Abstract
Acute myeloid leukemia (AML) represents a heterogeneous group of hematological neoplasms with marked heterogeneity in response to both standard therapy and survival. Hispidulin, a flavonoid compound that is anactive ingredient in the traditional Chinese medicinal herb Salvia plebeia R. Br, has recently been reported to have anantitumor effect against solid tumors in vitro and in vivo. The aim of the present study was to investigate the effects of hispidulin on the human leukemia cell line in vitro and the underlying mechanisms of its actions on these cells. Our results showed that hispidulin inhibits AML cell proliferation in a dose- and time-dependent manner, and induces cell apoptosis throughan intrinsic mitochondrial pathway. Our results also revealed that hispidulin treatment significantly inhibits extracellular matrix metalloproteinase inducer (EMMPRIN) expression in both tested AML cell lines in a dose-dependent manner, and that the overexpression of EMMPRIN protein markedly attenuates hispidulin-induced cell apoptosis. Furthermore, our results strongly indicated that the modulating effect of hispidulin on EMMPRIN is correlated with its inhibitory effect on both the Akt and STAT3 signaling pathways.
Keywords: Hispidulin, apoptosis, EMMPRIN, Akt, STAT3
Introduction
Acute myeloid leukemia (AML), a hematologic malignancy characterized by uncontrolled proliferation and accumulation of myeloblasts in the bone marrow (BM), blood, and other organs, represents a heterogeneous group of hematological neoplasms with marked heterogeneity in response to both standard therapy and survival [1]. The clinical outcomes of AML are very unpredictable, from survival of a few days to the complete cure, depending on the age, biology, cytogenetic features, deregulated genes, and classification of the disease [2]. Standard treatment options for AML are chemotherapy and hematopoietic stem cell transplantation, which help ensure long-term, disease-free survival for many patients; however, the majority of AML patients will ultimately be diagnosed with relapsed or refractory disease despite cytotoxic chemotherapy [3,4]. For AML patients who relapse or have a resistant disease, therapeutic options are limited. The long-term overall survival rates for AML patients <60 and ≥60 years old are 30-40% and <10%, respectively, [5] which makes AML especially challenging; therefore, developing new therapeutic agents that are less toxic and more specific to AML is imperative.
Extracellular matrix metalloproteinase inducer (EMMPRIN), encoded by a gene localized to 19p13.3, is a cell-surface glycoprotein belonging to the immunoglobulin superfamily. EMMPRIN has been implicated in many biologicalfunctions, including embryo implantation, spermatogenesis [6], retinal development [7], inflammation [8], wound healing [9], and tumor progression [10]. A number of studies have shown that elevated EMMPRIN expression is associated with clinically aggressive behavior and poor prognoses in a variety of solid tumors [11-13], which might be associated with the inducing effect on the synthesis of matrix metalloproteinase (MMP) in the surrounding stromal cells [14]. Within the context of hematological malignancy, Fu et al. [15] observed elevated expressions of EMMPRIN in the BM leukemia cells of patients with newly diagnosed AML. Our previous study also showed that shRNA-mediated EMMPRIN silencing inhibits AML U937 cell proliferation and increases chemosensitivity to Adriamycin, [16] which indicates that EMMPRIN might be a promising therapeutic target in AML.
Hispidulin (4’,5,7-trihydroxy-6-methoxyflavone), a naturally occurring flavonoid, is one of the major active ingredients of the traditional Chinese medicinal herb Salvia plebeia R. Br. [17,18]. In vivo and in vitro studies have provided evidence of the antifungal, anti-inflammatory, antioxidant, antithrombotic, antiepileptic, neuroprotective, and antiosteoporotic activities of this compound [19-22]. Recently, the antiproliferative effect of hispidulin has been reported in human malignancies, including pancreatic, gastric, ovarian, and glioblastoma cancer in vitro [23-26]. Our previous results also demonstrated that hispidulin induces apoptosis in hepatocellular carcinomacells [27] and we reported that hispidulin inhibits the proliferation and enhances the chemosensitivity of gallbladder cancer cells by targeting HIF-1α [28]; however, whether the antitumor effect of hispidulin in a solid tumor can be replicated in hematological malignancies has never been studied. Hence, the present study was conducted to investigate the effects of hispidulin on the human leukemia cell line in vitro and the underlying mechanisms for its action on these cells.
Materials and methods
Cell lines and cultures
Human AML U937 and HL-60 cell lines were obtained from the Shanghai Institutes for Biological Science (Shanghai, China). Cells were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium (Gibco, MD, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, MD, USA) and 1.0% L-glutamine in a 5.0% CO2-humidified incubator.
Primary culture of human acute myeloid leukemia cells
Leukemic blasts were obtained from eight AML patients (ranging from 35 to 65 years old) undergoing routine diagnostic aspirations. All patients provided written informed consent in accordance with the Declaration of Helsinki, and the study was approved by the Institutional Review Board of the University of Qingdao, College of Medicine, Shandong, China. Samples were separated by centrifugation over Ficoll/Hypaque (specific gravity: 1.077e1.081; Sigma-Aldrich Corporation, Shanghai, China) at 400 g at room temperature. The interface layer containing the primarily blasts was removed using a sterile Pasteur pipette and resuspended in medium containing 10% FBS. The purity of the blast population was confirmed to be 80% by morphologic evaluation of Wright-stained cytospun cell preparations. Cells exhibiting >95% viability by trypan blue exclusion were cultured in the presence of 50 ng/mL rhGM-CSF, 100 ng/mL rhIL-3, and 10 ng/mL rhG-CSF (Sigma-Aldrich Corporation, Shanghai, China).
Primary culture of human peripheral blood mononuclear and CD34+ mononuclear cells
Peripheral blood mononuclear cells (PBMCs) of three healthy donors were isolated by centrifugation over lymphocyte separation medium (Sigma-Aldrich Corporation, Shanghai, China). The study protocol was reviewed and approved by the Institutional Review Board of the University of Qingdao, College of Medicine. Written informed consent was obtained from each participant. After three washes in phosphate-buffered saline (PBS), PBMCs were counted and cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% FBS, 2.0 mg phytohemagglutinin per mL, 10 ng phorbol 12-myristate-13-acetate per mL, nonessential amino acids, 5.0 mM b-mercaptoethanol, 10 mM 2-hydroxyethyl-1-piperazineethanesulfonic acid (HEPES), 2.0 mM glutamine, and 1.0 mM sodium pyruvate (all purchased from Sigma-Aldrich Corporation, Shanghai, China). BM aspirates were obtained from three volunteer donors using protocols approved by the Institutional Review Board of the University of Qingdao, College of Medicine. Following informed consent, CD34+ cells were extracted using immunomagnetic beads conjugated with an anti-CD34 antibody (ThermoFisher, Norway, 11301D) following the manufacturer’s instructions.
In vitro proliferation assay
In vitro proliferation was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Sigma-Aldrich Corporation, St. Louis, MO, USA). Briefly, cells were plated at a density of 5.0 × 103 cells/well in 96-well culture plates. After treatment, 20 µL MTT solution (5.0 mg/mL in PBS) were added to each well and incubated for 2.0 h. MTT formazan was dissolved in 150 µL dimethyl sulfoxide (DMSO) and the absorbance was measured at 595 nm with an ELISA reader (Tecan Group Ltd, Männedorf, Switzerland). The value of absorbance was normalized to that of the untreated control group.
