Abstract
Maternally Expressed Gene 3 (Meg3) encodes a long non-coding RNA that has been recently shown to regulate tumorigenesis through its interaction with microRNA (miR). We have recently reported that miR-1297 might play a role in the regulation of PTEN/PI3k/Akt signaling pathway in testicular germ cell tumor (TGCT). However, a crosstalk between Meg3 and miR-1297 in TGCT has not been appreciated. Here, we analyzed the levels of Meg3, miR-1297 and PTEN in TGCT specimens, compared to paired adjacent non-tumor tissue (NT), and found that Meg3 levels were significantly decreased and miR-1297 levels were unchanged in TGCT. PTEN protein but not mRNA levels significantly decreased in TGCT. Bioinformatics analyses showed that miR-1297 bound to 3’-UTR of PTEN mRNA, while miR-1297 also bound to Meg3. Luciferase report assay showed that Meg3 overexpression abolished the effects of miR-1297 on 3’-UTR of PTEN mRNA, possibly through competitive binding, which was supported by double fluorescent in situ hybridization showing co-localization of intracellular Meg3 and miR-1297 signals in TGCT cells. Moreover, Meg3 overexpression abolished the inhibitory effects of miR-1297 on PTEN, resulting in deactivation of Akt and decreases in cell growth. Together, these data demonstrate a previous unappreciated pathway in which Crosstalk between Meg3 and miR-1297 regulates growth of TFCT through PTEN/PI3K/AKT signaling. Re-expression of Meg3 may be an attractive strategy for TGCT therapy.
Keywords: Testicular germ cell tumor (TGCT), Maternally Expressed Gene 3 (Meg3), miR-1297, PTEN/PI3K/AKT
Introduction
Testicular germ cell tumor (TGCT) is predominantly detected in young men aged from 25 to 34 years. TGCT originates from transformed primordial germ cells, while most patients with newly diagnosed TGCT are highly treatable [1]. However, some TGCTs have already reached advanced stage, and the therapeutic outcome of these tumors is not satisfactory [2]. Therefore, a better understanding of the molecular mechanisms underlying the carcinogenesis, tumor growth and progression is important for generating novel methods to treat TGCT [3-5].
MicroRNAs (miRNAs) are small, endogenous, non-coding RNAs of about 22 nucleotides. The miRNAs have been extensively studied recently and their potential roles in regulation of protein translation through targeting the 3’-untranslated region (3’-UTR) of the mRNA have been well established [6,7]. It is noteworthy that miRNAs regulate many biological events including cancer initiation, progression, outgrowth and metastases [8-11]. Among all miRNAs, the involvement of miR-1297 in development of various cancers has been also reported [12-15]. Specifically, we and other recently found that miR-1297 might regulate TGCT cell growth [16,17].
Phosphatase and tensin homolog (PTEN) gene has been identified as a tumor suppressor that is mutated in a large number of cancers at high frequency [18-28]. PTEN encodes a protein called phosphatidylinositol-3,4,5-trisphosphate 3-phosphatase, which is comprised of a tensin-like domain and a catalytic domain, like the dual specificity protein tyrosine phosphatases [29-31]. PTEN preferentially dephosphorylates phosphoinositide substrates, and negatively regulates intracellular levels of Phosphoinositide 3-kinase (PI3k) to inhibit PI3k/Akt signaling pathway, activation of which is critical for growth and survival of various types of cancers [29-31]. Previous reports have shown that suppression of PTEN may play a role in TGCT pathogenesis [32-34]. Most recently, we reported miR-1297 as a potential oncogene that may induce TGCT cell proliferation by targeting PTEN and inhibiting its protein translation [16]. However, the exact molecular mechanisms remain undetermined. Moreover, the clinical relevance is uncertain.
Maternally expressed gene 3 (Meg3) is an imprinted DLK1-MEG3 locus gene that is located at human chromosome 14q32.3 [35-37]. The Meg3 gene encodes a long noncoding RNA (lncRNA) that is widely expressed in various tissues, and is often absent in cancer cells [38-41]. The loss of Meg3 expression has been shown to be a consequence of gene deletion and promoter hypermethylation, while re-expression of Meg3 has been shown to suppress in cultured tumor cell growth [38-41]. Nevertheless, a possible role of Meg3 in the tumorigenesis of TGCT has not been appreciated.
Here, we found that Meg3 levels were significantly decreased and miR-1297 levels were unchanged in TGCT specimens. Moreover, PTEN protein but not mRNA levels significantly decreased. Bioinformatics analyses showed that miR-1297 bound to 3’-UTR of PTEN mRNA, while miR-1297 also bound to Meg3. Luciferase report assay showed that Meg3 overexpression abolished the effects of miR-1297 on 3’-UTR of PTEN mRNA, possibly through competitive binding, which was supported by double fluorescent in situ hybridization showing co-localization of intracellular Meg3 and miR-1297 signals in TGCT cells. Moreover, Meg3 overexpression abolished the inhibitory effects of miR-1297 on PTEN, resulting in deactivation of Akt and decreases in cell growth.
