover CD. Effective drug delivery by PEGylated drug conjugates. Adv Drug Deliv Rev. 2003;55:217–250. doi: 10.1016/s0169-409x(02)00180-1. [DOI] [PubMed] [Google Scholar]
- 38.Du J, Sun Y, Shi QS, Liu PF, Zhu MJ, Wang CH, Du LF, Duan YR. Biodegradable Nanoparticles of mPEG-PLGA-PLL Triblock Copolymers as Novel Non-Viral Vectors for Improving siRNA Delivery and Gene Silencing. Int J Mol Sci. 2012;13:516–533. doi: 10.3390/ijms13010516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kratz F. Albumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticles. J Control Release. 2008;132:171–183. doi: 10.1016/j.jconrel.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 40.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 41.Wohlfart S, Khalansky AS, Gelperina S, Maksimenko O, Bernreuther C, Glatzel M, Kreuter J. Efficient chemotherapy of rat glioblastoma using doxorubicin-loaded PLGA nanoparticles with different stabilizers. PLoS One. 2011;6:e19121. doi: 10.1371/journal.pone.0019121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kreuter J. Nanoparticulate systems for brain delivery of drugs. Adv Drug Deliv Rev. 2001;47:65–81. doi: 10.1016/s0169-409x(00)00122-8. [DOI] [PubMed] [Google Scholar]
- 43.Kreuter J. Drug delivery to the central nervous system by polymeric nanoparticles: What do we know? Adv Drug Deliv Rev. 2014;71C:2–14. doi: 10.1016/j.addr.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 44.Kreuter J. Mechanism of polymeric nanoparticle-based drug transport across the bloodbrain barrier (BBB) J Microencapsul. 2013;30:49–54. doi: 10.3109/02652048.2012.692491. [DOI] [PubMed] [Google Scholar]
- 45.Lalatsa A, Schatzlein AG, Uchegbu IF. Strategies to deliver peptide drugs to the brain. Mol Pharm. 2014;11:1081–1093. doi: 10.1021/mp400680d. [DOI] [PubMed] [Google Scholar]
- 46.Snyder EL, Dowdy SF. Recent advances in the use of protein transduction domains for the delivery of peptides, proteins and nucleic acids in vivo. Expert Opin Drug Deliv. 2005;2:43–51. doi: 10.1517/17425247.2.1.43. [DOI] [PubMed] [Google Scholar]
- 47.Zou LL, Ma JL, Wang T, Yang TB, Liu CB. Cell-penetrating Peptide-mediated therapeutic molecule delivery into the central nervous system. Curr Neuropharmacol. 2013;11:197–208. doi: 10.2174/1570159X11311020006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lo SL, Wang S. An endosomolytic Tat peptide produced by incorporation of histidine and cysteine residues as a nonviral vector for DNA transfection. Biomaterials. 2008;29:2408–2414. doi: 10.1016/j.biomaterials.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 49.Zhou J, Patel TR, Fu M, Bertram JP, Saltzman WM. Octa-functional PLGA nanoparticles for targeted and efficient siRNA delivery to tumors. Biomaterials. 2012;33:583–591. doi: 10.1016/j.biomaterials.2011.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drin G, Cottin S, Blanc E, Rees AR, Temsamani J. Studies on the internalization mechanism of cationic cell-penetrating peptides. J Biol Chem. 2003;278:31192–31201. doi: 10.1074/jbc.M303938200. [DOI] [PubMed] [Google Scholar]
- 51.Tian XH, Wei F, Wang TX, Wang P, Lin XN, Wang J, Wang D, Ren L. In vitro and in vivo studies on gelatin-siloxane nanoparticles conjugated with SynB peptide to increase drug delivery to the brain. Int J Nanomedicine. 2012;7:1031–1041. doi: 10.2147/IJN.S26541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xia H, Gao X, Gu G, Liu Z, Hu Q, Tu Y, Song Q, Yao L, Pang Z, Jiang X, Chen J, Chen H. Penetratin-functionalized PEG-PLA nanoparticles for brain drug delivery. Int J Pharm. 2012;436:840–850. doi: 10.1016/j.ijpharm.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 53.Bidwell GL 3rd, Perkins E, Hughes J, Khan M, James JR, Raucher D. Thermally targeted delivery of a c-Myc inhibitory polypeptide inhibits tumor progression and extends survival in a rat glioma model. PLoS One. 2013;8:e55104. doi: 10.1371/journal.pone.0055104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao H, Pang Z, Jiang X. Targeted delivery of nano-therapeutics for major disorders of the central nervous system. Pharm Res. 2013;30:2485–2498. doi: 10.1007/s11095-013-1122-4. [DOI] [PubMed] [Google Scholar]
