Abstract
Growing evidences suggest that there are close associations between anthocyanins and cardiac protection. However, little is known about the detailed roles of anthocyanins in regulating extracellular matrix (ECM) remodeling. Incubation of primary cultured fibroblasts with anthocyanins reduced both intracellular collagen expression and extracellular collagen secretion. Down-regulation of collagen production was also shown in infarcted cardiac tissues after permanent coronary artery ligation in mice treated with anthocyanins. The phosphorylation levels of Akt and/or P-38 were significantly increased by anthocyanins supplementation in primary cultured fibroblasts. Gelatin zymography analysis of matrix metalloproteinase-2 (MMP-2) activity in conditioned medium collected from fibroblasts demonstrated that anthocyanins treatment significantly reduced MMP-2 activity. These results demonstrated that anthocyanins play a role in mediating myocardial ECM remodeling and that the Akt/P-38 pathways mediate these protective effects on hearts.
Keywords: Extra cellular remodeling, MMPs, anthocyanins, infracted heart, fibroblasts
Introduction
Extracellular matrix (ECM) is no longer considered as a passive support structure. Instead, cardiac ECM is capable of fundamentally altering cardiomyocytes specificity and behaviors. Fibroblasts are the major contributors to the interstitial fibrosis that control the production and degradation of ECM components, especially collagen networks. 85% of the ECM proteins are constituted by collagens [1], which have a triple helix of polypeptide chains and globular domains, and comprise a family of proteins of at least 19 genetically distinct types, among which type I and type III constitute two-thirds. Up-regulated interstitial collagens account for the reduction of capillary density and oxygen diffusion, reduced contractility of cardiomyocytes, and increased structural rigidity of the left ventricular [2]. It was reported that infarcted myocardium was completely replaced with fibroblast accompanied by an increased deposition of collagen, and then replaced by connective tissue within 21 days after coronary artery ligation in rats. Thus, remodeling in the non-infarcted left ventricle is associated with the development of myocardial interstitial fibrosis with rearrangement of extracellular matrix architecture.
Although numerous enzymes are able to degrade individual ECM components, proteolytic process of ECM is primarily under the control of specific MMPs and their inhibitors (i.e., TIMPs) [3]. Until now, little information is known about the specific targeting of ECM components except some drugs (i.e abciximab, which is used to treat acute coronary syndrome) that target the major cellular receptors for ECM, integrins. Integrins are transmembrane heterodimeric glycoproteins composed of one a and one b subunit, which usually recognize only relative short peptide motifs in a molecule.
Anthocyanins, as one group of flavonoids, are widely distributed amongst blue and purple fruits and vegetables (e.g. berries and grapes), which have been shown to have various protective properties mainly due to their antioxidant capacity. The antioxidant properties of anthocyanins are determined by the number and position of hydroxyl group as substituents.
Growing evidences suggest that Anthocyanins are able to elicit beneficial effect on heart via attenuation of oxidative stress [4], inflammatory disorder [5], and atherosclerosis [6]. For example, Skemiene et al. [7] found that anthocyanins attenuated ischemic injury by blocking caspase activiation through reducing cytosolic cytochrome c. The relationship between anthocyanins and cardiac remodeling has seldom been investigated. One study reported that alcohol-free red wine extract (AFRW) was rich in 14 types of polyphenols and 4 types of anthocyanins. Feeding with AFRW for 8 weeks significantly decreased the amount of collagen type III in remodeling heart [8]. However, the detailed molecular mechanism mediating anthocyanins on collagen expression remained unknown, and the direct effect of anthocyanins on MMPs activity has not yet been determined.
In this study, using both animal and cell models, we aimed to investigate the effect of anthocyanins on cardiac ECM remodeling and the underlying mechanisms. It was found that anthocyanins significantly reduced expression and secretion of collagens, and the activities of MMPs, which was correlated with Akt/p38 pathways.
Materials and methods
Reagents
Malvidin-3-0-glucoside (malvidin, a kind of anthocyanins) was purchased from Indofine Chemical Co. (Hillsborough, NJ). The antibodies of Collagen, Akt, and p38 MAPK were purchased from Cell Signaling Technology, Inc. (Danvers, MA). Rabbit anti-type I or III collagen, and MT-MP1 antibodies were purchased from Abcam (Cambridge, MA).
Animals
All the animal experimental procedures were approved by the Committee on the Use of Live Animals of Hebei Medical University, and were carried out in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85-23, revised 1996).
