Abstract
Inadequate management of neuropathic pain results in poor clinical outcomes and reduces quality of life for the patient all over the world, but intricate interplay between wide variety of the pathophysiological mechanisms involved in the development and progression of neuropathic pain makes it difficult to design effective therapeutic strategies. The present study aims to elucidate the interaction of 5-HT1A receptors (5-HT1ARs), soluble guanylate cyclase (sGC) and NO/cGMP signaling pathway in the development of neuropathic pain. The results showed that after sciatic nerve crush procedure, the protein level of sGC in the spinal cord was greatly increased. The mechanical threshold in rats was significantly enhanced by the sGC inhibitor ODQ and neuronal NO synthase (nNOS) inhibitor SMTC, indicating the role of sGC and nNOS in the process of neuropathic pain. The treatment of NO donors (SNP and SIN-1) and cGMP-selective phosphodiesterase inhibitor (Zaprinast) all significantly decreased the mechanical threshold in rats, but the 5-HT1ARs inhibitor WAY100635 significantly increased the mechanical threshold in rats, demonstrating the role of NO/cGMP pathway and 5-HT1ARs in the development of neuropathic pain. Finally, the protein levels of sGC was greatly increased by SNP and Zaprinast but decreased by WAY100635 and SMTC, showing the regulation of NO/cGMP pathway and 5-HT1ARs on the protein expression of sGC. Taken together, it is suggested that sGC in the spinal cord regulates the neuropathic pain, which is mediated by 5-HT1ARs and NO/cGMP pathway.
Keywords: Neuropathic pain, soluble guanylate cyclase, 5-HT1A receptors, neuronal NO synthase, NO/cGMP pathway
Introduction
Neuropathic pain, characterized by allodynia and pain hypersensitivity from the partially denervated regions [1], is notoriously difficult to treat with available analgesics. Patients with neuropathic pain suffer complicated symptoms associated with ongoing spontaneous pain and evoked pain [2]. Inadequate management of neuropathic pain results in poor clinical outcomes and reduces quality of life for the patient all over the world. Although several drugs such as anticonvulsants, antidepressants, and opioids are used for neuropathic pain relief, an ideal treatment is not available yet. Intricate interplay between wide variety of the pathophysiological mechanisms involved in the development and progression of neuropathic pain makes it difficult to design effective therapeutic strategies [3]. If better treatment strategies are to be developed, the pathophysiology of neuropathic pain needs to be well investigated.
There is now a substantial body of evidence to support that nitric oxide (NO)-cyclic guanosine 3’,5’-cyclic monophosphate (cGMP) signaling pathway is involved in numerous neuropathic pain symptoms [4]. It has been shown that NO plays an important role in modulation of nociceptive transmission and plasticity in the spinal cord where the neuronal NO synthase (nNOS) is concentrated [5]. The expression of the inducible nitric oxide synthase (iNOS) is found be to increased in the spinal cord and dorsal root ganglia of nerve-injured animals [6]. The fact that systemic and intrathecal administration of NOS inhibitors reduces nociceptive responses to formalin-produced peripheral inflammation in rodents supports the theory that NO may modulate the neuropathic pain [7]. Furthermore, many studies revealed that NO donors caused a reduction in tail flick or paw withdrawal latency and facilitate nociceptive behaviors in the second phase induced by formalin [8,9], while an increase of NO level in the spinal cord was induced by injection of formalin into the rat paw [10]. The major action of NO is to activate the soluble form of the enzyme guanylate cyclase (sGC), which has been designated as a physiological NO receptor [11]. Many physiological functions of NO are mediated by sGC. SGC is a heme-containing protein found in the cytosolic fraction of virtually all mammalian cells and consists of one α (82 kDa) and one β (70 kDa) subunit [12]. Three different isoforms of α (α1, α2 and α3) and β subunits (β1, β2 and β3) have been identified from rat or human tissues [12]. The sGC activity increases more than 200-fold after it is activated by NO and converts guanosine-5’-triphosphate (GTP) to cGMP [13]. As an intracellular second messenger, cGMP modifies several intracellular processes including activation of cGMP-dependent protein kinases (PKG), cGMP-gated channels, and cGMP-regulated phosphodiesterases with most effects mediated by PKG [14]. One cGMP-dependent signaling pathway related to nociception is initiated by release of NO and cGMP production by sGC in neurons of the dorsal horn in spinal cord [15]. Studies with NOS or NO-GC inhibitors or knock-out mice devoid of NO-GC activity indicated that NO-mediated cGMP production played a key role in pain sensitization [15,16]. However, the possible involvement of the sGC and NO/cGMP signaling pathway in the development of sciatic nerve crush-induced neuropathic pain has not been well explored.
