Abstract
Cancer stem cells (CSCs) are responsible for the tumorigenesis and recurrence, so targeting CSCs is a potential effective method to cure cancers. Activated Hedgehog signaling pathway has been proved to be implicated in the maintenance of self-renewal of CSCs, and arsenic trioxide (As2O3) has been reported to inhibit Gli1, a key transcription factor of Hedgehog pathway. In this study, we evaluated whether As2O3 has inhibitory effects on cancer stem-like cells (CSLCs) in lung cancer and further explored the possible mechanism. CCK8 assay and colony formation assay were performed to demonstrate the ability of As2O3 to inhibit the growth of NCI-H460 and NCI-H446 cells, which represented non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC), respectively. Tumor sphere formation assay was carried out to evaluate the effects of As2O3 on stem cell-like subpopulations. The expression of stem cell biomarkers CD133 and stem cell transcription factors such as Sox2 and Oct4 were detected. Moreover, the effects of As2O3 on expression of Gli1 and its target genes were observed. We found that As2O3 inhibited the cell proliferation and reduced the colony formation ability. Importantly, As2O3 decreased the formation of tumor spheres. The expression of stem cell biomarker CD133 and stem cell transcription factors such as Sox2 and Oct4 were markedly reduced by As2O3 treatment. Furthermore, As2O3 decreased the expression of Gli1, N-myc and GAS1. Our results suggested that As2O3 is a promising agent to inhibit CSLCs in lung cancer. In addition, the mechanism of CSLCs inhibition might involve Gli1 down-regulation.
Keywords: Arsenic trioxide, cancer stem-like cells, lung cancer, Gli1
Introduction
Lung cancer is one of the most aggressive malignant tumors throughout the world, claiming millions of death each year [1]. Despite advances in chemotherapy and targeted drugs, the overall five-year survival rate among patients with advanced lung cancer remains poor, and the recurrence rate is high. It is reported that lung cancer stem cells (LCSCs) are closely correlated to the poor prognosis and unsuccessful clinical outcomes in lung cancer [2].
Cancer stem cells (CSCs) or cancer initiating cells are small subpopulations of cancer cells, which can differentiate and generate heterogeneous cell populations to constitute the tumor [3]. CSCs thus play a key role in the initiation and progression of malignant tumors [3]. CSCs are highly resistant to conventional chemotherapy and ionizing radiation [4]. This suggests that while many chemotherapeutic agents kill the tumor bulk population, CSCs can survive to generate new tumor and cause tumor recurrence. It is reported that CSCs form spherical colonies when they are cultured in serum-free medium in the presence of specific growth factors [5,6]. Besides, some researchers proposed that CD133+ cells have the characteristics of CSCs because they are more proliferative, clonogenic and tumorigenic than CD133- counterparts and express genes associated with stemness [7,8]. Oct4 and Sox2, initially known as key transcription factors for embryonic stem cells, are also involved in the maintenance of CSCs [9,10].
Aberrant Hedgehog signaling pathway is implicated in the initiation and progression of various types of tumors, including myeloid leukaemia [11], multiple myeloma [12], basal-cell carcinoma [13], glioma [14] as well as lung cancer [15,16]. Inhibition of Hedgehog signaling impedes clonogenic growth and tumor initiation ability of glioma stem cells [14]. It has been reported that GANT-61 (Smo antagonist) is able to reduce CSCs and profoundly impede pancreatic cancer metastatic spread [17]. In lung cancer, Gli1 expression is associated with poor overall survival [18]. Depletion of Gli1 significantly abolishes the growth of stem-like side populations from NSCLCs [19].
Arsenic trioxide (As2O3) has been used as a traditional remedy in China for thousands of years [20]. In recent decades, As2O3 has been proved to induce complete remission in acute promyelocytic leukemia (APL) with minimal toxicity [21], and it also strongly inhibited the self renewal of APL stem cells [22]. Furthermore, it was found that the combination of As2O3 and PI3K inhibitor PI-103 strongly diminishes acute myeloid leukemia stem cells while sparing normal hematopoietic stem cells [23]. Owing to its effects on patients with leukemia, researchers have turned their attention to utilization of As2O3 for treatment of various solid tumors. Studies have shown that As2O3 inhibits the cancer stem-like cells in gliomas via deregulation of Notch activation [24]. As2O3 has been found to induce differentiation of human hepatocellular carcinoma stem cells and prolong survival after hepatectomy in a mouse model [25]. Our team has previously demonstrated that As2O3 induces apoptosis and arrests cell cycle in lung cancer cells [26]. Additionally, As2O3 significantly inhibits the growth of lung cancer xenograft tumors and the formation of malignant pleural effusion in a mice model as a result of its antiangiogenic effects [27,28]. Moreover, As2O3 is clinically effective in the treatment of lung cancer complicated with malignant pleural effusion. Our findings suggest that As2O3 might be a new approach for the treatment of lung cancer. However, it is unclear whether As2O3 has the ability to inhibit cancer stem-like cells (CSLCs) in lung cancer.