Materials and methods
Patient specimens
Resected TGCT specimens from 33 patients were obtained together with the paired adjacent non-tumor tissues (NT) from 2009 to 2013 at Zhongshan Hospital of Fudan University. All patients provided signed, informed consent for their tissues to be used for scientific research. Ethical approval for the current study was obtained from Zhongshan Hospital of Fudan University. All diagnoses were based on pathological and/or cytological evidence. The histological features of the specimens were evaluated by senior pathologists according to the World Health Organization classification criteria. Obtained tissues were immediately frozen and stored at -70°C prior to RT-qPCR and Western blot.
TGCT cell lines
Human TGCT cell line NCCIT was established by Shinichi Teshima (National Cancer Institute, Tokyo, Japan) in 1985 from a mediastinal mixed germ cell tumor [42]. NCCIT was purchased from American Type Culture Collection (ATCC, Rockville, MD, USA) and cultured in RPMI 1640 medium supplemented with 20% heat-inactivated fetal bovine serum (FBS; Sigma-Aldrich, St Louis, MO, USA), 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA, USA) in a humidified atmosphere of 5% CO2 at 37°C.
Transfection
Meg3 transgene, short-hairpin interfering RNA for Meg3 (shMeg3), miR-1297 mimics (miR-1297), and control null sequence (null) were obtained from Origene (Beijing, China). These plasmids were used to modify either Meg3 levels or miR-1297 levels, and transfected into NCCIT cells at a concentration of 50 nmol/l using Lipofectamine-2000 (Invitrogen), according to the manufacturer’s instructions. Transfection efficiency was more than 95%, based on expression of a co-transgene, green fluorescent protein (GFP).
MTT assay
For assay of cell viability, cancer cells were seeded into 24 well-plate at 10000 cells per well and subjected to a MMT Kit (MTT, Roche, Indianapolis, IN, USA), according to the instruction of the manufacturer. The MTT assay is a colorimetric assay for assessing viable cell number, taking advantage that NADPH-dependent cellular oxidoreductase enzymes in viable cells reduce the tetrazolium dye 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to its insoluble formazan in purple readily being quantified by absorbance value (OD) at 570 nm. Experiments were performed 5 times.
MicroRNA target prediction and 3’-UTR luciferase-reporter assay
MiRNAs targets were predicted with the algorithms TargetSan (https://www.targetscan.org) [43]. The PTEN 3’-UTR reporter plasmid and mutatewere both purchased from Creative Biogene (Shirley, NY, USA). NCCIT cells were co-transfected with plasmidsusingLipofectamine 2000 (5×104 cells per well). Cells were collected 48 hours after transfection for assay using the dual-luciferase reporter assay system gene assay kit (Promega), according to the manufacturer’s instructions.
In situ hybridization
In situ hybridization analysis of Meg3 and miR-1297 mRNA in NCCIT cells was performed as has been previously described [44]. Briefly, the digoxigenin (DIG)-labeled and biotin-labeledriboprobes for Meg3 and miR-1297 were synthesized by in vitro transcription, using the fragments of Meg3 and miR-1297 amplified from NCCIT cells by PCR with the Expand High Fidelity PCR system (Roche Applied Science, Nutley, NJ, USA), followed by cloning into pGEM-T vector (Promega). The amplicons were confirmed by DNA sequencing.
Western blot
Protein was extracted from the resected specimens or cultured cells with RIPA lysis buffer (1% NP40, 0.1% Sodium dodecyl sulfate (SDS), 100 μg/ml phenylmethylsulfonyl fluoride, 0.5% sodium deoxycholate, in PBS) on ice. The supernatants were collected after centrifugation at 12000× g at 4°C for 20 min. Protein concentration was determined using a BCA protein assay kit (Bio-rad, China), and whole lysates were mixed with 4×SDS loading buffer (125 mmol/l Tris-HCl, 4% SDS, 20% glycerol, 100 mmol/l Dithiothreitol (DTT), and 0.2% bromophenol blue) at a ratio of 1:3. Samples were heated at 100°C for 5 min and were separated on SDS-polyacrylamide gels. The separated proteins were then transferred to a PVDF membrane. The membrane blots were first probed with a primary antibody. After incubation with horseradish peroxidase-conjugated second antibody, autoradiograms were prepared using the enhanced chemiluminescent system to visualize the protein antigen. The signals were recorded using X-ray film. Primary antibodies were rabbit anti-PTEN, anti-Akt and anti-α-tubulin (Cell Signaling, San Jose, CA, USA). Secondary antibody is HRP-conjugated anti-rabbit (Jackson ImmunoResearch Labs, West Grove, PA, USA). α-tubulin was used as a protein loading control. The protein levels were first normalized to α-tubulin, and then normalized to the experimental control.
Quantitative real-time PCR (RT-qPCR)
Total RNA were extracted with miRNeasy mini kit (Qiagen, Hilden, Germany). For cDNA synthesis, complementary DNA (cDNA) was randomly primed from 2 μg of total RNA using the Omniscript reverse transcription kit (Qiagen). RT-qPCR was subsequently performed in triplicate with a 1:4 dilution of cDNA. All primers were purchased from Qiagen. Data were collected and analyzed using 2-ΔΔCt method. Values of genes were first normalized against α-tubulin, and then compared to controls.
