Abstract
Silymarin, which derived from the milk thistle plant (silybum marianum), has been used for centuries as a natural remedy for diseases of the liver and biliary tract. Considering the therapeutic potential to liver disease, we tested efficacy of silymarin on hepatic steatosis with a high fat diet (HFD)-induced mouse model of non-alcoholic fatty liver disease (NAFLD), and investigated possible effects on lipid metabolic pathways. In our study, silymarin could attenuate the hepatic steatosis, which was proved by both Oil Red O staining and hepatic triglyceride (TG) level determination. Furthermore, compared with INT-747, a potent and selective FXR agonist, silymarin could preserve plasmatic high-density lipoprotein cholesterol (HDL-C) to a higher level and low-density lipoprotein cholesterol (LDL-C) to a lower level, which benefited more to the circulation system. Through real-time PCR analysis, we clarified a vital protective role of silymarin in mRNA regulation of genes involved in lipid metabolism and oxidative stress. It was also shown that silymarin had no effects on body weight, food intake, and liver transaminase. Taken together, silymarin could attenuate hepatic steatosis in a mouse model of NAFLD through regulation of lipid metabolism and oxidative stress, and benefit to the circulation system. All these findings shed new light on NAFLD treatment.
Keywords: Silymarin, hepatic steatosis, nonalcoholic fatty liver disease, circulation, lipid metabolism, oxidative stress
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a spectrum of liver disease including steatosis, fibrosis, cirrhosis or hepatocellular carcinoma in the absence of alcohol abuse [1]. In recent decades, NAFLD has been increasing worldwide, and its prevalence is estimated to rise from 20 or 30% to as high as 90% in morbidly obese individuals of Western populations [2]. While hepatic steatosis itself is generally considered a rather benign and reversible condition, persistent presence of steatosis in liver may lead to inflammation, which is the key feature of non-alcoholic steatohepatitis (NASH) and enables the development of more advanced stages of the disease [3].
Several medications have been proposed as NAFLD therapies, such as metformin [4], Ezetimibe [5], peroxisome proliferator-activated receptor-γ (PPARγ) agonists [6] and farnesoid X receptor (FXR) agonists [7], among which INT-747, also named obeticholic acid, is a potent and selective FXR agonist that reduces liver fat and fibrosis in animal models of NAFLD [8,9].
Silymarin, which derived from the milk thistle plant (silybum marianum), has been used for centuries as a natural remedy for diseases of the liver and biliary tract [10,11]. It has been reported to work as antioxidants scavenging free radicals and inhibiting lipid peroxidation. Studies also suggest that it protects against genomic injury, increases hepatocyte protein synthesis, decreases the activity of tumor promoters, and slows calcium metabolism [12]. Considering the therapeutic potential to liver disease, we tested efficacy of silymarin on hepatic steatosis with a high fat diet (HFD)-induced mouse model of NAFLD, and investigated the possible effects on lipid metabolic pathways.
Materials and methods
Animals
Male C57BL/6J mice (7 weeks) were purchased from SLAC (Shanghai, China). Animals were housed in a temperature-controlled room (22°C) with 12-h-light/12-h-dark cycling, and had free access to food and water. Mice were administered either an HFD (D12492; Research Diets, New Brunswick, NJ) or control diet (normal chow, SLAC). All animals received humane care, and all experimental protocols complied with the “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health (NIH) of the United States and were approved by the ethics committee of Nanjing University of Chinese Medicine.
Drugs
INT-747 (S7660) and Silymarin (S2358) were purchased from Selleck Chemicals (Houston, TX).
HFD-induced NAFLD model and treatment
After one week acclimation, the mice (7 weeks) were fed with HFD and fresh water, ad libitum, for 20 weeks. Typically, male C57BL/6J mice became obese, and steatosis appeared in the liverat the end of treatment. From week 27, diet-induced obesity (DIO) mice were divided into 3 groups according to body weight, and treated with vehicle (1% HPMC + 1% tween 20), INT-747 (30 mg/kg) or silymarin (30 mg/kg) for another 4 weeks (PO, QD). Lean mice were treated with vehicle only as a naïve control.
