Abstract
Previous studies had demonstrated that IL-13 and its receptor IL-13Rα2 participated in the process of onset and development of colorectal cancer, however, its detailed mechanism was still unclear. Herein, we demonstrated that IL-13 induced the expression of 11βHSD2 in an IL-13Rα2 dependent manner in colorectal cancer cells. Furthermore, we indicated 11βHSD2 was critical for IL-13 to induce the expression of COX2 and activated Akt, which was essential for IL-13 to promote the colony formation abilities and migration abilities of colorectal cancer cells. Inhibitor of 11βHSD2 glycyrrhizic acid (GA) significantly reduced the liver metastasis of colorectal cancers cells seeded in the Appendix serous of the nude mice. These results provide evidences to reveal the molecular mechanism in the process of colorectal cancer involving IL-13 and its receptor IL-13Rα2, and may provide new therapeutic target for treatment of colorectal cancer.
Keywords: IL-13, IL-13Rα2, 11βHSD2, colorectal cancer
Introduction
Colorectal cancer, one of the common malignancies with high morbidity and lethal rate, and liver metastasis is the prominent death-related cause of this disease [1,2]. It is critical for us to address the mechanism that is responsible for liver metastasis of colorectal cancer.
Interleukin-13 (IL-13) is one of the cytokines secreted by activated Th2 cells [3], which is previously indicated to participate in the regulation of inflammation and immune response [4]. Recently, IL-13 was found to be take part in the process of tumor onset and progression [5-7], including colorectal cancer [6,8]. High expression of IL-13 and its receptor in the colorectal cancer tissues was negative correlation with tumor-free survival of the patients [8]. Kanai and his colleagues found that overexpression of IL-13 reduced the adhesion of colo205 colorectal cancer cells [9]. To the present, studies found IL-13 elicits its function via its receptor on the surface of the cell. IL-13Rα1 and IL-13Rα2 are the two receptors of IL-13. IL-13Rα1 forms a heterodimer with IL-4 receptor, which transduces the signal from IL-13 via activating MAPK and STAT6 pathways [5]. IL-13Rα2 has a higher affinity with IL-13 than that of IL-13Rα1 [10], thus considering as specific receptor of IL-13. The expression of IL-13Rα2 has been reported significantly increased in many kinds of tumors, such as head and neck neoplasm [11], ovarian cancer [12], Kaposi’s sarcoma [13], pancreatic cancer [14,15], adrenocortical carcinoma [16] and colorectal cancer [6,8]. Although the pivotal role of IL13Rα2 in cancer onset and progression is due to the lacking of interacting domain with effect protein Box I/II Jak, the detail mechanisms through which IL-13Rα2 takes part in the process of cancer is still unclear.
11β-hydroxysteroid dehydrogenase type II (11βHSD2) is a key enzyme regulating the activities of endogenous glucocorticoids and participates in the regulation of inflammation [17]. Recently, 11βHSD2 has been reported involving in the regulation of tumor cell proliferation and chemo-resistance [9,17,18]. In our previous studies, we found that the expression of 11βHSD2 was increased in clinical colorectal cancer tissues and participated in the process of metastasis of colorectal cancer [19,20]. Although the role of 11βHSD2 in the tumorgenesis of cancer has been well studied, the mechanism regulating the expression of 11βHSD2 in cancers, especially in colorectal cancer, is still unclear.
In the study, we demonstrated that IL-13 could induce the expression 11βHSD2 in IL-13Rα2 dependent manner. 11βHSD2 is a crucial downstream molecule of IL-13, and mediates the activation of Akt as well as increase the expression of COX2 in CT26 and SW480 colorectal cancer cell lines. What’s more we used in vitro assay as well as in vivo assay to demonstrate that 11βHSD2 was essential for IL-13 to promote the colony formation abilities, migration abilities and metastasis abilities of colorectal cancer cells. Our results presented here may provide new experimental evidence as well as new therapeutic targets for colorectal cancer prevention and treatment.
Materials and methods
Cell culture and treatment
CT26 and SW480 cells were cultured in RPMI-1640 Medium (ATCC 30-2001), L-15 Medium (Catalog No. 30-2008), respectively, supplemented with 10% FBS and 1% penicillin-streptomycin and maintained at water-jacketed CO2 incubator at 37°C with 5% CO2. Transfections were performed with LipofectamineTM 2000 reagent (Invitrogen, Carlsbad, CA) per manufacturer’s instructions. For IL-13 and/or GA treatment, the culture medium was replaced with mediun containing 10 ng/ml IL-13 or culture mediun with 10 ng/ml IL-13 and 10 uM GA for indicated times.