Statistical analysis
All statistical analyses were carried out using the SPSS 18.0 statistical software package. All data were statistically analyzed using one-way ANOVA with a Bonferroni correction, followed by Fisher’s exact test. All values are depicted as mean ± standard deviation and are considered significant if p < 0.05.
Results
Meg3 levels and PTEN proteindecrease, while PTEN mRNA and miR-1297 levels are unchanged in TGCT
We recently reported miR-1297 as a potential oncogene that may induce TGCT cell proliferation by targeting PTEN and inhibiting its protein translation. In order to figure out clinical relevance and further elucidate the underlying mechanisms, we analyzed the levels of PTEN in the TGCT specimens from the patients, and compared to the paired adjacent non-tumor tissue (NT). We found that the mRNA levels of PTEN in TGCT specimens were not significantly changed (Figure 1A), but the protein levels of PTEN in TGCT were significantly lower than NT (Figure 1B), suggesting presence of the post-transcriptional control of PTEN. We hypothesized that miR-1297 may be a regulator of PTEN in TGCT based on our previous studies. However, we did not detect significant changes in miR-1297 levels in TGCT (Figure 1C). Interestingly, we found that another non-coding RNA, Meg3, significantly decreased in TGCT, compared to NT (Figure 1D).
Figure 1.
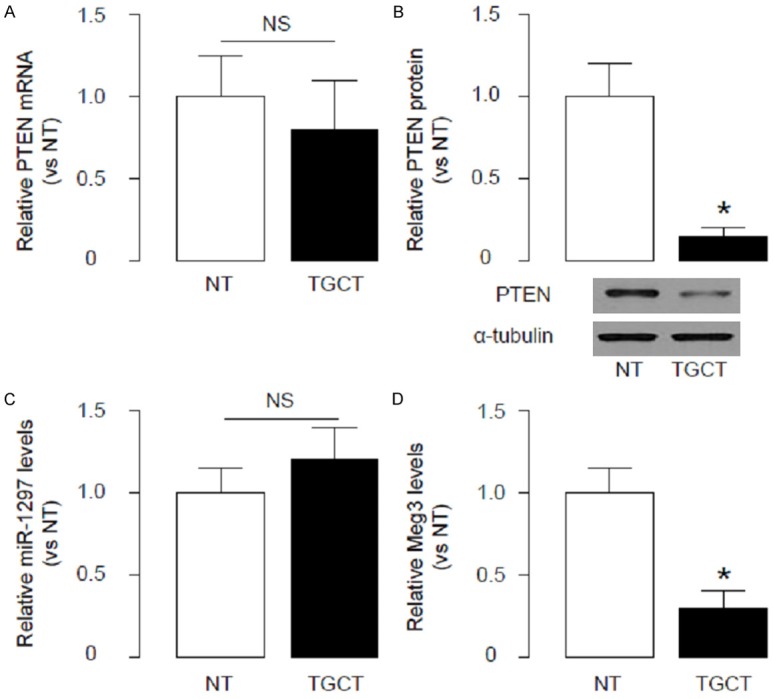
Meg3 levels and PTEN protein decrease, while PTEN mRNA and miR-1297 levels are unchanged in TGCT. We analyzed the levels of PTEN in the TGCT specimens from the patients, and compared to the paired adjacent non-tumor tissue (NT). (A, B) We found that the mRNA levels of PTEN in TGCT specimens were not significantly changed (A), but the protein levels of PTEN in TGCT were significantly lower than NT (B). (C) We did not detect significant changes in miR-1297 levels in TGCT. (D) We found that another non-coding RNA, Meg3, significantly decreased in TGCT, compared to NT. *p<0.05. NS: non-significant. N=33.
MiR-1297 binds to both PTEN mRNA and Meg3 in TGCT cells
Then, we performed bioinformatics analyses that showed that miR-1297 bound to 3’-UTR of PTEN mRNA (Figure 2A), while miR-1297 also bound to Meg3 (Figure 2B). Hence, we hypothesized that Meg3 may affect the binding between miR-1297 and 3’-UTR of PTEN mRNA.
Figure 2.
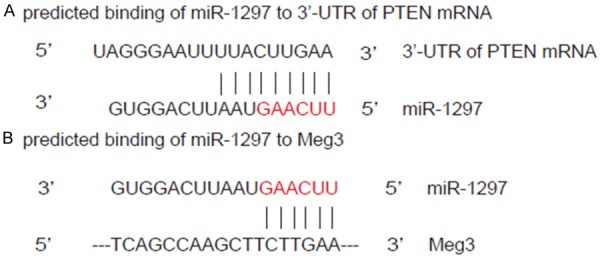
MiR-1297 binds to both PTEN mRNA and Meg3 in TGCT cells. (A, B) We performed bioinformatics analyses that showed that miR-1297 bound to 3’-UTR of PTEN mRNA (A), while miR-1297 also bound to Meg3 (B).