The serotonin (5-hydroxytryptamine, 5-HT) system has been implicated as one of the principal neurotransmitters in the processing of nociception [17]. Seven subtypes of 5-HT receptors, composed of six distinct heptahelical G-protein-coupled receptors and one ligand-gated ion channel, have now been identified [18]. Previous reports have indicated 5-HT1-5 receptors are involved in the modulation of nociception in the spinal cord [19]. Among the many subtypes of 5-HT receptors potentially contributing to medullo-spinal modulation of pain, the 5-HT1A receptors (5-HT1ARs) have a potentially significant role, as they are expressed both in supraspinal and spinal areas related with the modulation of nociception [20,21]. Administration of 5-HT1ARs agonists in the spinal cord has produced both pro- and antiallodynic effects [22]. However, the role of 5-HT1ARs in the regulation of sGC and NO/cGMP signaling pathway in the development of sciatic nerve crush-induced neuropathic pain is not well studied yet. Hence, the present study was designed to examine the role of 5-HT1ARs and sGC and NO/cGMP signaling pathway in the process of neuropathic pain in sciatic nerve-ligated rats, trying to elucidate the interaction of 5-HT1ARs, sGC and NO/cGMP signaling pathway in the development of neuropathic pain.
Materials and methods
Animals
Male, 240-to 250-gram Sprague-Dawley rats were purchased from Shanghai Experimental Animal Center and used in this research. The experimental protocol was approved by the Animal Care and Use Committee of Shanghai Jiaotong University and carried out in accordance with The Guide for the Care and Use of Laboratory Animals, 8th Edition (National Academies Press, Washington, DC, 2010). Rats were housed in cages in the Animal Center in International Peace Maternal and Child Health Hospital of Shanghai Jiaotong University, which was maintained under standard conditions (22°C room temperature, 33% humidity) with a 12 h light/dark cycle. They were provided with access to food and water ad libitum. All measures to minimize pain or discomfort were taken by the investigators.
Sciatic nerve crush procedure
The sciatic nerve crush procedure was similar to the study of Gibbons et al. [23]. Firstly, rats were anaesthetized with ketamine (100 mg/kg, i.m.) and xylazine (7.5 mg/kg, i.m.). The skin of the right hind limb was shaved and sterilized with 10% povidone iodine solution before surgery. When the anesthesia was confirmed by absence of response to paw clamp, an incision was made on the posterior thigh. The right sciatic nerve was exposed and crushed with a pair of forceps for 10 s then crushed again at a 90° angle for another 10 s. Finally, the incised skin and muscles were closed and the rats were given ampicillin and meloxicam to prevent post-surgical infection and pain. They were rested for 7 days before assessment of allodynia. In Control animals, the forceps were placed around the sciatic nerve but the nerve was not actually crushed.
Western blotting analysis
For the Western blot analysis, the spinal cord were harvested from rats and homogenized in ice-cold homogenizing buffer (Tris-HCl, pH=6.8). Protein was then extracted from the samples using a protein extraction buffer (15 mM HEPES, 10% glycerol, 0.5% NP-40, 250 mM NaCl, 1:1000 PMSF) and a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA). The samples were heated for 5 minutes at 92-95°C and 20 μg of them were resolved on 15% polyacrylamide sodium dodecyl sulfate gels. After they were transferred to nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA, USA), the membranes were blocked in 5% skimmed milk in PBS-Tween 20 (PBST) for 1 h at room temperature and incubated overnight at 4°C with antibodies (anti-sGC and anti-β-actin, Santa Cruz, CA, USA). Next, the membrane was rinsed with PBST, incubated with peroxidase-labelled secondary antibodies for 2 hours at room temperature, washed again in PBST, and visualized with 3,3’,5,5’-tetramethylbenzidine (Sigma-aldrich, St. Louis, MO, USA). The optical densities of the sGC bands were quantified using PC BAS 2.0 software.
Assessment of allodynia
Similarly to Gibbons et al. [23], allodynia was assessed with an electronic von Frey anesthesiometer (Stoelting, Wood Dale, IL, USA). It was used to provoke a flexion reflex followed by a flinch response and to record the mechanical threshold pressure. Assessments were carried out in triplicate at 5-min intervals every day for 14 days. The data on the first day was used as the baseline and the mechanical threshold was normalized to its respective baseline. The areas under the curves (AUC) were calculated according to the linear trapezoidal rule.
Minipump administration of drugs
For minipump administration of drugs, rats were anesthetized with ketamine (100 mg/kg, i.m.) and xylazine (7.5 mg/kg, i.m.), then mounted on a animal board. Next, rats were implanted with i.th catheters (8 cm polyethylene-10 tubing) so that the catheter terminated in the lumbar region of the spinal cord, as described previously [24]. The exteriorized end of the catheter was secured by exposing it to a mild flame. Each spinal infusion cannula was connected to a dorsally subcutaneously implanted Alzet® osmotic minipump model 2001 (200-µl reservoir, 1.0±0.04 µl/hr pumping rate, delivers for up to one week) with polyvinylchloride tubing. The osmotic minipumps were implanted prior to the sciatic nerve crush procedure and allowed to deliver their content over the next seven days. All the drugs (ODQ, L-NAME, SMTC, L-NIO, 1400 W, SNP, SIN-1, Zaprinast and WAY100635) were bought from Santa Cruz Biotechnology (CA, USA) and continually administrated by the implanted minipump.
Statistical analysis of data
Data were analyzed by analysis of variance (ANOVA) and post-hoc Bonferroni’s multiple comparison tests using the GraphPad Prism 4.0 software. Statistical differences were considered significant at P<0.05. Data were represented as Mean ± S.E.M.