Permanent coronary artery ligation surgery and anthocyanins treatment
6-8 wk C57BL/6J mice were administered with anthocyanins orally (10 mg/kg per day) [9], as shown in Figure 1. After one week’s treatment, the mice were subjected to permanent coronary artery ligation (CAL) surgery, as previously reported [10], in order to induce cardiac extra cellular matrix (ECM) remodeling. Briefly, mice were intraperitoneally anesthetized with a combination of Ketamine (90 mg/kg) and Xylazine (10 mg/kg). The mice were ventilated with oxygen and 2% isoflurane by oral endotracheal intubation. A left thoracotomy was performed for exposing the left anterior descending coronary artery (LAD). The LAD was ligated with 8-0 silk suture permanently. The mice were sacrificed for the following experiment at 7-d after the surgery. After surgery, the mice were still given with anthocyanins for another one week. Then the mice were sacrificed for serum and heart tissues collection. The left ventricles were divided in two parts: infracted area, non-infarcted area. The tissue homogenates prepared from non-infracted area were used in the following studies.
Figure 1.
Workflow of CAL and anthocyanin treatment. CAL: permanent coronary artery ligation surgery. Anthocyanins: 10 mg/kg per day, for two weeks.
Immunohistochemistry staining of collagen
The immunohistochemistry staining of type I collagen was conducted as described elsewhere [11]. Cryostat sections (5 to 7 μm) were washed 3 times with PBS and then blocked with 2% BSA solution for 60 minutes. Slides were incubated with the collagen I antibody (1:500 dilution) for overnight at 4°C. Slides were washed 3 times with BSA solution and incubated for 1 hour at RT with goat anti-rabbit secondary Ab. The images were captured by Olympus microscope (Japan).
Primary cardiac fibroblast culture
Neonatal cardiac fibroblasts (CFs) were isolated from 3-4 day old Wistar rats as previously described [12]. Briefly, excised hearts were digested with 0.15% trypsin for 1 h and neutralized with DMEM containing 10% FBS. The digested cells were incubated in cell culture incubator for 1 h and then the cardiomyocytes in suspension were removed. The remaining attached cells in plates were CFs. After CFs were passaged twice and grown to 100% confluence, they were starved with serum-free DMEM for at least 3 hours and then treated with anthocyanins.
Picrosirius Red staining
Conditioned medium from CFs was concentrated and analyzed as previously described [13]. Briefly, concentrated conditioned medium samples (50 mg) were loaded into 96-well plates and allowed to evaporate for 48 hr at 37°C in 5% CO2. Following staining with 0.1% Picrosirius Red for 1 hr and washing with 10 mM HCl, the stain was solubilized with 0.1 M NaOH. The OD of samples and collagen standard were then measured by spectrophotometry at an absorbance of 540 nm.
Western blotting
Protein samples from CFs were prepared according to previously mentioned methods [14]. Primary anti-phopho-Akt, anti-phopho-p38 MAPK, anti-total-Akt, anti-total p38 MAPK, anti-type I and III collagen were used at 1:1000 dilutions, and appropriate HRP-conjugated secondary antibodies were used at 1:10,000 dilutions.The signals of immune complexes were visualized using enhanced chemiluminesecence (ECL) system PLUS or ADVANCE. Quantitative analysis was performed after densitometric scanning by Bio Imaging Analyzer LAS 3000 (FUJIFILM, Tokyo, Japan).
Gelatin zymography
The conditioned culture medium collected from CFs was concentrated using Amicon filter (Millipore, Ontario, Canada) and protein content of the culture medium was measured by BCA method. Aliquots of culture media containing 25 μg of protein were then resolved on a 10% SDS polyacrylamide gel containing 0.1% gelatin. The gel was renatured in 2.5% Triton X-100 buffer for 1 h, followed by washing 4 times in ddH2O. The gel was then incubated for 20-72 h at 37°C with gentle shaking in 0.05 M Tris-HCl (pH 7.5) with 5 mM CaCl2 to allow activation of MMPs. The activation was stopped by incubation the gel with 100 mM EDTA for 15 min. Gels were then stained for 30 min in 0.5% Coomassie blue R-250, and destained in 40% methanol, 10% acetic acid to highlight protease activity of MMPs. Protease activity was quantified using densitometric scanning and Image J software (NIH).