Colony formation assay
HL-60 and U937 cells (3.0 × 104) suspended in 1.6 mL RPMI agarose medium (10% FBS and 0.33% agarose) containing the shogaol analogs or DMSO were plated in each well of a 6-well plate over a bottom layer of solidified RPMI agarose medium (10% FBS and 0.5% agarose). Cultures were maintained for 2.0 weeks without fresh medium feeding at 37°C in a humidified atmosphere of 95% air and 5.0% CO2, after which cell colonies >0.1 mm were enumerated and photographed using the Nikon Eclipse TE2000-U microscope fitted with Nikon Digital Camera DXM1200 at a 109 objective.
Trypan blue staining assay
The in vitro viability of both HL-60 and U937 cells was determined by trypan blue dye exclusion. The reduction in viable cell numbers was examined immediately after treatment with hispidulin for 48 h at the indicated concentrations. Cells without trypan blue stain were considered viable and the percentage of viable cells was calculated by the number of cells without the trypan blue stain divided by the number of total cells.
Cell-cycle analysis
Cell-cycle analysis was conducted 48 h after transfection using the Cell Cycle and Apoptosis Analysis Kit (Beyotime, Shanghai, China) following the manufacturer’s instructions. Briefly, cells were collected and fixed with 70% cold ethanol at 4.0°C overnight. DNA was stained with 0.05 mg/mL propidium iodide and 2.0 mg/mL RNase for 30 min at room temperature. Cells were then subjected to a FACScan cytometry (Beckman Coulter Inc., Miami, FL, USA) for cell-cycle analysis. The percentage of cells in G0/G1, S, and G2/M phases of the cell cycle was calculated using Cell Lab Quanta SC software.
Cell apoptosis analysis
The effect of hispidulin treatment on cell apoptosis was evaluated by flow cytometry using an FITC Annexin V apoptosis kit (BD Pharmingen, Franklin Lakes, NJ, USA) according to manufacturer’s instructions. In brief, cells were washed with ice-cold phosphate buffered saline (PBS) and resuspended in binding buffer (10 mM HEPES, pH 7.4, 140 mM sodium chloride, and 2.5 mM calcium chloride) at a concentration of 1.0 × 106 cells/mL. Cells were stained with annexin V-FITC and propidium iodide (PI) for 15 min in the dark before being analyzed by flow cytometry. Annexin V-FITC positive cells were considered to be undergoing apoptosis and those negative for FITC were considered to be alive.
Histone/DNA ELISA assay of acute myeloid leukemia cell apoptosis
After the cells were treated with hispidulin at the indicated concentrations, the cell death detection ELISA plus Kit (Sigma-Aldrich Corporation, Shanghai, China, 11684795910) was used for quantitatively analyzing apoptosis in the AML cells following the manufacturer’s instructions. Briefly, AML cells were lysed and the cell lysates were overlaid and incubated in microplate modules coated with antihistone antibody. Samples were then incubated with anti-DNA peroxidase followed by color development with 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid substrate. The absorbance of the samples was determined using a microplate reader at 405 nm, which was used as a quantitative measurement of cell apoptosis.
Western blotting analysis
Following treatment with hispidulin for 24 h, the cells were harvested from flasks and lysed with ice-cold lysis buffer (50 mM 2-amino-2-hydroxymethyl-propane-1,3-diol-hydrochloric acid [Tris-HCl], pH 7.4, 150 mM sodium chloride, 1.0 mM magnesium chloride, 100 μg/mL phenylmethanesulfonylfluoride, and 1.0 % Triton X-100) for 30 min on ice. Cell lysate was then collected after centrifugation at 4.0°C and 12000 rpm for 5.0 min. Equal amounts (50 μg) of lysate proteins were separated on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis gels after denaturing by boiling and transferred onto polyvinylidene difluoridemembranes (Millipore, MA, USA). Proteins were probed with specific antibodies following standard protocols. After washing, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (Beyotime Institute of Biotechnology, Shanghai, China, Product No. A0208). An enhanced chemiluminescence kit (Beyotime Institute of Biotechnology, Shanghai, China, Product No. P0018) was used for detection of the signals following the manufacturer’s instructions. The primary antibodies used were mouse monoclonal antibody to EMMPRIN (Santa Cruz, USA; 1:1000 dilution, sc-374101), rabbit polyclonal antibody to cyclin D1 (Santa Cruz, Santa Cruz, CA, USA; 1:1000 dilution, sc-753), rabbit polyclonal antibody to caspase-3 (Bioss, Beijing, China; 1:1000, bs-0081R), rabbit polyclonal antibody to caspase-8 (Bioss, Beijing, China; 1:1000, bs-0052R), rabbit polyclonal antibody to caspase-9 (Bioss, Beijing, China, 1:1000, bs-0050R), rabbit monoclonal antibody to phospho-Stat3 (1:1000, Cell Signaling, ON, Canada; C67E7), rabbit monoclonal antibody to phospho-Akt (1:1000, Cell Signaling, ON, Canada, C31E5E), and a rabbit polyclonal antibody to β-actin (Santa Cruz, Santa Cruz, CA, USA; 1:1000 dilution, sc-7210) used as a gel loading control (Santa Cruz, Santa Cruz, CA, USA; 1:1,000 dilution).
Measurement of mitochondrial membrane potential
Hl-60 and U937 cells were seeded in 96-well plates at a density of 1.0 × 105 cells/mL and treated with hispidulin at the indicated concentrations for 48 h. The mitochondrial membrane was monitored using the fluorescent dye rhodamine 123, which preferentially partitions active mitochondria based on the highly negative mitochondrial membrane potential, as previously described [37]. Depolarization of mitochondrial membrane potential results in the loss of rhodamine 123 from mitochondria and intracellular fluorescence is decreased. A final concentration of 10 μM rhodamine 123 were added to the harvested cells and analyzed using a flow cytometer with Summit ver. 4.3 (CyAn ADP, Beckman Coulter, Brea, CA, USA).
Quantitative reverse transcription polymerase chain reaction analysis
Total RNA was extracted from cells using the RNA simple Total RNA Kit (DP419) (TIANGEN Biotech Co., Beijing, China). The primer for EMMPRIN was synthesized based on the published sequence [29] as follows: forward primer: 5’-CAGCGGTTGGAGGTTGT-3’ and reverse primer 5’-TTTGAGGGTGGAGGTGG-3’. First-strand cDNA was synthesized from 1.0 μg RNA using areverse transcription (RT) system (TaKaRa Biotech Company, Dalian, China). The resulting 2.0 μg cDNA were subjected to polymerase chain reaction (PCR) amplification. For the PCR reaction, a master mix was prepared that comprised SYBR GREEN mastermix (Solarbio Co., Beijing, China) and a forward primer, reverse primer, and 10 ng template cDNA. The PCR conditions were 5.0 min at 95°C followed by 40 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 30 s. The products were then kept at 4.0°C.
Overexpression of EMMPRIN
To investigate the effects of EMMPRIN on the hispidulin-induced antitumor effect in leukemia cells, EMMPRIN was overexpressed in model AML cell lines as previously described. Briefly, the EMMPRIN-coding DNA sequences were obtained by RT-PCR and cloned into the pEGFP-N1 vector (Beyotime Institute of Biotechnology, Shanghai, China, D2602). The resulting plasmid was named “pEGFP-N1-EMMPRIN”. AML cells were transfected with pEGFP-N1-EMMPRIN; pEGFP-N1 vector was used as the control. Two days after transfection, G418 was added to the cells for selection of the clones stably transfected with pEGFP-N1-EMMPRIN. The resulting cells that stably expressed EMMPRIN were maintained in a medium containing G418. The transfection efficiency was approximately 75%. The overexpression of EMMPRIN was confirmed by RT-PCR and western blot.
