Abstract
Osteosarcoma is the most prevalent primary malignant bone tumor mainly endangering young adults. In this study, we explore whether polydatin (PD), a glycoside form of resveratrol, is effective for osteosarcoma. Our results showed that PD dose-dependently inhibited proliferation and promoted apoptosis in 143B and MG63 osteosarcoma cells, examined by MTT assay and Annexin V-FITC apoptosis detection. Further, we found PD increased expression of Bax and attenuated expression of Bcl-2, and consequently augmented caspase-3 activity. Moreover, PD also dose-dependently inhibited β-catenin signaling pathway as indicated by decreased β-catenin expression and activity, while overexpression of β-catenin by adenoviruses system could abrogate the anti-tumor effect of PD. Our finding indicated that PD could inhibit the proliferation by inhibiting the β-catenin signaling and induce apoptosis via upregulation the ratio of Bax/Bcl-2 in human osteosarcoma cells.
Keywords: Osteosarcoma, polydatin (PD), proliferation, apoptosis, β-catenin
Introduction
Osteosarcoma (OS) is one of the most frequent primary malignant tumors of the musculoskeletal, occurring mainly in children and adolescents with a high propensity for local invasion and distant metastasis. Since the use of multidrug chemotherapy and refined regional surgical technique in 1980s, the overall survival rate of non-metastatic OS has elevated from 10% to 65% [1]. However, despite the widespread use of neo-adjuvant therapy, the survival rate remains be in the plateau over the past two decades. Furthermore, OS with multiple metastases sustains to be grim with a poor survival rate of 10-20% [1,2]. Conventional chemotherapy contains three-drug backbone consisting of methotrexate, doxorubicin, and cisplatin (MAP) with other two possible inclusions of ifosfamide and etoposide [3]. Surprisingly, chemotherapeutic drugs used today seem to be wholly similar to what used three decades ago. And side-effects, such as pulmonary and renal toxicity, hearing impairment, encephalopathy and hemorrhagic cystitis can be caused by conventional chemotherapeutic drugs, most of which are life-threatening for OS [4]. Give the side effects and poor progress of orthodox chemotherapies in OS treatment, there is an urgent need for finding new chemotherapies with safer and more sufficient anti-cancer properties.
Polydatin (PD), also named piceid (3, 4’, 5-trihydroxystilbene-3-b-D-glucoside, Figure 1), is a stilbene compound originally extracted from the root and rhizome of Polygonum cuspidatum Sieb. et Zucc. (Polygonaceae). PD is a glycoside form of resveratrol with glucoside group bonded in position C-3 substituting a hydroxyl group, which renders PD properties of resistance to enzymatic oxidation, solubility in water and penetrating the cell actively through the glucose carriers [5]. PD has been proven with protective effects against sepsis [6], shock [7], renal injury [8], septic lung injury [9], ischemia/reperfusion-induced apoptosis [10], lipopolysaccharide/D-galactosamine-induced fulminant hepatic failure [11]. Numerous studies have suggested that PD, like resveratrol, may have the similar bioactivities such as inhibition of platelet aggregation [12], anticarcinogenic effects [13]. Consistently, Su et al. [14] and Liu et al. [15] found that PD could significantly kill several human tumor cell lines in a dose- and time-dependent manner, suggesting PD might be a promising anti-tumor drug.
Figure 1.
Chemical structure of polydatin.
Previous study has demonstrated resveratrol has an anti-osteosarcoma effect via inhibiting cell proliferation and vascular endothelial growth factor (VEGF) expression [16]. However, the anti-osteosarcoma effect of PD has not been acknowledged yet. In this current study, we evaluated the anti-osteosarcoma activity of PD and explored the underlying molecular mechanism in human osteosarcoma cell lines (143B and MG63).
Materials and methods
Chemical and reagents
PD (C20H22O8, MW: 390.40, purity ≥ 95%) was purchased from Nanjing Debjochem Co. Ltd. (Nanjing, China). Annexin V-FITC/propidium iodide (PI) apoptosis detection kit and the caspase-3 colorimetric assay kit were from KeyGEN Biotech (Nanjing, China). Bicinchoninic acid (BCA) protein assay kit was obtained from Pierce (Rockford, IL, USA). RPMI 1640 medium was purchased from Gibco BRL Life Technologies (CA, USA). FBS (fetal bovine serum) was obtained from HyClone Laboratories (Logan, UT, USA). Bcl-2, Bax, β-catenin, and GAPDH Antibody were purchased from Cell Signaling Technology (Boston, MA, USA). The dual luciferase assay reagent kit was from Promega (San Luis Obispo, USA). Dimethyl sulfoxide (DMSO), crystal violet and 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide (MTT) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). All other chemicals and solvents obtained from local area were of the highest analytical grade available.