We hypothesized that As2O3 could inhibit CSLCs in lung cancer through down-regulation of Gli1. In our studies, we employed two human lung cancer cell lines, NCI-H460 and NCI-H446, representing non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC), respectively.
Materials and methods
Cell culture and reagents
NCI-H460 cell line was obtained from Cell Bank of the Chinese Academy of Sciences (Shanghai, China). NCI-H446 cell line was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were cultured in RPMI 1640 (HyClone, Logan City, Utah, USA) plus 10% fetal bovine serum (FBS) (HyClone, Logan City, Utah, USA) and 1% penicillin-streptomycin (HyClone, Logan City, Utah, USA). The cells were maintained at 37°C in a humidified incubator containing 5% CO2. As2O3 was dissolved in PBS and diluted into indicated concentrations when needed.
Cell viability assay
Cell viability was determined by the CCK8 assay (beyotime, Shanghai, China). 3×103 cells were seeded per well in 96-well culture plates. After adhesion, the cells were treated with 1, 2, 4, 8, 16 and 32 μM As2O3, respectively. Cells treated with vehicle were used as controls. After incubation at 37°C for 24, 48 or 72 hours, the absorbance was measured by spectrophotometer at a wavelength of 450 nm. The cell viability was calculated as a percentage of the OD value in the control cells. Dose-response curves were made. The IC50 which represents the drug concentration that inhibits 50% of the growth of vehicle-treated control cells was calculated.
Colony formation assay
NCI-H460 and NCI-H446 cells were treated with 1, 2 and 4 μM As2O3 for 72 hours. Subsequently, 2×103 cells per well were counted and seeded in 6-well culture dishes. The cells were not exposed to As2O3 at this stage. After incubation at 37°C for 14 days, colonies were stained by Coomassie brilliant blue dying and photographed by a camera. Macroscopic colonies of each well were counted.
Tumor sphere formation assay
NCI-H460 and NCI-H446 cells were treated with different concentrations of As2O3 (1-4 μM) for 72 hours. Subsequently, 1×104 cells per well were counted and seeded in low-adherent 6-well culture plates (Corning, NY, USA) under serum-free conditions consisting of DMEM/F-12 (Life Technologies, Rockville, MD, USA), 20 μl/ml B27 (Life Technologies, Rockville, MD, USA), 20 ng/ml epidermal growth factor (EGF) (Invitrogen, Carlsbad, CA, USA), 20 ng/ml basic fibroblast growth factor (bFGF) (Invitrogen, Carlsbad, CA, USA) and 1% penicillin-streptomycin (HyClone, Logan City, Utah, USA). After incubation at 37°C for 5 days, pictures were taken under a microscope and the number of tumor spheres was counted in five separated 50× fields.
Quantitative real-time PCR (qPCR)
NCI-H460 and NCI-H446 cells were treated with different concentrations of As2O3 (1-4 μM) for 72 hours. The total RNA was extracted and then reverse transcribed into cDNA. RT-PCR analysis was performed using SYBR Premix Ex Taq (Takara, Otsu, Shiga, Japan). The following primers were used: CD133 forward 5’-TTTCAAGGACTTGCGAACTCTC-3’; CD133 reverse 5’-TGCTATTCAGCTGGCTTAGAGAC-3’; Oct4 forward 5’-CGACCATCTGCCGCTTTGAG-3’; Oct4 reverse 5’-CCCCCTGTCCCCCATTCCTA-3’; Sox2 forward 5’-GCGAACCATCTCTGTGGTCT-3’; Sox2 reverse 5’-GGAAAGTTGGGATCGAACAA-3’; Gli1 forward 5’-ATGAAACTGACTGCCGTTGG-3’; Gli1 reverse 5’-CTTCTCGCCAGTGTGTCTGC-3’; N-myc forward 5’-ACCACAAGGCCCTCAGTACC-3’; N-myc reverse 5’-GTGCATCCTCACTCTCCACG-3’; GAS1 forward 5’-CGGAGCTTGACTTCTTGGAC-3’; GAS1 reverse 5’-CCCAACCCTTCAAATTGCTA-3’; β-actin forward 5’-CCTGGCACCCAGCACAAT-3’; β-actin reverse 5’-GGGCCGGACTCGTCATACT-3’.