Western blot
The lysis used for western blot was prepared by adding RIPA (0.1% SDS, 1% NP-40, 1 mM MgCl2 and 10 mM Tris, PH 8.0) with 1×coktail proteinase inbitor directly to the cells with indicated treatment. Equal amount of protein was separated by SDS-PAGE and then transferred to PVDF membrane pretreated with methanol. Primary antibodies used in this study were purchased from Abcam. HRP-conjugated anti-mouse or anti-rabbit IgG was used and the bands were visualized by chemiluminescence.
Quantitative real-time reverse transcription PCR
Total RNAs were extracted from the cells with Trizol reagent (Invitrogen, Carlsbad, CA) according to manufacturer’s instructions. The first-strand cDNA was synthesised with MMLV reverse transcriptase (TAKARA, Dalian, China) with one microgram total RNAs. Real-time PCR was performed using iQ SYBR Green Supermix and the iCycler real-time PCR detection system (Bio-Rad), and the fold change in expression of each target RNA relative to U6 snRNA or beta-actin mRNA was calculated based on the threshold cycle (Ct) as 2-(ΔCt), where ΔCt = Cttarget- CtU6/actin, and Δ (ΔCt) = ΔCt sample -ΔCt control.
MTT assay
CT26 or SW480 cells were seeded in the 96-well plate and then the culture medium was replaced with culture medium containing 10 ng/ml IL-13 or culture mediun with 10 ng/ml IL-13 and 10 uM GA for indicated times. Following the treatments, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide was added to the medium to final concentration of 500 ug/ml, and the cells were stated at 37°C for four hours before culture medium was discarded and 100 ul DMSO was added. The absorbance at 570 nm wavelength was determined.
Colony formation assay
CT26 and SW480 cells were seeded at 24-well plate at 300 cells per well 300 cells and treated with 10 ng/ml IL-13 or 10 ng/ml IL-13 and 10 mM GA. The culture medium was changed every other day for seven times before the cells were fixed with paraformaldehyde and stained with crystal violet. The number of colones with more than 50 cells was counted, and the colony formation rate was calculated as (number of colones/300)*100%.
Transwell migration assays
The 24-well Boyden chamber with 8 μm pore size polycarbonate membrane (Corning, Cambridge, MA) was used to analyze the migration and invasion of tumor cells. Briefly, about 5×105 cells, resuspended in culture medium without FBS but with/wihout 10 ng/ml IL-13 and/or 10 nM GA, were seeded in the chamber of the well, and then the well was put into 24-well with 600 ul culture medium containing 20% FBS. About 48 hours later, the cells were fixed with paraformaldehyde and then stained with crystal violet, and the cells went through the membrane were counted.
In vivo tumor metastasis assay
About 5×105 CT26 cells were seeded in the Appendix serous of nude mice. When the in situ tumors were formed, GA was administered via drinking water for 24 days. The mice were then sacrificed and the metastasis sites in the liver were counted. All the animals used in this study were cared in accordance with American Association for the Accreditation of Laboratory Animal Care guidelines for human treatment of animals and adhered to national and international standards.
Results
IL-13 induces 11βHSD2 expression in colorectal cancer cells
Previous studies have demonstrated that IL-13 induced the expression of 11βHSD2 in lung epithelial cells [21]. To investigate whether IL-13 induces 11βHSD2 expression in colorectal cancer cells, we treated mouse colorectal cancer cell line CT26 or human colorectal cancer cell SW480 with IL-13. The expression of 11βHSD2 mRNA and protein were detected by qRT-PCR and western blot, respectively. The results indicate that IL-3 increased the mRNA level of 11βHSD2 in time dependent manner in both cell lines (Figure 1A and 1B). Ant the results of western blot further support that IL-13 increased the expression of 11βHSD2 in colorectal cancer cells (Figure 1C and 1D). Together, these results demonstrated that IL-13 increased the expression of 11βHSD2 in colorectal cancer cell lines.
Figure 1.
