Abstract
Background: Osteosarcoma is a kind of highly malignant tumor and the growth and metastasis is closely related to angiogenesis. Vascular endothelial growth factor (VEGF) is an important angiogenesis-promoting factor. In the current study, we investigated the effects of suppressed VEGF on osteosarcoma and its molecular mechanism provided for a basis by targeting angiogenesis. Material/Methods: We established bearing human osteosarcoma Wistar rats model by subcutaneous inoculation of human SaOS-2 cells and the adenovirus vector Ad-VEGF-siRNA was constructed for further study. We assessed the efficiency of VEGF silencing and its influence on SaOS-2 cells. The expression of mRNA and protein were detected by RT-PCR and western blotting, respectively. Intratumoral microvessel density (MVD), VEGF and CD31 were evaluated by immunohistochemistry. We detected the cell apoptotic rates by flow cytometry. Results: Our results indicated that Ad-VEGF-siRNA could effectively suppressed the expression of VEGF expression, inhibited the proliferation capability and promoted apoptosis of SaOS-2 cells in vitro. Silencing of VEGF expression also suppress osteosarcoma tumor growth and reduce osteosarcoma angiogenesis in the Wistar rats model in vivo. Furthermore, We found that phosphoinositide 3-kinase (PI3K) and protein kinase B (AKT) activation were considerably reduced while inhibition VEGF expression in SaOS-2 cells. Conclusion: Our data demonstrated that VEGF silencing could suppress cells proliferation, promote cells apoptosis and reduce osteosarcoma angiogenesis through inactivation of VEGF/PI3K/AKT signaling pathway.
Keywords: Osteosarcoma, vascular endothelial growth factor (VEGF), adenovirus, siRNA, angiogenesis
Introduction
Osteosarcoma is one of the most commonly malignant bone cancer in adolescents [1]. Tumor resection, neoadjuvant chemotherapy and/or adjuvant radiotherapy were the conventional treatment for osteosarcoma, which has achieved 70% 5-year survival and 90% limb salvage rate [2,3]. Despite treated the patients with limb salvage therapy and neoadjuvant chemotherapy to improve the limb salvage rate and survival rate, but the malignancy degree of osteosarcoma continually strengthen and the age of onset constantly decreased, it still seriously affect the physical and mental health of patient [4]. Therefore, it is still urgent to find a new therapeutic approach to fight against osteosarcoma.
The occurrence and development of malignant osteosarcoma is a process of genetic damage and pathological changes, of which angiogenesis is involved in the entire process of osteosarcoma growth, invasion and metastasis [5]. In addition, osteosarcoma is a kind of highly vascular malignancy, and the growth and metastasis of osteosarcoma is also closely related to tumor angiogenesis. S U et al. reported that many pro-angiogenic factors play a crucial role in the process of tumor angiogenesis, including vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF) and insulin-like growth factor (IGF), of which particularly VEGF plays the most important role [6].
VEGF was an important regulatory factor in tumor angiogenesis via directly promote angiogenesis, vascular remodeling and increase vascular permeability, which lead to the formation of the tumor stroma [7,8]. VEGF also can promote tumor growth and metastasis via interaction with metal protease. The human VEGF gene is located on chromosome 6p21.3, length of 14 kb, which includes eight exons and seven introns [9]. It has been reported that VEGF is a selective endothelial cell mitogen, can increase the permeability of microvascular and selectively stimulate division of endothelial cell. VEGF also can promote angiogenesis by binding with the corresponding receptor in normal endothelial cells [10]. However, Matsumoto reported that multiple variants of VEGF and its corresponding receptors were expressed in osteosarcoma cells, and which were co-located in the tumor vascular endothelial cells and tumor cells, suggesting that VEGF plays an important role in tumor evolution [11,12]. The news report of Baptista indicated that there are six VEGF variants in osteosarcoma cells could combine different transmembrane receptors and then start the signal transmission system, finally promote the tumor angiogenesis [13]. In addition, Goi demonstrated that blocking of VEGF expression could inhibit tumor angiogenesis and eventually starve the tumors [14]. VEGF is considered to be the most important factor to promote angiogenesis, thereby inhibited VEGF expression becomes the main mean of anti-angiogenic therapy [14]. Since Tirumani proposed that tumor angiogenesis could be as a therapeutic target for tumor [15], many scholars focused on anti-angiogenic therapy in osteosarcoma to block the transfer of osteosarcoma cells in the source and inhibit the development of tumor metastasis foci, thus improve the clinical efficacy of osteosarcoma [16,17].
In the current study, we constructed the adenovirus vector carrying small interfering RNA (Ad-VEGF-siRNA) against human VEGF. And we also built the solid tumor model in Wistar rats bearing human osteosarcoma SaOS-2 cells. Subsequently, Ad-VEGF-siRNA was intratumorally injected and the tumorigenicity was determined. We also observed the difference between treatment group and the control group, clarifying the effect of Ad-VEGF-siRNA on the tumor angiogenesis in Wistar rats. Moreover, we detected the expression of VEGF in vitro and in vivo by RT-PCR and western blotting. Taken together, our data will provides a relationship of VEGF inhibition and tumor growth, osteosarcoma angiogenesis, which associated with the down-regulated of PI3K/AKT signaling pathway. We want to provide a theoretical and experimental basis for successfully beating the osteosarcoma by anti-tumor angiogenesis.
Materials and methods
Cell culture and main reagents
Human osteosarcoma cell line SaOS-2 came from ATCC was provided by the China Center for Type Culture Collection (CCTCC). RPMI-1640 medium, trypsin-EDTA mixture and PBS buffer was purchased from Hangzhou Gino company. Fetal bovine serum and DMSO was purchased from Beijing Dingguo Changsheng Biotechnology Company. The anti-CD31 antibody, anti-human VEGF antibody and SABC kit was purchased from Abcam.