Western blotting
NCI-H460 and NCI-H446 cells were treated with various concentrations of As2O3 (1-4 μM) for 72 hours. Proteins were extracted, electrophoretically separated, and then transferred onto PVDF membranes. The membranes were blocked with a solution containing 5% skim milk for 1 h, and were incubated overnight at 4°C with the primary antibodies. The primary antibodies were as follows: CD133 (1:1000, ABclonal, Cambridge, MA, USA), Sox2 (1:1000, Cell Signal technology, Danvers, MA, USA), Oct4 (1:000, Cell Signal technology, Danvers, MA, USA), Gli1 (1:1000, ABclonal, Cambridge, MA, USA), N-myc (1:1000, ABclonal, Cambridge, MA, USA), GAS1 (1:1000, Abcam, Cambridge, UK), and β-actin (1:1000, ABclonal, Cambridge, MA, USA). Finally, the membranes were incubated with the appropriate secondary antibody and visualized using the ECL detection reagents.
Statistical analysis
All data were analyzed using the SPSS 22.0 software. The measured data were presented as the means ± SD, and were analyzed by a one-way ANOVA, followed by Dunnett’s test. A value of P<0.05 was considered to be statistically significant.
Results
As2O3 inhibited lung cancer cell growth
Firstly, we used CCK8 assay to examine the growth inhibitory effects of As2O3 on NCI-H460 and NCI-H446 cells. Cells were treated with 1, 2, 4, 8, 16 and 32 μM As2O3 for 24, 48 or 72 h. Our data showed that As2O3 could inhibit the proliferation of NCI-H460 and NCI-H446 cells in a concentration- and time-dependent manner (Figure 1). The IC50s of NCI-H460 and NCI-H446 cells after treatment for 72 h were 5.610 and 4.252 μM, respectively.
Figure 1.
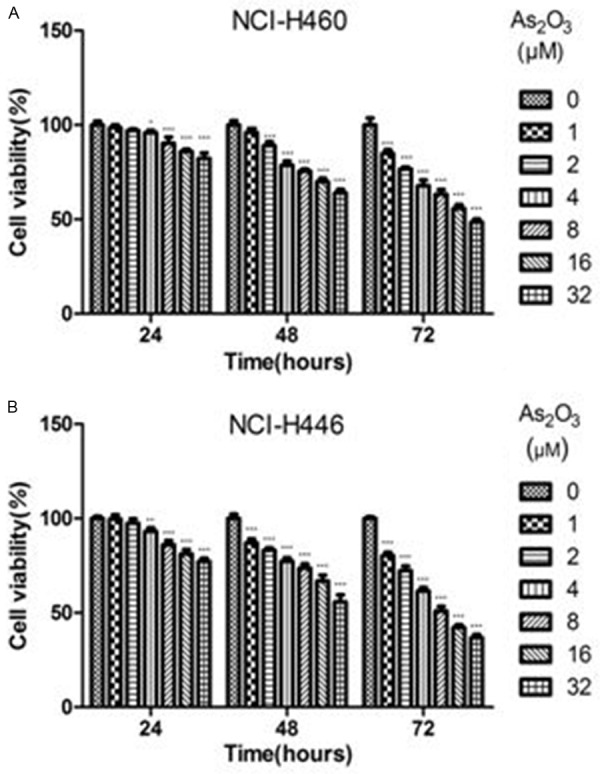
Inhibitory effects of As2O3 on lung cancer cell growth. NCI-H460 and NCI-H446 cells were treated with different concentrations of As2O3 for 24, 48 or 72 h. CCK8 assay was used to determine the cell viability. The control group was treated with PBS. A. Concentration- and time-dependent growth inhibitory effects of As2O3 on NCI-H460 cells. B. As2O3 inhibited the growth of NCI-H446 cells in a concentration- and time-dependent manner. Columns, mean; Error bars, SD. *P<0.05, **P<0.01, ***P<0.001 compared to the control.