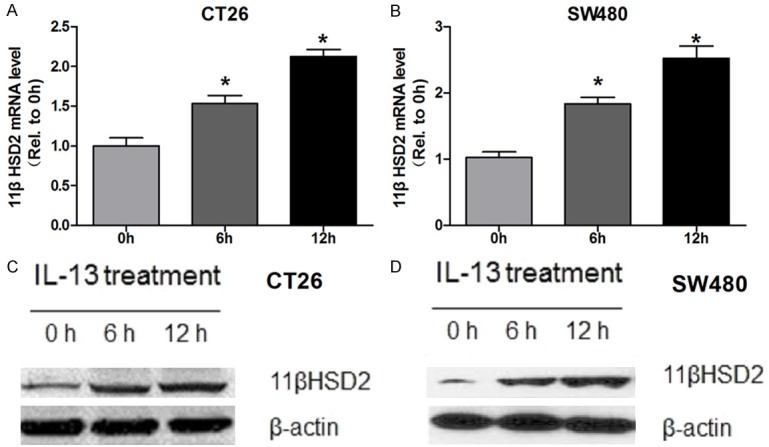
IL-13 induces 11βHSD2 expression in colorectal cancer cells. A. Mouse colorectal cancer cells CT26 were treated with 10 ng/ml IL-13 for indicated time before total RNAs were extracted. The mRNA level of 11βHSD2 was determined by qRT-PCR, mRNA level of beta-actin was detected to serve as an inner control. Relative expression level of 11βHSD2 was calculated using 2-Δ(ΔCt) method with that of 0 h set to one. * p≤0.05. B. Human colorectal cancer cells SW480 were treated with 10 ng/ml IL-13 for indicated time before total RNAs were extracted. The mRNA level of 11βHSD2 was determined by qRT-PCR, mRNA level of beta-actin was detected to serve as an inner control. Relative expression level of 11βHSD2 was calculated using 2-Δ(ΔCt) method with that of 0 h set to one. * p≤0.05. C. Mouse colorectal cancer cells CT26 were treated with 10 ng/ml IL-13 for indicated time before the cells were lysised with RAPI. The protein level of 11βHSD2 was determined by western blot, GAPDH served as a loading control. D. Human colorectal cancer cells CT26 were treated with 10 ng/ml IL-13 for indicated time before the cells were lysised with RAPI. The protein level of 11βHSD2 was determined by western blot, GAPDH served as a loading control.
IL-13Rα2 is essential for IL-13 to induce the expression of 11βHSD2 in colorectal cancer cells
In previous studies, we had demonstrated that 11βHSD2 promotes the migration and invasion of colorectal cancer cells via modulating COX2/AKT pathway. IL-13Rα2 is highly expressed in colorectal cancer and promotes the invasion of the cancer cells in PI3K/AKT pathway dependent manner [8]. Based on these studies, we speculated that IL-13 might regulate the expression of 11βHSD2 in IL-13Rα2 dependent manner in colorectal cancer cells. To validate this, we constructed colorectal cell line that stable knockdown of IL-13Rα2 (IL-13Rα2#23 and IL-13Rα2#28 for CT26 cell, IL-13Rα2#09 and IL-13Rα2#12 for SW480 cell). As shown in Figure 2A and 2B, the expression of IL13Rα2 was significantly reduced in the stable cells. The promoting effects of IL-13 on the expression of 11βHSD2 were abrogated by knockdown of IL-13Rα20 (Figure 2C-F). These results indicated that IL-13 induced the expression of 11βHSD2 in IL-13Rα2 dependent manner.
Figure 2.
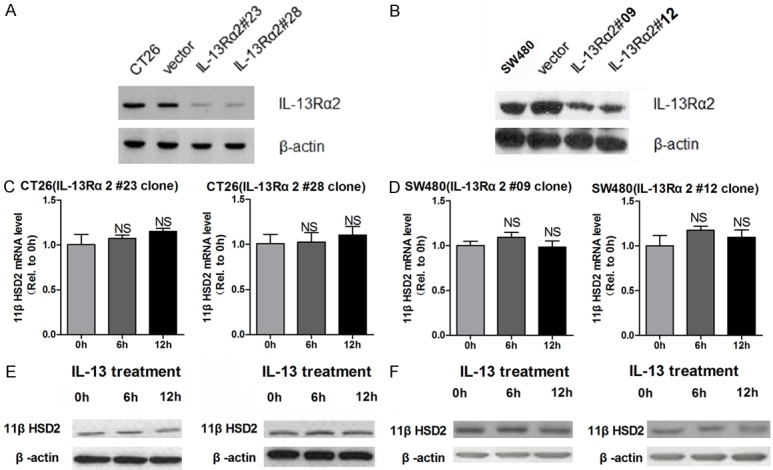
IL-13Rα2 is essential for IL-13 to induce the expression of 11βHSD2 in colorectal cancer cells. A and B. The protein level of IL-13Rα2 in CT26 (vector, IL-13Rα2#23 and IL-13Rα2#28) and SW480 (vector, IL-13Rα2#09 and IL-13Rα2#12) cells stable transfected with IL-13Rα2 konckdown plasmid or respective control was determined by western blot, GAPDH served as a loading control. C and D. Stable cell lines indicated above were treated with 10 ng/ml IL-13 for indicated time before total RNAs were extracted. The mRNA level of 11βHSD2 was determined by qRT-PCR, mRNA level of beta-actin was detected to serve as an inner control. Relative expression level of 11βHSD2 was calculated using 2-Δ(ΔCt) method with that of 0 h set to one. NS non-significant. E and F. Stable cell lines indicated above were treated with 10 ng/ml IL-13 for indicated time before the cells were lysised with RAPI. The protein level of 11βHSD2 was determined by western blot, GAPDH served as a loading control.