Statistical analyses
All of the experiments were performed in triplicate and were repeated three times. The data are presented as the mean ± SD of the values obtained. Results were evaluated by one-way analysis of variance using SPSS13.0 (SPSS Inc., Chicago, Il, USA). P<0.05 was considered as statistically significant.
Results
Hispidulin inhibits the proliferation of leukemia cell lines in a dose-dependent manner
To investigate the anti-proliferative effect of hispidulin on leukemia cells, the leukemia cell lines HL-60 and U937 were treated with hispidulin at the indicated concentrations for 12, 24, and 48 h, and cell proliferation was determined by MTT assay. As shown in Figure 1A, hispidulin treatment inhibited cell proliferation in both tested cell lines in a dose-dependent manner. Following treatment with hispidulin at 12.5, 25.0, and 50.0 µM for 12 h, HL-60 cell proliferation was reduced to 82.1±9.2%, 61.5±7.8%, and 45.3±4.2%, respectively, compared that of cells in the control group. In comparison, U937 cells were less sensitive to hispidulin than HL-60 cells when treated with hispidulin at the same concentrations for 24 h. Cell proliferation decreased further with hispidulin treatment for 48 h but U937 cells were still less sensitive to hispidulin treatment than HL-60 cells. To demonstrate the inhibitory effect of hispidulin on cell growth, a colony-formation assay was also performed. As shown in Figure 1B, colony number and size were reduced in U937 and HL-60 cells treated with hispidulin compared with thosein the control. Trypan blue assay was also performed to determine the inhibitory effect of hispidulin on cell survival. As shown in Figure 1C, treatment with hispidulin for 48 h resulted in a dose-dependent loss of cell viability in both tested cell lines. The effect of hispidulin on primary AML cells was also tested. As shown in Figure 1D, hispidulin at a concentration of 25 and 50 µM significantly suppressed the survival of primary AML cells. Moreover, our results also showed that hispidulin at a concentration as high as 50 µM did not cause a significant change in the survival of peripheral blood mononuclear cells (PMBCs) and normal CD34+ hematopoieticcells (Figure 1D). Taken together, these results demonstrate that hispidulin has selective antileukemiapropertiesthat do not harm healthy cells.
Figure 1.
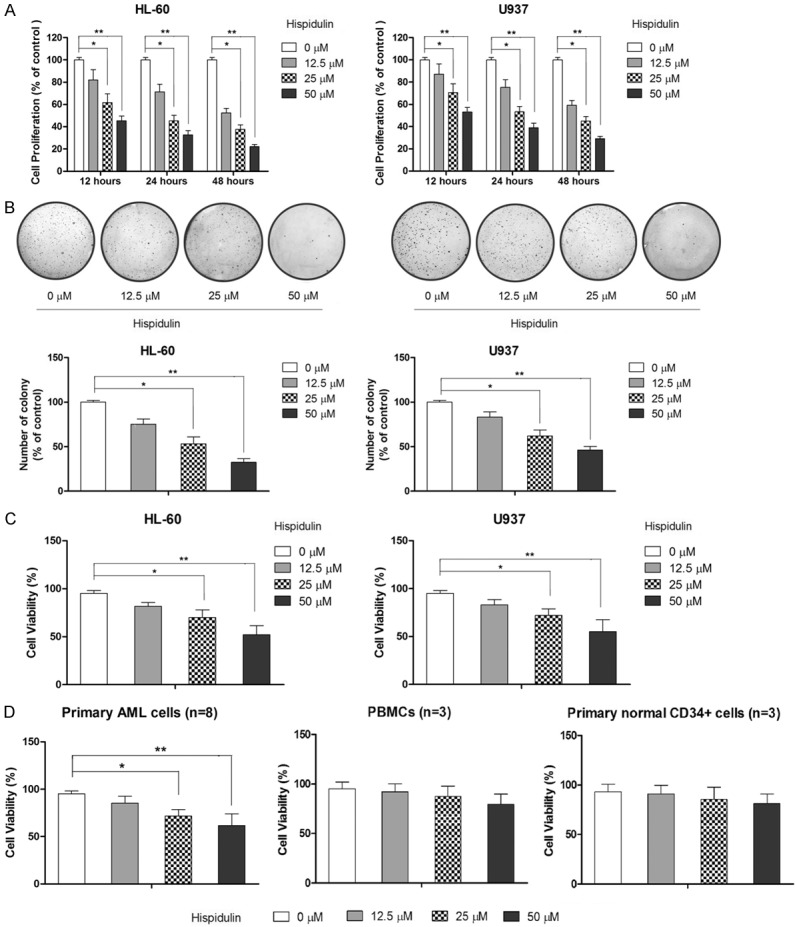
Hispidulin exerts anti-proliferative effects and inhibits survival in HL-60 and U937 cells in a dose- and time-dependent manner. A. HL-60 and U937 cells were incubated with hispidulin at the indicated concentrations for the indicated times before 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assays were performed to determine the effect of hispidulin on cell proliferation. B. Colony-formation assays were performed to determine the effect of hispidulin on cell growth. C. Trypan blue assayswere performed to determine the inhibitory effect of hispidulin on cell survival. D. The effect of hispidulin on cell viability of primary acute myeloid leukemia (AML) cells, peripheral blood mononuclear cells (PMBCs), and CD34+ cells was determined by trypan blue assay. *P<0.05, **P<0.01.
Hispidulin induces cell-cycle arrest
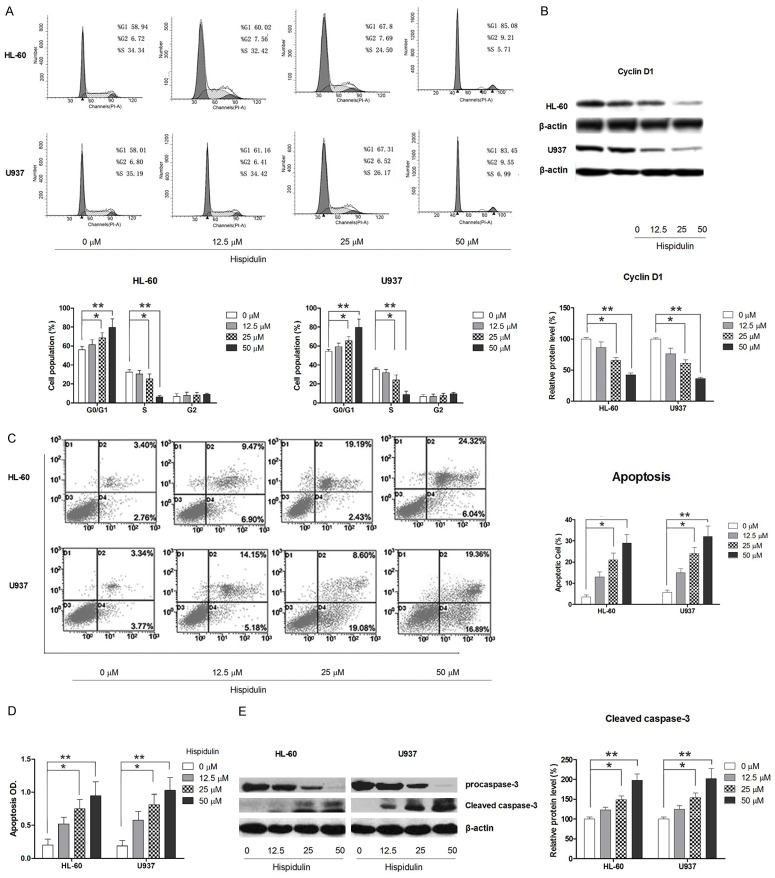
To further elucidate the mechanism by which cell growth is inhibited, the cell-cycle distribution was examined following hispidulin treatment. Following flow cytometry analysis, our results showed that hispidulin treatment results in the accumulation of cells inthe G0/G1 phase, as shown in Figure 2A. In addition, the increase in cells in the G0/G1 phase was accompanied with a concomitant decrease of cells in the S-phase in both cell lines treated with hispidulin without a significant change in the cell population in the G2 phase. Because cyclin D1 plays an important role in regulating G1 progression and G1-S transition in vertebrate cells, the level of cyclin D1 was examined to investigate whether hispidulin treatment could induce changes in the expression of cell cycle-related protein. As shown in Figure 2B, cyclin D1 levels in both U937 and HL-60 cells were remarkably downregulated by hispidulin treatment. Collectively, these results suggested that hispidulin treatment results in a decrease of cells in the G1 phase and an increase in the population of cells in the proliferative S phase by regulating cyclin D1 expression in AML cells.