Construction of adenovirus vector carrying specific siRNA target for VEGF
We designed the 19nt target sequence corresponding to siRNA design principles and according to the sequence of VEGF in Genebank, the sequence are as follows: VEGF-siRNA#1, Sense: GCACAUAGGAGAGAUGAGCUUdTdT, Antisense: AAGCUCAUCUCUCCUAUGUGCUGdTdT; VEGF-siRNA#2, Sense: UGAAGUUCAUGGAUGUCUAdTdT, Antisense: UAGACAUCCAUGAACUUCAdTdT; VEGF-siRNA#3, Sense: GCCUUGCCUUGCUGCUCUAdTdT, Antisense: UAGAGCAGCAAGGCAAGGCdTdT. The adenoviral vector Ad-VEGF-siRNA and Ad-Control-siRNA were constructed by ourselves and packaging the corresponding virus.
Establishment of wistar rats model bearing human osteosarcoma
Wistar rats were purchased from the Animal Center of Chinese Academy of Medical Sciences, fed in SPF level and aged 4-8 weeks, male and female each half. The animals manipulate was performed as describe under the approved agreement. Osteosarcoma cell line SaOS-2 were incubated of 5% CO2 and saturated humidity at 37°C, and cultured in RPMI-1640 medium containning 10% fetal bovine serum. When the cells grew to confluence, digestion of SaOS-2 cells with 0.25% trypsin supplemental 0. 02% EDTA, and then collected cells into centrifuge tube, 1000 rpm centrifugated 5 min, the supernatant was removed. Preparation of SaOS-2 single cell suspension, and adjusted the cell density of 1.5×107/mL. 3×106 SaOS-2 cells were suspended in 50 μL PBS and subcutaneously injected into the left axillary in Wistar rats.
Experimental grouping, administration and tumor growth index determination
The Wistar rats were randomly divided into three groups, and each group contained 5 Wistar rats. Group A was treated with Ad-VEGF-siRNA, injection of Ad-VEGF-siRNA 100 μL from tail vein since fourteenth days after inoculation. Injected every other day, 3 times a week and were injected 9 times for a total of 2×1010 PFU. Group B was treated with Ad-Control-siRNA, injection of Ad-Control-siRNA 100 μL from tail vein since fourteenth days after inoculation. Injected every other day, 3 times a week and were injected 9 times for a total of 2×1010 PFU. Group C was treated with PBS 100 μL from tail vein, injected every other day, 3 times a week and were injected 9 times. Tumor volume and tumor weight were detected according to the formula, V=ab2/2 which a represent the length and b was the width of the tumor [25]. Draw the tumor growth curve and calculate the inhibition rate of tumor. Each group was sacrificed on the seventeenth day after administration, and the tumors were weighed.
Immunohistochemical staining procedure
Immunohistochemical staining procedure according to the requirements of the operating instructions. (1) dewaxed paraffin with water; (2) antigen retrieval, each movie three times, each two minutes; (3) PBS wash 5 minutes for 3 times, 3% hydrogen peroxide at room temperature for 15 minutes, to remove the endogenous peroxidase activity; (4) PBS wash 5 minutes for 3 times, 5% goat serum blocking solution treatment at room temperature 30 minutes. Remove excess liquid; (5) added primary antibody, CD31 (1:200), VEGF (1:100), incubated overnight at 4°C in the refrigerator; (6) 37°C incubated for 1 hour. (7) PBS wash 5 minutes for 3 times, adding universal secondary antibody treatment 30 minutes; (8) PBS wash 5 minutes for 3 times, adding HRP streptavidin working solution for 30 minutes; (9) PBS wash 5 minutes for 3 times, DAB reagent color, color side view in the edge under a microscope. washing 5-15 minutes to terminate the color reaction; (10) hematoxylin, dehydrated and transparent, were mounted.
Quantification of the intratumoral MVD
10% formalin-fixed and paraffin-embedded tumor tissues were cut 4 μM sections. Sections were de-paraffinized in xylene and rehydrated in ethanol. Then, antigen retrieval was accomplished and slides were respectively cultured with VEGF and CD31 primary antibody (both purchased from Abcam) overnight at 4°C in a humidified chamber. DAB used to visualize the tissue slide and stained with haematoxylin. The blinded operator was performed to quantitative the tumor angiogenesis.
Cell proliferation assay
The cell proliferative potential was evaluated by Cell Titer Blue assay according to manufacturer’ s procedure. At first, 1×103 cells were infected with Ad-VEGF-siRNA, Ad-Control-siRNA and PBS, respectively. After infected with 1-7 days, 10 μL Cell Titer Blue/well was added to each plate and were cultured for another 2 h, the absorbance at 450 nm was read by multi-well spectrophotometer (Bio-Rad).
Reverse transcriptase PCR and quantitative reverse transcriptase PCR
Samples total RNAs were extracted using Trizol reagent (Sigma) according to the requirements of the operating instructions. Then, PrimeScript™ RT reagent Kit (TaKaRa) was used for reverse transcription reaction following the manufacturer’s instruction. Real-time PCR (RT-PCR) was used to detect the expression of VEGF, CD31 and Bax, the reaction was applied CFX96 Real-Time PCR System (Bio-Rad). GAPDH mRNA was served as a normalization.