- 55.Gelperina S, Maksimenko O, Khalansky A, Vanchugova L, Shipulo E, Abbasova K, Berdiev R, Wohlfart S, Chepurnova N, Kreuter J. Drug delivery to the brain using surfactantcoated poly(lactide-co-glycolide) nanoparticles: influence of the formulation parameters. Eur J Pharm Biopharm. 2010;74:157–163. doi: 10.1016/j.ejpb.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 56.Petri B, Bootz A, Khalansky A, Hekmatara T, Muller R, Uhl R, Kreuter J, Gelperina S. Chemotherapy of brain tumour using doxorubicin bound to surfactant-coated poly(butyl cyanoacrylate) nanoparticles: revisiting the role of surfactants. J Control Release. 2007;117:51–58. doi: 10.1016/j.jconrel.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 57.Kreuter J, Ramge P, Petrov V, Hamm S, Gelperina SE, Engelhardt B, Alyautdin R, von Briesen H, Begley DJ. Direct evidence that polysorbate-80-coated poly(butylcyanoacrylate) nanoparticles deliver drugs to the CNS via specific mechanisms requiring prior binding of drug to the nanoparticles. Pharm Res. 2003;20:409–416. doi: 10.1023/a:1022604120952. [DOI] [PubMed] [Google Scholar]
- 58.Chaturvedi M, Molino Y, Sreedhar B, Khrestchatisky M, Kaczmarek L. Tissue inhibitor of matrix metalloproteinases-1 loaded poly(lactic-co-glycolic acid) nanoparticles for delivery across the blood-brain barrier. Int J Nanomedicine. 2014;9:575–588. doi: 10.2147/IJN.S54750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martell LA, Agrawal A, Ross DA, Muraszko KM. Efficacy of transferrin receptor-targeted immunotoxins in brain tumor cell lines and pediatric brain tumors. Cancer Res. 1993;53:1348–1353. [PubMed] [Google Scholar]
- 60.Jones AR, Shusta EV. Blood-brain barrier transport of therapeutics via receptor-mediation. Pharm Res. 2007;24:1759–1771. doi: 10.1007/s11095-007-9379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ulbrich K, Hekmatara T, Herbert E, Kreuter J. Transferrin- and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood-brain barrier (BBB) Eur J Pharm Biopharm. 2009;71:251–256. doi: 10.1016/j.ejpb.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 62.Cui Y, Xu Q, Chow PK, Wang D, Wang CH. Transferrin-conjugated magnetic silica PLGA nanoparticles loaded with doxorubicin and paclitaxel for brain glioma treatment. Biomaterials. 2013;34:8511–8520. doi: 10.1016/j.biomaterials.2013.07.075. [DOI] [PubMed] [Google Scholar]
- 63.Lalani J, Raichandani Y, Mathur R, Lalan M, Chutani K, Mishra AK, Misra A. Comparative receptor based brain delivery of tramadolloaded poly(lactic-co-glycolic acid) nanoparticles. J Biomed Nanotechnol. 2012;8:918–927. doi: 10.1166/jbn.2012.1462. [DOI] [PubMed] [Google Scholar]
- 64.Tosi G, Badiali L, Ruozi B, Vergoni AV, Bondioli L, Ferrari A, Rivasi F, Forni F, Vandelli MA. Can leptin-derived sequence-modified nanoparticles be suitable tools for brain delivery? Nanomedicine (Lond) 2012;7:365–382. doi: 10.2217/nnm.11.98. [DOI] [PubMed] [Google Scholar]
- 65.Tosi G, Vergoni AV, Ruozi B, Bondioli L, Badiali L, Rivasi F, Costantino L, Forni F, Vandelli MA. Sialic acid and glycopeptides conjugated PLGA nanoparticles for central nervous system targeting: In vivo pharmacological evidence and biodistribution. J Control Release. 2010;145:49–57. doi: 10.1016/j.jconrel.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 66.Tosi G, Costantino L, Rivasi F, Ruozi B, Leo E, Vergoni AV, Tacchi R, Bertolini A, Vandelli MA, Forni F. Targeting the central nervous system: in vivo experiments with peptide-derivatized nanoparticles loaded with Loperamide and Rhodamine-123. J Control Release. 2007;122:1–9. doi: 10.1016/j.jconrel.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 67.Li J, Feng L, Fan L, Zha Y, Guo L, Zhang Q, Chen J, Pang Z, Wang Y, Jiang X, Yang VC, Wen L. Targeting the brain with PEG-PLGA nanoparticles modified with phage-displayed peptides. Biomaterials. 2011;32:4943–4950. doi: 10.1016/j.biomaterials.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guo L, Ren J, Jiang X. Perspectives on brain-targeting drug delivery systems. Curr Pharm Biotechnol. 2012;13:2310–2318. doi: 10.2174/138920112803341770. [DOI] [PubMed] [Google Scholar]