Colony formation analysis
To determine whether As2O3 could inhibit the colony formation capacity, we subsequently performed the colony formation assay. In NCI-H460 cells, control group, 1 μM As2O3-treated group, 2 μM As2O3-treated group and 4 μM As2O3-treated group formed an average of 309, 304, 213 and 179 colonies, respectively (Figure 2). In NCI-H446 cells, control group formed an average of 642 colonies. However, these numbers decreased to 477, 236 and 137 when treated with 1 μM, 2 μM and 4 μM As2O3, respectively (Figure 2). Our data showed that the clonogenic capacity of NCI-H460 and NCI-H446 cells could be remarkably inhibited by As2O3 treatment in a dose-dependent manner.
Figure 2.
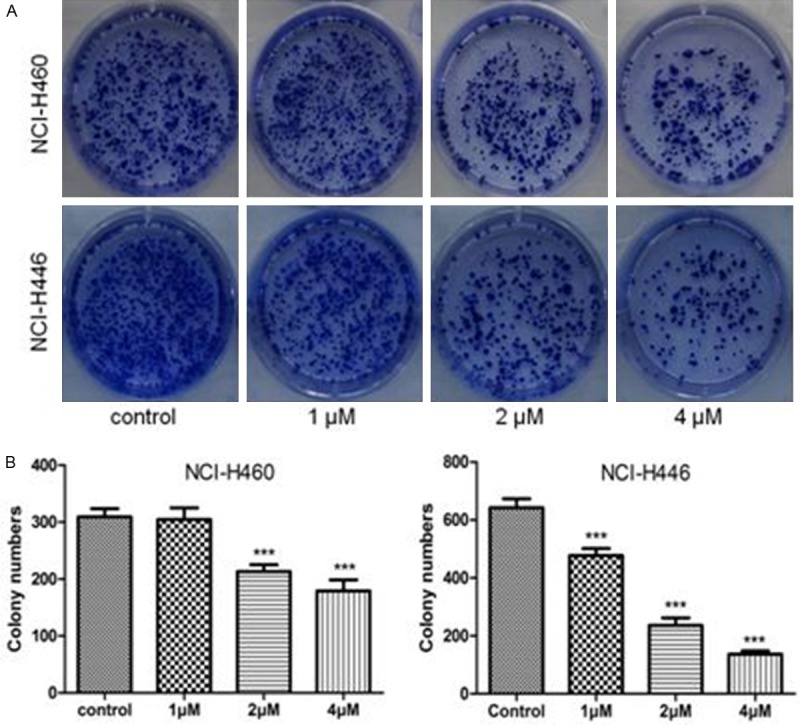
As2O3 inhibited the colony formation of lung cancer cells. NCI-H460 and NCI-H446 cells were treated with 1-4 μM As2O3 for 72 h. A. Images revealed that As2O3 could inhibit colony formation in NCI-H460 and NCI-H446 cells. B. The clonony numbers were significantly reduced by As2O3 treatment. Columns, mean; Error bars, SD. ***P<0.001 compared to the control.
As2O3 reduced the formation of tumor spheres
Tumor spheres maintain stem cell-like subpopulations [5,6]. Therefore, we investigated the effects of As2O3 on the formation of tumor spheres. As expected, As2O3 was able to significantly decrease the number and the size of the tumor spheres. Cells treated with vehicle formed more numerous and larger tumor spheres. However, As2O3-treated cells merely formed smaller and lesser tumor spheres. Additionally, the growth inhibitory effects of As2O3 on tumor spheres were concentration-dependent (Figure 3). These results suggested that the stem cell-like subpopulations were sensitive to As2O3 treatment.
Figure 3.