Western blotting
Total proteins were separated by SDS-PAGE on an 8-10% gel. After electrophoresis, gels were transferred onto 0.45 μm PVDF membrane (Millipore Corp) and blocked with 2% BSA for 2-4 h at room temperature. The membranes were incubated with the following primary antibodies, VEGF (Abcam), p-PI3K and p-AKT (Santa Cruz), total-PI3K (Abcam), total-AKT (Cell Signaling Technology) and GAPDH (Santa Cruz). Secondary antibody is HRP-conjugated anti-rabbit (Jackson Labs). The bound antibody was detected by chemo-fluorescence detection kit (Amersham, Piscataway, NJ) according to the manufacturer’ instructions. GAPDH was used as a loading control and the representative images were shown.
Analyze the cell apoptotic rates by flow cytometry
SaOS-2 cells were separately treated with Ad-VEGF-siRNA, Ad-Control-siRNA and PBS. 1×106 SaOS-2 cells were harvested after treatment 24 hours, and double-stained with fluorescein APC-labeled annexin V and PI (Becton Dickinson). The percentage of apoptotic cells were detected by flow cytometry (Becton Dickinson) after staining. Analysis of the apoptotic rates in different groups and the representative images were shown.
Statistical analysis
All results were presented as mean ± SD. Statistical significance was determined by SAS9.0 or analysis of one-way ANOVA using the SPSS15.0 program. p < 0.05 was considered as statistically significant difference.
Results
Effectively inhibit VEGF expression by adenovirus vector mediated siRNA in SaOS-2 cells
In order to quested the role of VEGF in osteosarcoma tumorigenesis, at first, we investigated the biological effects of VEGF on SaOS-2 cells. In the current study, we designed of the 19nt target sequence corresponding to siRNA design principles and the sequence of VEGF in Genebank. Then, we constructed three adenoviral vectors Ad-VEGF-siRNA-1, Ad-VEGF-siRNA-2 and Ad-VEGF-siRNA-3 to inhibit the expression of VEGF. Detected the inhibition efficiency of the Ad-VEGF-siRNA by RT-PCR and western blotting assays. Our results indicated that expression of VEGF mRNA (Figure 1A) and protein (Figure 1B) were dramatically decreased in Ad-VEGF-siRNA-1 group, Ad-VEGF-siRNA-2 group and Ad-VEGF-siRNA-3 group, compared with untreated group and/or Ad-Control-siRNA group. In the three interfering with VEGF adenovirus vector, especially Ad-VEGF-siRNA-3 group exhibited the most significant inhibition effect.
Figure 1.
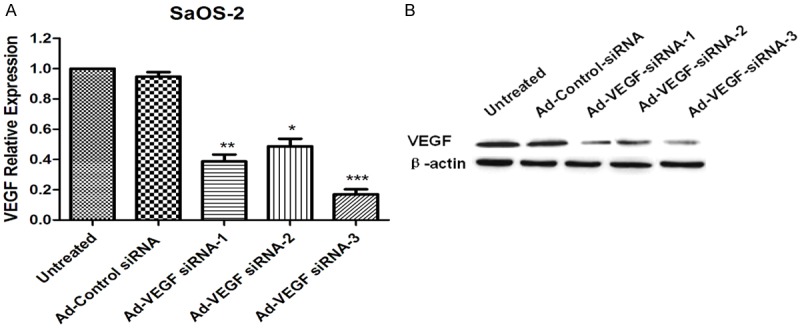
Effectively inhibit VEGF expression by adenovirus vectors mediated VEGF-siRNA in osteosarcoma cell line SaOS-2. A. RT-PCR data displayed that the expression of VEGF mRNA was obviously down-regulated in Ad-VEGF-siRNA-1 group, Ad-VEGF-siRNA-2 group and Ad-VEGF-siRNA-3 group, compared with untreated group and/or Ad-Control-siRNA group. B. The expression of VEGF protein were detected by western blotting assay.
Inhibition of VEGF expression could impair the cell proliferation of SaOS-2 cells
To illustrate that anti-tumor effects of VEGF on SaOS-2 cells in vitro, we treated SaOS-2 cells with Ad-VEGF-siRNA-3, Ad-Control-siRNA and/or untreated. expression of VEGF mRNA were detected by RT-PCR assay at different time points. The results showed that VEGF mRNA (Figure 2A) were significantly decreased at 48 h, the VEGF expression was further reduced with the extension of the time after infection. CCK-8 assay was applied to detected the proliferation of SaOS-2 cells. The data indicated that the proliferative potential of SaOS-2 cells was markedly inhibited in Ad-VEGF-siRNA-3 group compared with untreated group and/or Ad-Control-siRNA group (Figure 2B). The data in vitro can effectively support that VEGF play an important roles in human osteosarcoma cells by inhibited cell proliferation.
Figure 2.
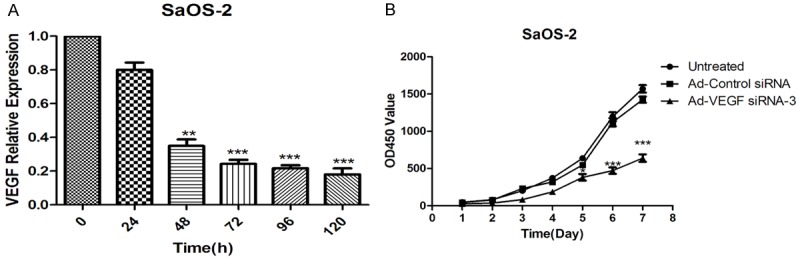
Inhibition of VEGF expression impaired the proliferation of SaOS-2 cells. A. The expression of VEGF mRNA were detected by RT-PCR assay. B. Cell Titer Blue assay shown that the proliferation of SaOS-2 cells was significantly reduced after infected with Ad-VEGF-siRNA-3, compared with untreated group and/or Ad-Control-siRNA-3 group. Untreated group cells were used as normalized. *p < 0.05, **p < 0.01, and ***p < 0.001.