Sample collection
After 4 weeks of treatment, all mice were euthanized by CO2 inhalation. Blood was obtained by cardiac puncture and collected into EDTA-containing tubes, then centrifuged at 5,000 RPM for 10 min to get plasma. Livers were removed, weighed, and cut into 4 parts, including three parts for TG/TC/FFA determination and one part for histological analysis. Liver for histology was fixed in 4% formalin in room temperature until further analysis.
Biochemical analysis
Plasmatic levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), triglyceride (TG), total cholesterol (TC), high density lipoprotein-cholesterol (HDL-C), and low density lipoprotein-cholesterol (LDL-C) were determined by using Hitachi 7180 autoanalyzer (Hitachi, Tokyo, Japan) with Cobas kits (Roche Diagnostics, Indianapolis, IN). TG, TC, and free fatty acid (FFA) levels in liver were determined with Sigma kits (St. Louis, MO) according to the instructions of manufacture.
Histopathologic evaluation
For hematoxylin and eosin (H&E) staining, formalin-fixed liver was embedded into paraffin and cut into 4 μm sections. For Oil Red-O staining, liver tissue was dehydrated before embedded into OCT compound (Sakura, Tokyo, Japan), and cut into 10 μm frozen sections. Commercially available kits (Beyotime, Shanghai, China) were used to stain sections. An expert pathologist evaluated the slides in a blinded fashion with Olympus light microscope in 100× (for Oil Red-O) or 200× (for H&E) augmentation, and assigned scores for steatosis, ballooning, and inflammation as follows [13] (Table 2):
Table 2.
Assigned scores for steatosis, ballooning, and inflammation
| Steatosis | Ballooning | Inflammation | |
|---|---|---|---|
| 0 | < 5% | None | < 2 foci per 200× field |
| 1 | % | Few balloon cells | 2-4 foci per 200× field |
| 2 | 33%-66% | Manycells/prominent ballooning | > 4 foci per 200× field |
| 3 | > 66 % |
Real-time polymerase chain reaction
Total RNA was extracted from the liver using Trizol reagent (Invitrogen, Carlsbad, CA). Quantitative RT-PCR was performed using the ABI Prism 7000 Sequence Detection System with PCR Master Mix Reagent (Applied Biosystems, Foster City, CA). Primers used were given as below:
ACC1: Forward 5’-TGAGATTGGCATGGTAGCCTG-3’, Reverse 5’-CTCGGCCATCTGGATATTCAG-3’; CAT: Forward 5’-GGAGGCAGAAACTTTCCCATT-3’, Reverse 5’-GGCCAAACCTTGGTCAGATC-3’; CPT1A: Forward 5’-CCTGCATTCCTTCCCATTTG-3’, Reverse 5’-TGCCCATGTCCTTGTAATGTG-3’; FAS: Forward 5’-CCTGGATAGCATTCCGAACCT-3’, Reverse 5’-AGCACATCTCGAAGGCTACACA-3’; MTP: Forward 5’-GCCTTTATCCTGCACCTCTG-3’, Reverse 5’-AATAGCCAGATGCCTGCAAGGC-3’; p40phox: Forward 5’-CAGCCAACATCGCTGACA-TC-3’, Reverse 5’-CAAAGTGGCTGGTGAAGCCC-3’; p47phox: Forward 5’-ACTCTCACTGAATACTTCAACG-3’, Reverse 5’-TCATCAGGCCGCACTTT-3’; p67phox: Forward 5’-AAGCAAAAAGAGCCCAAGGAA-3’, Reverse 5’-CATGTAAGGCATAGGCACGCT-3’; PPARα: Forward 5’-AGGAAGCCGTTCTGTGACAT-3’, Reverse 5’-AATCCCCTCCTGCAACTTCT-3’; SOD1: Forward 5’-GCAGGACCTCATTTTAATCCTCACT-3’, Reverse 5’-AGGTCTCCAACATGCCTCTCTTC-3’; SREBP1c: Forward 5’-GGCACTAAGTGCCCTCAACCT-3’, Reverse 5’-GCCACATAGATCTCTGCCAGTGT-3’.
Statistical analysis
Data were presented as Mean ± SEM. Significance of difference between groups was analyzed by performing two-way RM ANOVA for time course study, or one-way ANOVA with Dunnett’s multiple comparison test for other studies. P value less than 0.05 was considered statistically significant. Data were analyzed and graphed by Prism 6.0 (GraphPad Software, La Jolla, CA).