Results
Protein levels of sGC in brain and spinal cord tissues
Figure 1 shows the protein levels of sGC in the brain (hippocampus, cerebellum and cortex) and spinal cord tissues. Representive sGC bands from Western Blot experiments are shown Figure 1A. Samples in control groups were from the tissues of rats who received no sciatic nerve crush procedure; Samples in the NC groups were from tissues of rats who received sciatic nerve crush procedure. Quantification of optical densities was shown Figure 1B. There was no significant change in the hippocampus, cerebellum or cortex after sciatic nerve crush procedure, but in the spinal cord, the protein levels of sGC was greatly increased (P<0.05 compared to control).
Figure 1.
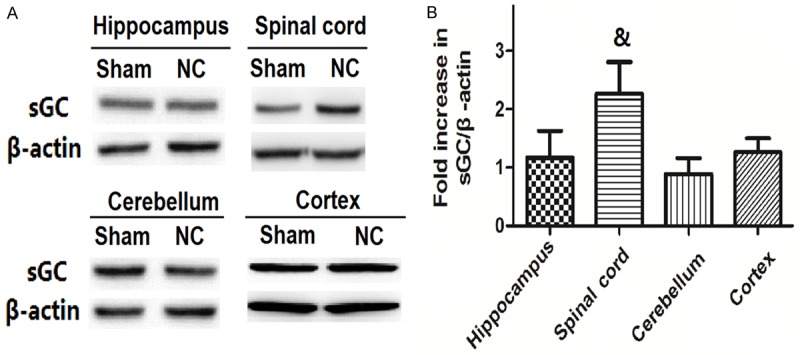
The changes of protein levels of SGC in brain and spinal cord tissues after nerve crush. Representive sGC bands from Western Blot experiments are shown in A. Quantification of optical densities was shown B. Values are expressed as the Mean ± S.E.M. &p<0.05 compared to the Sham group.
Time course of changes in mechanical threshold when treated with sGC inhibitor and their AUCs
Figure 2A compares the time course of changes in mechanical threshold in different groups: Control; nerve crush (NC) alone; 1 μg ODQ + NC, 5 μg ODQ + NC; 10 μg ODQ + NC. ODQ was continuous given with the osmotic minipump for seven days after the sciatic nerve crush procedure. Compared to the control group, the thresholds of the NC group continued to fall until stabilizing at 50% lower than the control threshold. Treatment with 1 μg ODQ had no effect on the thresholds, but 5 μg ODQ and 10 μg ODQ treatment both significantly enhanced the thresholds. As shown in Figure 2B, the AUC in the NC group was significantly smaller compared to the control group (P<0.05). The AUCs in the 5 μg ODQ + NC and 10 μg ODQ + NC groups were significantly increased compared to the NC group (P<0.05).
Figure 2.
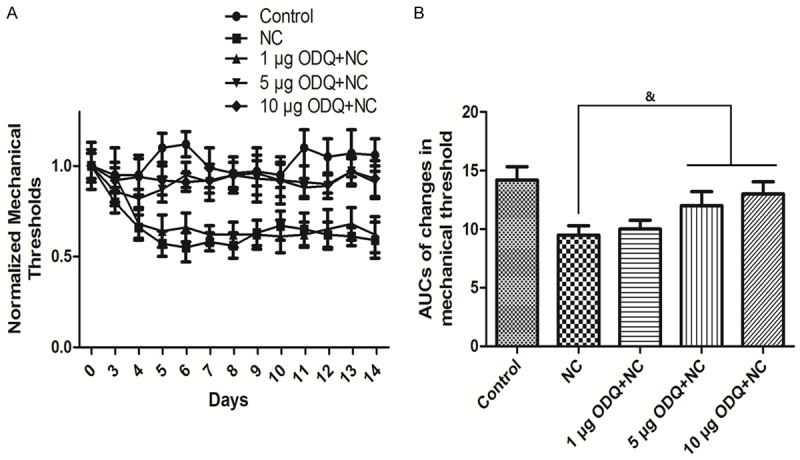
Time course of changes in mechanical threshold when treated with sGC inhibitor and their AUCs. Time course of changes in mechanical threshold is shown in A. Quantification of optical densities is shown in B. Values are expressed as the Mean ± S.E.M. &p<0.05 compared to the NC group.
Time course of changes in mechanical threshold when treated with NOS inhibitors and their AUCs
Figure 3A compares the time course of changes in mechanical threshold in groups treated with different NOS inhibitors (L-NAME: non-specific NOS inhibitor; SMTC: nNOS inhibitor; L-NIO: eNOS inhibitor; 1400 W: iNOS inhibitor). All the inhibitors were continuous given with the osmotic minipump for seven days after the sciatic nerve crush procedure. Compared to the NC group, the thresholds of the L-NIO and 1400 W groups had no effect on the thresholds, but L-NAME and SMTC treatment both significantly enhanced the thresholds. As shown in Figure 3B, the AUCs in the L-NAME + NC and SMTC + NC groups were significantly increased compared to the NC group (P<0.05).
Figure 3.