VEGF silencing efficiently inhibited the growth of osteosarcoma in Wistar rats model
To further investigated the effects of VEGF silencing on osteosarcoma cells in vivo, The human osteosarcoma cell line SaOS-2 cells were applied to generate a xenograft osteosarcoma model in Wistar rats. When the subcutaneous tumors reached approximately 0.5 cm in length, the tumor volume and tumor weight were determined. The Wistar rats were randomly divided into three groups, and each group contains 5 Wistar rats. Group A was treated with Ad-VEGF-siRNA-3, injection of Ad-VEGF-siRNA-3 100 μL from tail vein since fourteenth days after inoculation. Injected every other day, 3 times a week and were injected 9 times for a total of 2×1010 PFU. Group B and C were treated with PBS and Ad-Control-siRNA 100 μL from tail vein, respectively. Figure 3A indicated that inhibition of VEGF expression could significantly reduced the growth of tumor in Wistar rats. In addition, the tumor volume and tumor weight were obviously lower in Ad-VEGF-siRNA-3 group compared with PBS group and/or Ad-Control-siRNA group at day 25 (Figure 3B, 3C). The results also indicated that tumorigenic proportion of SaOS-2 cells infected with Ad-VEGF-siRNA-3 was lower than PBS group and/or Ad-Control-siRNA group (Figure 3D). VEGF is one of the most effective angiogenic growth factors, we determined the expression of VEGF mRNA in Wistar rats tumor model. The data showed that expression of VEGF mRNA was markedly suppressed in Ad-VEGF-siRNA-3 group compared with PBS group and/or Ad-Control-siRNA group (Table 1).
Figure 3.
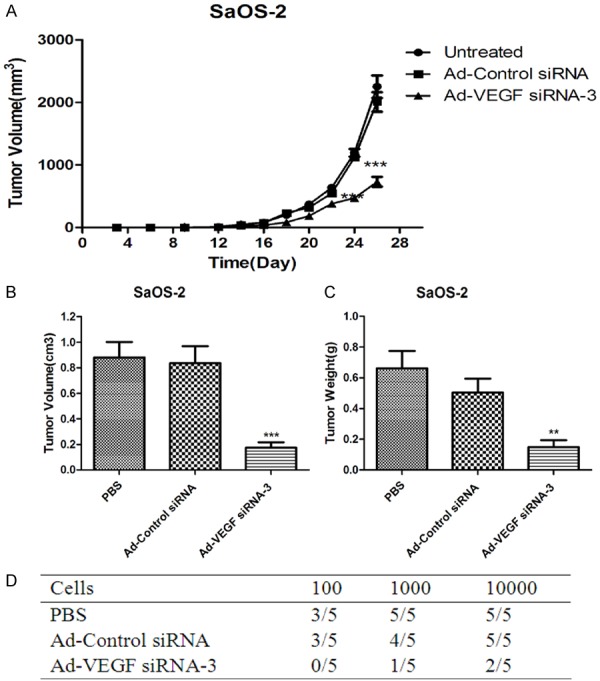
Inhibition of VEGF expression impaired the proliferation of SaOS-2 cells in Wistar rats. (A) Ad-VEGF-siRNA group cells markedly reduced the proliferative capacity of tumor versus with PBS group and/or Ad-Control-siRNA group. *p < 0.05, ***p < 0.001. (B, C) The tumor volume (B) and tumor weight (C) of Ad-VEGF-siRNA-3 cells were significantly reduced compared with PBS group and/or Ad-Control-siRNA group. (D) VEGF silencing could effectively suppressed the tumorigenicity of SaOS-2 cells in Wistar rats.
Table 1.
The expression levels of VEGF within osteosarcoma tissues after Wistar rats infected with adenovirus (positive area × OD) (x̅±s)
| Groups | Number of slices | VEGF | p1 | p2 |
|---|---|---|---|---|
| Ad-VEGF-siRNA-3 group | 8 | 341646.16±131753.84 | > 0.05 | |
| Ad-Control-siRNA group | 9 | 1325485.62±452693.48 | < 0.05 | |
| PBS group | 7 | 1452542.18±624145.49 | < 0.05 |
Note: The differences between Ad-VEGF-siRNA group and Ad-Control-siRNA group, Ad-Control-siRNA group were analyzed and shown in p1. The difference between Ad-Control-siRNA group and PBS group was analyzed and shown in p2.
Silencing of VEGF expression effectively inhibits angiogenesis of osteosarcoma xenografd in Wistar rat model
Our previously study shown that silencing of VEGF expression could efficiently inhibited the growth of osteosarcoma in Wistar rats model. Afterwards, we further investigated the factors which caused inhibited the growth of osteosarcoma cells. Angiogenesis is an important factor in promoting the growth of osteosarcoma. MVD is an important indicator for evaluating tumor angiogenesis, so we assayed the effect of VEGF silencing on osteosarcoma angiogenesis. The data showed that Ad-VEGF-siRNA-3 could decrease the counts of intratumoral MVD compared with PBS group and/or Ad-Control-siRNA group (Figure 4A, p < 0.001; Table 2). VEGF and CD31 were used to label the vascular endothelial cells. The results indicated that expression of VEGF (Figure 4B, p < 0.01) and CD31 (Figure 4C, p < 0.001) were also decreased in Ad-VEGF-siRNA-3 group compared with PBS group and/or Ad-Control-siRNA group. The representative images were shown in Figure 4D. All together, these results demonstrated that the angiogenesis was inhibited obviously in silencing of VEGF expression.
Figure 4.