Data analysis
All results were derived from at least three sets of repeated experiments. The statistical calculations were performed by Student’s t-test or ANOVA followed by Tukey multiple comparisons using Prism version 5 (GraphPad Software, San Diego, CA, USA). All values were presented as means ± SEM. In all statistical comparisons, a P value less than 0.05 was used to indicate significant differences.
Results
Supplementation with anthocyanins attenuated interstitial collagen deposition in heart
In order to examine whether anthocyanin elicited an effect on extra cellular matrix (ECM) redmodeling in heart, the mice were subjected to CAL. After intragastrically feeding with anthocyanin for two weeks, the mice were sacrificed for heart tissues collection. The non-infarcted sections of left ventricle were subjected to type I collagen staining for assessment of degree of cardiac fibrosis. As shown in Figure 2, interstitial collagen deposition was significantly reduced after two week’s treatment with anthocyanins.
Figure 2.
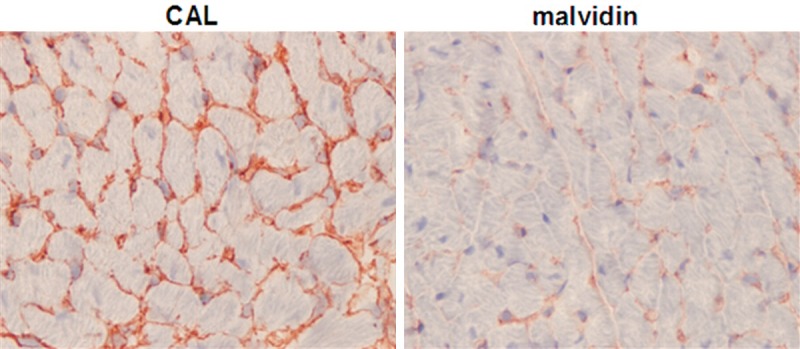
Anthocyanins reduced cardiac collagen deposition after myocardial infarction. The degree of cardiac fibrosis was accessed by staining type I collagen using immunohistochemistry method. CAL: wild type mice subjected to left anterior descending coronary artery ligation. malvidin: the CAL mice were treated with anthocyanins for 4 weeks. Representative images from each group are shown. Magnification, 400×.
Anthocyanins reduced fibrillar collagen expression and secretion in primary cultured fibroblasts
Next, the effect of anthocyanins on regulation of both extracellular and intracellular collagen production in fibroblasts was studied. Using Picrosirius Red staining method, it was found that extracellular secretion of collagen was stimulated by 3 ug/ml of anthocyanins at 24-h Figure 3Aa. Furthermore, dose dependent experiments were investigated as shown in Figure 3Ab, indicating that treatment with 1 or 3 ug/ml of anthocyanin for 24 h significantly increased the accumulation of fibrillar collagen in conditioned medium by ~1.30- or ~1.59-fold, respectively.
Figure 3.
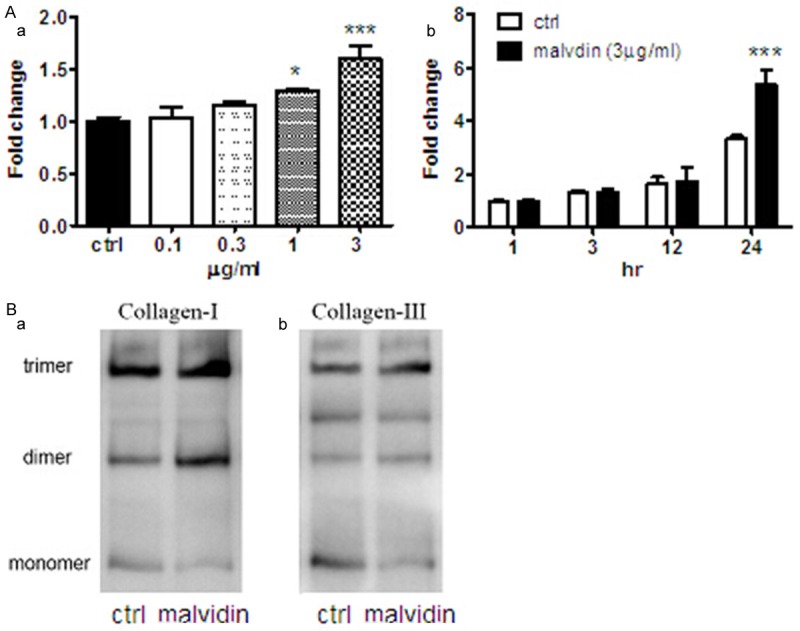
Anthocyanins stimulated collagen expression and secretion in cardiac fibroblast cells. A. (a) After treatment with anthocyanins for 24-h, the extracellular medium of fibroblast was collected and concentrated for measurement of collagen content by Picrosirius Red staining. The results are presented as fold change against the control value. (b) The extracellular fibrillar collagen was measured after treatment with 3 ug/ml anthocyanins for various time points. The results are presented as fold change against the control value at 1-h. ***P < 0.001; *P < 0.05 vs corresponding ctrl, n=6. B. Protein samples derived from fibroblast cells following treatment with anthocyanin for 24-h were subjected to Western blot analysis and probed with rabbit anti-type I collagen antibody (a) and rabbit anti-type III antibody (b). Data shown are representative results from three independent experiments.