Results
Silymarin had no effects on body weight and food intake
None of the mice died during the experiment and no adverse events observed in any mouse. During the 4-week treatment, there was a slight decrease in body weight of silymarin-treated group compared with that of vehicle group, but statistical significance did not appear (Figure 1A, 1C). Likewise, silymarin also had no significant effects on food intake and cumulative food intake change (Figure 1B, 1D) as well as liver weight (Figure 1E).
Figure 1.
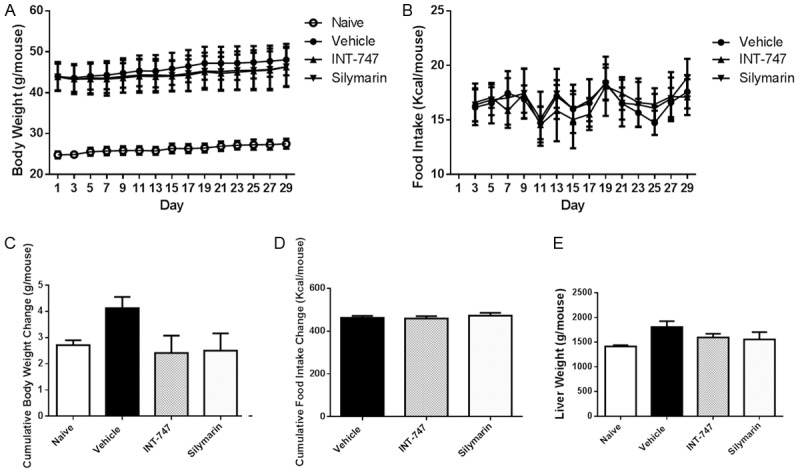
Silymarin had no effects on body weight and food intake. C57BL/6J mice (7 weeks) were fed with HFD for 20 weeks, and treated with vehicle (1% HPMC + 1% tween 20), INT-747 (30 mg/kg) or silymarin (30 mg/kg) for another 4 weeks (PO, QD). Lean mice were treated with vehicle only as a naïve control. Data were presented as Mean ± SEM. N=10.
Silymarin had no effects on liver transaminases
In order to investigate whether silymarin might cause liver injury, we determined plasmatic levels of ALT, AST, and ALP. Compared with the levels in lean mice, those in DIO mice were slightly elevated, however, there was no significant difference between vehicle and silymarin groups (Figure 2), which showed that silymarin did not lead to liver injury.
Figure 2.
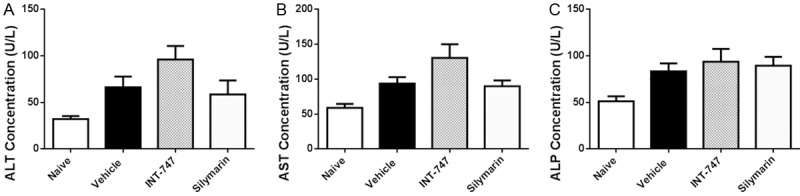
Silymarin had no effects on liver transaminases. At the termination of treatment, blood was obtained by cardiac puncture, and plasmatic ALT, AST, and ALP were determined by using autoanalyzer. Data were presented as Mean ± SEM. N=10.
Silymarin decreased plasmatic levels of TC, HDL-C and LDL-C
Plasmatic TC, HDL-C, LDL-C, and TG were determined to analyze the effects of silymarin on lipid metabolic pathways. We found that silymarin significantly (P < 0.001) reversed HFD-induced elevation of TC level (Figure 3A) as well as HDL-C/LDL-C levels (Figure 3B, 3C). Furthermore, silymarin could promote LDL-C to a lower level compared with INT-747 did, and preserve HDL-C to a higher level (P < 0.05 vs. INT-747 group), suggesting that silymarin benefited more to circulation system. On the contrary, both silymarin and INT-747 had no effects on TG level (Figure 3D).
Figure 3.