IL-13 promotes the expression of COX2 and activates Akt in 11βHSD2 dependent manner
Previous studies had found that both IL-13 and 11βHSD2 activate PI3K/Akt pathway. The above results demonstrated that IL-13 could induce the expression of 11βHSD2 in IL-13Rα2 dependent manner. We inferred that IL-13 may activate Akt via enhancing the expression of 11βHSD2. To address this question, we used glycyrrhizic acid (GA) to inhibit the activities of 11βHSD2 in the cells to evaluate its role in the activation of Akt by IL-13. Treatment of CT26 cells and SW480 cells with IL-13 significantly increased the expression of COX2 (Figure 3A and 3B), IL-13 also activates Akt in both cell lines (Figure 3A and 3B). Simultaneously treated the cells with GA abrogated the impacts of IL-13 on the expression of COX2 and the activation of Akt (Figure 3A and 3B). The results indicated that in colorectal cancer cells IL-13 activated Akt in 11βHSD2 dependent manner.
Figure 3.
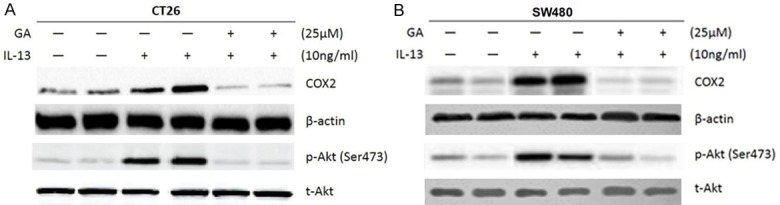
IL-13 promotes the expression of COX2 and activates Akt in 11βHSD2 dependent manner. A. Mouse colorectal cancer cells CT 26 were treated with IL-13 alone or simultaneously treated with IL-13 and 11βHSD2 inhibitor glycyrrhizic acid (GA). The protein level of COX2 was determined with western blot, and GAPDH served as a loading control. For determining the activation of Akt, phosphorylation specific antibody to Akt was used for blocking, and total Akt served as a loading control. B. Human colorectal cancer cells CT 26 were treated with IL-13 alone or simultaneously treated with IL-13 and 11βHSD2 inhibitor glycyrrhizic acid (GA). The protein level of COX2 was determined with western blot, and GAPDH served as a loading control. For determining the activation of Akt, phosphorylation specific antibody to Akt was used for blocking, and total Akt served as a loading control.
11βHSD2 is essential for IL-13 to promote the malignancy of colorectal cancer cells
The above results manifested that 11βHSD2 was essential for IL-13 to activate Akt in colorectal cancer cells. We sought to find out whether IL-13 affected the malignancy of colorectal cancer in 11βHSD2 dependent manner. The results of MTT assay demonstrated that in contrast to vehicle treated cells, As a result, IL-13 significantly increased the viabilities of CT26 and SW480 cells (Figure 4A and 4B). Meanwhile, inhibited the activities of 11βHSD2 with GA abrogated the promoting effects of IL-13 on activities of both cell lines (Figure 4A and 4B). We further used soft agar colony formation assay to demonstrate that IL-13 elicits its promoting impacts on colorectal cancer cells in 11βHSD2 dependent manner. In accordance with the results of MTT assay, IL-13 significantly increased the colony formation abilities of CT26 and SW480 cells, while GA treatment obviously reduced the promoting effects of IL-13 (Figure 4C and 4D).
Figure 4.