To examine intracellular collagen expression, we used western blotting to measure alterations of collagen-I and -III expression following anthocyanins administration. It was shown that treatment with anthocyanin for 24-h could significantly increased multimerization of collagen-I and -III in primary cultured cardiac fibroblast Figure 3B.
Anthocyanins increased MMPs proteolytic activity in primary cultured fibroblasts
Using gelatin zymography, the effect of anthocyanins on MMPs activity in primary cultured fibroblasts was investigated. After treatment with anthocyanins for specific time, the conditioned medium was collected and concentrated. 25 ul of concentrated medium was loaded into gelatin contained SDS-PAGE and the bands of MMPs were visualized by staining the gels with coomassie blue. It was found that MMP-2 activity was significantly increased following 3 ug/ml anthocyanins treatment for 24 h by 2.03 fold, while 48-h treatment showed no effect Figure 4.
Figure 4.
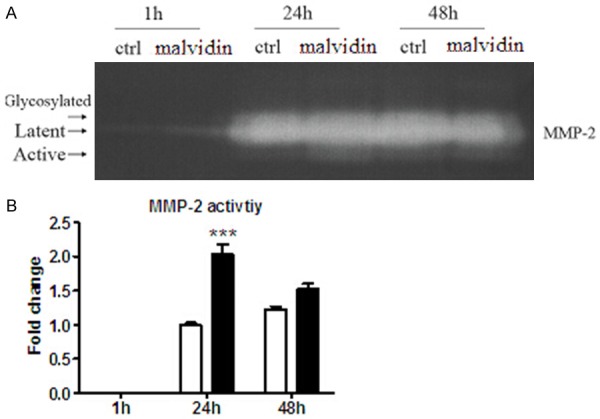
Gelatin zymographic analysis of the effect of anthocyanins on MMPs activity. Through the analysis of conditioned medium obtained from CFs, it was demonstrated that anthocyanins increased MMP-2 activity at 24-h post treatment. A. Representative zymography gel images of MMP-2 (glycosylated form corresponding to ~72 kDa, latent form corresponding to ~68 kDa and active form corresponding to ~62 kDa) are shown. B. Proteolytic activity was measured by densiotometric scanning and quantitative analysis of the active form of MMP2 for n=6 experiments performed under the conditions indicated. The results are presented as fold change against the control value at 24-h. ***P < 0.001 vs ctrl, n=6.
Anthocyanins increased MMP-2 proteolytic activity via up-regulation of MT1-MMP
The effect of anthocyanins on MT1-MMP, a membrane bound MMP isoform and a known activator of MMP-2, was also investigated. As shown in Figure 5A, treatment with anthocaynins increased MT1-MMP expression level by 1.45-fold, which suggested that anthocyanins stimulated MMP-2 proteolytic activity via up-regulation of MT1-MMP.
Figure 5.