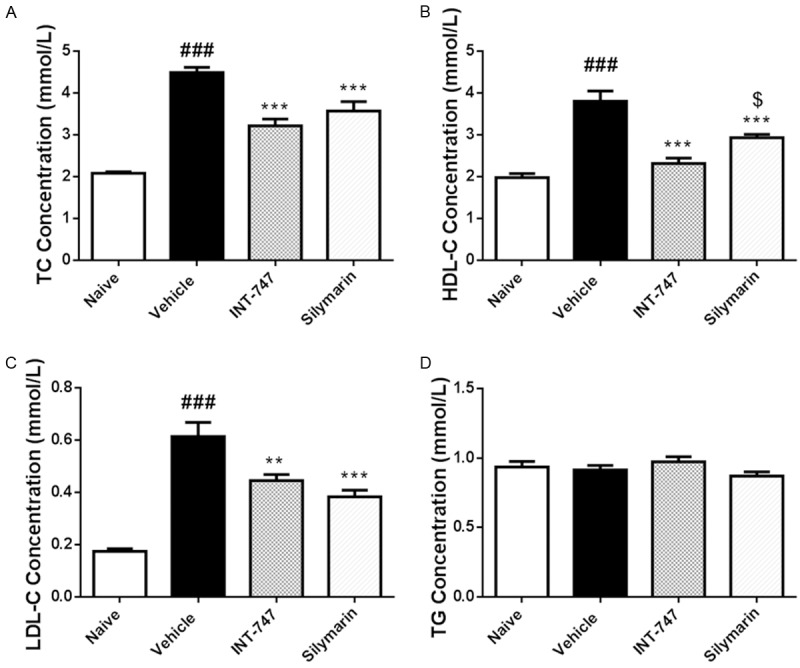
Silymarin decreased plasmatic levels of TC, HDL-C and LDL-C. At the termination of treatment, blood was obtained by cardiac puncture, and plasmatic TG, TC, HDL-C, and LDL-C were determined by using autoanalyzer. Data were presented as Mean ± SEM. N=10. ###P < 0.001 vs. naïve group, ***P < 0.001 vs. vehicle group, **P < 0.01 vs. vehicle group, $P < 0.05 vs. INT-747 group.
Silymarin decreased hepatic TG level
24-week administration of HFD could induce the lipid accumulation in liver, including TG, TC, and FFA (Figure 4). 4-week treatment of silymarin significantly reduced the TG level from 89.8 ± 8.2 μmol/g to 21.7 ± 5.0 μmol/g (P < 0.001). However, there were no changes on the levels of TC and FFA after silymarin treatment in DIO mice.
Figure 4.
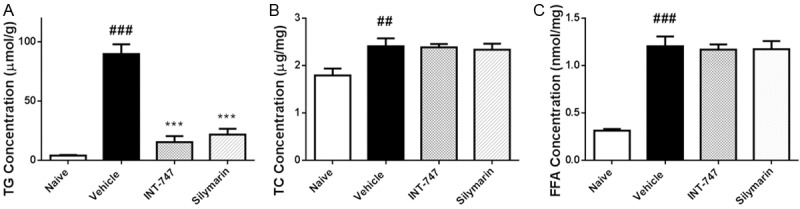
Silymarin decreased hepatic TG level. At the termination of treatment, livers were removed, weighed, and TG/TC/FFA levels were determined by using commercially available kits. Data were presented as Mean ± SEM. N=10. ###P < 0.001 vs. naïve group, ##P < 0.01 vs. naïve group, ***P < 0.001 vs. vehicle group.
Silymarin attenuated hepatic steatosis
We performed histopathologic evaluation to conform the effects of silymarin on hepatic TG level. Oil red O staining was used to determine the steatosis status in liver, and it was shown that silymarin reduced the score from 2.4 ± 0.2 to 1.4 ± 0.2 (P < 0.001), suggesting the hepatic steatosis was significantly attenuated after silymarin treatment (Figure 5A, 5B, Table 1). H&E staining showed ballooning degenerated hepatocytes and inflammation foci in the liver lobule were nearly no changed after silymarin treatment in DIO mice (Figure 5A, 5C, 5D, Table 1). Taken together, silymarin significantly reduced NAFLD activity score (NAS), which means steatosis grade + ballooning grade + inflammation grade (Figure 5A, 5E, Table 1).
Figure 5.