Cell culture and treatment
Osteosarcoma cells 143B and MG63 were obtained from American Type Culture Collection (ATCC, Rockville, MD), and cultured in RPMI-1640 supplemented with 10% FBS and 1% penicillin/streptomycin. All cells were routinely incubated in an atmosphere of 5% CO2 at 37°C. All cell experiments were done using cells in exponential cell growth. Incubated for twenty-four hours after seeding, cells were treated with culture medium containing various concentrations of PD (10-4, 10-5, 10-6 M).
MTT assay for proliferation assay
MTT Assay was performed to evaluate the proliferation of osteosarcoma cells. MTT assay is a rapid and sensitive procedure for accessing cellular toxicity of compounds in-vitro. Cells were seeded into 96-well plates at a concentration of 105/ml and plates were sealed and cultured for 6 hours before treatment of PD. Cells were incubated for 24, 48 and 72 hours after treatment. Following incubation, 20 μl of MTT was added to each well, and the cells were incubated for an additional four hours. Subsequently, media was carefully discarded and 100 μl of dimethyl sulfoxide were added to dissolve the formazan crystals, then the 96-well plates were put on a horizontal oscillator for ten minutes. The absorbance values were measured at a wavelength of 570 and a reference wavelength of 620 nm. Each experiment was conducted in triplicate, and the data are presented as mean values.
Cell viability detection
Crystal violet assay was used to determine the cytotoxicity of PD on osteosarcoma cells. Cells were seeded and grown in 200 μl of growth medium in 96-well plates at a concentration of 105/ml. After incubated with various doses of PD for 72 h, the wells were carefully washed with PBS and the adherent cells were fixed with 2% (wt/vol) paraformaldehyde for 15 min at room temperature (RT). Then after washing, the cells were incubated and were slightly shook at RT with 100 μl 2% crystal violet solution (in 19.2% ethanol containing 0.8% ammonium oxalate) for 30 min. Next, the plate was washed twice with distilled water and air dried completely. Finally, the remained cells were solubilized in 1% (wt/vol) sodium dodecyl sulfate in 50% (vol/vol) ethanol the absorbance was read with enzyme-linked immunosorbent assay (ELISA) plate reader at a wavelength of 570 nm. Each experiment was conducted in triplicate and the data are presented as mean values.
FACS assay of apoptosis
For apoptosis analysis, Annexin V-FITC/propidium iodide (PI) staining using an Annexin V-FITC apoptosis detection kit (KeyGEN Biotech, Nanjing, China) was performed by flow cytometry according to the manufacturer’s guidelines. Briefly, after 48 h of PD treatment, the cells were washed with cold phosphate buffered saline (PBS), incubated with Annexin V-FITC/PI at room temperature for 5 min in the dark. The fluorescence of the cells was detected by the flow cytometry using a FITC signal detector (FL1) and a PI signal detector (FL2). According to the method, Annexin V-FITC (-)/PI (-) indicates survived cells, Annexin V-FITC (+)/PI (-) indicates cells of apoptosis in the early stage, and Annexin V-FITC (+)/PI (+) indicates cells of apoptosis or necrosis in the late stage. Each experiment was performed in triplicate and reproducible results were achieved.
Caspase-3 activity assay
The activity of caspase-3 was detected in vitro using a caspase-3 colorimetric assay kit according to the manufacturer’s instructions. In short, following the treatment, the collected cells were lysed and centrifuged at 12000 g for 15 minutes at 4°C. The supernatant were collected and the protein concentrations were measured by the BCA method. Then supernatant containing 50 µg of total protein were incubated with 5 µl caspase substrate in the 100 µl reaction buffer at 37°C for 4 h in the dark. The activity of caspase 3 was determined by a microplate reader at 405 nm, and calculated as a percentage of OD in PD treatment cells relative to the control that were not treated with the PD.
Quantitative real time PCR
Total RNA was extracted from the cells using Trizol (Invitrogen, USA) according to the manufacturer’s protocol. The primer sequences were as follows: β-catenin (Forward: 5’-ATGGAGCCGGACAGAAAAGC-3’; Reverse: 5’-CTTGCCACTCAGGGAAGGA-3’), GAPDH (Forward: 5’-AGGTCGGTGTGAACGGAT TTG-3’; Reverse: 5’-TGTAGACCATGTAGTTGAGGTCA-3’). Real-time PCR was carried out using Maxima SYBR Green/ROX qPCR Master Mix (Fermentas) according to the manufacturer’s instructions. 7500 Real Time PCR System (Applied Biosystems) was employed for the thermal cycling reactions. After the normalizing with GAPDH, relative change in gene expression of β-catenin was determined by the comparative Ct method (2-ΔΔC method).
Protein isolation and western blot
Cells were washed with PBS and lysed in cell lysis buffer. The lysate was centrifuged at 12000 g at 4°C for 10 min. The supernatant was collected and protein concentration was determined by BCA method. 40 µg total protein from each treated cell group was fractionated on 10% polyacrylamide-sodium dodecyl sulfate (SDS) gel and then transferred to polyvinylidene fluoride membranes. The membranes were blocked with 5% (w/v) fat-free milk in Tris-buffered saline (TBS) containing 0.05% Tween-20, followed by incubation with a primary antibody at 4°C overnight. Then after washing with TBST for three times, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies. Antibody binding was visualized using enhanced ECL chemiluminescence system and short exposure of the membrane to X-ray films (Kodak, Japan).