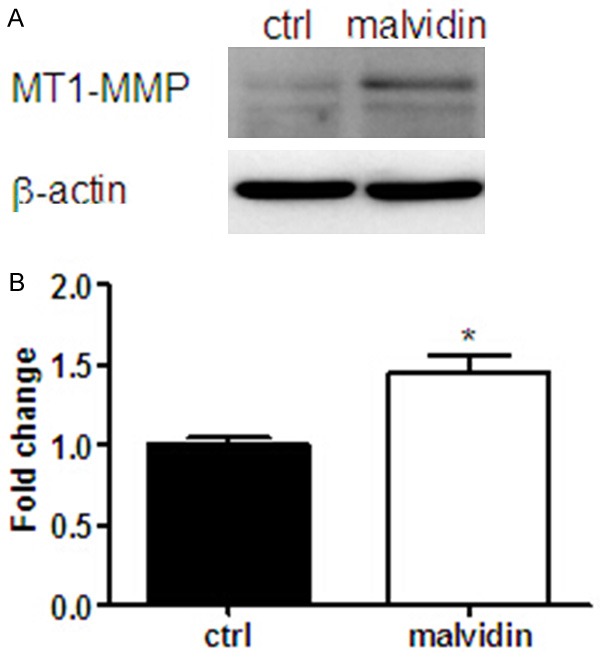
Anthocyanins increased intracellular MT1-MMP expression level in CFs. A. The protein expression level of MT1-MMP following anthocyanin (3 ug/ml) treatment for 24-h was checked by Western blot. B. The bands corresponding to ~65 kDa were measured by densitometric scanning and the results were normalized by b-actin values and presented as fold change against control values. *P < 0.05 vs ctrl, n=6.
Anthocyanins enhanced Akt and/or p38 phosphorylation levels in fibroblast cells
In order to elucidate what the molecular mechanism underlying the regulation of anthocyanin on extra cellular remodeling, the level of p-Akt or P38 in cultured fibroblast cells were investigated by Western blot. As shown in Figure 6A, both 1 and 3 ug/ml of anthocyanins could significantly decreased p-Akt level at 24-h, by 45% and 57% respectively. Similarly, it was shown that anthocyanin with the dose of 1 and 3 ug/ml attenuated p-p38 levels by 28% and 45%.
Figure 6.
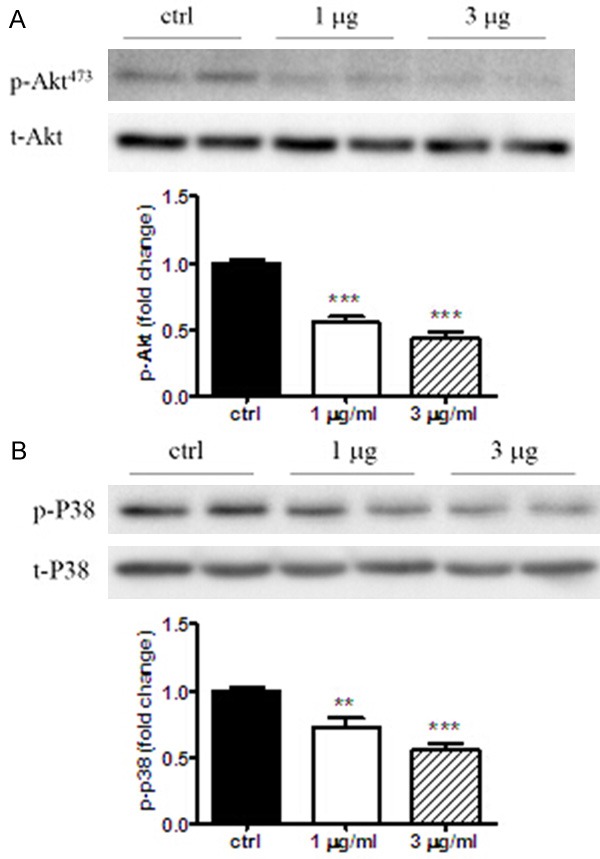
Anthocyanins reduced p-Akt and p-p38 levels in cardiac fibroblast cells. The phosphorylation level of Akt (A) or p38 (B) induced by anthocyanins treatment for 24-h was checked by Western blot. The corresponding bands were measured by densitometric scanning and the results were normalized by t-Akt or t-p38 values. All the results are presented as fold change against ctrl values. ***P < 0.001; **P < 0.01 vs ctrl. n=6.
Discussion
Although most literatures supported that anthocyanins may protect the heart against ischemic injury by mechanisms related to their antioxidant activities, the detailed molecular mechanism is still unknown. One of the novel findings of our study was that anthocyanins play a role in regulating of cardiac ECM remodeling, which was demonstrated both in vivo and vitro models.
Anthocyanins, either single active ingredient (i.e., cyaniding 3-O-beta-glucopyranoside) [15], or fruit/plant extracts [8], have been implicated to reduced collagen synthesis in various tissues. On the other hand, anthocyanins were also demonstrated to play a role in reducing the activities of MMP-2 [16] or -9 [17] in cancer cells metastasis. However, we have no idea whether anthocyanins elicit a regulative effect on cardiac ECM remodeling, induced by CAL.