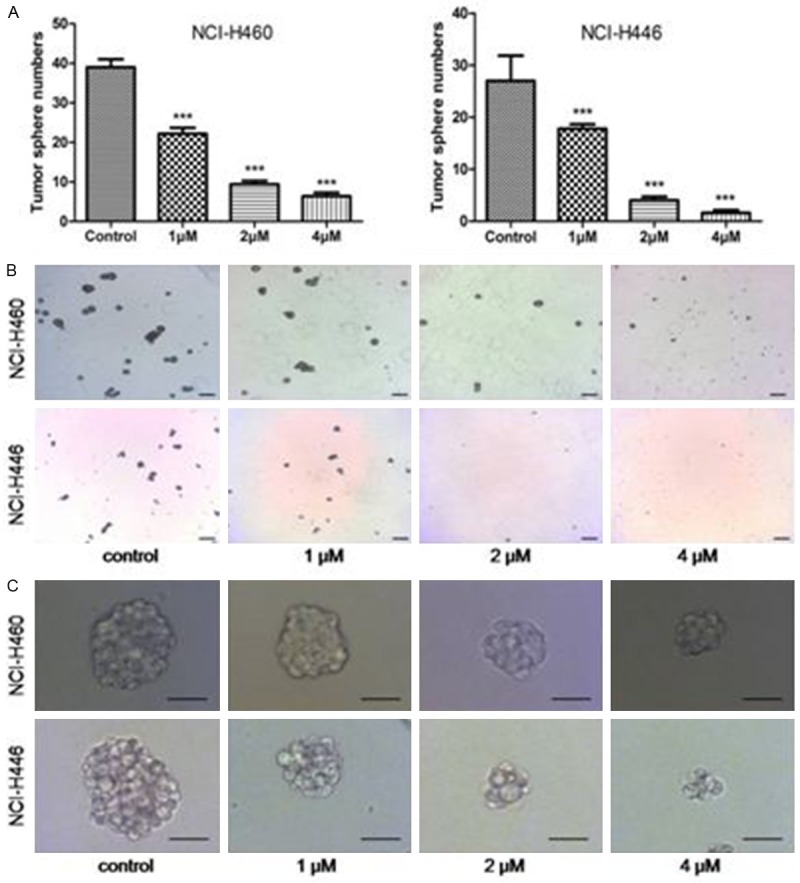
The effects of As2O3 on the tumor sphere formation. Equal numbers of viable NCI-H460 and NCI-H446 cells were cultured in serum free conditions following 72 h of As2O3 treatment. A. The number of tumor spheres was significantly decreased following As2O3 treatment. B. Representative images revealed that As2O3 could reduce the number of tumor spheres. Bar, 200 μm. C. Typical images showed that the size of tumor spheres in As2O3-treated group was smaller than that in control group. Bar, 50 μm. Columns, mean; bars, SD. ***P<0.001 compared to the control.
The expression of CD133, Sox2 and Oct4 were reduced by As2O3 treatment
To further examine the effects of As2O3 on CSLCs, we used qPCR and Western blot analysis to measure the expression levels of CD133 after treatment with As2O3 for 72 h. As2O3 significantly suppressed the expression of CD133 at both mRNA and protein levels in a dose-dependent manner (Figure 4A and 4B). Besides, we detected the effects of As2O3 on Sox2 and Oct4 which are very important transcription factors in regulating self-renewal and multipotency of CSCs. As shown in Figure 4A and 4B, the expression levels of Sox2 and Oct4 were also decreased by As2O3 treatment.
Figure 4.
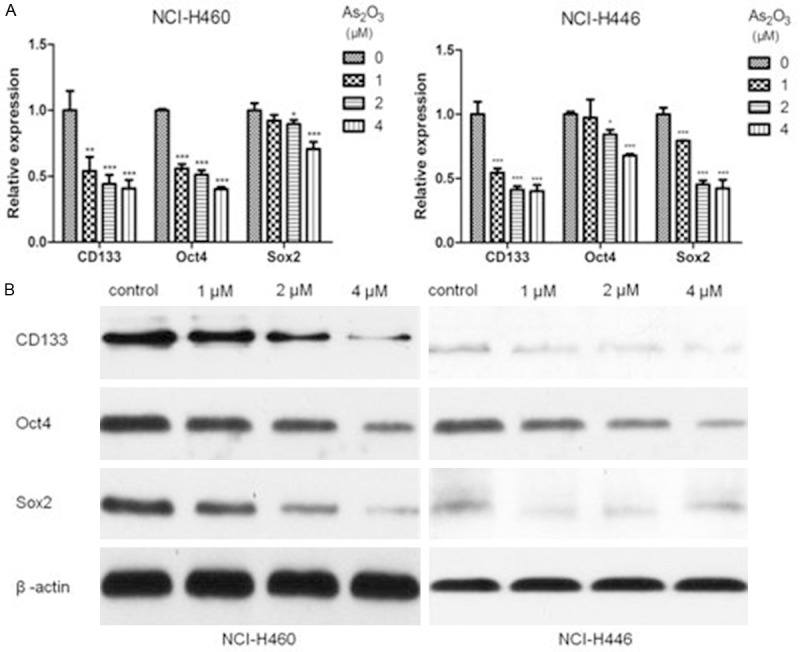
As2O3 treatment down-regulated the expression of stem cell markers. NCI-H460 and NCI-H446 cells were treated with different concentrations of As2O3 (1 μM, 2 μM and 4 μM) for 72 h. A. qPCR analysis showed that As2O3 significantly suppressed CD133, Oct4 and Sox2 mRNA expression in a dose-dependent manner. B. Western blot showed that reductions in CD133, Oct4 and Sox2 at protein levels were seen in As2O3 treated group. β-actin served as internal control. Columns, mean; Error bars, SD. *P<0.05, **P<0.01, ***P<0.001 compared to the control.