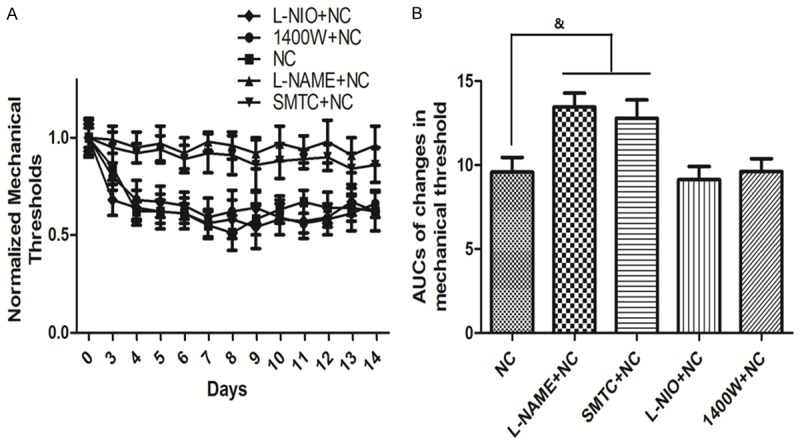
Time course of changes in mechanical threshold when treated with NOS inhibitors and their AUCs. Time course of changes in mechanical threshold is shown in A. Quantification of optical densities is shown in B. Values are expressed as the Mean ± S.E.M. &p<0.05 compared to the NC group.
Time course of changes in mechanical threshold when treated with NO donors and Zaprinast
Figure 4A compares the time course of changes in mechanical threshold in groups treated with NO donors (SNP and SIN-1) and Zaprinast. Zaprinast is a cGMP-selective phosphodiesterase inhibitor which promotes cGMP accumulation. Compared to the NC group, the thresholds of the SNP + NC, SIN-1 + NC and Zaprinast + NC groups were all significantly decreased. As shown in Figure 4B, the AUCs in the SNP + NC, SIN-1 + NC and Zaprinast + NC groups were significantly decreased compared to the NC group (P<0.05).
Figure 4.
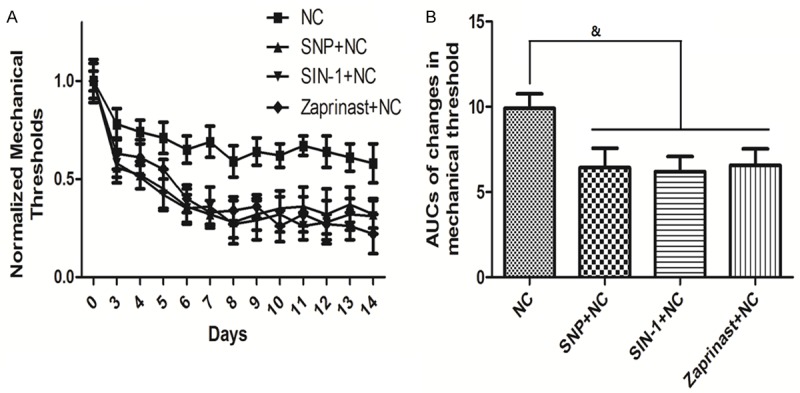
Time course of changes in mechanical threshold and their AUCs when treated with NO donors and Zaprinast. Time course of changes in mechanical threshold is shown in A. Quantification of optical densities is shown in B. Values are expressed as the Mean ± S.E.M. &p<0.05 compared to the NC group.
Time course of changes in mechanical threshold when treated with 5-HT1ARs inhibitor and their AUCs
Figure 5A demonstrates the time course of changes in mechanical threshold in groups treated with different dosages of 5-HT1ARs inhibitor WAY100635. Compared to the NC group, the thresholds of the 12.5 μg WAY100635 did not change significantly, but treatments with 25 μg, 50 μg and 100 μg WAY100635 significantly increased the thresholds. As shown in Figure 5B, the AUCs in the 25 μg, 50 μg and 100 μg WAY100635 groups were significantly higher than NC group (P<0.05).
Figure 5.
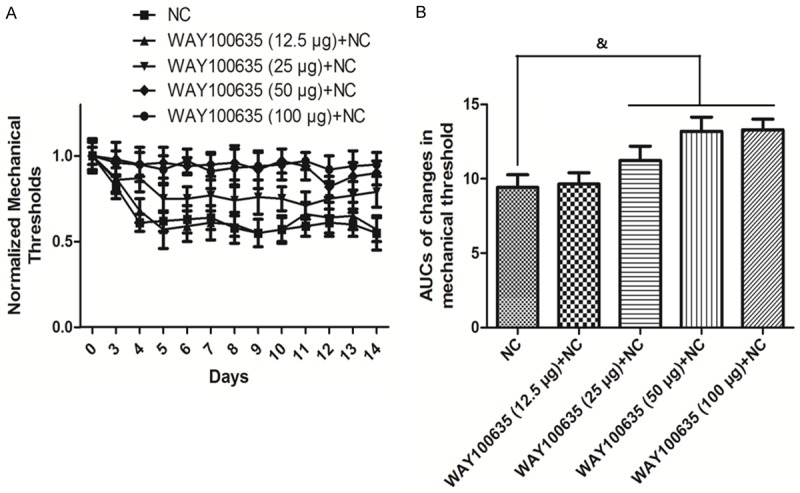
Time course of changes in mechanical threshold when treated with 5-HT1ARs inhibitor and their AUCs. Time course of changes in mechanical threshold is shown in A. Quantification of optical densities is shown in B. Values are expressed as the Mean ± S.E.M. &p<0.05 compared to the NC group.