It has been reported that Type III procollagen mRNA levels were already increased at day 2 after myocardial infarction (MI), whereas type I procollagen mRNA followed this response at day 4 after MI. This increase was sustained for at least 21 days in the infarcted left ventricle for type III procollagen mRNA, whereas type 1 procollagen mRNA levels were keeping elevating till 90 days after MI [18]. In the present study, we found that the protein expression level of collagen III was dramatically enhanced on Day 7 after CAL, which could be attenuated by anthocyanins treatment. In primary cultured CFs, anthocyanins treatment significantly reduced MMP-2 activity, which might be mediated by MT1-MP.
p38 is one sub-family of mitogen-activated protein kinases (MAPKs) and activated by inflammatory cytokines, osmotic stress and apoptotic signals [19]. p38 has been shown to be involved in the regulation of MMPs, but its effect on MMPs is still contradicting. For example, IL-1a upregulated the expression of both MMP-9 and -13 in liver myofibroblast cell line, and the induction of MMP-13 but not MMP-9 was in p38 dependent manner [20]. Similarly, TNFα and TGFβ induced expression of MMP-13 and MMP-1 was abolished by p38 inhibitor, SB203580 [21]. On the other hand, another study showed that p38 alpha could inhibit ERK1/2 with a subsequent suppression of MMP-1 [22]. Activation of p38 beta2 could inhibit platelet-derived growth factor-BB-mediated MMP activation [23]. Tumor suppressor gene product, retinoblastoma, negatively regulates p38 activation, leading to decreased MMP-1 secretion [24]. The above results might imply that p38 regulates MMP-1 gene expression bidirectionally depending on the stimuli. Our results showed that anthocyanins significantly reduced phosphorylation level of p38, thereby inhibiting MMP-2 activity.
The relationship between Akt pathway and collagen expression is quite consistent. Hubchak et al. found that TGF-β stimulated collagen-I expression in mesangial cell through a PI3K/Akt mechanism [25]. Exposure of lung fibroblasts to Bleomycin (a known inducer of fibrosis) resulted in a rapid activation of PI3K/Akt and a parallel increase in collagen production [26]. In adult cardiac fibroblasts, PI3K/Akt and Smad pathways were involved in TGF-β induced lysyl oxidase (a key enzyme to form mature collagens) gene, protein and activity up-regulation [27]. These data are in line with ours, which showing that anthocyanins significantly reduced the levels of p-p38 and collagens.
The effect of Akt pathway on MMPs expression or activity remains nearly unknown. Blockade of PI3Kγ resulted in increased levels of cAMP and expression of MMPs, including MMP2 [28]. Inhibition of Akt by various strategies leaded to down-regulation of collagen-I production but up-regulation of MMP1 in human dermal fibroblasts [29]. In this regard, it might be possible that anthocyanins induced dephosphorylation of Akt will result in activation of MMP-2 and -9. Additional studies are required further to address these potential mechanisms.
In summary, in myocardial infarction model induced by permanent coronary artery ligation, we demonstrated for the first time that anthocyanins attenuated ECM remodeling by reducing collagens expression and secretion, and MMP activity, which might be mediated by Akt/p38 MAPK pathways. Supplementation with anthocyanins has a therapeutic effect on ischemic heart via modulating of ECM remodeling.
Disclosure of conflict of interest
None.