Luciferase reporter assay
To investigate the activation of β-catenin signaling pathway modulated by PD, TOPFlash (4 × TCF binding sites) luciferase reporter was used as reported previously [17]. Luciferase activities were analyzed by the dual-luciferase reporter assay system (Promega, Madison, WI) with normalization to the control Renilla.
Adenovirus infection
Recombinant adenoviruses expressing β-catenin was used according to procedure described. The expression of RFP was as a marker for monitoring transfection efficiency. An analogous adenovirus expressing only RFP (Ad-RFP) was used as a control, and expression efficiency was determined by real-time PCR, western blotting, and functional assays of β-catenin signaling pathway.
Statistical analysis
The results were expressed as the means ± standard deviation (SD), either Student’s t-test or one-way ANOVA was used to achieve data analyses. A two-tailed P value of less than 0.05 was considered significant difference.
Results
PD inhibited the proliferation and viability of osteosarcoma cells
The proliferation and viability of 143B and MG63 cells were determined by MTT and crystal violet viability assay, respectively, following treatment with concentrations of 10-6, 10-5, and 10-4 M of PD for 24, 48 and 72 h. We found that PD inhibited the proliferation of these human cells in a dose-dependent manner based on the MTT assay (Figure 2A). In lines with MTT assay, PD also exhibited the inhibitive effects on these osteosarcoma cells in a dose-dependent manner determined by crystal violet viability assay (Figure 2B).
Figure 2.
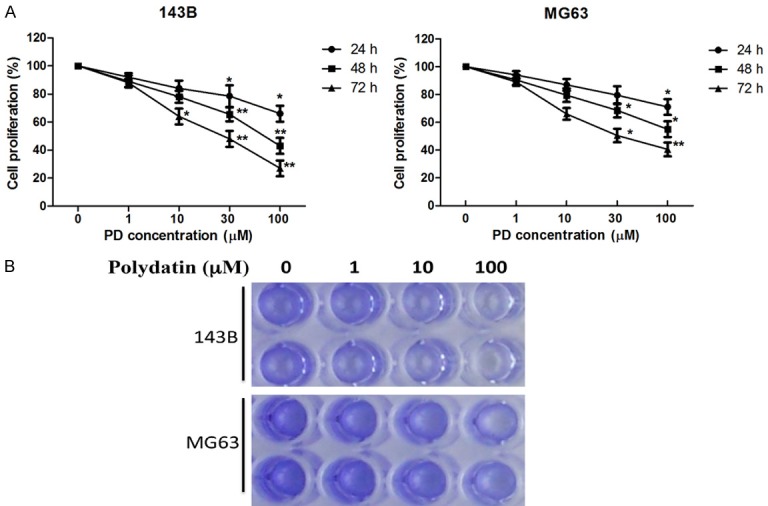
Effect of polydatin on proliferation and viability of human osteosarcoma cells. 143B and MG63 osteosarcoma cells were treated with polydatin at indicated concentration. A. The proliferation was measured by MTT 24, 48, and 72 h after treatment. B. The viability was evaluated by Crystal violet 72 h after treatment. Each value is mean ± S.D. *P<0.05, **P<0.01.
PD induced apoptosis in osteosarcoma cells
The apoptosis of MG63 induced by PD was quantified by flow cytometry using Annexin V-FITC/PI staining. As shown in Figure 3, the apoptosis were significantly increased in the groups treated with PD by comparison with control groups, and dose-dependent effects were found.
Figure 3.