Figure 2.
Hispidulin treatment leads to cell-cycle blockage and apoptosis in HL-60 and U937 cells. HL-60 and U937 cells were incubated with hispidulin at the indicated concentrations for 48 h. A. Cell cycle distribution was assessed with flow cytometry analysis. B. Effects of hispidulin on cyclin D1 as determined by western blot. C. Cell apoptosis as determined by flow cytometry. D. Cell apoptosis as determined by histone/DNA ELISA assay. E. Activation of caspase-3 as determined by western blot. The western blot and the images of flow cytometric assay represent three independently conducted experiments. *P<0.05, **P<0.01.
Hispidulin induces mitochondrial apoptosis in leukemia cells
Apoptotic cell death was determined by both flow cytometry analysis and histone-DNA ELISA assay. As shown in Figure 2C and 2D, results from both assays showed that treatment with hispidulin for 48 h induces dose-dependent apoptosis in both tested cell lines compared to that in the control group. (Early apoptosis of HL-60 might be accurately presented by flow cytometric assay because they do not readily expose PS at the outer cell membrane during early apoptosis). Apoptotic markers including caspase-3 cleavage were also observed in hispidulin-treated cells, which further suggested that hispidulin-induced cell death is mainly a result of apoptosis (Figure 2E). Apoptosis is mainly mediated by the death receptor-triggered extrinsic pathway and the mitochondrial-initiated intrinsic pathway, prompted by the activation of caspase-8 and caspase-9, respectively [30]. To determine the mechanism by which hispidulin induces apoptosis in AML cells, the activation status of both caspase-8 and caspase-9 was examined. As shown in Figure 3A and 3B, although no marked activation of capase-8 was triggered by hispidulin at any dose, hispidulin did cause dose-dependent activation of caspase-9 in both HL-60 and U937 cells, suggesting that hispidulin induces apoptosis through an intrinsic mitochondrial pathway. To further confirm the role of mitochondria in hispidulin-induced apoptosis, the change in the mitochondrial membrane potential and the release of cytochrome c from the mitochondria to the cytosol were examined. The results demonstrated that hispidulin treatment causes a marked increase in the cells with a loss in mitochondrial membrane potential relative to the corresponding control, as shown in Figure 3C, and western blot showed an increase in cytosol cytochrome c (Figure 3D), both supporting the role of mitochondria in hispidulin-induced apoptosis.
Figure 3.
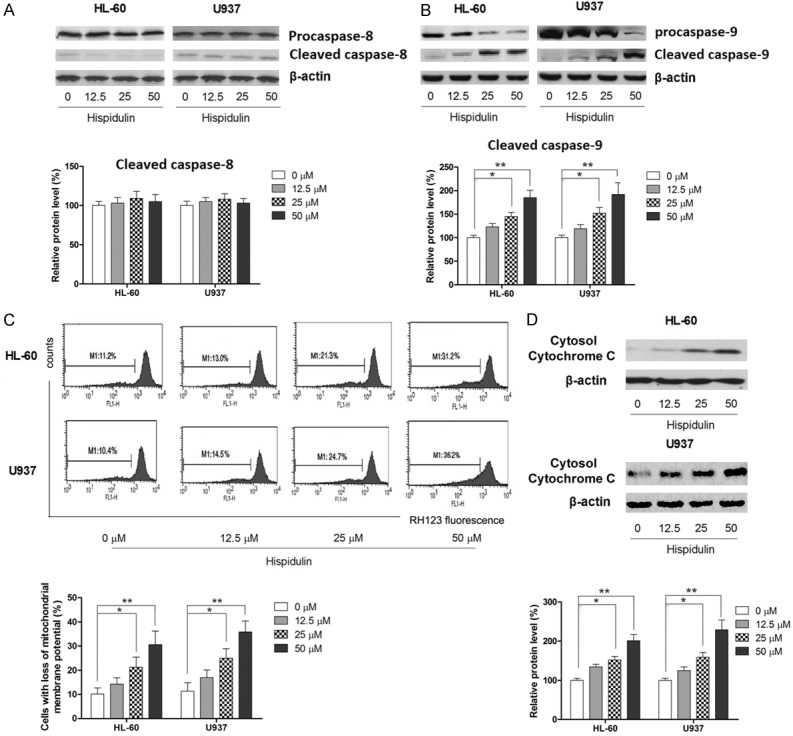
Hispidulin induces mitochondrial apoptosis in HL-60 and U937 cells. HL-60 and U937 cells were incubated with hispidulin at the indicated concentrations for 48 h. Effects of hispidulin on activation of caspase-8 and -9, respectively (A and B) as determined by western blot. (C) Mitochondrial membrane potential as determined by flow cytometry analysis. (D) Effects of hispidulin on cytochrome c as determined by western blot. Western blot and the images of flow cytometric assay represent three independently conducted experiments. *P<0.05, **P<0.01.