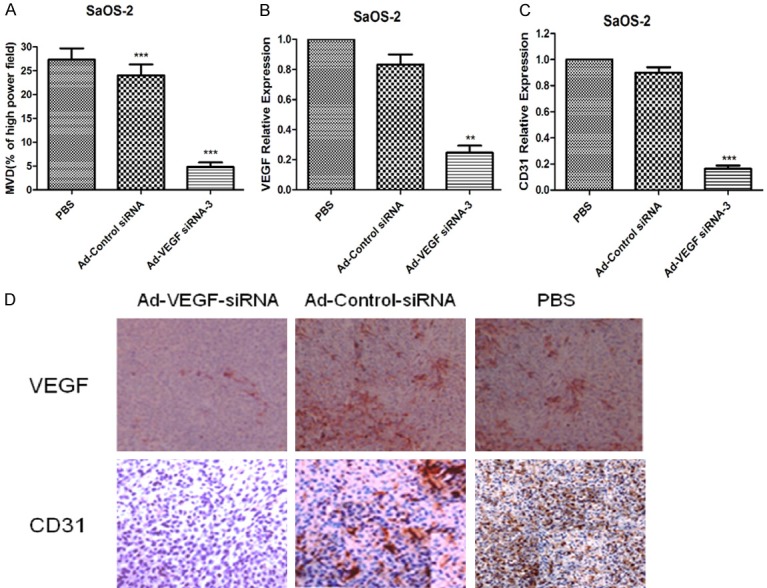
Silencing of VEGF expression effectively inhibits xenografted osteosarcoma angiogenesis in Wistar rats model. (A) Quantification of the intratumoral MVD in 10 high power fields. ***p < 0.001. (B, C) Expression of VEGF mRNA (B) and CD31 mRNA (C) was determined by RT-PCR in tumor tissues of Wistar rats. **p < 0.01, ***p < 0.001. (D) Expression of VEGF and CD31 protein were analysed by immunohistochemistry in tumor tissues of different Wistar rats.
Table 2.
The VEGF levels and MVD value in Wistar rats with osteosarcoma (x̅±s)
| Groups | VEGF | MVD |
|---|---|---|
| Ad-VEGF-siRNA-3 group | 287632.03±125212.54 | 6.38±0.39 |
| Ad-Control-siRNA group | 1023744.93±219364.36 | 12.47±1.63 |
| PBS group | 1143239.52±193745.41 | 11.95±1.79 |
Silencing of VEGF expression could promote the apoptosis of SaOS-2 cells in osteosarcoma
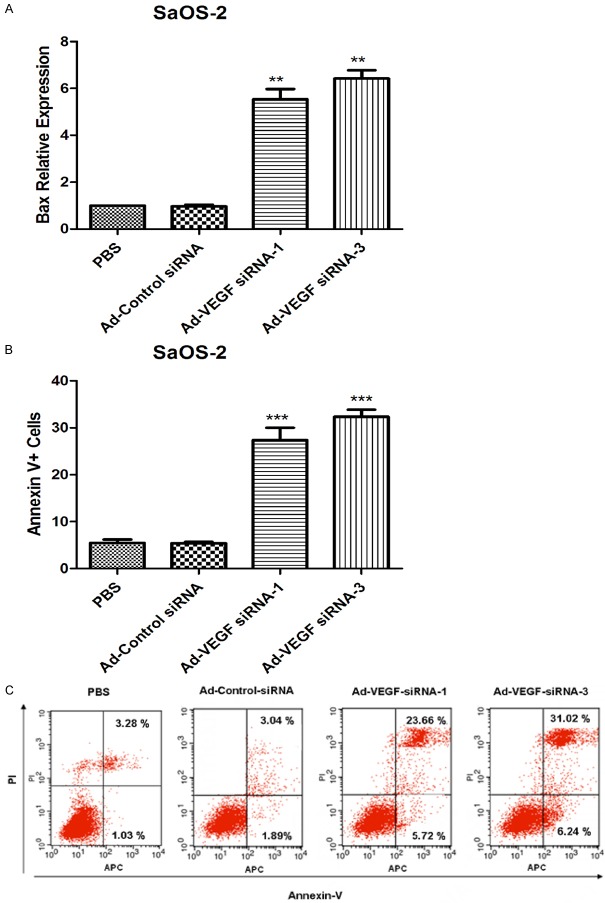
To further investigated that whether VEGF plays an important role in human SaOS-2 cells via induced cell apoptosis. Bax is a crucial pro-apoptotic gene, so we detected the expression of Bax by RT-PCR. The results indicated that expression of Bax mRNA were significantly up-regulated in Ad-VEGF-siRNA-1 group and Ad-VEGF-siRNA-3 group. APC-AnnexinV/PI double-labeled was also used to detected the cell apoptotic rates by flow cytometry in human SaOS-2 cells. The results showed that the apoptotic rates of SaOS-2 cells were obviously increased after infected with Ad-VEGF-siRNA-1 and Ad-VEGF-siRNA-3, compared with PBS group and/or Ad-Control-siRNA group (Figure 5B, p < 0.001). The representative images were shown in Figure 5C. These data implied that VEGF silencing could suppress the cell survival of SaOS-2 cells by inducing cell apoptosis.
Figure 5.
Silencing of VEGF expression promote apoptosis of SaOS-2 cells. A. Expression of Bax mRNA was assayed by RT-PCR in SaOS-2 cells, **p < 0.01. B. The cell apoptotic rate was cauculated by flow-cytometry (FCM) in SaOS-2 cells after treated with Ad-VEGF-siRNA-1 and Ad-VEGF-siRNA-3 for 24 h, PBS and/or Ad-Control-siRNA group cells were considered as normalizion, ***p < 0.001. C. The representative images were shown in FCM detection.