Meg3 competes with PTEN mRNA for binding to miR-1297
Thus, we prepared miR-1297-expressing plasmids, Meg3-expressing plasmids and Meg3-depleting plasmids (shMeg3), as well as plasmids carrying 3’-UTR of PTEN mRNA and plasmids carrying 3’-UTR of PTEN mRNA with spot mutation at miR-1297-binding sites. First, the modulation of miR-1297 levels by miR-1297-expressing plasmids in a human TGCT cell line NCCIT was confirmed by RT-qPCR (Figure 3A), and the modulation of Meg3 levels by either Meg3-expressing plasmids or Meg3-depleting plasmids was also confirmed by RT-qPCR (Figure 3B). Then we performed Luciferase report assay. We found that miR-1297 overexpression significantly decreased the bioluminescence of NCCIT cells transfected with 3’-UTR of PTEN mRNA, but not changed the bioluminescence of NCCIT cells transfected with 3’-UTR of PTEN mRNA mutation (Figure 3C). Moreover, Overexpression of Meg3 abolished the suppressive effects of miR-1297 on bioluminescence, while depletion of Meg3 augmented it (Figure 3C). All these data suggest that Meg3 may compete with PTEN mRNA for binding to miR-1297.
Figure 3.
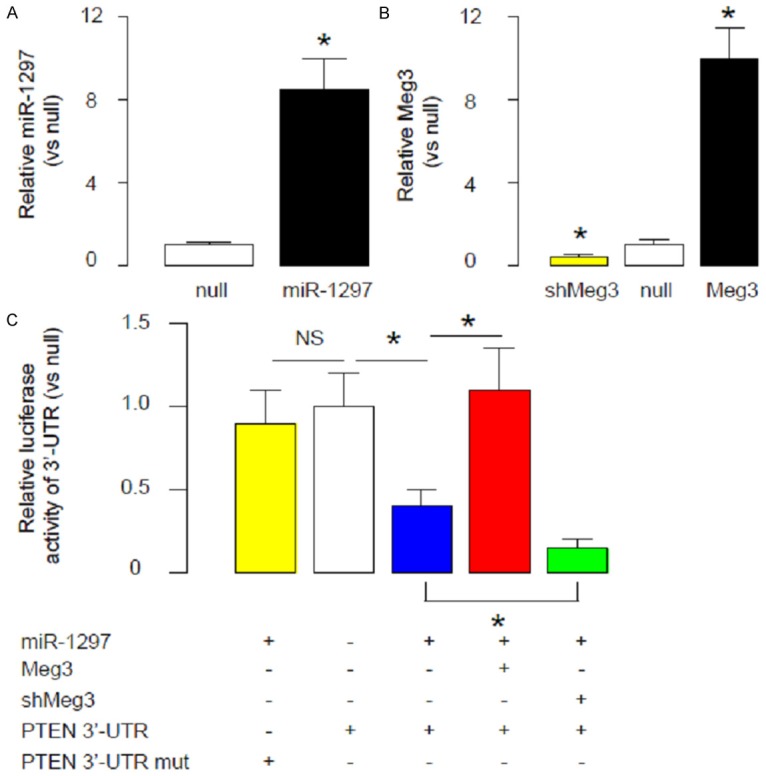
Meg3 competes with PTEN mRNA for binding to miR-1297. We prepared miR-1297-expressing plasmids, Meg3-expressing plasmids and Meg3-depleting plasmids (shMeg3), as well as plasmids carrying 3’-UTR of PTEN mRNA and plasmids carrying 3’-UTR of PTEN mRNA with spot mutation at miR-1297-binding sites. (A, B) The modulation of miR-1297 levels by miR-1297-expressing plasmids in a human TGCT cell line NCCIT was confirmed by RT-qPCR (A), and the modulation of Meg3 levels by either Meg3-expressing plasmids or Meg3-depleting plasmids was also confirmed by RT-qPCR (B). (C) We performed Luciferase report assay. We found that miR-1297 overexpression significantly decreased the bioluminescence of NCCIT cells transfected with 3’-UTR of PTEN mRNA, but not changed the bioluminescence of NCCIT cells transfected with 3’-UTR of PTEN mRNA mutation. Moreover, Overexpression of Meg3 abolished the suppressive effects of miR-1297 on bioluminescence, while depletion of Meg3 augmented it. *p<0.05. NS: non-significant. N=5.
Co-localization of Meg3 and miR-1297 in NCCIT cells
To confirm the physical binding between Meg3 and miR-1297, we performed double fluorescent in situ hybridization to study the association of miR-1297 and Meg3 in NCCIT cells. We found that the signals of Meg3 and miR-1297 co-localized in NCCIT cells that were co-transfected with Meg3 and miR-1297 (Figure 4). Together, these data suggest that Meg3 and miR-1297 indeed physically bind to each other.
Figure 4.