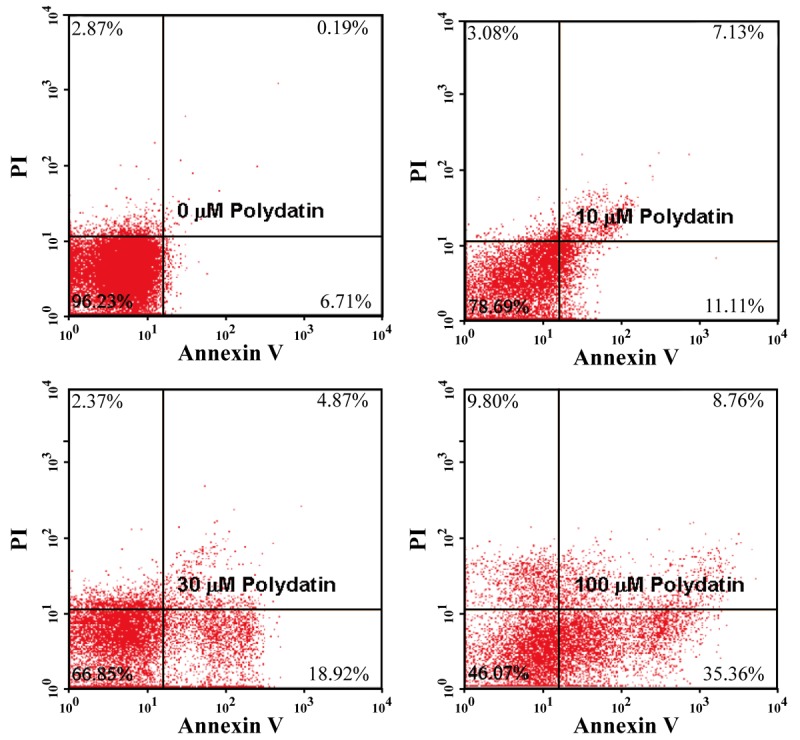
Effect of polydatin on apoptosis of human osteosarcoma cells. 143B osteosarcoma cells were treated with 0, 10, 30, and 100 μM polydatin for 48 h, apoptosis was detected using annexin V-FITC/PI dual staining by flow cytometry.
PD enhanced the activation of caspase-3 in osteosarcoma cells
The caspase-cascade system plays a critical role in the initiation, transduction, and amplification of cell apoptosis [18]. To investigate the cleaved effector caspase in the apoptotic pathway, the activation of caspase-3 were measured by the colorimetric assay. As shown in Figure 4, compared with untreated group, exposure of PD can evidently and dose-dependently induce activation of caspase-3 in 143B osteosarcoma cells.
Figure 4.

Effect of polydatin on caspase-3 activity in human osteosarcoma cells. 143B osteosarcoma cells were treated with 0, 10, 30, and 100 μM polydatin for 48 h, caspase-3 activity was measured by the colorimetric assay. Each value is mean ± S.D. *P<0.05, **P<0.01.
PD up-regulated expression of pro-apoptotic protein (Bax) and down-regulated anti-apoptotic protein (Bcl-2)
The Bcl-2 family proteins are important regulators of apoptosis, including both pro-apoptotic protein Bax and anti-apoptotic protein Bcl-2, and the ratio of Bax/Bcl-2 determined the fate of cell [19]. To further study the potential mechanism by which the PD induces apoptosis of human osteosarcoma cells, the effect of PD on the expression of Bcl-2 and Bax in 143B osteosarcoma cells was examined. As shown in Figure 5, PD profoundly up-regulated Bax and down-regulated Bcl-2 in a dose-dependent manner.
Figure 5.
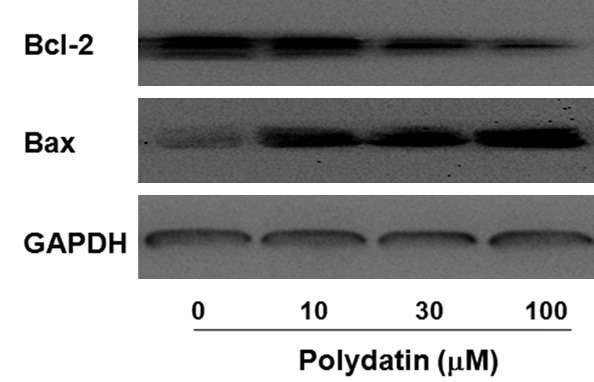
Effect of polydatin on expression of Bcl-2 and Bax in human osteosarcoma cells. 143B osteosarcoma cells were treated with 0, 10, 30, and 100 μM polydatin for 48 h, expression of Bcl-2 and Bax were determined by western blotting.
PD suppresses β-catenin signal pathway
The expression of β-catenin is obviously higher than normal tissue [20], subsequently; it can induce over activation of several downstream target genes including c-Myc and c-Jun, leading to increased cell proliferation and survival [21]. Therefore, Quantitative Real-Time PCR and western blotting were employed to examine the mRNA and protein expression of β-catenin. Treatment of PD obviously decreased β-catenin mRNA in a dose-dependent manner, as presented in Figure 6A, and the similar results were found in the pattern of protein expression of β-catenin (Figure 6B). Additionally, luciferase assay with β-catenin reporter presented that PD suppressed β-catenin activity in a dose-dependent manner in the osteosarcoma cell line (Figure 6C).
Figure 6.