Silencing of VEGF expression could inhibit activation of VEGF/PI3K/AKT signaling pathway in SaOS-2 cells
VEGF was one of the crucial regulator in tumor angiogenesis, playing an important role in tumor cells survival. PI3K/AKT signaling pathway was the main downstream of VEGF, participating in differently cellular biological processes. More and more reports supported that VEGF/PI3K/AKT signaling pathway was an necessary in tumor initiation, progression and prognosis. In the current study, we firstly detected the expression of VEGF in SaOS-2 cells after infected with Ad-VEGF-siRNA, Ad-Control-siRNA or PBS. Expression of VEGF protein was markedly inhibited in Ad-VEGF-siRNA-3 group. Phosphorylation of PI3K and AKT was indicated as the activation PI3K/AKT signaling pathway. In SaOS-2 cells, we further assayed the expression of total-PI3K, total-AKT, p-PI3K, and p-AKT by western blotting. The results demonstrated that expression of total-PI3K or total-AKT was not influenced by Ad-VEGF-siRNA-3 treatment. However, phosphorylation of PI3K and AKT was notablely decreased in SaOS-2 cells compared with PBS group and/or Ad-Control-siRNA group (Figure 6). In a word, our these results indicated that effects of silencing VEGF expression on SaOS-2 cells proliferation and angiogenesis was closely interrelated with inactivation of VEGF/PI3K/AKT signaling pathway.
Figure 6.
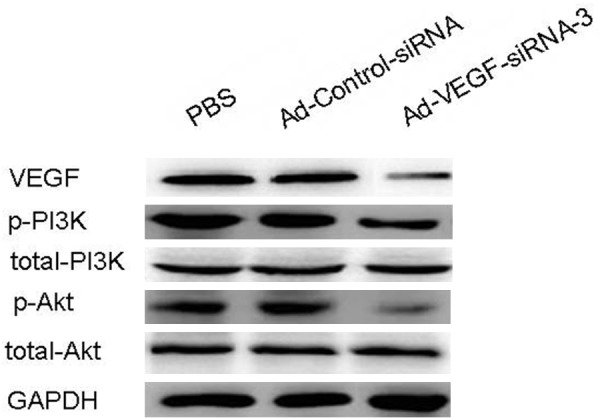
Silencing of VEGF expression suppressed the phosphorylation of PI3K and AKT in osteosarcoma cell line SaOS-2. The levels of VEGF, total-PI3K, p-PI3K, total-AKT and p-AKT were detected by western blotting in PBS group, Ad-Control-siRNA group and Ad-VEGF-siRNA-3 group. PBS and/or Ad-Control-siRNA group cells were considered as normalizion.
Discussion
Osteosarcoma is a highly malignant tumor rich in blood vessels, and tumor angiogenesis was closely related to tumor growth, invasion and metastasis [18,19]. VEGF is known to the strongest pro-angiogenic factor, and generally considered that tumor cells can self-secrete VEGF to improve the formation of its own vascular system [20]. The new blood vessels are the foundation for malignant tumor growth, invasion and metastasis, but also are important indicator of tumor malignancy degree. JL reported that VEGF expression was negative correlated to the prognosis of osteosarcoma [21]. Yu et al. has analyzed retrospectively 56 cases with stage IIB osteosarcoma, results showed that cumulative 5-year survival rate was 42.5% in the patients with less than 25% positive VEGF, while cumulative 5-year survival rate was only 8.3 % in the patients with more than 25% positive [22], furtherly confirmed that it had a close relationship between the VEGF expression and osteosarcoma.
Qiao reported that the tumor vessel wall was directly constituted by the tumor cells without attachment of endothelial cells, and tumor cells could release proteolytic enzymes to degrade the basement membrane within the pipe, then directly contact with the blood circulation, which is known as “vasculogenicm imicry” [23]. The vasculogenicm imicry may be more conducive to tumor growth, invasion and metastasis, implying that suppressed tumor angiogenesis may be an more effective strategy in the treatment of tumor [24].
Osteosarcoma cells play an critical role in tumor blood supply and tumor development. Moreover, VEGF may act on multiple kinds of cells in tumor development, including endothelial cells and endothelial precursor cells, to form typed-vascular pipeline and eventually participate in the process of osteosarcoma SaOS-2 cells proliferation and apoptosis [25]. Therefore, VEGF secreted by osteosarcoma cells may be an important initiating factor in the original tumors and metastases. In our study, the results showed that the microvascular was thin and dense with strong expression of VEGF in Ad-Control-siRNA group and PBS group, while the microvascular was thick and thin with low expression of VEGF in the Ad-VEGF-siRNA group, suggested that MVD and VEGF had a strong positive correlation, which were consistent with our subsequently correlation analysis (r=0.9992, p=0.0209, p < 0.05). Our data showed that formation of fine and dense capillaries were stimulated by strong expression of VEGF in osteosarcoma, which is the rapid formation and immature tumor angiogenesis. Our results also demonstrated that tumor microvascular usually distributed in the irregular edges of osteosarcoma. In addition, our findings also confirmed that there were no significant correlation between MVD and age, onset time or histological subtype in osteosarcoma. Subsequently, our data further demonstrated that there was a positive correlation between tumor MVD and VEGF expression (r=0.9990).
Tumor MVD is an important indicator for evaluating tumor angiogenesis and treatment effect of anti-angiogenesis [26-28]. CD31 is a membrane protein embedded onto the surface of vascular endothelial cells [29], implying that CD31 staining have a high specificity for vascular endothelial cells. Therefore, CD31 staining was used to detect the tumor microvascular of endothelial cells, and MVD was calculated by the shading in the vision. The results showed that MVD value were 6.38±0.39 in Ad-VEGF-siRNA-3 group, apparently lower than in PBS group (11.95±1.79) and/or Ad-Control-siRNA group (12.47±1.63).