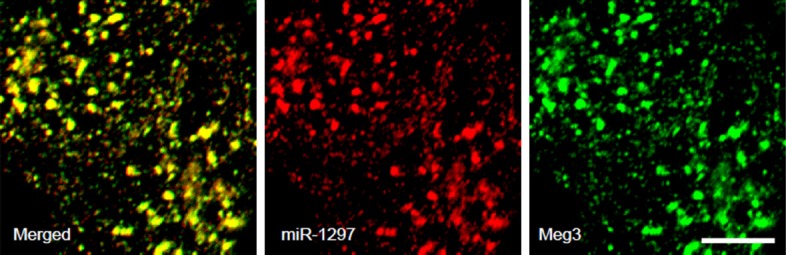
Co-localization of Meg3 and miR-1297 in NCCIT cells. We performed double fluorescent in situ hybridization to study the association of miR-1297 and Meg3 in NCCIT cells. We found that the signals of Meg3 and miR-1297 co-localized in NCCIT cells that were co-transfected with Meg3 and miR-1297. Scale bars are 1 µm.
Meg3 contradicts the inhibitory effects of miR-1297 on PTEN to regulate Akt-mediated cell growth
Finally, we examined the NCCIT cells transfected with miR-1297, or miR-1297 and Meg3, or miR-1297 and shMeg3 for PTEN and Akt. We found that miR-1297 significantly decreased PTEN levels, and increased Akt. The effects of miR-1297 on PTEN and Akt were abolished by Meg3 overexpression, and augmented by Meg depletion (Figure 5A). Moreover, in an MTT assay, miR-1297 significantly increased cell growth. The effects of miR-1297 on cell growth were abolished by Meg3 overexpression, and augmented by Meg depletion (Figure 5B). Together, these data confirm our findings using luciferase reporter assay, and demonstrate that Meg3 may contradict the inhibitory effects of miR-1297 on PTEN to regulate Akt-mediated cell growth in TGCT (Figure 6).
Figure 5.
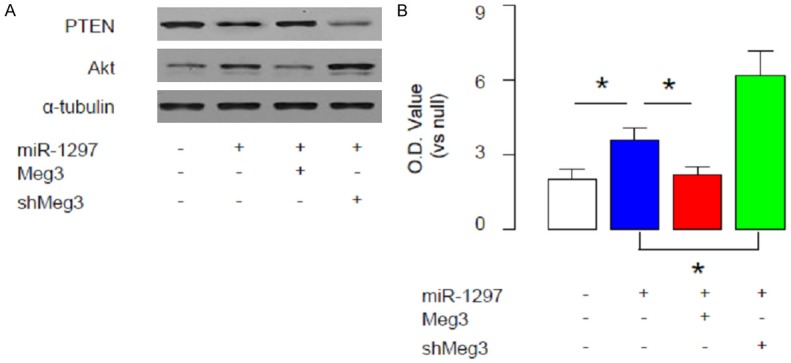
Meg3 contradicts the inhibitory effects of miR-1297 on PTEN to regulate Akt-mediated cell growth. We examined the NCCIT cells transfected with miR-1297, or miR-1297 and Meg3, or miR-1297 and shMeg3 for PTEN and Akt. A. We found that miR-1297 significantly decreased PTEN levels, and increased Akt. The effects of miR-1297 on PTEN and Akt were abolished by Meg3 overexpression, and augmented by Meg depletion. B. In an MTT assay, miR-1297 significantly increased cell growth. The effects of miR-1297 on cell growth were abolished by Meg3 overexpression, and augmented by Meg depletion. *p<0.05. N=5.
Figure 6.
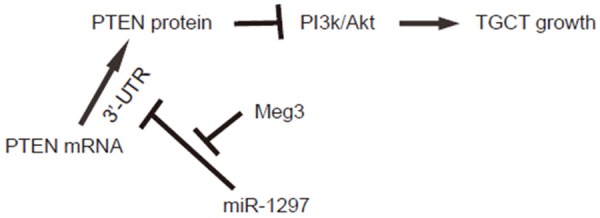
Schematic of the model. Meg3 may contradict the inhibitory effects of miR-1297 on PTEN to regulate Akt-mediated cell growth in TGCT.
Discussion
Identification of novel molecular regulatory pathways involved in the carcinogenesis of TGCT may provide novel strategiesfor clinical treatment of TGCT. Meg3 is an lncRNA and is often lost in various cancers [38-41]. Multiple mechanisms have been predicted to explain the regulation of cell growth by Meg3, e.g. throughinduction of apoptosisby p53. Maternal deletion of the Meg3 gene in mice has been studied and shown that the mice are suffered from skeletal muscle defects and perinatal death. Moreover, inactivation of Meg3 has been reported to result in a significant increase in angiogenesis-promoting genes and microvessel formation in the brain. These evidence highlights Meg3 as a novel lncRNA tumor suppressor [37].
In the current study, by using bioinformatics analyses, luciferase report assay implanted with loss-of-function and gain-of-function experiments, and double in situ hybridization, we provided strong evidence showing that Meg3 may contradict the effects of miR-1297 on the PTEN mRNA. The net effects on PTEN, which is a potential inhibitor of PI3k/Akt pathway, decide the activating levels of this signaling pathway for cell growth control. These in vitro data well explained our clinical findings, showing that miR-1297 did not alter in TGCT tissue, compared to NT. The loss of Meg3 in TGCT may weaken the suppressive effects on the binding of miR-1297 to PTEN mRNA, resulting in amplification of miR-1297 effects and the consequent decreases in PTEN protein translation.