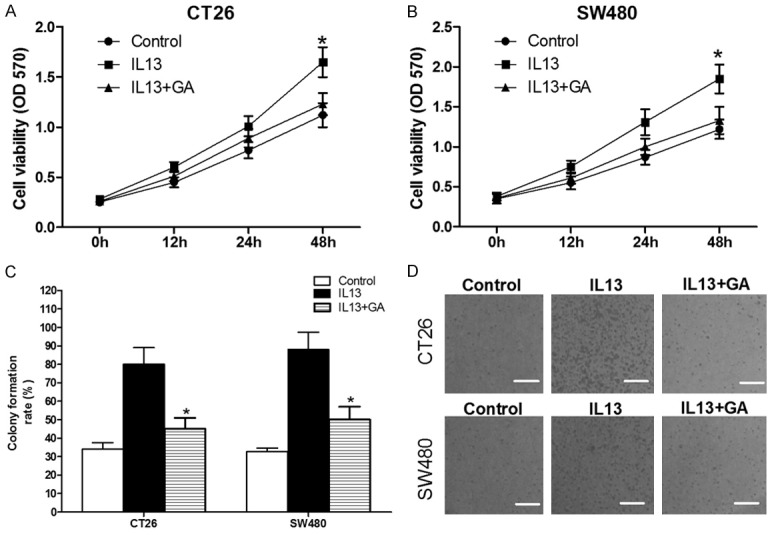
IL-13 increases cellular viabilities and colony formation abilities of CT26 and SW480 colorectal cancer cells in 11βHSD2 dependent manner. A and B. CT26 or SW480 colorectal cancer cells were seeded in 96-well plate and then treated with IL-13 alone or simultaneously with IL-13 and GA for indicating time, the viabilities of the cell were determined by MTT assay. Data were presented as absolute absorbance at wavelength of 570 nm. *p≤0.05. C and D. 300 cells were seeded in 24-well and treated with IL-13 or IL-13 and GA as indicated. The culture medium was changed every other day for seven times before the cells were fixed with paraformaldehyde and stained with crystal violet. The number of colonies with more than 50 cells were counted, and the colony formation rate was calculated as (number of colonies/300)*100%. *p≤0.05.
Next, we used transwell migration assay to assess the impacts of IL-13 on the migration abilities of colorectal cancer cells, and to evaluate the role of 11βHSD2 during this process. IL-13 significantly increased the migration abilities of CT26 and SW480 cells (Figure 5A and 5B). Simultaneously treated the cells with GA to inhibit the activities of 11βHSD2 abolished the promoting effects of IL-13 on the migration of colorectal cancer cells (Figure 5A and 5B). We further used scrape healing assay to validate the results of transwell migration assay. The same as the results of transwell assay, the healing rates of IL-13 treated cells were grater than that of control cells (Figure 5C and 5D), GA treatment abrogated the promoting effects of IL-13 (Figure 5C and 5D). Together, these results indicated that IL-13 conducts its tumor promoting effects in part dependent on the activities of 11βHSD2 in colorectal cancer cell.
Figure 5.
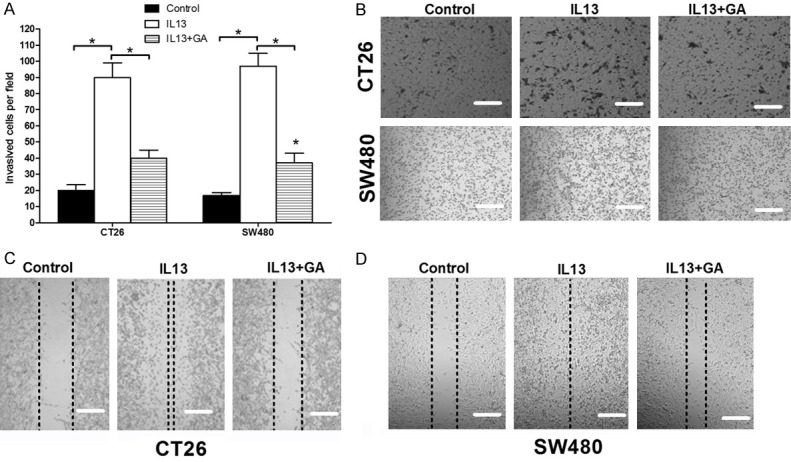
IL-13 increased the migration abilities of CT26 and SW480 colorectal cancer cells in 11βHSD2 dependent manner. (A and B) 5*105 CT26 or SW480 cells were seeded in the upper chamber of tanswell device, and then treated with IL-13 or simultaneously treated with IL-13 and GA for 48 hours before the well was fixed and then stained with crystal voilet. The cells passed through the well were counted. The histogram indicates the mean number of the cell perfiled (A), and the representative images were showed in (B). *p≤0.05. (C and D) CT26 or SW480 cells were seeded in 12-well plate, and when the cells came to about 90% confluence, a scrape was made with a tip, and then washed the cell twice with culture medium. The cells were then cultured with medium containing IL-13 and/or GA, the healling rates of the scrapes were measured 48 hours post treatment.
11βHSD2 is critical for the metastasis of colorectal cancer in mouse model
To further investigate the role 11βHSD2 in the process of colorectal cancer metastasis, we used a mouse model to test the impacts of GA treatment on the migration of colorectal cancer cells in vivo. Treatment with GA significantly reduced the metastasis of colorectal cancer cells to the liver in compared with that of the control group (Figure 6A). This results further support that 11βHSD2 is critical during the process of metastasis of colorectal cancers.