References
- 1.Brown L. Cardiac extracellular matrix: a dynamic entity. Am J Physiol Heart Circ Physiol. 2005;289:H973–4. doi: 10.1152/ajpheart.00443.2005. [DOI] [PubMed] [Google Scholar]
- 2.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–8. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 3.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–39. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 4.Zafra-Stone S, Yasmin T, Bagchi M, Chatterjee A, Vinson JA, Bagchi D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol Nutr Food Res. 2007;51:675–83. doi: 10.1002/mnfr.200700002. [DOI] [PubMed] [Google Scholar]
- 5.Dalgard C, Nielsen F, Morrow JD, Enghusen-Poulsen H, Jonung T, Horder M, de Maat MP. Supplementation with orange and blackcurrant juice, but not vitamin E, improves inflammatory markers in patients with peripheral arterial disease. Br J Nutr. 2009;101:263–9. doi: 10.1017/S0007114508995660. [DOI] [PubMed] [Google Scholar]
- 6.Monagas M, Khan N, Andres-Lacueva C, Casas R, Urpi-Sarda M, Llorach R, Lamuela-Raventos RM, Estruch R. Effect of cocoa powder on the modulation of inflammatory biomarkers in patients at high risk of cardiovascular disease. Am J Clin Nutr. 2009;90:1144–50. doi: 10.3945/ajcn.2009.27716. [DOI] [PubMed] [Google Scholar]
- 7.Skemiene K, Rakauskaite G, Trumbeckaite S, Liobikas J, Brown GC, Borutaite V. Anthocyanins block ischemia-induced apoptosis in the perfused heart and support mitochondrial respiration potentially by reducing cytosolic cytochrome c. Int J Biochem Cell Biol. 2013;45:23–9. doi: 10.1016/j.biocel.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 8.Palfi A, Bartha E, Copf L, Mark L, Gallyas F Jr, Veres B, Kalman E, Pajor L, Toth K, Ohmacht R, Sumegi B. Alcohol-free red wine inhibits isoproterenol-induced cardiac remodeling in rats by the regulation of Akt1 and protein kinase C alpha/beta II. J Nutr Biochem. 2009;20:418–25. doi: 10.1016/j.jnutbio.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Tan D, Shi L, Liu X, Zhang Y, Tong C, Song D, Hou M. Blueberry Anthocyanins-Enriched Extracts Attenuate Cyclophosphamide-Induced Cardiac Injury. PLoS One. 2015;10:e0127813. doi: 10.1371/journal.pone.0127813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eguchi M, Xu G, Li RK, Sweeney G. Diabetes influences cardiac extracellular matrix remodelling after myocardial infarction and subsequent development of cardiac dysfunction. J Cell Mol Med. 2012;16:2925–34. doi: 10.1111/j.1582-4934.2012.01613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins-Hooper H, Sartori R, Macharia R, Visanuvimol K, Foster K, Matsakas A, Flasskamp H, Ray S, Dash PR, Sandri M, Patel K. Propeptide-mediated inhibition of myostatin increases muscle mass through inhibiting proteolytic pathways in aged mice. J Gerontol A Biol Sci Med Sci. 2014;69:1049–59. doi: 10.1093/gerona/glt170. [DOI] [PubMed] [Google Scholar]
- 12.Palanivel R, Fang X, Park M, Eguchi M, Pallan S, De Girolamo S, Liu Y, Wang Y, Xu A, Sweeney G. Globular and full-length forms of adiponectin mediate specific changes in glucose and fatty acid uptake and metabolism in cardiomyocytes. Cardiovasc Res. 2007;75:148–57. doi: 10.1016/j.cardiores.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Flack EC, Lindsey ML, Squires CE, Kaplan BS, Stroud RE, Clark LL, Escobar PG, Yarbrough WM, Spinale FG. Alterations in cultured myocardial fibroblast function following the development of left ventricular failure. J Mol Cell Cardiol. 2006;40:474–83. doi: 10.1016/j.yjmcc.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 14.Dadson K, Chasiotis H, Wannaiampikul S, Tungtrongchitr R, Xu A, Sweeney G. Adiponectin mediated APPL1-AMPK signaling induces cell migration, MMP activation, and collagen remodeling in cardiac fibroblasts. J Cell Biochem. 2014;115:785–93. doi: 10.1002/jcb.24722. [DOI] [PubMed] [Google Scholar]