Although here we describe a model in which Meg3 competes with PTEN mRNA for binding to miR-1297 in TGCT cells, we do not exclude the possibility of other mechanisms may also contribute to the difference in the changes in mRNA and protein levels of PTEN in TGCT cells, e.g. miRNAs other than miR-1297, or modification of protein degradation through phosphorylation, SUMOylation, acetylation, and ubiquitination. These possibilities may be analyzed in future studies.
To summarize, here we reported a novel molecular regulatory pathway among miR-1297, Meg3, and PTEN mRNA. We propose that Meg3 may be a promising target to treat TGCT and deserves further investigation.
Acknowledgements
The project was sponsored by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry and Youth Fund of Zhongshan Hospital (No. 2015ZSQN66).
Disclosure of conflict of interest
None.
References
- 1.van de Geijn GJ, Hersmus R, Looijenga LH. Recent developments in testicular germ cell tumor research. Birth Defects Res C Embryo Today. 2009;87:96–113. doi: 10.1002/bdrc.20140. [DOI] [PubMed] [Google Scholar]
- 2.Cost NG, Adibi M, Lubahn JD, Romman A, Raj GV, Sagalowsky AI, Margulis V. Effect of testicular germ cell tumor therapy on renal function. Urology. 2012;80:641–648. doi: 10.1016/j.urology.2012.04.064. [DOI] [PubMed] [Google Scholar]
- 3.Hayashi T, Yamada T, Kageyama Y, Kihara K. Expression failure of the notch signaling system is associated with the pathogenesis of testicular germ cell tumor. Tumour Biol. 2004;25:99–105. doi: 10.1159/000079140. [DOI] [PubMed] [Google Scholar]
- 4.Chung CC, Kanetsky PA, Wang Z, Hildebrandt MA, Koster R, Skotheim RI, Kratz CP, Turnbull C, Cortessis VK, Bakken AC, Bishop DT, Cook MB, Erickson RL, Fossa SD, Jacobs KB, Korde LA, Kraggerud SM, Lothe RA, Loud JT, Rahman N, Skinner EC, Thomas DC, Wu X, Yeager M, Schumacher FR, Greene MH, Schwartz SM, McGlynn KA, Chanock SJ, Nathanson KL. Meta-analysis identifies four new loci associated with testicular germ cell tumor. Nat Genet. 2013;45:680–685. doi: 10.1038/ng.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cambareri GM, Reiley EA, Hensle TW. Germ cell tumor in an adolescent with extensive testicular microlithiasis: concerns regarding future management. Urology. 2013;82:454–457. doi: 10.1016/j.urology.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Di Leva G, Croce CM. miRNA profiling of cancer. Curr Opin Genet Dev. 2013;23:3–11. doi: 10.1016/j.gde.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pereira DM, Rodrigues PM, Borralho PM, Rodrigues CM. Delivering the promise of miRNA cancer therapeutics. Drug Discov Today. 2013;18:282–289. doi: 10.1016/j.drudis.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Mei Q, Li F, Quan H, Liu Y, Xu H. Busulfan inhibits growth of human osteosarcoma through miR-200 family microRNAs in vitro and in vivo. Cancer Sci. 2014;105:755–762. doi: 10.1111/cas.12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang F, Xiao W, Sun J, Han D, Zhu Y. MiRNA-181c inhibits EGFR-signaling-dependent MMP9 activation via suppressing Akt phosphorylation in glioblastoma. Tumour Biol. 2014;35:8653–8658. doi: 10.1007/s13277-014-2131-6. [DOI] [PubMed] [Google Scholar]
- 10.Liu G, Jiang C, Li D, Wang R, Wang W. MiRNA-34a inhibits EGFR-signaling-dependent MMP7 activation in gastric cancer. Tumour Biol. 2014;35:9801–9806. doi: 10.1007/s13277-014-2273-6. [DOI] [PubMed] [Google Scholar]
- 11.Jin Y, Lu J, Wen J, Shen Y, Wen X. Regulation of growth of30 family regulates non-attachmeny miR-192. Tumour Biol. 2015;36:3791–3797. doi: 10.1007/s13277-014-3020-8. [DOI] [PubMed] [Google Scholar]
- 12.Ouzounova M, Vuong T, Ancey PB, Ferrand M, Durand G, Le-Calvez Kelm F, Croce C, Matar C, Herceg Z, Hernandez-Vargas H. MicroRNA miR-30 family regulates non-attachment growth of breast cancer cells. BMC Genomics. 2013;14:139. doi: 10.1186/1471-2164-14-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kao CJ, Martiniez A, Shi XB, Yang J, Evans CP, Dobi A, deVere White RW, Kung HJ. miR-30 as a tumor suppressor connects EGF/Src signal to ERG and EMT. Oncogene. 2014;33:2495–2503. doi: 10.1038/onc.2013.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu F, Deng H, Yao H, Liu Q, Su F, Song E. Mir-30 reduction maintains self-renewal and inhibits apoptosis in breast tumor-initiating cells. Oncogene. 2010;29:4194–4204. doi: 10.1038/onc.2010.167. [DOI] [PubMed] [Google Scholar]