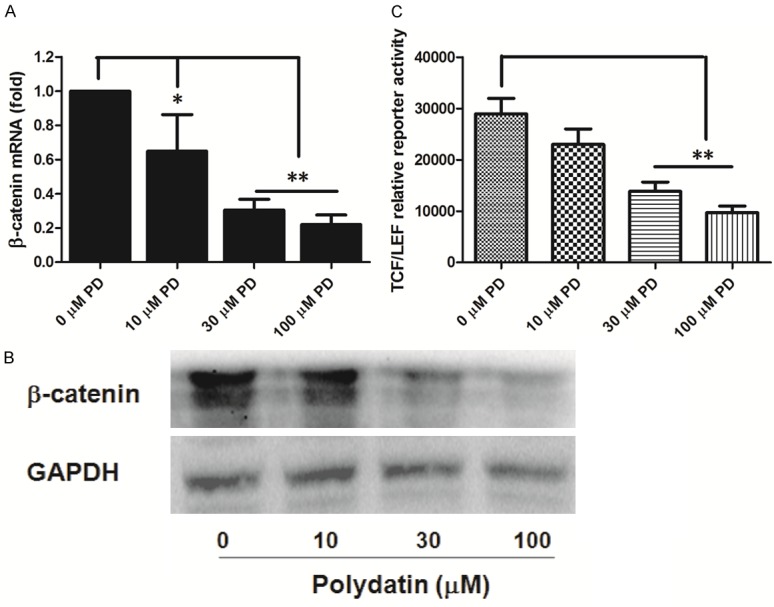
Effect of polydatin on β-catenin mRNA and protein expression and TCF/LEF activity in human osteosarcoma cells. A and B. 143B osteosarcoma cells were treated with 0, 10, 30, and 100 μM polydatin, β-catenin mRNA and protein expression were determined at 48 h posttreatment. C. 143B cells were transfected with the β-catenin-TCF/LEF reporter pTOP-Luc for 24 h, and treated with the indicated concentrations of PD. Luciferase activity was determined at 48 h posttreatment. Each value is mean ± S.D. *P<0.05, **P<0.01.
β-catenin mediated the beneficial effect of PD
In order to verify whether inhibiting β-catenin pathway is responsible for the antitumor activity of PD, overexpression of β-catenin by adenoviruses vector was used. As shown in Figure 7, overexpression of β-catenin abolished the inhibitory of PD on cell proliferation in osteosarcoma cells.
Figure 7.
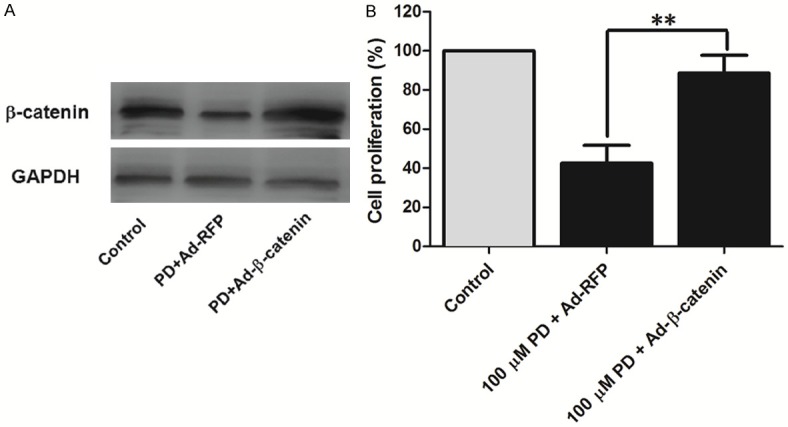
Overexpression of β-catenin blocked the anti-proliferation of PD in human osteosarcoma cells. 143B cells were infected with Ad-β-catenin or Ad-RFP. At 24 h after infection, cells were treated with 100 μM polydatin for 48 h. A. Total protein was obtained for western blotting to determine β-catenin expression. B. Cell proliferation was measured by MTT. Each value is mean ± S.D. *P<0.05, **P<0.01.
Discussion
Osteosarcoma is the most common type of malignant bone tumor. Benefited from the advance in multidrug chemotherapy and surgical technique, the survival rate of patients with osteosarcoma has increased. Recent years, a 5-year survival rate for the treatment of surgical alone was 20%, which was considered to be accepted for approximately 80% the patients suffered from pulmonary metastasis at the time of presentation [22,23]. However, the effectiveness of chemotherapeutic drugs attenuated because of required chemoresistance, moreover, many currently used drugs have been confirmed to be intrinsic and potent toxic to normal cells, which renders their long-term use and effectiveness limited [19]. Therefore, new therapeutic agents are desperately needed to improve the prognosis of osteosarcoma.
In this study, the results revealed that PD had the anti-tumor effect on human osteosarcoma lines. We found that PD induced apoptosis and inhibited proliferation of 143B and MG63 cells in a dose dependent manner. Additionally, we confirmed that enhanced caspase-3 activation, up-regulation of Bax, down-regulation of Bcl-2, and reduced activation of β-catenin signaling were involved. According to previous reports, PD has favorable effects on many human tumor cells, including human cervical carcinoma HeLa cells, hepatoma cell line SMMC-7721 cells, epidermal carcinoma A-431 cells, nasopharyngeal carcinoma CNE cell, and it was identified that PD could induce apoptosis of nasopharyngeal carcinoma CNE cell via mitochondrial disruption, endoplasmic reticulum stress and down-regulation of Akt phosphorylation [15]. Additionally, it was documented that PD could inhibit the formation of capillary-like tube networks (angiogenesis) of human umbilical vein endothelial cells (HUVECs) and suppress DNA synthesis in Lewis lung carcinoma [24]. These findings indicate that PD has an anti-tumor effect in human osteosarcoma cells, and it is also a promising anti-tumor candidate for other tumors.