- 69.Geldenhuys W, Wehrung D, Groshev A, Hirani A, Sutariya V. Brain-targeted delivery of doxorubicin using glutathione-coated nanoparticles for brain cancers. Pharm Dev Technol. 2015;20:497–506. doi: 10.3109/10837450.2014.892130. [DOI] [PubMed] [Google Scholar]
- 70.Herve F, Ghinea N, Scherrmann JM. CNS delivery via adsorptive transcytosis. AAPS J. 2008;10:455–472. doi: 10.1208/s12248-008-9055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bilensoy E. Cationic nanoparticles for cancer therapy. Expert Opin Drug Deliv. 2010;7:795–809. doi: 10.1517/17425247.2010.485983. [DOI] [PubMed] [Google Scholar]
- 72.Jaruszewski KM, Ramakrishnan S, Poduslo JF, Kandimalla KK. Chitosan enhances the stability and targeting of immuno-nanovehicles to cerebro-vascular deposits of Alzheimer’s disease amyloid protein. Nanomedicine. 2012;8:250–260. doi: 10.1016/j.nano.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim JH, Park JS, Yang HN, Woo DG, Jeon SY, Do HJ, Lim HY, Kim JM, Park KH. The use of biodegradable PLGA nanoparticles to mediate SOX9 gene delivery in human mesenchymal stem cells (hMSCs) and induce chondrogenesis. Biomaterials. 2011;32:268–278. doi: 10.1016/j.biomaterials.2010.08.086. [DOI] [PubMed] [Google Scholar]
- 74.Patil Y, Panyam J. Polymeric nanoparticles for siRNA delivery and gene silencing. Int J Pharm. 2009;367:195–203. doi: 10.1016/j.ijpharm.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blum JS, Saltzman WM. High loading efficiency and tunable release of plasmid DNA encapsulated in submicron particles fabricated from PLGA conjugated with poly-L-lysine. J Control Release. 2008;129:66–72. doi: 10.1016/j.jconrel.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Basarkar A, Singh J. Nanoparticulate systems for polynucleotide delivery. Int J Nanomedicine. 2007;2:353–360. [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang S, Zhao B, Jiang H, Wang B, Ma B. Cationic lipids and polymers mediated vectors for delivery of siRNA. J Control Release. 2007;123:1–10. doi: 10.1016/j.jconrel.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 78.Little SR, Lynn DM, Ge Q, Anderson DG, Puram SV, Chen J, Eisen HN, Langer R. Poly-beta amino ester-containing microparticles enhance the activity of nonviral genetic vaccines. Proc Natl Acad Sci U S A. 2004;101:9534–9539. doi: 10.1073/pnas.0403549101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Little SR, Lynn DM, Puram SV, Langer R. Formulation and characterization of poly (beta amino ester) microparticles for genetic vaccine delivery. J Control Release. 2005;107:449–462. doi: 10.1016/j.jconrel.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 80.Baoum A, Dhillon N, Buch S, Berkland C. Cationic surface modification of PLG nanoparticles offers sustained gene delivery to pulmonary epithelial cells. J Pharm Sci. 2010;99:2413–2422. doi: 10.1002/jps.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bhardwaj V, Ankola DD, Gupta SC, Schneider M, Lehr CM, Kumar MN. PLGA nanoparticles stabilized with cationic surfactant: safety studies and application in oral delivery of paclitaxel to treat chemical-induced breast cancer in rat. Pharm Res. 2009;26:2495–2503. doi: 10.1007/s11095-009-9965-4. [DOI] [PubMed] [Google Scholar]