Figure 6.
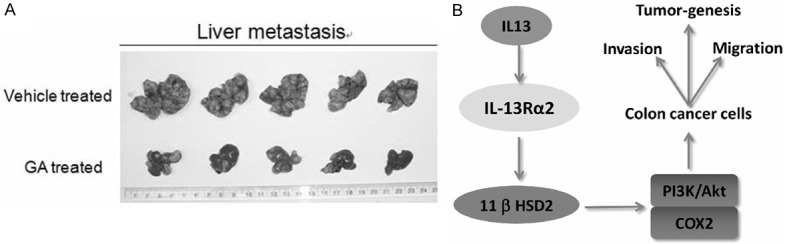
11βHSD2 is critical for the metastasis of colorectal cancer in vivo. A. About 5*105 CT26 cells were seeded in the Appendix serous of nude mice. When the in situ tumors were formed, GA was injected into the enterocoelia every other day for 24 days. The mice were then sacrificed and the metastasis sites in the liver were counted. Showing was the liver of the mice. B. A schematic showing our main findings in this study, and the mechanism through which IL-13 modulates the malignancy of colorectal cancer cells.
In conclusion, we demonstrated that IL-13 induced the expression of 11βHSD2 in IL-13Rα2 dependent manner in colorectal cancer cells. And we indicated 11βHSD2 was essential for IL-13 to activate PI3K/Akt pathway and took part in the regulation of the malignancy of colorectal cancer cells (Figure 6B). Our findings shed new light on the mechanism through which IL-13 regulates the malignancy of colorectal cancers.
Discussion
In this study, we used series of experiments to demonstrate that in colorectal cancer cells IL-13 induced the expression of 11βHSD2 in IL-13Rα2 dependent manner, which was critical for tumor promoting effects of IL-13. The inhibitor of 11BHSD2 could abrogate the function of IL-13 on colorectal cancer cells further supporting its role in IL-13 related pathway in colorectal cancer.
Due to lack effectors’ protein interacting domain existed in IL-13Rα1, IL-13Rα2 was considered as a “decoy receptor”, which negative regulates the activities of IL-13 [10,22], for a long time. But, recently IL-13Rα2 was found activating signaling pathway in many kinds of cells, such AP1/TGF-β1/Smad3 pathway in activated lung fibroblast, MEK/ERK/NOX1/ROS pathway in intestine epithelial cells [23], and ERK/AP-1 pathway in pancreatic cancer and ovarian cancer [12,15], implicating the pluripotential function of IL-13Rα2. Here, we found that IL-13 could induce the expression of 11βHSD2 in colorectal cancer cells. IL-13Rα2 is an essential mediator of IL-13 to induce the expression of 11βHSD2, as knocking down the expression of IL-13Rα2 abrogated the influences of IL-13 on the expression of 11βHSD2. Our results further support that IL-13Rα2 is potent receptor of IL-13, and participates in many aspects of IL-13 function.
11βHSD2 is a critical enzyme, regulating the activities of endogenous glucocorticoids, which has been reported to participate in the process of oncogenesis [9,18,24]. In a previous report, the researchers found that IL-13 could induce the expression of 11βHSD2 in lung epithelial cells during asthma [21], while, our findings here further support the role of IL-13 in the regulation of 11βHSD2 expression.
Both IL-13 and IL-13R has been reported highly expressing in cancer tissues compared with non-cancerous tissues [6,8,25]. Herein, we found that IL-13 could significantly increase the cellular viabilities, colony formation abilities as well as the migration abilities of CT26 and SW480 cells, and this is in accordance with previous reports [7,26]. 11βHSD2 inhibitor GA could reverse the impacts of IL-13 on the viabilities and colony formation abilities of colorectal cancer cells, indicating that 11βHSD2 functions downstream of IL-13 during the process of oncogenesis.
Activation of PI3K/Akt pathway is one of the mechanisms responsible for the tumor promoting effects of IL-13Rα2. For a while, the details how IL-13Rα2 activates PI3K/Akt pathway are unclear. Herein, we demonstrated that 11βHSD2 was the downstream molecule of IL-13Rα2 mediating the activation of Akt. This supports the crucial role of 11βHSD2 in the function of IL-13. When we inhibited endogenous 11βHSD2 with it inhibitor GA, the metastasis of colorectal cancer to the liver was reduced supporting its tumor promoting effects.
Although we provided evidences demonstrated that IL-13 could induce the expression of 11βHSD2 in IL-13Rα2 dependent manner, the detail mechanism through which IL-13Rα2 induces the expression of 11βHSD2 is still unknown. Efforts should be made in finding out the effecter proteins of IL-13Rα2 responsible for the induction of 11βHSD2.