Overexpression of EMMRPIN attenuates hispidulin-induced cell apoptosis
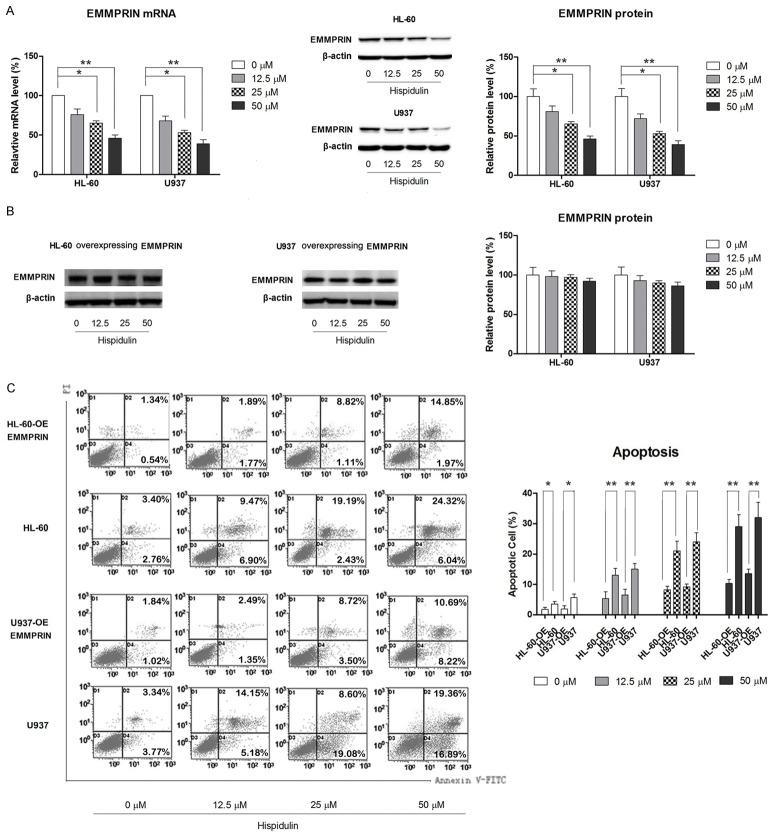
Previous research has suggested that EMMPRIN might be a promising therapeutic target for AML [16]; therefore, the effect of hispidulin treatment on the expression of EMMPRIN was investigated. As shown in Figure 4A, the expression of EMMPRIN is remarkably reduced in a dose-dependent manner at both the mRNA and protein levels.
Figure 4.
Hispidulin induces apoptosis in HL-60 AND U937 cells by targeting EMMPRIN. HL-60 AND U937 cells were incubated with hispidulin at the indicated concentrations (or 50 μM if not marked) for 48 h. A. mRNA expression of EMMPRIN determined by quantitative reverse transcription-polymerase chain reaction. B. Effects of hispidulin on EMMPRIN levels as determined by western blot. C. Flow cytometric analysis of theapoptosis-inducing effects of hispidulin in EMMPRIN overexpressing HL-60 and U937 cells. Western blot and the images of flow cytometric assay represent three independently conducted experiments. *P<0.05, **P<0.01.
To explore the role of EMMPRIN in hispidulin-induced apoptosis, both HL-60 and U937 cells were transfected with an EMMPRIN-overexpressing plasmid and the effect of hispidulin on EMMPRIN-overexpressing AML cells was examined. As shown in Figure 4B, both HL-60 and U937 cell lines transfected with EMMPRIN-overexpressing plasmid were significantly more resistant to hispidulin-induced apoptosis, suggesting an involvement of EMMPRIN modulation in hispidulin-triggered mitochondrial apoptosis.
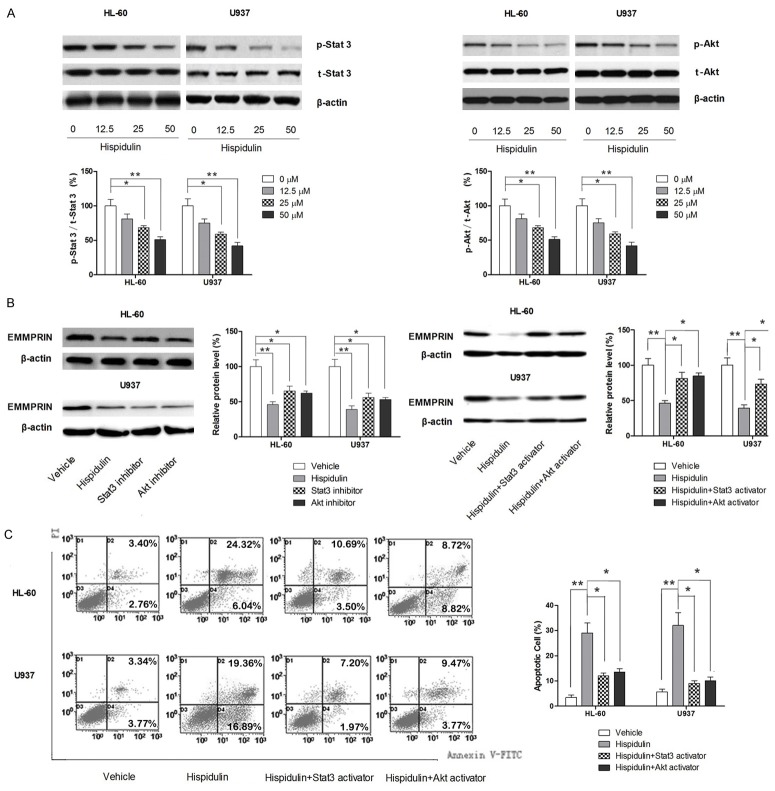
Hispidulin inhibits the expression of EMMPRIN through inhibiting both Akt and STAT3 signaling
The aforementioned results showed that hispidulin induces apoptosis in AML cells by modulating EMMPRIN, and our previous studies showed that hispidulin can suppress the activation of STAT3 and Akt signaling [27,31,32]; therefore, the question was raised as to whether hispidulin modulates the expression of EMMPRIN by affecting these two signaling pathways. As shown in Figure 5A, both STAT3 and Akt signaling is inhibited in AML cell lines in a dose-dependent manner when treated with hispidulin. Next, specific STAT3 inhibitor Stattic and Akt signaling inhibitor LY294002 were used as positive controls to examine the role of EMMPRIN expression on inhibiting the signaling pathway. As shown in Figure 5B, inhibition of either STAT3 or Akt signaling resulted in significantly suppressed expression of EMMPRIN, while hispidulin at 50 μM led to a slightly more profound suppressing effect on EMMPRIN by inhibiting both signaling pathways. In addition, activators of both signaling pathways (IL-6 and IGF-I for STAT3 and Akt signaling, respectively) were used in combination with hispidulin to further demonstrate the mechanism by which hispidulin modulates the expression of EMMEPRIN. As shown in Figure 5B, the inhibiting effect of hispidulin on EMMPRIN levels in both AML cell lines was significantly attenuated by either activator of these two signaling pathways. The attenuating effects on EMMPRIN levels were also reflected in the ability to induce apoptosis. As shown in Figure 5C, either STAT3 or Akt activator was able to significantly abolish the apoptosis-inducing effect of hispidulin. Taken together, our results suggested that hispidulin regulates the level of EMMPRIN by inhibiting both STAT3 and Akt signaling.
Figure 5.
Hispidulin modulates EMMPRIN level by inhibiting Akt and STAT3 signaling in HL-60 and U937 cells. HL-60 and U937 cells were incubated with hispidulin at the indicated concentrations (or 50 μM if not marked) for 48 h. A. Effects of hispidulin on activation of Akt and STAT3 signaling as determined by western blot. B. Involvement of Akt and STAT3 signaling in the modulatory effect of hispidulin on EMMPRIN level as determined by western blot. C. Involvement of Akt and STAT3 signaling in the apoptosis-inducing effect of hispidulin as determined by flow cytometric analysis. Western blot and the images of flow cytometric assay represent three independently conducted experiments. *P<0.05, **P<0.01.
Discussion
Epidemiological studies have suggested that high consumption of flavonoids decreases the risk of a variety of cancers [33]. While investigating the anticancer properties of flavonoids, a few recent studies have focused on a small flavonoid, hispidulin [23-27]. This study was designed to evaluate the therapeutic effects of hispidulin on AML and to investigate the underlying mechanism(s) of its action.