As2O3 down-regulated Gli1 and its target genes expression
Aberrant activated Hedgehog signaling is implicated in the initiation and propagation of lung cancer [15,18,29-32]. Blockade of Hedgehog signaling leads to a reduction in stem cell-like subpopulations [19,33]. To further explore the underlying mechanism of the inhibitory effects of As2O3 on CSLCs, we used qPCR analysis to measure the expression levels of Hedgehog signaling molecules after treatment with As2O3 at the indicated concentrations. The Gli1 mRNA levels were significantly decreased by As2O3 in a concentration-dependent manner. Additionally, downstream genes of Gli1 such as N-myc and GAS1 were reduced by As2O3 treatment (Figure 5A). These results were confirmed at protein levels by Western blot (Figure 5B).
Figure 5.
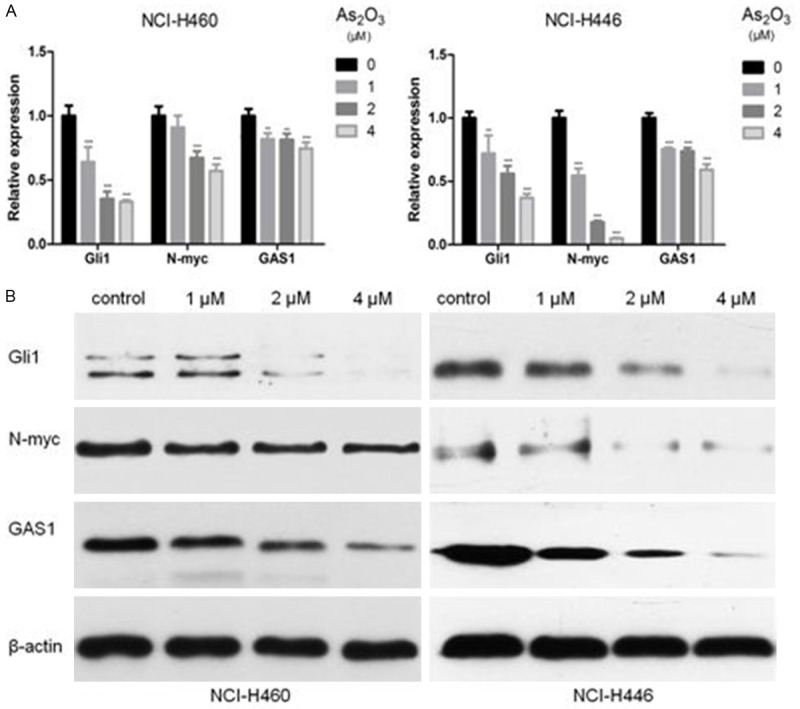
As2O3 inhibited the expression of Gli1 and its target genes such as N-myc and GAS1. NCI-H460 and NCI-H446 cells were treated with 1-4 μM As2O3 for 72 h. A. As2O3 decreased the Gli1, N-myc and GAS1 mRNA levels in NCI-H460 and NCI-H446 cells. B. As2O3 also influenced the expression of Gli1, N-myc and GAS1 at protein levels. Columns, mean; Error bars, SD. **P<0.01, ***P<0.001 compared to the control.