In conclusion, here we clarified that 11βHSD2, induction by IL-13 in IL-13Rα2 dependent manner, is an essential downstream component of IL-13/IL-3 Rs pathway in respect to the malignancy of colorectal cancers. Our study may shed new light on the role of IL-13Rα2 in transducing the signal of IL-13 in colorectal cancer, and may provide new targets for prevention and treatment of colorectal cancer.
Acknowledgements
The Study was support by NSFC(Natural Science Foundation of China, #No. 81472705).
References
- 1.Sovich JL, Sartor Z, Misra S. Developments in Screening Tests and Strategies for Colorectal Cancer. Biomed Res Int. 2015;2015:326728. doi: 10.1155/2015/326728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marz L, Piso P. Treatment of peritoneal metastases from colorectal cancer. Gastroenterol Rep (Oxf) 2015;3:298–302. doi: 10.1093/gastro/gov044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu J. T helper 2 (Th2) cell differentiation, type 2 innate lymphoid cell (ILC2) development and regulation of interleukin-4 (IL-4) and IL-13 production. Cytokine. 2015;75:14–24. doi: 10.1016/j.cyto.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown KD, Zurawski SM, Mosmann TR, Zurawski G. A family of small inducible proteins secreted by leukocytes are members of a new superfamily that includes leukocyte and fibroblast-derived inflammatory agents, growth factors, and indicators of various activation processes. J Immunol. 1989;142:679–687. [PubMed] [Google Scholar]
- 5.Hallett MA, Venmar KT, Fingleton B. Cytokine stimulation of epithelial cancer cells: the similar and divergent functions of IL-4 and IL-13. Cancer Res. 2012;72:6338–6343. doi: 10.1158/0008-5472.CAN-12-3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandal D, Levine AD. Elevated IL-13Ralpha2 in intestinal epithelial cells from ulcerative colitis or colorectal cancer initiates MAPK pathway. Inflamm Bowel Dis. 2010;16:753–764. doi: 10.1002/ibd.21133. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Park JM, Terabe M, van den Broeke LT, Donaldson DD, Berzofsky JA. Unmasking immunosurveillance against a syngeneic colon cancer by elimination of CD4+ NKT regulatory cells and IL-13. Int J Cancer. 2005;114:80–87. doi: 10.1002/ijc.20669. [DOI] [PubMed] [Google Scholar]
- 8.Barderas R, Bartolome RA, Fernandez-Acenero MJ, Torres S, Casal JI. High expression of IL-13 receptor alpha2 in colorectal cancer is associated with invasion, liver metastasis, and poor prognosis. Cancer Res. 2012;72:2780–2790. doi: 10.1158/0008-5472.CAN-11-4090. [DOI] [PubMed] [Google Scholar]
- 9.Sai S, Nakagawa Y, Yamaguchi R, Suzuki M, Sakaguchi K, Okada S, Seckl JR, Ohzeki T, Chapman KE. Expression of 11beta-hydroxysteroid dehydrogenase 2 contributes to glucocorticoid resistance in lymphoblastic leukemia cells. Leuk Res. 2011;35:1644–1648. doi: 10.1016/j.leukres.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lupardus PJ, Birnbaum ME, Garcia KC. Molecularmailto basis for shared cytokine recognition revealed in the structure of an unusually high affinity complex between IL-13 and IL-13Ralpha2. Structure. 2010;18:332–342. doi: 10.1016/j.str.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao J, Wu Y, Su Z, Amoah Barnie P, Jiao Z, Bie Q, Lu L, Wang S, Xu H. Infiltration of alternatively activated macrophages in cancer tissue is associated with MDSC and Th2 polarization in patients with esophageal cancer. PLoS One. 2014;9:e104453. doi: 10.1371/journal.pone.0104453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujisawa T, Joshi BH, Puri RK. IL-13 regulates cancer invasion and metastasis through IL-13Ralpha2 via ERK/AP-1 pathway in mouse model of human ovarian cancer. Int J Cancer. 2012;131:344–356. doi: 10.1002/ijc.26366. [DOI] [PubMed] [Google Scholar]
- 13.Husain SR, Obiri NI, Gill P, Zheng T, Pastan I, Debinski W, Puri RK. Receptor for interleukin 13 on AIDS-associated Kaposi's sarcoma cells serves as a new target for a potent Pseudomonas exotoxin-based chimeric toxin protein. Clin Cancer Res. 1997;3:151–156. [PubMed] [Google Scholar]