Previous studies have shown that hispidulin exerts anti-proliferative effects on solid tumors, including glioblastoma, pancreatic cancer, gastric cancer, ovarian cancer, hepatocellular carcinoma, gallbladder cancer, and renal cell carcinoma in vitro [23,25,27,34-36]. Consistent with these previous reports, our results demonstrated that hispidulin treatment inhibits the proliferation of AML cells in a dose- and time-dependent manner, as confirmed by MTT assay and colony formation tests. Moreover, our results revealed that hispidulin can arrest the AML cell cycle. In the present study, hispidulin treatment led to a cell-cycle blockat the G0/G1 phase. Given that suppression of cyclin D1 blocks the S-entry phase without altering the length of the cell cycle [37,38], the level of cell cycle-related protein cyclin D1 was evaluated to further elucidate the underlying mechanisms by which hispidulin mediates cell-cycle arrest in AML cells. Our results revealed a lower expression of cyclin D1 in both HL-60 and U937 cells treated with hispidulin at all tested concentrations, suggesting another mechanism by which hispidulin inhibits the proliferation of AML cells. In the present study, hispidulin induced an increase in the G0/G1 cell population with a decrease in both the S and G2/M phase cell populations, which agrees with our previous findings using hepatocellular carcinoma and gallbladder cancer cells [27,35]. In contrast, Yu et al. [26] reported that hispidulin induced G1/S arrest, as shown by the increase in both G0/G1 and S phase cells. It appears that the effect of hispidulin on cell-cycle progression is cell specific, the proof of which would need further study on more cancer cell lines.
Apoptosis, also referred to as programmed cell death, has been proposed as the major mechanism by which cancer is treated [39] and the mechanism by which many anticancer agents achieve therapeutic efficacy [40]. In our study, it was also revealed that hispidulin at tested dosages significantly increases apoptosis in AML cells. Apoptosis mainly involves two signaling pathways-the death receptor extrinsic pathway and the mitochondrial intrinsic pathway [30]. Caspase-8 activation and BH3 interacting-domain proteins are involved in the execution of the extrinsic pathway, whereas the intrinsic pathway requires activation of caspase-9 [30]. In this study, it was found that the apoptosis induced by hispidulin in AML cells occurs only through the intrinsic pathway, as evidenced by caspase-9 activation, loss of mitochondrial membrane potential, and release of cytochrome c from the mitochondria to the cytosol. The results in the present study coincided with our results of the study on hepatocellular carcinoma cells and Yu’s results of the study on gastric cancer cells, both also demonstrating the inducing effect of hispidulin on mitochondrial apoptosis [26,27]; however, in contrast to these findings, an early study showed that hispidulin activates both the intrinsic and extrinsic apoptosis pathways by inducing p53 expression human glioblastoma multiforme cells [24]. The different apoptosis mechanisms induced by hispidulin might be explained by the use of different cancer cells tested and/or the difference in target molecule expressions.
EMMPRIN is a glycoprotein highly expressed on the surface of various malignant tumor cells and whose function in inducing the secretion of matrix metalloproteinases (MMPs; mainly MMP-1, MMP-2, and MMP-9) and thus promoting invasion and metastasis has been well established [41]. Besides its function in invasion and metastasis, recent evidence has revealed the association between EMMPRIN and apoptosis in a variety of human tumor cells [41]. In particular, our recent work showed that shRNA-mediated EMMPRIN silencing inhibits AML U937 cell proliferation and increases chemosensitivity to adriamycin [16], which also suggests that EMMPRIN has the potential to serve as a target molecule for antitumor chemotherapeutic agents. In this study, our results revealed that the apoptosis-inducing effect of hispidulin on the AML cell lines HL60 and U937 is mediated, at least partly, by suppressing EMMPRIN expressions, which is well correlated with the results of our previous work. On the other hand, EMMPRIN has been found to be involved inthe regulation of autophagy or autophagic cell death in tumor cells [42] and agents that downregulate EMMPRIN have been shown to induce autophagic cell death in human hepatoma cells [43,44]; therefore, by downregulating EMMPRIN, hispidulin might also increase autophagy of AML cells. Coupled with the established inhibitory effect on cell migration and the invasion of EMMPRIN, hispidulin might be able to exert an antitumor effect on AML cells by causing changes in multiple facets of cellular behaviors by downregulating EMMPRIN.
The expression of EMMPRIN is regulated by a number of signaling pathways that are involved in the survival or metastasis of cancer cells, including PI3K/Akt, STAT3, ERK, and JNK [45,46]. In our previous studies, we showed that hispidulin induces apoptosis in hepatocellular carcinoma cells and that it prevents hypoxia-induced EMT in colon cancer cells, which are mediated, at least in part, by inhibiting the PI3k/Akt signaling pathway [27,47]. Another of our studies showed that hispidulin synergizes with sunitinib in renal carcinoma in vitro and in vivo by inhibiting STAT3 signaling [36]. We then postulated whether hispidulin modulates EMMPRIN expression by working on both signaling pathways. The results show that hispidulin causes simultaneous dose-dependent inhibition in both signaling pathways. Moreover, by using an activator of both signaling pathways, inhibition of both using hispidulin is involved in hispidulin-induced modulation of EMMPRIN levels and apoptosis in both AML cell lines. In the present study, we found that STAT3 acts upstream and plays a role in regulating EMMPRIN expression; however, it has also been reported that STAT3 signaling can be activated by EMMPRIN overexpression and can mediate pancreatic cancer development through CD44 [48]. In this case, it is possible that a more complicated association between EMMPRIN and STAT3 signaling is present in tumor cells, which needs to be investigated in future studies.
Conclusions
The results of the present study showed that hispidulin exerts potent antitumor effects against AML cell lines by inducing mitochondrial apoptosis. Other studies also revealed that hispidulin downregulates EMMPRIN in AML cells and induces apoptosis by modulating EMMPRIN. Furthermore, the results strongly indicated that the modulating effect of hispidulin on EMMPRIN is correlated with its inhibitory effect on both Akt and STAT3 signaling. Given the low toxicity of hispidulin to healthy organs and tissues as a natural compound, our results suggested that hispidulin might be a promising agent for the treatment of AML, although more data must be collected.
Acknowledgements
This work was supported by National Natural Science Foundation (No. 31470570), Chongqing Natural Science Foundation (No. cstc2014jcyjA80013), Chongqing Creative Programe for Graduate Students (CYS15155) and Science Foundation of Chongqing Education Commission (No. kj1400534).
Disclosure of conflict of interest
None.