- 15.Martinez I, Cazalla D, Almstead LL, Steitz JA, DiMaio D. miR-29 and miR-30 regulate B-Myb expression during cellular senescence. Proc Natl Acad Sci U S A. 2011;108:522–527. doi: 10.1073/pnas.1017346108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang NQ, Zhang J, Tang QY, Guo JM, Wang GM. miRNA-1297 induces cell proliferation by targeting phosphatase and tensin homolog in testicular germ cell tumor cells. Asian Pac J Cancer Prev. 2014;15:6243–6246. doi: 10.7314/apjcp.2014.15.15.6243. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Wang HL, Peng X, Zhou HF, Wang X. miR-1297 mediates PTEN expression and contributes to cell progression in LSCC. Biochem Biophys Res Commun. 2012;427:254–260. doi: 10.1016/j.bbrc.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 18.Yan SY, Chen MM, Li GM, Wang YQ, Fan JG. MiR-32 induces cell proliferation, migration, and invasion in hepatocellular carcinoma by targeting PTEN. Tumour Biol. 2015;36:4747–4755. doi: 10.1007/s13277-015-3124-9. [DOI] [PubMed] [Google Scholar]
- 19.Qi H, Lou M, Chen Y, Liu X, Chen N, Shan J, Ling Z, Shen J, Zhu L, Yen Y, Zheng S, Shao J. Non-enzymatic action of RRM1 protein upregulates PTEN leading to inhibition of colorectal cancer metastasis. Tumour Biol. 2015;36:4833–4842. doi: 10.1007/s13277-015-3137-4. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Wu Z, Ma Z, Liu H, Wu Y, Zhang Q. WWP1 as a potential tumor oncogene regulates PTEN-Akt signaling pathway in human gastric carcinoma. Tumour Biol. 2015;36:787–798. doi: 10.1007/s13277-014-2696-0. [DOI] [PubMed] [Google Scholar]
- 21.Yang X, Cheng Y, Li P, Tao J, Deng X, Zhang X, Gu M, Lu Q, Yin C. A lentiviral sponge for miRNA-21 diminishes aerobic glycolysis in bladder cancer T24 cells via the PTEN/PI3K/AKT/mTOR axis. Tumour Biol. 2015;36:383–391. doi: 10.1007/s13277-014-2617-2. [DOI] [PubMed] [Google Scholar]
- 22.Xiao WZ, Han DH, Wang F, Wang YQ, Zhu YH, Wu YF, Liu NT, Sun JY. Relationships between PTEN gene mutations and prognosis in glioma: a meta-analysis. Tumour Biol. 2014;35:6687–6693. doi: 10.1007/s13277-014-1885-1. [DOI] [PubMed] [Google Scholar]
- 23.Shen L, Chen XD, Zhang YH. MicroRNA-128 promotes proliferation in osteosarcoma cells by downregulating PTEN. Tumour Biol. 2014;35:2069–2074. doi: 10.1007/s13277-013-1274-1. [DOI] [PubMed] [Google Scholar]
- 24.de Assis LV, Isoldi MC. The function, mechanisms, and role of the genes PTEN and TP53 and the effects of asbestos in the development of malignant mesothelioma: a review focused on the genes’ molecular mechanisms. Tumour Biol. 2014;35:889–901. doi: 10.1007/s13277-013-1210-4. [DOI] [PubMed] [Google Scholar]
- 25.Tural D, Batur S, Erdamar S, Akar E, Kepil N, Mandel NM, Serdengecti S. Analysis of PTEN, BRAF and PI3K status for determination of benefit from cetuximab therapy in metastatic colorectal cancer patients refractory to chemotherapy with wild-type KRAS. Tumour Biol. 2014;35:1041–1049. doi: 10.1007/s13277-013-1138-8. [DOI] [PubMed] [Google Scholar]
- 26.Chen Q, Qin R, Fang Y, Li H. Berberine Sensitizes Human Ovarian Cancer Cells to Cisplatin Through miR-93/PTEN/Akt Signaling Pathway. Cell Physiol Biochem. 2015;36:956–965. doi: 10.1159/000430270. [DOI] [PubMed] [Google Scholar]
- 27.Dou L, Wang S, Sui X, Meng X, Shen T, Huang X, Guo J, Fang W, Man Y, Xi J, Li J. MiR-301a mediates the effect of IL-6 on the AKT/GSK pathway and hepatic glycogenesis by regulating PTEN expression. Cell Physiol Biochem. 2015;35:1413–1424. doi: 10.1159/000373962. [DOI] [PubMed] [Google Scholar]
- 28.Xu C, Kong X, Wang H, Zhang N, Kong X, Ding X, Li X, Yang Q. MTDH mediates estrogen-independent growth and tamoxifen resistance by down-regulating PTEN in MCF-7 breast cancer cells. Cell Physiol Biochem. 2014;33:1557–1567. doi: 10.1159/000358719. [DOI] [PubMed] [Google Scholar]