Protein levels of sGC in the spinal cord with treatments of SNP, WAY100635, SMTC and Zaprinast
Figure 6 shows the protein levels of sGC in the spinal cord with treatments of SNP, WAY100635, SMTC and Zaprinast. Samples in Control groups were from the spinal cord of rats who received no sciatic nerve crush procedure. Rats in the Vehicle group received sciatic nerve crush procedure and saline. The rest groups received sciatic nerve crush procedure and drug administration. Representive sGC bands from Western Blot experiments are shown in Figure 6A. Quantification of optical densities was shown in Figure 6B. Compared to the Vehicle group, the protein levels of sGC was significantly increased in the SNP and Zaprinast groups, but decreased in the WAY100635 and SMTC groups (P<0.05 compared to Vehicle).
Figure 6.
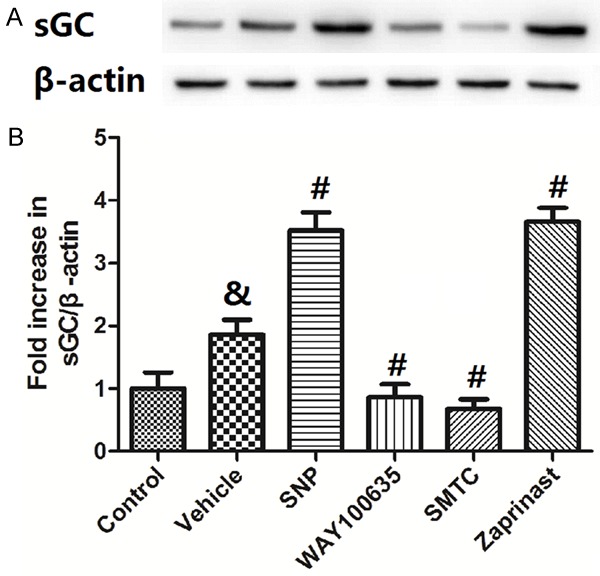
Protein levels of sGC in the spinal cord with treatments of NO donor or inhibition of 5-HT1ARs, nNOS or Zaprinast. Representive sGC bands from Western Blot experiments are shown in A. Quantification of optical densities was shown B. Values are expressed as the Mean ± S.E.M. &p<0.05 compared to the Control group; #p<0.05 compared to the Vehicle group.
Discussion
Sciatic nerve crush is a well accepted model to produce neuropathic pain. In this model, neuropathic pain is caused by a prolonged combination of mechanical compression and inflammation of nerves and results in a persistent increase in hyperalgesia and allodynia. After the sciatic nerve crush is accomplished, the nerve lesion induces neuronal and immunological changes and modifies gene and protein expression, both in the peripheral nervous system and in the spinal cord [25]. However, the exact mechanism under the development of neuropathic pain is not clear yet. The present study aims to examine the roles and interaction of 5-HT1ARs, sGC and NO/cGMP signaling pathway in the development of neuropathic pain. The results showed that after sciatic nerve crush procedure, the protein level of sGC in the spinal cord was greatly increased. The mechanical threshold in rats was significantly enhanced by the sGC inhibitor ODQ, nNOS inhibitor SMTC, indicating the role of sGC and nNOS in the process of neuropathic pain. The treatment of NO donors (SNP and SIN-1) and cGMP-selective phosphodiesterase inhibitor (Zaprinast) all significantly decreased the mechanical threshold in rats, but the 5-HT1ARs inhibitor WAY100635 significantly increased the mechanical threshold in rats, demonstrating the role of NO/cGMP pathway and 5-HT1ARs in the development of neuropathic pain. Finally, the protein levels of sGC was greatly increased by SNP and Zaprinast but decreased by WAY100635 and SMTC, showing the regulation of NO/cGMP pathway and 5-HT1ARs on the protein expression of sGC.
Previous studies have reported a variety of mechanisms triggering the emergence of pain after nerve injuries, including sensitization of pain receptors, spontaneous and ectopic firing of nociceptive neurons, and changes in gene expression of ion channels and receptors in nociceptive axons and dorsal root ganglia (DRG) neurons [26,27]. However, the role of sGC was seldom explored. Functional sGC is a heterodimer comprising one α and one β subunit [28], only α1β1 and α2β1 heterodimers have been found in the CNS [29]. The α1β1 heterodimer is the predominant isoform of sGC that is obligatory for catalytic activity. NO binds to the ferrous heme at histidine 105 of the β1 subunit and leads to an increase in sGC activity and cGMP production of at least 200-fold. Ding and Weinberg studied the organization of sGC in the superficial dorsal horn of rat and found out that almost all neurokinin 1 receptor-positive neurons in lamina I and many local circuit neurons in laminae I-II were strongly immunopositive for sGC [30]. We studied the protein levels of sGC in the brain and spinal cord tissues and found out that there was no significant change of sGC in the hippocampus, cerebellum or cortex after sciatic nerve crush procedure, but in the spinal cord, the protein levels of sGC was greatly increased. We also revealed that treatment with 5 μg ODQ and 10 μg ODQ treatment both significantly enhanced the mechanical thresholds. ODQ is a highly selective, irreversible inhibitor of sGC [31]. Its ability to antagonize sciatic nerve crush induced nociception supports the contention that activation of sGC was involved in the mechanism.