References
- 1.Estey E, Dohner H. Acute myeloid leukaemia. Lancet. 2006;368:1894–1907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- 2.Smith ML, Hills RK, Grimwade D. Independent prognostic variables in acute myeloid leukaemia. Blood Rev. 2011;25:39–51. doi: 10.1016/j.blre.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Robak T, Wierzbowska A. Current and emerging therapies for acute myeloid leukemia. Clin Ther. 2009;31 Pt 2:2349–2370. doi: 10.1016/j.clinthera.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 4.Meijer E, Cornelissen JJ. Allogeneic stem cell transplantation in acute myeloid leukemia in first or subsequent remission: weighing prognostic markers predicting relapse and risk factors for non-relapse mortality. Semin Oncol. 2008;35:449–457. doi: 10.1053/j.seminoncol.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Fiedler W, Staib P, Kuse R, Duhrsen U, Flasshove M, Cavalli F, Hossfeld DK, Berdel WE. Role of angiogenesis inhibitors in acute myeloid leukemia. Cancer J. 2001;7(Suppl 3):S129–133. [PubMed] [Google Scholar]
- 6.Igakura T, Kadomatsu K, Kaname T, Muramatsu H, Fan QW, Miyauchi T, Toyama Y, Kuno N, Yuasa S, Takahashi M, Senda T, Taguchi O, Yamamura K, Arimura K, Muramatsu T. A null mutation in basigin, an immunoglobulin superfamily member, indicates its important roles in peri-implantation development and spermatogenesis. Dev Biol. 1998;194:152–165. doi: 10.1006/dbio.1997.8819. [DOI] [PubMed] [Google Scholar]
- 7.Fadool JM, Linser PJ. 5A11 antigen is a cell recognition molecule which is involved in neuronal-glial interactions in avian neural retina. Dev Dyn. 1993;196:252–262. doi: 10.1002/aja.1001960406. [DOI] [PubMed] [Google Scholar]
- 8.Gabison EE, Hoang-Xuan T, Mauviel A, Menashi S. EMMPRIN/CD147, an MMP modulator in cancer, development and tissue repair. Biochimie. 2005;87:361–368. doi: 10.1016/j.biochi.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 9.Zhu P, Ding J, Zhou J, Dong WJ, Fan CM, Chen ZN. Expression of CD147 on monocytes/macrophages in rheumatoid arthritis: its potential role in monocyte accumulation and matrix metalloproteinase production. Arthritis Res Ther. 2005;7:R1023–1033. doi: 10.1186/ar1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nabeshima K, Iwasaki H, Koga K, Hojo H, Suzumiya J, Kikuchi M. Emmprin (basigin/CD147): matrix metalloproteinase modulator and multifunctional cell recognition molecule that plays a critical role in cancer progression. Pathol Int. 2006;56:359–367. doi: 10.1111/j.1440-1827.2006.01972.x. [DOI] [PubMed] [Google Scholar]
- 11.Jin JS, Yao CW, Loh SH, Cheng MF, Hsieh DS, Bai CY. Increasing expression of extracellular matrix metalloprotease inducer in ovary tumors: tissue microarray analysis of immunostaining score with clinicopathological parameters. Int J Gynecol Pathol. 2006;25:140–146. doi: 10.1097/01.pgp.0000189244.57145.84. [DOI] [PubMed] [Google Scholar]
- 12.Zheng HC, Takahashi H, Murai Y, Cui ZG, Nomoto K, Miwa S, Tsuneyama K, Takano Y. Upregulated EMMPRIN/CD147 might contribute to growth and angiogenesis of gastric carcinoma: a good marker for local invasion and prognosis. Br J Cancer. 2006;95:1371–1378. doi: 10.1038/sj.bjc.6603425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Xu J, Chen L, Zhong WD, Zhang Z, Mi L, Zhang Y, Liao CG, Bian HJ, Jiang JL, Yang XM, Li XY, Fan CM, Zhu P, Fu L, Chen ZN. HAb18G (CD147), a cancer-associated biomarker and its role in cancer detection. Histopathology. 2009;54:677–687. doi: 10.1111/j.1365-2559.2009.03280.x. [DOI] [PubMed] [Google Scholar]
- 14.Ranuncolo SM, Armanasco E, Cresta C, Bal De Kier Joffe E, Puricelli L. Plasma MMP-9 (92 kDa-MMP) activity is useful in the follow-up and in the assessment of prognosis in breast cancer patients. Int J Cancer. 2003;106:745–751. doi: 10.1002/ijc.11288. [DOI] [PubMed] [Google Scholar]
- 15.Fu J, Fu J, Chen X, Zhang Y, Gu H, Bai Y. CD147 and VEGF co-expression predicts prognosis in patients with acute myeloid leukemia. Jpn J Clin Oncol. 2010;40:1046–1052. doi: 10.1093/jjco/hyq098. [DOI] [PubMed] [Google Scholar]
- 16.Gao H, Jiang Q, Han Y, Peng J, Wang C. shRNA-mediated EMMPRIN silencing inhibits human leukemic monocyte lymphoma U937 cell proliferation and increases chemosensitivity to adriamycin. Cell Biochem Biophys. 2015;71:827–835. doi: 10.1007/s12013-014-0270-4. [DOI] [PubMed] [Google Scholar]
- 17.Way TD, Lee JC, Kuo DH, Fan LL, Huang CH, Lin HY, Shieh PC, Kuo PT, Liao CF, Liu H, Kao JY. Inhibition of epidermal growth factor receptor signaling by Saussurea involucrata, a rare traditional Chinese medicinal herb, in human hormone-resistant prostate cancer PC-3 cells. J Agric Food Chem. 2010;58:3356–3365. doi: 10.1021/jf903793p. [DOI] [PubMed] [Google Scholar]
- 18.Yin Y, Gong FY, Wu XX, Sun Y, Li YH, Chen T, Xu Q. Anti-inflammatory and immunosuppressive effect of flavones isolated from Artemisia vestita. J Ethnopharmacol. 2008;120:1–6. doi: 10.1016/j.jep.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 19.Kavvadias D, Sand P, Youdim KA, Qaiser MZ, Rice-Evans C, Baur R, Sigel E, Rausch WD, Riederer P, Schreier P. The flavone hispidulin, a benzodiazepine receptor ligand with positive allosteric properties, traverses the blood-brain barrier and exhibits anticonvulsive effects. Br J Pharmacol. 2004;142:811–820. doi: 10.1038/sj.bjp.0705828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niu X, Chen J, Wang P, Zhou H, Li S, Zhang M. The Effects of Hispidulin on Bupivacaine-Induced Neurotoxicity: Role of AMPK Signaling Pathway. Cell Biochem Biophys. 2014;70:241–9. doi: 10.1007/s12013-014-9888-5. [DOI] [PubMed] [Google Scholar]
- 21.Zhou R, Wang Z, Ma C. Hispidulin Exerts Anti-osteoporotic Activity in Ovariectomized Mice via Activating AMPK Signaling Pathway. Cell Biochem Biophys. 2014;69:311–7. doi: 10.1007/s12013-013-9800-8. [DOI] [PubMed] [Google Scholar]
- 22.Nepal M, Choi HJ, Choi BY, Yang MS, Chae JI, Li L, Soh Y. Hispidulin attenuates bone resorption and osteoclastogenesis via the RANKL-induced NF-kappaB and NFATc1 pathways. Eur J Pharmacol. 2013;715:96–104. doi: 10.1016/j.ejphar.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Yang JM, Hung CM, Fu CN, Lee JC, Huang CH, Yang MH, Lin CL, Kao JY, Way TD. Hispidulin sensitizes human ovarian cancer cells to TRAIL-induced apoptosis by AMPK activation leading to Mcl-1 block in translation. J Agric Food Chem. 2010;58:10020–10026. doi: 10.1021/jf102304g. [DOI] [PubMed] [Google Scholar]
- 24.Lin YC, Hung CM, Tsai JC, Lee JC, Chen YL, Wei CW, Kao JY, Way TD. Hispidulin potently inhibits human glioblastoma multiforme cells through activation of AMP-activated protein kinase (AMPK) J Agric Food Chem. 2010;58:9511–9517. doi: 10.1021/jf1019533. [DOI] [PubMed] [Google Scholar]