PI3K/AKT signaling pathway was one of the main downstream of VEGF, participating in differently cellular biological processes [30,31]. So we further explore the molecular mechanism of suppressed osteosarcoma by inhibition of VEGF expression, and assessed the effect of VEGF silencing on PI3K/AKT signaling pathway. Our data suggested that along with obvious down-regulation of VEGF, level of p-PI3K and p-AKT was subsequently decreased in SaOS-2 cells after infected with Ad-VEGF-siRNA. However, the level of total-PI3K and total-AKT remained constant, which implies that VEGF may affect the occurrence and development of osteosarcoma by regulating PI3K/AKT signaling pathway. These results will provide a basis for developing a therapeutic strategy for targeting osteosarcoma angiogenesis.
In the process of targeting VEGF gene therapy, tumor may still escape suppression owing to other angiogenic factors will be expressed in the mutated tumor and other cells outside the tumor also can produce VEGF into blood circulation [32]. Moreover, how the long-term effects of Ad-VEGF-siRNA on osteosarcoma, and how the side effects of Ad-VEGF-siRNA on the experimenter themselves. The impact of these processes on osteosarcoma gene therapy still needs further study. But the anti-angiogenesis therapy has become a new model of cancer therapy, provides a new target for anti-angiogenic gene therapy for osteosarcoma.
References
- 1.Chou AJ, Geller DS, Gorlick R. Therapy for osteosarcoma: where do we go from here? Paediatr Drugs. 2008;10:315–27. doi: 10.2165/00148581-200810050-00005. [DOI] [PubMed] [Google Scholar]
- 2.Wang RC, Wen MC, Wang J, Ho SC, Jan YJ. Osteosarcoma arising in a long-standing uterine leiomyoma: a case report and literature review. Int J Surg Pathol. 2011;19:99–103. doi: 10.1177/1066896908327037. [DOI] [PubMed] [Google Scholar]
- 3.Hang JF, Chen PC. Parosteal osteosarcoma. Arch Pathol Lab Med. 2014;138:694–99. doi: 10.5858/arpa.2013-0030-RS. [DOI] [PubMed] [Google Scholar]
- 4.Mortus JR, Zhang Y, Hughes DP. Developmental pathways hijacked by osteosarcoma. Adv Exp Med Biol. 2014;804:93–118. doi: 10.1007/978-3-319-04843-7_5. [DOI] [PubMed] [Google Scholar]
- 5.Zhou W, Hao M, Du X, Chen K, Wang G, Yang J. Advances in targeted therapy for osteosarcoma. Discov Med. 2014;17:301–7. [PubMed] [Google Scholar]
- 6.S U, R A, P A. The knowledge, attitude and practice towards blood donation among voluntary blood donors in chennai, India. J Clin Diagn Res. 2013;7:1043–1046. doi: 10.7860/JCDR/2013/4851.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradshaw M, Mansfield A, Peikert T. The role of vascular endothelial growth factor in the pathogenesis, diagnosis and treatment of malignant pleural effusion. Curr Oncol Rep. 2013;15:207–16. doi: 10.1007/s11912-013-0315-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kozłowska A, Mackiewicz J, Mackiewicz A. Therapeutic gene modified cell based cancer vaccines. Gene. 2013;525:200–7. doi: 10.1016/j.gene.2013.03.056. [DOI] [PubMed] [Google Scholar]
- 9.Wang E, Chen Y. Intravitreal anti-vascular endothelial growth factor for choroidal neovascularization secondary to pathologic myopia: systematic review and meta-analysis. Retina. 2013;33:1375–92. doi: 10.1097/IAE.0b013e31827d260a. [DOI] [PubMed] [Google Scholar]
- 10.Lee M, Keener J, Xiao J, Long Zheng X, Rodgers GM. ADAMTS13 and its variants promote angiogenesis via upregulation of VEGF and VEGFR2. Cell Mol Life Sci. 2015;72:349–56. doi: 10.1007/s00018-014-1667-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsumoto G, Hirohata R, Hayashi K, Sugimoto Y, Kotani E, Shimabukuro J, Hirano T, Nakajima Y, Kawamata S, Mori H. Control of angiogenesis by VEGF and endostatin-encapsulated protein microcrystals and inhibition of tumor angiogenesis. Biomaterials. 2014;35:1326–33. doi: 10.1016/j.biomaterials.2013.10.051. [DOI] [PubMed] [Google Scholar]
- 12.Shantha Kumara HM, Cabot JC, Yan X, Herath SA, Luchtefeld M, Kalady MF, Feingold DL, Baxter R, Whelan RL. Minimally invasive colon resection is associated with a persistent increase in plasma PlGF levels following cancer resection. Surg Endosc. 2011;25:2153–8. doi: 10.1007/s00464-010-1514-z. [DOI] [PubMed] [Google Scholar]
- 13.Baptista AM, Camargo AF, Filippi RZ, Oliveira CR, Azevedo Neto RS, Camargo OP. Correlation between the expression of vegf and survival in osteosarcoma. Acta Ortop Bras. 2014;22:250–5. doi: 10.1590/1413-78522014220500978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goi T, Nakazawa T, Hirono Y, Yamaguchi A. The anti-tumor effect is enhanced by simultaneously targeting VEGF and PROK1 in colorectal cancer. Oncotarget. 2015;6:6053–61. doi: 10.18632/oncotarget.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tirumani SH, Fairchild A, Krajewski KM, Nishino M, Howard SA, Baheti AD, Rosenthal MH, Jagannathan JP, Shinagare AB, Ramaiya NH. Anti-VEGF Molecular Targeted Therapies in Common Solid Malignancies: Comprehensive Update for Radiologists. Radiographics. 2015;35:455–74. doi: 10.1148/rg.352140119. [DOI] [PubMed] [Google Scholar]