- 82.Zhan C, Lu W. The blood-brain/tumor barriers: challenges and chances for malignant gliomas targeted drug delivery. Curr Pharm Biotechnol. 2012;13:2380–2387. doi: 10.2174/138920112803341798. [DOI] [PubMed] [Google Scholar]
- 83.Hovanessian AG, Soundaramourty C, El Khoury D, Nondier I, Svab J, Krust B. Surface expressed nucleolin is constantly induced in tumor cells to mediate calcium-dependent ligand internalization. PLoS One. 2010;5:e15787. doi: 10.1371/journal.pone.0015787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maletinska L, Blakely EA, Bjornstad KA, Deen DF, Knoff LJ, Forte TM. Human glioblastoma cell lines: levels of low-density lipoprotein receptor and low-density lipoprotein receptorrelated protein. Cancer Res. 2000;60:2300–2303. [PubMed] [Google Scholar]
- 85.Chang J, Paillard A, Passirani C, Morille M, Benoit JP, Betbeder D, Garcion E. Transferrin adsorption onto PLGA nanoparticles governs their interaction with biological systems from blood circulation to brain cancer cells. Pharm Res. 2012;29:1495–1505. doi: 10.1007/s11095-011-0624-1. [DOI] [PubMed] [Google Scholar]
- 86.Lei C, Cui Y, Zheng L, Chow PK, Wang CH. Development of a gene/drug dual delivery system for brain tumor therapy: potent inhibition via RNA interference and synergistic effects. Biomaterials. 2013;34:7483–7494. doi: 10.1016/j.biomaterials.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 87.Wang B, Lv L, Wang Z, Zhao Y, Wu L, Fang X, Xu Q, Xin H. Nanoparticles functionalized with Pep-1 as potential glioma targeting delivery system via interleukin 13 receptor alpha2-mediated endocytosis. Biomaterials. 2014;35:5897–5907. doi: 10.1016/j.biomaterials.2014.03.068. [DOI] [PubMed] [Google Scholar]
- 88.Ireson CR, Kelland LR. Discovery and development of anticancer aptamers. Mol Cancer Ther. 2006;5:2957–2962. doi: 10.1158/1535-7163.MCT-06-0172. [DOI] [PubMed] [Google Scholar]
- 89.Guo J, Gao X, Su L, Xia H, Gu G, Pang Z, Jiang X, Yao L, Chen J, Chen H. Aptamer-functionalized PEG-PLGA nanoparticles for enhanced anti-glioma drug delivery. Biomaterials. 2011;32:8010–8020. doi: 10.1016/j.biomaterials.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 90.Gao GH, Li Y, Lee DS. Environmental pHsensitive polymeric micelles for cancer diagnosis and targeted therapy. J Control Release. 2013;169:180–184. doi: 10.1016/j.jconrel.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 91.Yavuz MS, Cheng Y, Chen J, Cobley CM, Zhang Q, Rycenga M, Xie J, Kim C, Song KH, Schwartz AG, Wang LV, Xia Y. Gold nanocages covered by smart polymers for controlled release with near-infrared light. Nat Mater. 2009;8:935–939. doi: 10.1038/nmat2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Du JZ, Mao CQ, Yuan YY, Yang XZ, Wang J. Tumor extracellular acidity-activated nanoparticles as drug delivery systems for enhanced cancer therapy. Biotechnol Adv. 2014;32:789–803. doi: 10.1016/j.biotechadv.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 93.Chhabra R, Grabrucker AM, Veratti P, Belletti D, Boeckers TM, Vandelli MA, Forni F, Tosi G, Ruozi B. Characterization of lysosome-destabilizing DOPE/PLGA nanoparticles designed for cytoplasmic drug release. Int J Pharm. 2014;471:349–357. doi: 10.1016/j.ijpharm.2014.05.054. [DOI] [PubMed] [Google Scholar]
- 94.Gu G, Xia H, Hu Q, Liu Z, Jiang M, Kang T, Miao D, Tu Y, Pang Z, Song Q, Yao L, Chen H, Gao X, Chen J. PEG-co-PCL nanoparticles modified with MMP-2/9 activatable low molecular weight protamine for enhanced targeted glioblastoma therapy. Biomaterials. 2013;34:196–208. doi: 10.1016/j.biomaterials.2012.09.044. [DOI] [PubMed] [Google Scholar]
- 95.Wang H, Su W, Wang S, Wang X, Liao Z, Kang C, Han L, Chang J, Wang G, Pu P. Smart multifunctional core-shell nanospheres with drug and gene co-loaded for enhancing the therapeutic effect in a rat intracranial tumor model. Nanoscale. 2012;4:6501–6508. doi: 10.1039/c2nr31263h. [DOI] [PubMed] [Google Scholar]