- 25.He L, Wu Y, Lin L, Wang J, Chen Y, Yi Z, Liu M, Pang X. Hispidulin, a small flavonoid molecule, suppresses the angiogenesis and growth of human pancreatic cancer by targeting vascular endothelial growth factor receptor 2-mediated PI3K/Akt/mTOR signaling pathway. Cancer Sci. 2011;102:219–225. doi: 10.1111/j.1349-7006.2010.01778.x. [DOI] [PubMed] [Google Scholar]
- 26.Yu CY, Su KY, Lee PL, Jhan JY, Tsao PH, Chan DC, Chen YL. Potential Therapeutic Role of Hispidulin in Gastric Cancer through Induction of Apoptosis via NAG-1 Signaling. Evid Based Complement Alternat Med. 2013;2013:518301. doi: 10.1155/2013/518301. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Gao H, Wang H, Peng J. Hispidulin induces apoptosis through mitochondrial dysfunction and inhibition of P13k/Akt signalling pathway in HepG2 cancer cells. Cell Biochem Biophys. 2014;69:27–34. doi: 10.1007/s12013-013-9762-x. [DOI] [PubMed] [Google Scholar]
- 28.Gao H, Xie J, Peng J, Han Y, Jiang Q, Han M, Wang C. Hispidulin inhibits proliferation and enhances chemosensitivity of gallbladder Cancer cells by targeting HIF-1α. Exp Cell Res. 2015;332:236–46. doi: 10.1016/j.yexcr.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 29.Sato M, Nakai Y, Nakata W, Yoshida T, Hatano K, Kawashima A, Fujita K, Uemura M, Takayama H, Nonomura N. EMMPRIN promotes angiogenesis, proliferation, invasion and resistance to sunitinib in renal cell carcinoma, and its level predicts patient outcome. PLoS One. 2013;8:e74313. doi: 10.1371/journal.pone.0074313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dias N, Bailly C. Drugs targeting mitochondrial functions to control tumor cell growth. Biochem Pharmacol. 2005;70:1–12. doi: 10.1016/j.bcp.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 31.Gao H, Jiang Q, Han Y, Peng J, Wang C. Hispidulin Potentiates the Antitumor Effect of Sunitinib Against Human Renal Cell Carcinoma in Laboratory Models. Cell Biochem Biophys. 2015;71:757–64. doi: 10.1007/s12013-014-0260-6. [DOI] [PubMed] [Google Scholar]
- 32.Gao H, Xie J, Peng J, Han Y, Jiang Q, Han M, Wang C. Hispidulin inhibits proliferation and enhances chemosensitivity of gallbladder cancer cells by targeting HIF-1alpha. Exp Cell Res. 2015;71:757–64. doi: 10.1016/j.yexcr.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 33.Yao H, Xu W, Shi X, Zhang Z. Dietary flavonoids as cancer prevention agents. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2011;29:1–31. doi: 10.1080/10590501.2011.551317. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Liu W, He X, Fei Z. Hispidulin enhances the anti-tumor effects of temozolomide in glioblastoma by activating AMPK. Cell Biochem Biophys. 2015;71:701–706. doi: 10.1007/s12013-014-0252-6. [DOI] [PubMed] [Google Scholar]
- 35.Gao H, Xie J, Peng J, Han Y, Jiang Q, Han M, Wang C. Hispidulin inhibits proliferation and enhances chemosensitivity of gallbladder cancer cells by targeting HIF-1alpha. Exp Cell Res. 2015;332:236–246. doi: 10.1016/j.yexcr.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 36.Gao H, Jiang Q, Han Y, Peng J, Wang C. Hispidulin potentiates the antitumor effect of sunitinib against human renal cell carcinoma in laboratory models. Cell Biochem Biophys. 2015;71:757–764. doi: 10.1007/s12013-014-0260-6. [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Wang Q, Cui Y, Liu ZY, Zhao W, Wang CL, Dong Y, Hou L, Hu G, Luo C, Chen J, Lu Y. Knockdown of cyclin D1 inhibits proliferation, induces apoptosis, and attenuates the invasive capacity of human glioblastoma cells. J Neurooncol. 2012;106:473–484. doi: 10.1007/s11060-011-0692-4. [DOI] [PubMed] [Google Scholar]
- 38.Grana X, Reddy EP. Cell cycle control in mammalian cells: role of cyclins, cyclin dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs) Oncogene. 1995;11:211–219. [PubMed] [Google Scholar]
- 39.Amaravadi RK, Lippincott-Schwartz J, Yin XM, Weiss WA, Takebe N, Timmer W, DiPaola RS, Lotze MT, White E. Principles and current strategies for targeting autophagy for cancer treatment. Clin Cancer Res. 2011;17:654–666. doi: 10.1158/1078-0432.CCR-10-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Safarzadeh E, Sandoghchian Shotorbani S, Baradaran B. Herbal medicine as inducers of apoptosis in cancer treatment. Adv Pharm Bull. 2014;4:421–427. doi: 10.5681/apb.2014.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuang YH, Chen X, Su J, Wu LS, Liao LQ, Li D, Chen ZS, Kanekura T. RNA interference targeting the CD147 induces apoptosis of multidrug resistant cancer cells related to XIAP depletion. Cancer Lett. 2009;276:189–195. doi: 10.1016/j.canlet.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 42.Gou X, Ru Q, Zhang H, Chen Y, Li L, Yang H, Xing J, Chen Z. HAb18G/CD147 inhibits starvation-induced autophagy in human hepatoma cell SMMC7721 with an involvement of Beclin 1 down-regulation. Cancer Sci. 2009;100:837–843. doi: 10.1111/j.1349-7006.2009.01113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hou Q, Tang X, Liu H, Tang J, Yang Y, Jing X, Xiao Q, Wang W, Gou X, Wang Z. Berberine induces cell death in human hepatoma cells in vitro by downregulating CD147. Cancer Sci. 2011;102:1287–1292. doi: 10.1111/j.1349-7006.2011.01933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X, Tang X, Liu H, Li L, Hou Q, Gao J. Autophagy induced by baicalin involves downregulation of CD147 in SMMC-7721 cells in vitro. Oncol Rep. 2012;27:1128–1134. doi: 10.3892/or.2011.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lv C, Kong H, Dong G, Liu L, Tong K, Sun H, Chen B, Zhang C, Zhou M. Antitumor efficacy of alpha-solanine against pancreatic cancer in vitro and in vivo. PLoS One. 2014;9:e87868. doi: 10.1371/journal.pone.0087868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen PS, Shih YW, Huang HC, Cheng HW. Diosgenin, a steroidal saponin, inhibits migration and invasion of human prostate cancer PC-3 cells by reducing matrix metalloproteinases expression. PLoS One. 2011;6:e20164. doi: 10.1371/journal.pone.0020164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xie J, Gao H, Jianjun P, Yantao H, Xuehong C, Qixiao J, Chunbo W. Hispidulin prevents hypoxia-induced epithelial-mesenchymal transition in human colon carcinoma cells. Am J Cancer Res. 2015;5:15. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Li L, Tang W, Wu X, Karnak D, Meng X, Thompson R, Hao X, Li Y, Qiao XT, Lin J, Fuchs J, Simeone DM, Chen ZN, Lawrence TS, Xu L. HAb18G/CD147 promotes pSTAT3-mediated pancreatic cancer development via CD44s. Clin Cancer Res. 2013;19:6703–6715. doi: 10.1158/1078-0432.CCR-13-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]