Apoptosis is characterized by shrinkage, cytoplasmic membrane blebbing, chromatin condensation, DNA fragmentation and finally destruction of the cells. In mammals, two pathways are involved in apoptosis process. One is the extrinsic pathway, which is activated by cell-surface death receptors and consequent activation of caspase-8 occurs. The other one is intrinsic pathway containing mitochondrial disruption by pro-apoptotic Bcl-2 family members and subsequent release of cytochrome c and activation caspase-9 [25]. Both activated caspase-8 and caspase-9 can activate caspase-3, subsequently initiate the apoptotic program through cleavage of plenty of vital proteins [26]. In our study, PD dose dependently enhanced the activation of caspase-3 and in parallel with the identified increase rate of apoptosis in the osteosarcoma cells, suggesting that enhanced activation of caspase-3 might play critical roles in PD-induced apoptosis.
Bcl-2 family proteins, key regulators of mitochondrion-mediated apoptosis, consist of anti-apoptotic protein such as Bcl-2 and pro-apoptotic proteins such as Bax [27], and regulate apoptosis through alteration of permeability of mitochondrial outer membrane (MOM) following homo- or hetero-association [28]. It was reported that chemotherapeutic agents influenced the participation of Bcl-2 family members in the regulation of apoptosis [27]. By immunoblotting, we found that PD dose-dependently unregulated Bax and downregulated Bcl-2 in the osteosarcoma cell lines. As is expected, the activation of caspase-3 increased in parallel with the upregulation of Bax and downregulation of Bcl-2. Bax, a pro-apoptotic Bcl-2 family protein, promotes apoptosis by the release of cytochrome c from mitochondria and downstream caspase activation [29]. However, Bcl-2 inhibits cell apoptosis by obstructing the pro-apoptotic effects of Bax and blocking the release of cytochrome c from the mitochondria [30]. The balance of the anti- and pro-apoptotic Bcl-2 family protein controls the tissue homeostasis and lower Bax/Bcl-2 ratio commonly exits in various cancers. Furthermore, higher Bax/Bcl-2 is associated with increased activation of caspase-3 and consequent susceptibility of a cell to apoptosis [31]. Therefore, we can conclude that PD induced apoptosis in osteosarcoma cells through caspase-3 signaling via increasing the ratio of Bax/Bcl-2.
Aberrant over activation of Wnt-β-catenin pathway plays an essential role in OS pathogenesis, and it seems to be positively associated with lung metastasis and chemotherapeutic resistance [21,32]. Accumulated nuclear β-catenin binds to the T-cell factor/lymphoid enhancer factor and subsequently promotes the target genes activity including Myc, cyclin D1, Axin 2 and Lef1, leading to increased cell survival against apoptosis induced by chemotherapeutic agents and proliferation [33,34]. In the current study, by Quantitative Real-Time PCR and western blotting, we found that PD dose-dependently decreased the expression of β-catenin mRNA and protein, which presented a negative association with cell proliferation. In order to identify the activation of β-catenin, luciferase reporter assay were used after treatment of PD. The results presented that the reduction of activated β-catenin paralleled with the protein and mRNA expression, but inversely related to osteosarcoma cell proliferation. Furthermore, by overexpression of β-catenin adenoviruse vector, we found that the inhibitive effect of PD on osteosarcoma cell proliferation was abrogated, indicating that β-catenin signal participated in the beneficial effect of PD. Taking together, our results demonstrate that PD inhibits osteosarcoma cell proliferation by inhibiting β-catenin signaling.
In conclusion, our data for the first time demonstrated that PD induced apoptosis through caspase-3 via upregulation the ratio of Bax/Bcl-2, and inhibited the proliferation of osteosarcoma cells by attenuating the β-catenin signaling. These results suggested that PD might become a potential novel chemotherapeutic agent for the treatment of osteosarcoma.
Acknowledgements
This study was supported by the Natural Science Foundation Project of CQ (CSTC, 2010AC5028).
Disclosure of conflict of interest
None.