The involvement of NO/cGMP pathway in the development of neuropathic pain has been intensively studied by other investigator, but its role in the modulation of nociception/antinociception remains controversial. A study of Gediz et al. suggested that phosphodiesterase type-5 (PDE5) inhibitor vardenafil produced a dose-dependent antinociceptive effect, resulting from L-arginine/NO-cGMP pathway activation [32]. Another PDE5 inhibitor tadalafil-induced anti-hyperalgesic effect also involves NO/cGMP pathway [33]. It was also reported that the modulation of NO/cGMP/K+ATP pathway plays an important role in the antinociceptive mechanism of dizocilpine [34]. However, Makuch et al. revealed that the NOS inhibitors enhanced morphine antinociception in acute pain and in chronic constriction injury-exposed rats [35]. It was also demonstrated that intrathecal administration of the non-selective NOS inhibitor (L-NAME) produced a dose-dependent reduction of thermal hyperalgesia and nNOS likely played a role during the injury, suggesting the nociceptive role of NO and nNOS [35]. In the present study, the mechanical threshold in rats was significantly enhanced by the L-NAME and nNOS inhibitor SMTC, indicating the role of NO and nNOS in the process of neuropathic pain. The treatment of NO donors (SNP and SIN-1) and Zaprinast all significantly decreased the mechanical threshold in rats, demonstrating the role of NO/cGMP pathway in the development of neuropathic pain. The fact that the mechanical threshold in rats was significantly enhanced by nNOS inhibitor SMTC but not eNOS inhibitor L-NIO or iNOS inhibitor 1400W, indicating the special role of nNOS-produced NO in the process of neuropathic pain. It seems that NO has a dual effect on the modulation of nociception/antinociception, and the location of NOS may be an important factor. In most reports, cerebral NOS produced antinociceptive effects, while the NOS in the spinal cord usually produced nociceptive effects. Moreover, the NO donor SNP and cGMP-selective phosphodiesterase inhibitor Zaprinast significantly increased the protein levels of sGC, but the nNOS inhibitor SMTC decreased the protein levels of sGC, indicating the regulation of nNOS/NO/GMP pathways on sGC expression.
Previous studies have shown that 5-HT1ARs, which are expressed both in supraspinal and spinal areas, play an important role in the medullo-spinal modulation of pain [20,21]. However, its role in the modulation of nociception/antinociception is not yet clear. Administration of 5-HT1ARs agonists in the spinal cord has produced both pro- and antiallodynic effects [22], which seems to depend on many factors such as the dose, duration of treatment, and type of nociceptive stimuli [36,37]. Here, the results that 5-HT1ARs inhibitor WAY100635 significantly increased the mechanical threshold in rats indicates the pro-allodynic effects of 5-HT1ARs. To further investigate the mechanism of the pro-allodynic effects of 5-HT1ARs, we measured the protein level of sGC with the treatment of WAY100635. The results showed that WAY100635 significantly reduced the protein level of sGC, indicating the regulation of 5-HT1ARs on sGC expression, which may be a mechanism of the action of 5-HT1ARs. It still remains to be studied why in the sciatic nerve crush induced neuropathic pain 5-HT1ARs promoted allodynia and increased sGC expression. A study of Zhang et al. showed that 5-HT1ARs agonist and antagonist and 5-HT reuptake inhibitor were able to regulate the hippocampal nNOS expression [38]. It is also possible in the present study that sciatic nerve crush induces activation of 5-HT1ARs, which sequentially activates nNOS and produces sufficient NO and stimulates the protein expression of sGC. The NO/sGC/cGMP pathway is eventually activated and the neuropathic pain is developed. However, further exploration is required to examine this hypothesis in the future.
Acknowledgements
This study was sponsored by the Science and Technology Commission of Shanghai Municipality, China (No. 124119a3401) and the Interdisciplinary Program of Shanghai Jiao Tong University (NO. YG2015MS45).
Disclosure of conflict of interest
None.