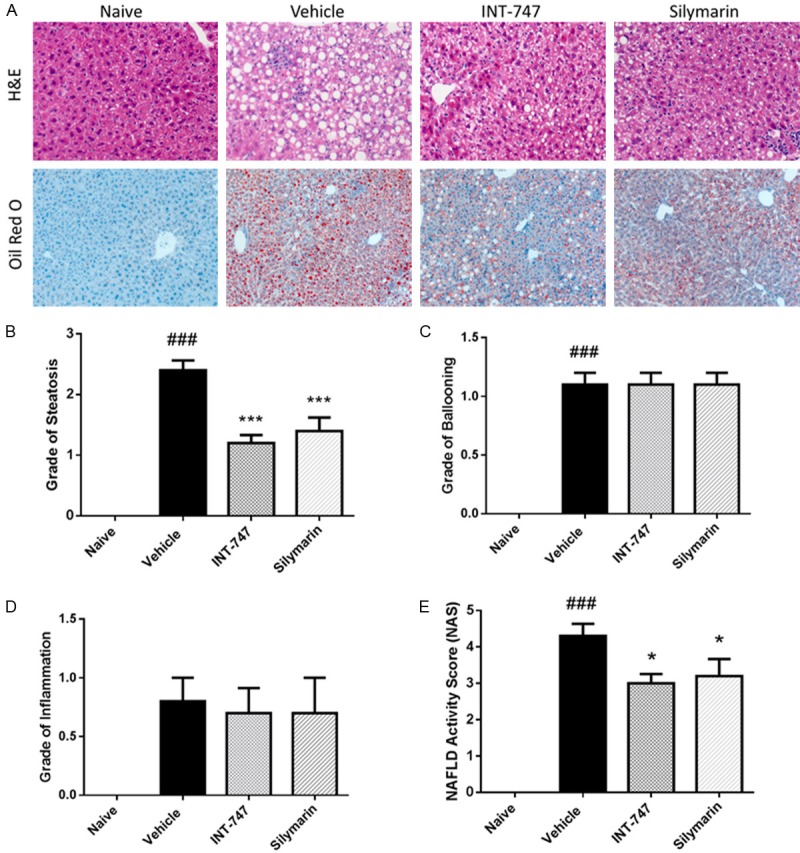
Silymarin attenuated hepatic steatosis. At the termination of treatment, livers were removed and fixed. For hematoxylin and eosin (H&E) staining, formalin-fixed liver was embedded into paraffin and cut into 4 μm sections. For Oil Red-O staining, liver tissue was dehydrated before embedded into OCT compound, and cut into 10 μm frozen sections. Commercially available kits were used to stain sections. A. 100× (for Oil Red-O) or 200× (for H&E) augmentation. B-E. Steatosis, ballooning, inflammation, and NAFLD activity scores assigned by an expert pathologist in a blinded fashion. Data were presented as Mean ± SEM. N=10. ###P < 0.001 vs. naïve group, ***P < 0.001 vs. vehicle group, *P < 0.05 vs. vehicle group.
Table 1.
Histopathologic evaluation on NAFLD activity score (NAS)
| Naïve | Vehicle | INT-747 | Silymarin | |
|---|---|---|---|---|
| Steotosis | 0 | 2.4 ± 0.2### | 1.2 ± 0.1*** | 1.4 ± 0.2*** |
| Ballooning | 0 | 1.1 ± 0.1### | 1.1 ± 0.2 | 1.1 ± 0.1 |
| Inflammation | 0 | 0.8 ± 0.3 | 0.7 ± 0.2 | 0.7 ± 0.3 |
| NAS | 0 | 4.3 ± 0.3### | 3.0 ± 0.3* | 3.2 ± 0.5* |
Data were presented as Mean ± SEM. N=10.
P < 0.001 vs. naïve group;
P < 0.001 vs. vehicle group;
P < 0.05 vs. vehicle group.
Role of silymarin in lipid metabolism and oxidative stress in liver
Next we determined the mRNA expression using real time-PCR to investigate the mechanism involved in lipid metabolism and oxidative stress. Expression of mRNAs for de novo lipogenesis (sterol regulatory element binding protein 1c, fatty acid synthase and acetyl-CoA carboxylase 1) was markedly increased in liver from DIO mice compared to lean mice, however, could be preserved to a lower level by silymarin treatment (Figure 6A). In addition, increased expression of mRNAs for fatty acid oxidation (peroxisome proliferator-activated receptor-α, carnitine palmitoyltransferase 1A, and mitochondrial trifunctional protein) in liver from DIO mice also could be attenuated by silymarin treatment (Figure 6B). Similarly, increased mRNAs expression of the NADPH oxidase components (p40phox, p47phox, p67phox) and antioxidant enzymes (superoxide dismutase 1 and catalase) in the DIO mice was reduced after treatment (Figure 6C, 6D). Taken together, silymarin played a vital role in regulation of lipid metabolism and oxidative stress in liver.