- 29.Hopkins BD, Parsons RE. Molecular pathways: intercellular PTEN and the potential of PTEN restoration therapy. Clin Cancer Res. 2014;20:5379–5383. doi: 10.1158/1078-0432.CCR-13-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim HJ, Crowe P, Yang JL. Current clinical regulation of PI3K/PTEN/Akt/mTOR signalling in treatment of human cancer. J Cancer Res Clin Oncol. 2015;141:671–689. doi: 10.1007/s00432-014-1803-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Worby CA, Dixon JE. Pten. Annu Rev Biochem. 2014;83:641–669. doi: 10.1146/annurev-biochem-082411-113907. [DOI] [PubMed] [Google Scholar]
- 32.Andreassen KE, Kristiansen W, Karlsson R, Aschim EL, Dahl O, Fossa SD, Adami HO, Wiklund F, Haugen TB, Grotmol T. Genetic variation in AKT1, PTEN and the 8q24 locus, and the risk of testicular germ cell tumor. Hum Reprod. 2013;28:1995–2002. doi: 10.1093/humrep/det127. [DOI] [PubMed] [Google Scholar]
- 33.Kimura T, Suzuki A, Fujita Y, Yomogida K, Lomeli H, Asada N, Ikeuchi M, Nagy A, Mak TW, Nakano T. Conditional loss of PTEN leads to testicular teratoma and enhances embryonic germ cell production. Development. 2003;130:1691–1700. doi: 10.1242/dev.00392. [DOI] [PubMed] [Google Scholar]
- 34.Karlsson R, Andreassen KE, Kristiansen W, Aschim EL, Bremnes RM, Dahl O, Fossa SD, Klepp O, Langberg CW, Solberg A, Tretli S, Magnusson PK, Adami HO, Haugen TB, Grotmol T, Wiklund F. Investigation of six testicular germ cell tumor susceptibility genes suggests a parent-of-origin effect in SPRY4. Hum Mol Genet. 2013;22:3373–3380. doi: 10.1093/hmg/ddt188. [DOI] [PubMed] [Google Scholar]
- 35.Mondal T, Subhash S, Vaid R, Enroth S, Uday S, Reinius B, Mitra S, Mohammed A, James AR, Hoberg E, Moustakas A, Gyllensten U, Jones SJ, Gustafsson CM, Sims AH, Westerlund F, Gorab E, Kanduri C. MEG3 long noncoding RNA regulates the TGF-beta pathway genes through formation of RNA-DNA triplex structures. Nat Commun. 2015;6:7743. doi: 10.1038/ncomms8743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balik V, Srovnal J, Sulla I, Kalita O, Foltanova T, Vaverka M, Hrabalek L, Hajduch M. MEG3: a novel long noncoding potentially tumour-suppressing RNA in meningiomas. J Neurooncol. 2013;112:1–8. doi: 10.1007/s11060-012-1038-6. [DOI] [PubMed] [Google Scholar]
- 37.Zhou Y, Zhang X, Klibanski A. MEG3 noncoding RNA: a tumor suppressor. J Mol Endocrinol. 2012;48:R45–53. doi: 10.1530/JME-12-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan J, Guo X, Xia J, Shan T, Gu C, Liang Z, Zhao W, Jin S. MiR-148a regulates MEG3 in gastric cancer by targeting DNA methyltransferase 1. Med Oncol. 2014;31:879. doi: 10.1007/s12032-014-0879-6. [DOI] [PubMed] [Google Scholar]
- 39.Sun M, Xia R, Jin F, Xu T, Liu Z, De W, Liu X. Downregulated long noncoding RNA MEG3 is associated with poor prognosis and promotes cell proliferation in gastric cancer. Tumour Biol. 2014;35:1065–1073. doi: 10.1007/s13277-013-1142-z. [DOI] [PubMed] [Google Scholar]
- 40.Benetatos L, Voulgaris E, Vartholomatos G. DLK1-MEG3 imprinted domain microRNAs in cancer biology. Crit Rev Eukaryot Gene Expr. 2012;22:1–15. doi: 10.1615/critreveukargeneexpr.v22.i1.10. [DOI] [PubMed] [Google Scholar]
- 41.Braconi C, Kogure T, Valeri N, Huang N, Nuovo G, Costinean S, Negrini M, Miotto E, Croce CM, Patel T. microRNA-29 can regulate expression of the long non-coding RNA gene MEG3 in hepatocellular cancer. Oncogene. 2011;30:4750–4756. doi: 10.1038/onc.2011.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teshima S, Shimosato Y, Hirohashi S, Tome Y, Hayashi I, Kanazawa H, Kakizoe T. Four new human germ cell tumor cell lines. Lab Invest. 1988;59:328–336. [PubMed] [Google Scholar]
- 43.Coronnello C, Benos PV. ComiR: Combinatorial microRNA target prediction tool. Nucleic Acids Res. 2013;41:W159–164. doi: 10.1093/nar/gkt379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pinaud R, Mello CV, Velho TA, Wynne RD, Tremere LA. Detection of two mRNA species at single-cell resolution by double-fluorescence in situ hybridization. Nat Protoc. 2008;3:1370–1379. doi: 10.1038/nprot.2008.115. [DOI] [PubMed] [Google Scholar]