References
- 1.Gilron I, Baron R, Jensen T. Neuropathic pain: principles of diagnosis and treatment. Mayo Clin Proc. 2015;90:532–545. doi: 10.1016/j.mayocp.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 2.Truini A, Garcia-Larrea L, Cruccu G. Reappraising neuropathic pain in humans--how symptoms help disclose mechanisms. Nat Rev Neurol. 2013;9:572–582. doi: 10.1038/nrneurol.2013.180. [DOI] [PubMed] [Google Scholar]
- 3.Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9:807–819. doi: 10.1016/S1474-4422(10)70143-5. [DOI] [PubMed] [Google Scholar]
- 4.Ji RR, Gereau RW 4th, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Rev. 2009;60:135–148. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saito S, Kidd GJ, Trapp BD, Dawson TM, Bredt DS, Wilson DA, Traystman RJ, Snyder SH, Hanley DF. Rat spinal cord neurons contain nitric oxide synthase. Neuroscience. 1994;59:447–456. doi: 10.1016/0306-4522(94)90608-4. [DOI] [PubMed] [Google Scholar]
- 6.De Alba J, Clayton NM, Collins SD, Colthup P, Chessell I, Knowles RG. GW274150, a novel and highly selective inhibitor of the inducible isoform of nitric oxide synthase (iNOS), shows analgesic effects in rat models of inflammatory and neuropathic pain. Pain. 2006;120:170–181. doi: 10.1016/j.pain.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 7.Roche AK, Cook M, Wilcox GL, Kajander KC. A nitric oxide synthesis inhibitor (L-NAME) reduces licking behavior and Fos-labeling in the spinal cord of rats during formalin-induced inflammation. Pain. 1996;66:331–341. doi: 10.1016/0304-3959(96)03025-4. [DOI] [PubMed] [Google Scholar]
- 8.Tao YX, Johns RA. Activation of cGMP-dependent protein kinase Ialpha is required for Nmethyl-D-aspartate- or nitric oxide-produced spinal thermal hyperalgesia. Eur J Pharmacol. 2000;392:141–145. doi: 10.1016/s0014-2999(00)00129-1. [DOI] [PubMed] [Google Scholar]
- 9.Shibuta S, Mashimo T, Zhang P, Ohara A, Yoshiya I. A new nitric oxide donor, NOC-18, exhibits a nociceptive effect in the rat formalin model. J Neurol Sci. 1996;141:1–5. doi: 10.1016/0022-510x(96)00102-5. [DOI] [PubMed] [Google Scholar]
- 10.Okuda K, Sakurada C, Takahashi M, Yamada T, Sakurada T. Characterization of nociceptive responses and spinal releases of nitric oxide metabolites and glutamate evoked by different concentrations of formalin in rats. Pain. 2001;92:107–115. doi: 10.1016/s0304-3959(00)00476-0. [DOI] [PubMed] [Google Scholar]
- 11.Furuyama T, Inagaki S, Takagi H. Localizations of alpha 1 and beta 1 subunits of soluble guanylate cyclase in the rat brain. Brain Res Mol Brain Res. 1993;20:335–344. doi: 10.1016/0169-328x(93)90060-3. [DOI] [PubMed] [Google Scholar]
- 12.Hobbs AJ, Ignarro LJ. Nitric oxide-cyclic GMP signal transduction system. Methods Enzymol. 1996;269:134–148. doi: 10.1016/s0076-6879(96)69016-8. [DOI] [PubMed] [Google Scholar]
- 13.Qian Y, Chao DS, Santillano DR, Cornwell TL, Nairn AC, Greengard P, Lincoln TM, Bredt DS. cGMP-dependent protein kinase in dorsal root ganglion: relationship with nitric oxide synthase and nociceptive neurons. J Neurosci. 1996;16:3130–3138. doi: 10.1523/JNEUROSCI.16-10-03130.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knowles RG, Palacios M, Palmer RM, Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci U S A. 1989;86:5159–5162. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidtko A, Gao W, König P, Heine S, Motterlini R, Ruth P, Schlossmann J, Koesling D, Niederberger E, Tegeder I, Friebe A, Geisslinger G. cGMP produced by NO-sensitive guanylyl cyclase essentially contributes to inflammatory and neuropathic pain by using targets different from cGMP-dependent protein kinase I. J Neurosci. 2008;28:8568–76. doi: 10.1523/JNEUROSCI.2128-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo ZD, Cizkova D. The role of nitric oxide in nociception. Curr Rev Pain. 2000;4:459–466. doi: 10.1007/s11916-000-0070-y. [DOI] [PubMed] [Google Scholar]
- 17.Andrews N, O’Neill MF. It might be a big family but the pain remains-last chance saloon for selective 5-HT receptor ligands? Curr Opin Pharmacol. 2011;11:39–44. doi: 10.1016/j.coph.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 19.Jeong CY, Choi JI, Yoon MH. Roles of serotonin receptor subtypes for the antinociception of 5-HT in the spinal cord of rats. Eur J Pharmacol. 2004;502:205–211. doi: 10.1016/j.ejphar.2004.08.048. [DOI] [PubMed] [Google Scholar]
- 20.Azmitia EC, Gannon PJ, Kheck NM, Whitaker-Azmitia PM. Cellular localization of the 5-HT1A receptor in primate brain neurons and glial cells. Neuropsychopharmacology. 1996;14:35–46. doi: 10.1016/S0893-133X(96)80057-1. [DOI] [PubMed] [Google Scholar]