References
- 1.Bakhshi S, Radhakrishnan V. Prognostic markers in osteosarcoma. Expert Rev Anticancer Ther. 2010;10:271–287. doi: 10.1586/era.09.186. [DOI] [PubMed] [Google Scholar]
- 2.Allison DC, Carney SC, Ahlmann ER, Hendifar A, Chawla S, Fedenko A, Angeles C, Menendez LR. A meta-analysis of osteosarcoma outcomes in the modern medical era. Sarcoma. 2012;2012:704872. doi: 10.1155/2012/704872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gill J, Ahluwalia MK, Geller D, Gorlick R. New targets and approaches in osteosarcoma. Pharmacol Ther. 2013;137:89–99. doi: 10.1016/j.pharmthera.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 4.D’Adamo DR. Appraising the current role of chemotherapy for the treatment of sarcoma. Semin Oncol. 2011;38(Suppl 3):S19–29. doi: 10.1053/j.seminoncol.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Ravagnan G, De Filippis A, Carteni M, De Maria S, Cozza V, Petrazzuolo M, Tufano MA, Donnarumma G. Polydatin, a natural precursor of resveratrol, induces beta-defensin production and reduces inflammatory response. Inflammation. 2013;36:26–34. doi: 10.1007/s10753-012-9516-8. [DOI] [PubMed] [Google Scholar]
- 6.Li XH, Wu MJ, Zhang LN, Zheng JJ, Zhang L, Wan JY. [Effects of polydatin on ALT, AST, TNFalpha, and COX-2 in sepsis model mice] . Zhongguo Zhong Xi Yi Jie He Za Zhi. 2013;33:225–228. [PubMed] [Google Scholar]
- 7.Wang X, Song R, Chen Y, Zhao M, Zhao KS. Polydatin--a new mitochondria protector for acute severe hemorrhagic shock treatment. Expert Opin Investig Drugs. 2013;22:169–179. doi: 10.1517/13543784.2013.748033. [DOI] [PubMed] [Google Scholar]
- 8.Chen L, Lan Z, Lin Q, Mi X, He Y, Wei L, Lin Y, Zhang Y, Deng X. Polydatin ameliorates renal injury by attenuating oxidative stress-related inflammatory responses in fructose-induced urate nephropathic mice. Food Chem Toxicol. 2013;52:28–35. doi: 10.1016/j.fct.2012.10.037. [DOI] [PubMed] [Google Scholar]
- 9.Li XH, Gong X, Zhang L, Jiang R, Li HZ, Wu MJ, Wan JY. Protective effects of polydatin on septic lung injury in mice via upregulation of HO-1. Mediators Inflamm. 2013;2013:354087. doi: 10.1155/2013/354087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang LP, Ma HJ, Bu HM, Wang ML, Li Q, Qi Z, Zhang Y. Polydatin attenuates ischemia/reperfusion-induced apoptosis in myocardium of the rat. Sheng Li Xue Bao. 2009;61:367–372. [PubMed] [Google Scholar]
- 11.Wu MJ, Gong X, Jiang R, Zhang L, Li XH, Wan JY. Polydatin protects against lipopolysaccharide-induced fulminant hepatic failure in D-galactosamine-sensitized mice. Int J Immunopathol Pharmacol. 2012;25:923–934. doi: 10.1177/039463201202500410. [DOI] [PubMed] [Google Scholar]
- 12.Orsini F, Pelizzoni F, Verotta L, Aburjai T, Rogers CB. Isolation, synthesis, and antiplatelet aggregation activity of resveratrol 3-O-beta-D-glucopyranoside and related compounds. J Nat Prod. 1997;60:1082–1087. doi: 10.1021/np970069t. [DOI] [PubMed] [Google Scholar]
- 13.Soleas GJ, Goldberg DM, Grass L, Levesque M, Diamandis EP. Do wine polyphenols modulate p53 gene expression in human cancer cell lines? Clin Biochem. 2001;34:415–420. doi: 10.1016/s0009-9120(01)00246-6. [DOI] [PubMed] [Google Scholar]
- 14.Su D, Cheng Y, Liu M, Liu D, Cui H, Zhang B, Zhou S, Yang T, Mei Q. Comparision of piceid and resveratrol in antioxidation and antiproliferation activities in vitro. PLoS One. 2013;8:e54505. doi: 10.1371/journal.pone.0054505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H, Zhao S, Zhang Y, Wu J, Peng H, Fan J, Liao J. Reactive oxygen species-mediated endoplasmic reticulum stress and mitochondrial dysfunction contribute to polydatin-induced apoptosis in human nasopharyngeal carcinoma CNE cells. J Cell Biochem. 2011;112:3695–3703. doi: 10.1002/jcb.23303. [DOI] [PubMed] [Google Scholar]
- 16.Liu Z, Li Y, Yang R. Effects of resveratrol on vascular endothelial growth factor expression in osteosarcoma cells and cell proliferation. Oncol Lett. 2012;4:837–839. doi: 10.3892/ol.2012.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu W, Li X, Chu ES, Go MY, Xu L, Zhao G, Li L, Dai N, Si J, Tao Q. Paired box gene 5 is a novel tumor suppressor in hepatocellular carcinoma through interaction with p53 signaling pathway. Hepatology. 2011;53:843–853. doi: 10.1002/hep.24124. [DOI] [PubMed] [Google Scholar]