Figure 6.
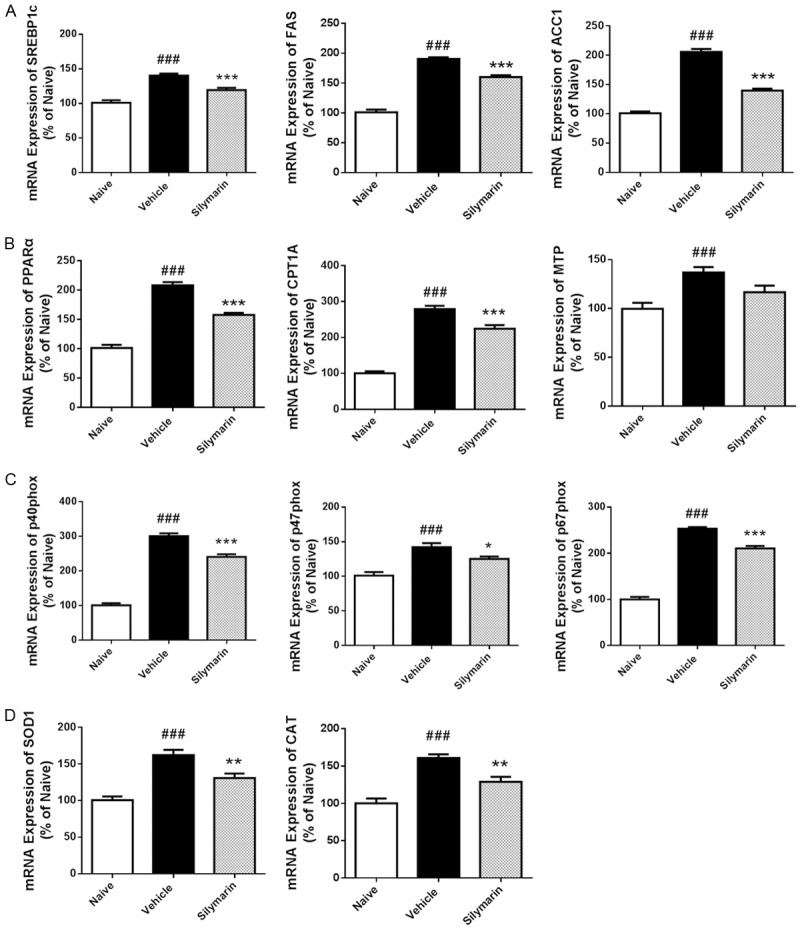
Role of silymarin in lipid metabolism and oxidative stress in liver. At the termination of treatment, livers were removed and homogenated for real-time PCR analysis of mRNA expression. ACC1, acetyl-CoA carboxylase 1; CAT, catalase; CPT1A, carnitine palmitoyltransferase 1A; FAS, fatty acid synthase; MTP, mitochondrial trifunctional protein; PPARα, peroxisome proliferator-activated receptor-α; SOD1, superoxide dismutase 1; SREBP1c, sterol regulatory element binding protein 1c. Data were presented as Mean ± SEM. N=8. ###P < 0.001 vs. naïve group, ***P < 0.001 vs. vehicle group, **P < 0.01 vs. vehicle group, *P < 0.05 vs. vehicle group.
Discussion
Obesity is the main causal factor in the development of the metabolic syndrome, including atherosclerotic cardiovascular disease, diabetes, and NAFLD [14]. For now, many animal models have been developed to investigate the mechanism and therapy of NAFLD, comprising genetic models, such as defective leptin signaling, defective hepatic lipogenesis, defective β-oxidation, defective NFκB/TNF signaling, and defective cholesterol signaling, and nutritional models, such as high fat diet, methionine- and choline- deficient diet, atherogenic diet, and fructose diet [3]. Since superabundant fat intake is the main cause leading to obesity and NAFLD in most developed countries, HFD model could mimic natural development of this disease, therefore, it was chosen in our study.