- 21.Marlier L, Teilhac JR, Cerruti C, Privat A. Autoradiographic mapping of 5-HT1, 5-HT1A, 5-HT1B and 5-HT2 receptors in the rat spinal cord. Brain Res. 1991;550:15–23. doi: 10.1016/0006-8993(91)90400-p. [DOI] [PubMed] [Google Scholar]
- 22.Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 23.Gibbons CR, Liu S, Zhang Y, Sayre CL, Levitch BR, Moehlmann SB, Shirachi DY, Quock RM. Involvement of brain opioid receptors in the anti-allodynic effect of hyperbaric oxygen in rats with sciatic nerve crush-induced neuropathic pain. Brain Res. 2013;6:111–116. doi: 10.1016/j.brainres.2013.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tumati S, Roeske WR, Largent-Milnes T, Wang R, Vanderah TW, Varga EV. Sustained morphine-mediated pain sensitization and antinociceptive tolerance are blocked by intrathecal treatment with Raf-1-selective siRNA. Br J Pharmacol. 2010;161:51–64. doi: 10.1111/j.1476-5381.2010.00869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moalem G, Tracey DJ. Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev. 2006;51:240–264. doi: 10.1016/j.brainresrev.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Navarro X, Vivo M, Valero-Cabre A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82:163–201. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Sah DW, Ossipo MH, Porreca F. Neurotrophic factors as novel therapeutics for neuropathic pain. Nat Rev Drug Discov. 2003;2:460–472. doi: 10.1038/nrd1107. [DOI] [PubMed] [Google Scholar]
- 28.Gibb BJ, Wykes V, Garthwaite J. Properties of NO-activated guanylyl cyclases expressed in cells. Br J Pharmacol. 2003;139:1032–1040. doi: 10.1038/sj.bjp.0705318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koesling D, Russwurm M, Mergia E, Mullershausen F, Friebe A. Nitric oxide-sensitive guanylyl cyclase: structure and regulation. Neurochem Int. 2004;45:813–819. doi: 10.1016/j.neuint.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 30.Ding JD, Weinberg RJ. Localization of soluble guanylyl cyclase in the superficial dorsal horn. J Comp Neurol. 2006;495:668–678. doi: 10.1002/cne.20901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garthwaite J, Southam E, Boulton CL, Nielsen EB, Schmidt K, Mayer B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4] oxadiazolo[4,3-a] quinoxalin-1-one. Mol Pharmacol. 1995;48:184–188. [PubMed] [Google Scholar]
- 32.Gediz EI, Nacitarhan C, Minareci E, Sadan G. Antinociceptive Effect of Vardenafil on Carrageenan-Induced Hyperalgesia in Rat: involvement of Nitric Oxide/Cyclic Guanosine Monophosphate/Calcium Channels Pathway. Iran J Pharm Res. 2015;14:1137–1143. [PMC free article] [PubMed] [Google Scholar]
- 33.Otari KV, Upasani CD. Involvement of NO-cGMP pathway in anti-hyperalgesic effect of PDE5 inhibitor tadalafil in experimental hyperalgesia. Inflammopharmacology. 2015;23:187–194. doi: 10.1007/s10787-015-0240-5. [DOI] [PubMed] [Google Scholar]
- 34.Vuckovic S, Srebro D, Savic Vujovic K, Prostran M. The antinociceptive effects of magnesium sulfate and MK-801 in visceral inflammatory pain model: The role of NO/cGMP/K(+)ATP pathway. Pharm Biol. 2015;53:1621–1627. doi: 10.3109/13880209.2014.996821. [DOI] [PubMed] [Google Scholar]
- 35.Makuch W, Mika J, Rojewska E, Zychowska M, Przewlocka B. Effects of selective and non-selective inhibitors of nitric oxide synthase on morphine- and endomorphin-1-induced analgesia in acute and neuropathic pain in rats. Neuropharmacology. 2013;75:445–457. doi: 10.1016/j.neuropharm.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 36.Bonnefont J, Chapuy E, Clottes E, Alloui A, Eschalier A. Spinal 5-HT1A receptors differentially influence nociceptive processing according to the nature of the noxious stimulus in rats: effect of WAY-100635 on the antinociceptive activities of paracetamol, venlafaxine and 5-HT. Pain. 2005;114:482–490. doi: 10.1016/j.pain.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 37.Bardin L, Assié MB, Pélissou M, Royer-Urios I, Newman-Tancredi A, Ribet JP, Sautel F, Koek W, Colpaert FC. Dual, hyperalgesic, and analgesic effects of the high-efficacy 5-hydroxytryptamine 1A (5-HT1A) agonist F 13640 [(3-chloro-4-fluoro-phenyl)-[4-fluoro-4-{[(5-methyl-pyridin-2-ylmethyl)-amino] -me thyl}piperidin-1-yl] methanone, fumaric acid salt] : relationship with 5-HT1A receptor occupancy and kinetic parameters. J Pharmacol Exp Ther. 2005;312:1034–1042. doi: 10.1124/jpet.104.077669. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J, Huang XY, Ye ML, Luo CX, Wu HY, Hu Y, Zhou QG, Wu DL, Zhu LJ, Zhu DY. Neuronal nitric oxide synthase alteration accounts for the role of 5-HT1A receptor in modulating anxietyrelated behaviors. J Neurosci. 2010;30:2433–2441. doi: 10.1523/JNEUROSCI.5880-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