- 18.Fan TJ, Han LH, Cong RS, Liang J. Caspase family proteases and apoptosis. Acta Biochim Biophys Sin (Shanghai) 2005;37:719–727. doi: 10.1111/j.1745-7270.2005.00108.x. [DOI] [PubMed] [Google Scholar]
- 19.Wu G, Chen X, Peng J, Cai Q, Ye J, Xu H, Zheng C, Li X, Ye H, Liu X. Millimeter wave treatment induces apoptosis via activation of the mitochondrial-dependent pathway in human osteosarcoma cells. Int J Oncol. 2012;40:1543–1552. doi: 10.3892/ijo.2012.1330. [DOI] [PubMed] [Google Scholar]
- 20.Zhou L, Park BH, Park JH, Jang KY, Park HS, Wagle S, Lee KB, Kim JR. Overexpression of the prolyl isomerase PIN1 promotes cell growth in osteosarcoma cells. Oncol Rep. 2013;29:193–198. doi: 10.3892/or.2012.2112. [DOI] [PubMed] [Google Scholar]
- 21.Qiang YW, Hu B, Chen Y, Zhong Y, Shi B, Barlogie B, Shaughnessy JD Jr. Bortezomib induces osteoblast differentiation via Wntindependent activation of beta-catenin/TCF signaling. Blood. 2009;113:4319–4330. doi: 10.1182/blood-2008-08-174300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang CZ, Zhang JK, Shi Z, Liu B, Shen CQ, Tao HM. Matrine induces caspase-dependent apoptosis in human osteosarcoma cells in vitro and in vivo through the upregulation of Bax and Fas/FasL and downregulation of Bcl-2. Cancer Chemother Pharmacol. 2012;69:317–331. doi: 10.1007/s00280-011-1699-4. [DOI] [PubMed] [Google Scholar]
- 23.Yan F, Liu Y, Wang W. Matrine inhibited the growth of rat osteosarcoma UMR-108 cells by inducing apoptosis in a mitochondrial-caspase-dependent pathway. Tumour Biol. 2013;34:2135–2140. doi: 10.1007/s13277-013-0744-9. [DOI] [PubMed] [Google Scholar]
- 24.Kimura Y, Okuda H. Effects of naturally occurring stilbene glucosides from medicinal plants and wine, on tumour growth and lung metastasis in Lewis lung carcinoma-bearing mice. J Pharm Pharmacol. 2000;52:1287–1295. doi: 10.1211/0022357001777270. [DOI] [PubMed] [Google Scholar]
- 25.Aggarwal BB, Bhardwaj U, Takada Y. Regulation of TRAIL-induced apoptosis by ectopic expression of antiapoptotic factors. Vitam Horm. 2004;67:453–483. doi: 10.1016/S0083-6729(04)67023-3. [DOI] [PubMed] [Google Scholar]
- 26.Launay S, Hermine O, Fontenay M, Kroemer G, Solary E, Garrido C. Vital functions for lethal caspases. Oncogene. 2005;24:5137–5148. doi: 10.1038/sj.onc.1208524. [DOI] [PubMed] [Google Scholar]
- 27.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaux DL, Korsmeyer SJ. Cell death in development. Cell. 1999;96:245–254. doi: 10.1016/s0092-8674(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 29.Finucane DM, Bossy-Wetzel E, Waterhouse NJ, Cotter TG, Green DR. Bax-induced caspase activation and apoptosis via cytochrome c release from mitochondria is inhibitable by BclxL. J Biol Chem. 1999;274:2225–2233. doi: 10.1074/jbc.274.4.2225. [DOI] [PubMed] [Google Scholar]
- 30.Ackler S, Mitten MJ, Foster K, Oleksijew A, Refici M, Tahir SK, Xiao Y, Tse C, Frost DJ, Fesik SW, Rosenberg SH, Elmore SW, Shoemaker AR. The Bcl-2 inhibitor ABT-263 enhances the response of multiple chemotherapeutic regimens in hematologic tumors in vivo. Cancer Chemother Pharmacol. 2010;66:869–880. doi: 10.1007/s00280-009-1232-1. [DOI] [PubMed] [Google Scholar]
- 31.Salakou S, Kardamakis D, Tsamandas AC, Zolota V, Apostolakis E, Tzelepi V, Papathanasopoulos P, Bonikos DS, Papapetropoulos T, Petsas T, Dougenis D. Increased Bax/Bcl-2 ratio up-regulates caspase-3 and increases apoptosis in the thymus of patients with myasthenia gravis. In Vivo. 2007;21:123–132. [PubMed] [Google Scholar]
- 32.Li C, Shi X, Zhou G, Liu X, Wu S, Zhao J. The canonical Wnt-beta-catenin pathway in development and chemotherapy of osteosarcoma. Front Biosci (Landmark Ed) 2013;18:1384–1391. doi: 10.2741/4187. [DOI] [PubMed] [Google Scholar]
- 33.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 34.Valenta T, Hausmann G, Basler K. The many faces and functions of beta-catenin. EMBO J. 2012;31:2714–2736. doi: 10.1038/emboj.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]