- 16.Abd El-Rehim DM, Osman NA. Expression of a disintegrin and metalloprotease 8 and endostatin in human osteosarcoma: Implication in tumor progression and prognosis. J Egypt Natl Canc Inst. 2015;27:1–9. doi: 10.1016/j.jnci.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Xu M, Xie Y, Sheng W, Miao J, Yang J. Adenovirus-mediated ING4 Gene Transfer in Osteosarcoma Suppresses Tumor Growth via Induction of Apoptosis and Inhibition of Tumor Angiogenesis. Technol Cancer Res Treat. 2015;14:617–26. doi: 10.7785/tcrt.2012.500424. [DOI] [PubMed] [Google Scholar]
- 18.Kubo T, Shimose S, Fujimori J, Furuta T, Arihiro K, Ochi M. Does expression of glucose transporter protein-1 relate to prognosis and angiogenesis in osteosarcoma? Clin Orthop Relat Res. 2015;473:305–10. doi: 10.1007/s11999-014-3910-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohba T, Cates JM, Cole HA, Slosky DA, Haro H, Ichikawa J, Ando T, Schwartz HS, Schoenecker JG. Pleiotropic effects of bisphosphonates on osteosarcoma. Bone. 2014;63:110–20. doi: 10.1016/j.bone.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Evans JJ, Chitcholtan K, Dann JM, Guilford P, Harris G, Lewis LK, Nagase J, Welkamp AA, Zwerus R, Sykes PH. Adrenomedullin interacts with VEGF in endometrial cancer and has varied modulation in tumours of different grades. Gynecol Oncol. 2012;125:214–9. doi: 10.1016/j.ygyno.2011.12.429. [DOI] [PubMed] [Google Scholar]
- 21.Yang JL. Investigation of osteosarcoma genomics and its impact on targeted therapy: an international collaboration to conquer human osteosarcoma. Chin J Cancer. 2014;33:575–80. doi: 10.5732/cjc.014.10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu XW, Wu TY, Yi X, Ren WP, Zhou ZB, Sun YQ, Zhang CQ. Prognostic significance of VEGF expression in osteosarcoma: a meta-analysis. Tumour Biol. 2014;35:155–60. doi: 10.1007/s13277-013-1019-1. [DOI] [PubMed] [Google Scholar]
- 23.Qiao L, Liang N, Zhang J, Xie J, Liu F, Xu D, Yu X, Tian Y. Advanced research on vasculogenic mimicry in cancer. J Cell Mol Med. 2015;19:315–26. doi: 10.1111/jcmm.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu MS, Xu LB, Zeng H, Shi XD, Wu WR, Liu C. Association of Notch1 with vasculogenic mimicry in human hepatocellular carcinoma cell lines. Int J Clin Exp Pathol. 2014;7:5782–91. [PMC free article] [PubMed] [Google Scholar]
- 25.Dong-Ju Z, Ai-Ju X, Yun-Jiao T, Ming-Qiu Z. Polymorphisms of vascular endothelial growth factor on prognosis in osteosarcoma patients. Pak J Med Sci. 2014;30:1072–6. doi: 10.12669/pjms.305.5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun H, Xu Y, Yang Q, Wang W. Assessment of tumor grade and angiogenesis in colorectal cancer: whole-volume perfusion CT. Acad Radiol. 2014;21:750–7. doi: 10.1016/j.acra.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Recher C, Ysebaert L, Beyne-Rauzy O, Mansat-De Mas V, Ruidavets JB, Cariven P, Demur C, Payrastre B, Laurent G, Racaud-Sultan C. Expression of focal adhesion kinase in acute myeloid leukemia is associated with enhanced blast migration, increased cellularity, and poor prognosis. Cancer Res. 2004;64:3191–7. doi: 10.1158/0008-5472.can-03-3005. [DOI] [PubMed] [Google Scholar]
- 28.Vanchinathan V, Mirzamani N, Kantipudi R, Schwartz EJ, Sundram UN. The vascular marker CD31 also highlights histiocytes and histiocyte-like cells within cutaneous tumors. Am J Clin Pathol. 2015;143:177–85. doi: 10.1309/AJCPRHM8CZH5EMFD. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan HC, Edgar MA, Cohen C, Kovach CK, HooKim K, Reid MD. The utility of ERG, CD31 and CD34 in the cytological diagnosis of angiosarcoma: an analysis of 25 cases. J Clin Pathol. 2015;68:44–50. doi: 10.1136/jclinpath-2014-202629. [DOI] [PubMed] [Google Scholar]
- 30.Geng J, Li X, Zhou Z, Wu CL, Dai M, Bai X. EZH2 promotes tumor progression via regulating VEGF-A/AKT signaling in non-small cell lung cancer. Cancer Lett. 2015;359:275–287. doi: 10.1016/j.canlet.2015.01.031. [DOI] [PubMed] [Google Scholar]
- 31.Kim GD. Hesperetin Inhibits Vascular Formation by Suppressing of the PI3K/AKT, ERK, and p38 MAPK Signaling Pathways. Prev Nutr Food Sci. 2014;19:299–306. doi: 10.3746/pnf.2014.19.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tucci M, Stucci S, Passarelli A, Giudice G, Dammacco F, Silvestris F. The immune escape in melanoma: role of the impaired dendritic cell function. Expert Rev Clin Immunol. 2014;10:1395–1404. doi: 10.1586/1744666X.2014.955851. [DOI] [PubMed] [Google Scholar]