- 14.Formentini A, Prokopchuk O, Strater J, Kleeff J, Grochola LF, Leder G, Henne-Bruns D, Korc M, Kornmann M. Interleukin-13 exerts autocrine growth-promoting effects on human pancreatic cancer, and its expression correlates with a propensity for lymph node metastases. Int J Colorectal Dis. 2009;24:57–67. doi: 10.1007/s00384-008-0550-9. [DOI] [PubMed] [Google Scholar]
- 15.Fujisawa T, Joshi B, Nakajima A, Puri RK. A novel role of interleukin-13 receptor alpha2 in pancreatic cancer invasion and metastasis. Cancer Res. 2009;69:8678–8685. doi: 10.1158/0008-5472.CAN-09-2100. [DOI] [PubMed] [Google Scholar]
- 16.Jain M, Zhang L, He M, Patterson EE, Nilubol N, Fojo AT, Joshi B, Puri R, Kebebew E. Interleukin-13 receptor alpha2 is a novel therapeutic target for human adrenocortical carcinoma. Cancer. 2012;118:5698–5708. doi: 10.1002/cncr.27629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chapman K, Holmes M, Seckl J. 11betahydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action. Physiol Rev. 2013;93:1139–1206. doi: 10.1152/physrev.00020.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabbitt EH, Ayuk J, Boelaert K, Sheppard MC, Hewison M, Stewart PM, Gittoes NJ. Abnormal expression of 11 beta-hydroxysteroid dehydrogenase type 2 in human pituitary adenomas: a prereceptor determinant of pituitary cell proliferation. Oncogene. 2003;22:1663–1667. doi: 10.1038/sj.onc.1206293. [DOI] [PubMed] [Google Scholar]
- 19.Jiang L, Yang S, Yin H, Fan X, Wang S, Yao B, Pozzi A, Chen X, Harris RC, Zhang MZ. Epithelial-specific deletion of 11beta-HSD2 hinders Apcmin/+ mouse tumorigenesis. Mol Cancer Res. 2013;11:1040–1050. doi: 10.1158/1541-7786.MCR-13-0084-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang MZ, Xu J, Yao B, Yin H, Cai Q, Shrubsole MJ, Chen X, Kon V, Zheng W, Pozzi A, Harris RC. Inhibition of 11beta-hydroxysteroid dehydrogenase type II selectively blocks the tumor COX-2 pathway and suppresses colon carcinogenesis in mice and humans. J Clin Invest. 2009;119:876–885. doi: 10.1172/JCI37398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Josephson MB, Jiao J, Xu S, Hu A, Paranjape C, Grunstein JS, Grumbach Y, Nino G, Kreiger PA, McDonough J, Grunstein MM. IL-13-induced changes in endogenous glucocorticoid metabolism in the lung regulate the proasthmatic response. Am J Physiol Lung Cell Mol Physiol. 2012;303:L382–390. doi: 10.1152/ajplung.00125.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawakami K, Taguchi J, Murata T, Puri RK. The interleukin-13 receptor alpha2 chain: an essential component for binding and internalization but not for interleukin-13-induced signal transduction through the STAT6 pathway. Blood. 2001;97:2673–2679. doi: 10.1182/blood.v97.9.2673. [DOI] [PubMed] [Google Scholar]
- 23.Fichtner-Feigl S, Young CA, Kitani A, Geissler EK, Schlitt HJ, Strober W. IL-13 signaling via IL-13R alpha2 induces major downstream fibrogenic factors mediating fibrosis in chronic TNBS colitis. Gastroenterology. 2008;135:2003–2013. 2013.e1–7. doi: 10.1053/j.gastro.2008.08.055. [DOI] [PubMed] [Google Scholar]
- 24.Koyama K, Myles K, Smith R, Krozowski Z. Expression of the 11beta-hydroxysteroid dehydrogenase type II enzyme in breast tumors and modulation of activity and cell growth in PMC42 cells. J Steroid Biochem Mol Biol. 2001;76:153–159. doi: 10.1016/s0960-0760(00)00157-6. [DOI] [PubMed] [Google Scholar]
- 25.Kanai T, Watanabe M, Hayashi A, Nakazawa A, Yajima T, Okazawa A, Yamazaki M, Ishii H, Hibi T. Regulatory effect of interleukin-4 and interleukin-13 on colon cancer cell adhesion. Br J Cancer. 2000;82:1717–1723. doi: 10.1054/bjoc.2000.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Endo Y, Marusawa H, Kou T, Nakase H, Fujii S, Fujimori T, Kinoshita K, Honjo T, Chiba T. Activation-induced cytidine deaminase links between inflammation and the development of colitis-associated colorectal cancers. Gastroenterology. 2008;135:889–898. 898.e1–3. doi: 10.1053/j.gastro.2008.06.091. [DOI] [PubMed] [Google Scholar]