The hepatic protection of silymarin has been investigated and attributed to antioxidant mechanism for many years [12,15,16]. However, possible mechanism on lipid metabolic pathways was little discovered. Since NAFLD is mainly caused by lipid accumulation in the liver, we tested efficacy of silymarin on hepatic steatosis, and determined the lipid metabolism in the treatment. Histological score and hepatic TG level are two main factors to determine the severity of NAFLD [17,18]. In our study, silymarin could attenuate the hepatic steatosis, which was proved by both Oil Red O staining and hepatic TG level determination. Furthermore, there was positive correlation between histological score and hepatic TG level, instead of levels of TC and FFA, in each sample (data not shown), suggesting hepatic TG level could be used as a biomarker which predicted histological steatosis.
We investigated the plasmatic levels of TG and TC to test effects of silymarin on circulation system. It was shown that silymarin did not change the TG level in blood, although it reduced that in liver. Furthermore, silymarin treatment reduced plasmatic level of TC, which is main risk factor leading to atherosclerosis [19,20]. As we known, HDL-C is regarded as “good” cholesterol, while LDL-C as “bad” cholesterol [21]. In our study, silymarin could preserve HDL-C to a higher level and LDL-C to a lower level compared with INT-747 did. In a phase III FLINT trial, compared with placebo, treatment with INT-747 was associated with higher concentrations of serum TC and LDL-C, and a decrease in HDL-C, which implied a potential risk in cardiovascular system [7]. Therefore, considering the benefits of silymarin to circulation, silymarin should be further investigated in future work as an alternative to INT-747.
In our study, there was slight, but not significant, elevation of liver transaminases, including ALT, AST, and ALP, in DIO mice suffering NAFLD. INT-747 could promote these enzymes to higher levels, while silymarin nearly had no effects on enzyme levels. It was also reported that treatment with INT-747 was associated with a higher concentration of serum ALP [7]. In contrast, silymarin behaved fewer side effects in the NAFLD treatment, which might contribute to the antioxidant activity of this drug [22,23].
Taken together, we tested efficacy of silymarin in a mouse model of NAFLD, and proved silymarin could attenuate hepatic steatosis in the treatment. Furthermore, we clarified the vital role of silymarin in regulation of lipid metabolism and oxidative stress, and also found that, compared with INT-747, silymarin benefited more to the circulation system. All these findings shed new light on NAFLD treatment.
Disclosure of conflict of interest
None.
References
- 1.Adams LA, Lindor KD. Nonalcoholic fatty liver disease. Ann Epidemiol. 2007;17:863–869. doi: 10.1016/j.annepidem.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Kotronen A, Westerbacka J, Bergholm R, Pietiläinen KH, Yki-Järvinen H. Liver fat in the metabolic syndrome. J Clin Endocrinol Metab. 2007;92:3490–3497. doi: 10.1210/jc.2007-0482. [DOI] [PubMed] [Google Scholar]
- 3.Hebbard L, George J. Animal models of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2011;8:35–44. doi: 10.1038/nrgastro.2010.191. [DOI] [PubMed] [Google Scholar]
- 4.Nair S, Diehl AM, Wiseman M, Farr GH, Perrillo RP. Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Aliment Pharmacol Ther. 2004;20:23–28. doi: 10.1111/j.1365-2036.2004.02025.x. [DOI] [PubMed] [Google Scholar]
- 5.Park H, Shima T, Yamaguchi K, Mitsuyoshi H, Minami M, Yasui K, Itoh Y, Yoshikawa T, Fukui M, Hasegawa G. Efficacy of long-term ezetimibe therapy in patients with nonalcoholic fatty liver disease. J Gastroenterol. 2011;46:101–107. doi: 10.1007/s00535-010-0291-8. [DOI] [PubMed] [Google Scholar]