- 15.Bendia E, Benedetti A, Baroni GS, Candelaresi C, Macarri G, Trozzi L, Di Sario A. Effect of cyanidin 3-O-beta-glucopyranoside on hepatic stellate cell proliferation and collagen synthesis induced by oxidative stress. Dig Liver Dis. 2005;37:342–8. doi: 10.1016/j.dld.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Fan MJ, Wang IC, Hsiao YT, Lin HY, Tang NY, Hung TC, Quan C, Lien JC, Chung JG. Anthocyanins from black rice (Oryza sativa L. ) demonstrate antimetastatic properties by reducing MMPs and NF-kappaB expressions in human oral cancer CAL 27 cells. Nutr Cancer. 2015;67:327–38. doi: 10.1080/01635581.2015.990576. [DOI] [PubMed] [Google Scholar]
- 17.Lee SJ, Hong S, Yoo SH, Kim GW. Cyanidin-3-Osambubioside from Acanthopanax sessiliflorus fruit inhibits metastasis by downregulating MMP-9 in breast cancer cells MDA-MB-231. Planta Med. 2013;79:1636–40. doi: 10.1055/s-0033-1350954. [DOI] [PubMed] [Google Scholar]
- 18.Cleutjens JP, Verluyten MJ, Smiths JF, Daemen MJ. Collagen remodeling after myocardial infarction in the rat heart. Am J Pathol. 1995;147:325–38. [PMC free article] [PubMed] [Google Scholar]
- 19.Garrington TP, Johnson GL. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol. 1999;11:211–8. doi: 10.1016/s0955-0674(99)80028-3. [DOI] [PubMed] [Google Scholar]
- 20.Lee HS, Miau LH, Chen CH, Chiou LL, Huang GT, Yang PM, Sheu JC. Differential role of p38 in IL-1alpha induction of MMP-9 and MMP-13 in an established liver myofibroblast cell line. J Biomed Sci. 2003;10:757–65. doi: 10.1159/000073963. [DOI] [PubMed] [Google Scholar]
- 21.Johansson N, Ala-aho R, Uitto V, Grenman R, Fusenig NE, Lopez-Otin C, Kahari VM. Expression of collagenase-3 (MMP-13) and collagenase-1 (MMP-1) by transformed keratinocytes is dependent on the activity of p38 mitogenactivated protein kinase. J Cell Sci. 2000;113 Pt 2:227–35. doi: 10.1242/jcs.113.2.227. [DOI] [PubMed] [Google Scholar]
- 22.Westermarck J, Li SP, Kallunki T, Han J, Kahari VM. p38 mitogen-activated protein kinase-dependent activation of protein phosphatases 1 and 2A inhibits MEK1 and MEK2 activity and collagenase 1 (MMP-1) gene expression. Mol Cell Biol. 2001;21:2373–83. doi: 10.1128/MCB.21.7.2373-2383.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Endo H, Utani A, Shinkai H. Activation of p38 MAPK suppresses matrix metalloproteinase-1 gene expression induced by platelet-derived growth factor. Arch Dermatol Res. 2003;294:552–8. doi: 10.1007/s00403-002-0364-5. [DOI] [PubMed] [Google Scholar]
- 24.Bradley K, Scatizzi JC, Fiore S, Shamiyeh E, Koch AE, Firestein GS, Gorges LL, Kuntsman K, Pope RM, Moore TL, Han J, Perlman H. Retinoblastoma suppression of matrix metalloproteinase 1, but not interleukin-6, through a p38-dependent pathway in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2004;50:78–87. doi: 10.1002/art.11482. [DOI] [PubMed] [Google Scholar]
- 25.Hubchak SC, Sparks EE, Hayashida T, Schnaper HW. Rac1 promotes TGF-beta-stimulated mesangial cell type I collagen expression through a PI3K/Akt-dependent mechanism. Am J Physiol Renal Physiol. 2009;297:F1316–23. doi: 10.1152/ajprenal.00345.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu Y, Azad N, Wang L, Iyer AK, Castranova V, Jiang BH, Rojanasakul Y. Phosphatidylinositol-3-kinase/akt regulates bleomycin-induced fibroblast proliferation and collagen production. Am J Respir Cell Mol Biol. 2010;42:432–41. doi: 10.1165/rcmb.2009-0002OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voloshenyuk TG, Landesman ES, Khoutorova E, Hart AD, Gardner JD. Induction of cardiac fibroblast lysyl oxidase by TGF-beta1 requires PI3K/Akt, Smad3, and MAPK signaling. Cytokine. 2011;55:90–7. doi: 10.1016/j.cyto.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 28.Guo D, Kassiri Z, Basu R, Chow FL, Kandalam V, Damilano F, Liang W, Izumo S, Hirsch E, Penninger JM, Backx PH, Oudit GY. Loss of PI3Kgamma enhances cAMP-dependent MMP remodeling of the myocardial N-cadherin adhesion complexes and extracellular matrix in response to early biomechanical stress. Circ Res. 2010;107:1275–89. doi: 10.1161/CIRCRESAHA.110.229054. [DOI] [PubMed] [Google Scholar]
- 29.Bujor AM, Pannu J, Bu S, Smith EA, Muise-Helmericks RC, Trojanowska M. Akt blockade downregulates collagen and upregulates MMP1 in human dermal fibroblasts. J Invest Dermatol. 2008;128:1906–14. doi: 10.1038/jid.2008.39. [DOI] [PubMed] [Google Scholar]