- 6.Tiikkainen M, Häkkinen AM, Korsheninnikova E, Nyman T, Mäkimattila S, Yki-Järvinen H. Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes. 2004;53:2169–2176. doi: 10.2337/diabetes.53.8.2169. [DOI] [PubMed] [Google Scholar]
- 7.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pellicciari R, Fiorucci S, Camaioni E, Clerici C, Costantino G, Maloney PR, Morelli A, Parks DJ, Willson TM. 6α-Ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J Med Chem. 2002;45:3569–3572. doi: 10.1021/jm025529g. [DOI] [PubMed] [Google Scholar]
- 9.Fiorucci S, Clerici C, Antonelli E, Orlandi S, Goodwin B, Sadeghpour BM, Sabatino G, Russo G, Castellani D, Willson TM. Protective effects of 6-ethyl chenodeoxycholic acid, a farnesoid X receptor ligand, in estrogen-induced cholestasis. J Pharmacol Exp Ther. 2005;313:604–612. doi: 10.1124/jpet.104.079665. [DOI] [PubMed] [Google Scholar]
- 10.Cacciapuoti F, Scognamiglio A, Palumbo R, Forte R, Cacciapuoti F. Silymarin in non alcoholic fatty liver disease. World J Hepatol. 2013;5:109–113. doi: 10.4254/wjh.v5.i3.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parmar M, Gandhi T. Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine-A review. Pharmacognosy Reviews. 2008;2:102. [Google Scholar]
- 12.Flora K, Hahn M, Rosen H, Benner K. Milk thistle (Silybum marianum) for the therapy of liver disease. Am J Gastroenterol. 1998;93:139–143. doi: 10.1111/j.1572-0241.1998.00139.x. [DOI] [PubMed] [Google Scholar]
- 13.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 14.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 15.Lettéron P, Labbe G, Degott C, Berson A, Fromenty B, Delaforge M, Larrey D, Pessayre D. Mechanism for the protective effects of silymarin against carbon tetrachloride-induced lipid peroxidation and hepatotoxicity in mice: evidence that silymarin acts both as an inhibitor of metabolic activation and as a chainbreaking antioxidant. Biochem Pharmacol. 1990;39:2027–2034. doi: 10.1016/0006-2952(90)90625-u. [DOI] [PubMed] [Google Scholar]
- 16.Shaker E, Mahmoud H, Mnaa S. Silymarin, the antioxidant component and Silybum marianum extracts prevent liver damage. Food Chem Toxicol. 2010;48:803–806. doi: 10.1016/j.fct.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 17.Clapper JR, Hendricks MD, Gu G, Wittmer C, Dolman CS, Herich J, Athanacio J, Villescaz C, Ghosh SS, Heilig JS. Diet-induced mouse model of fatty liver disease and nonalcoholic steatohepatitis reflecting clinical disease progression and methods of assessment. Am J Physiol Gastrointest Liver Physiol. 2013;305:G483–G495. doi: 10.1152/ajpgi.00079.2013. [DOI] [PubMed] [Google Scholar]
- 18.Fujii M, Shibazaki Y, Wakamatsu K, Honda Y, Kawauchi Y, Suzuki K, Arumugam S, Watanabe K, Ichida T, Asakura H. A murine model for non-alcoholic steatohepatitis showing evidence of association between diabetes and hepatocellular carcinoma. Med Mol Morphol. 2013;46:141–152. doi: 10.1007/s00795-013-0016-1. [DOI] [PubMed] [Google Scholar]
- 19.Brown MS, Goldstein JL. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- 20.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 21.Lu H, Daugherty A. Atherosclerosis: cell biology and lipoproteins. Curr Opin Lipidol. 2014;25:157–158. doi: 10.1097/MOL.0000000000000061. [DOI] [PubMed] [Google Scholar]
- 22.Ahmad I, Shukla S, Kumar A, Singh BK, Kumar V, Chauhan AK, Singh D, Pandey HP, Singh C. Biochemical and molecular mechanisms of N-acetyl cysteine and silymarin-mediated protection against maneb-and paraquat-induced hepatotoxicity in rats. Chem Biol Interact. 2013;201:9–18. doi: 10.1016/j.cbi.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 23.Hermenean A, Stan M, Ardelean A, Pilat L, Mihali CV, Popescu C, Nagy L, Deák G, Zsuga M, Kéki S. Antioxidant and hepatoprotective activity of milk thistle (Silybum marianum L. Gaertn.) seed oil. Open Life Sciences. 2015:10. [Google Scholar]