Discussion
The CSC theory indicates that malignant tumor is made up of heterogeneous neoplastic cells, among which a subpopulation exhibits unlimited self-renewal capacity and cell division potential to generate heterogeneous offspring [34]. This group of cancer cells is known as cancer stem cells or cancer initiating cells which propagate cancer initiation, development, invasion and metastasis [35]. Differing from the bulk population of tumor cells, CSCs display particular resistance to multiple cytotoxic chemotherapy drugs and are responsible for unsatisfied clinical outcomes and tumor relapse [35,36]. Therefore, a novel therapy that targets CSCs has the potential to be a more powerful cancer therapeutic strategy. So far, the best example involving inhibition of CSCs is treatment of APL with As2O3 which could effectively eradicate APL-derived stem cells and lead to improved overall survival [22,37,38]. Moreover, researchers found that As2O3 was effective in inhibition of CSCs in various solid tumors such as gliomas [24,39,40], hepatocellular carcinoma [25] and pancreatic cancer [41]. Our data showed that As2O3 significantly inhibited the survival of lung cancer cells in a dose- and time-dependent manner. In colony formation assay, NCI-H460 and NCI-H446 cells were treated with different concentrations of As2O3 for 72 hours; then equal numbers of viable cells per well were counted and seeded in 6-well culture plates. After incubation for 14 days, we observed that number of macroscopic colonies of As2O3-treated group was lesser than control group. So we speculated that As2O3 might inhibit CSLCs in NSCLC and SCLC cell lines. It is reported that CSCs has the ability of forming spherical colonies when cultured in serum-free medium, termed tumor spheres. Tumor sphere cells show increased proliferation, clonogenic potential, tumorigenic capacity as well as drug-resistant properties compared with monolayer cells [5,6]. Therefore, we performed sphere formation assay to validate our hypotheses. Our results revealed that low dose of As2O3 could dramatically decrease the size and the number of the tumor spheres, indicating the effects of As2O3 on stem cell-like subpopulations. Some researchers found that CD133+ lung cancer cells also display a spectrum of features consistent with CSCs, including clonogenic ability, tumorigenic capacity, multipotency and multidrug-resistant properties [7,42]. Patients with CD133+ tumors have shorter median progression-free survival and higher recurrence risks than patients with CD133- tumors [43-45]. We found that the expression of CD133 was significantly suppressed by As2O3 treatment. Additionally, As2O3 significantly decreased the expression of stem cell transcription factors, Oct4 and Sox2, which play crucial roles in the maintenance of multipotency and self-renewal of CSCs [9,46]. In a word, we found that low dose of As2O3 could inhibit stem cell-like subpopulations in lung cancer. It is very exciting for the potential clinical use of As2O3 in lung cancer treatment because of this drug’s minimal toxicity and lower economic burden for patients.
Hedgehog signaling pathway specifies the proliferation, differentiation and migration of normal stem cell [47,48]. Aberrant activated Hedgehog signaling pathway also plays an important role in the initiation and development of lung cancer and is required for the maintenance of LCSCs [15,18,29-32]. This pathway is composed of Hh ligands, Hh receptors (Ptch), Smoothened (Smo) and Gli protein [49]. Gli1 protein is the vital transcription factor of Hedgehog signaling pathway and contributes to activation of Hedgehog downstream genes. At present, a majority of Hedgehog antagonists exert its effects by binding to Smo. However, they displayed some limitations because constitutively activated mutations in Smo [13] and mutations in the downstream of Smo such as inactivated mutations in the inhibitory factor Sufu [50] or increased Gli expression [51,52] can lead to loss-of-function of these Hedgehog antagonists. Therefore, Gli1 may be a more effective target for blockade of Hedgehog signaling pathway. A previous study reported that As2O3 could block Hedgehog signaling by directly interaction with Gli1 protein [53]. Consistent with these results, our data showed that As2O3 down-regulated the expression levels of Gli1 and its target genes such as N-myc and GAS1. Besides, we showed that As2O3 caused degradation of Gli1 protein. A previous study established that As2O3 could directly bind to cysteine residues in the zinc fingers and induce PML oligomerization as well as ubiquitination, which lead to degradation of PML-RAR fusion protein [54]. It is highly plausible that As2O3 also binds to cysteine residues in the zinc finger domains in Gli1, thus causing Gli1 protein degradation. Furthermore, it has been reported that Gli1 appeared to regulate the stem cell transcription factors Sox2 and Oct4 [19,55]. Thus, we speculate that As2O3 modulate Sox2 and Oct4 expression through Gli1 blockade, leading to the inhibitory effects on CSLCs. However, this mechanism should be further investigated.
Therefore, when all of the data presented here are taken in whole, it suggests that: (a) As2O3 could inhibit the proliferation and colony formation ability of lung cancer cells. (b) The formation of tumor spheres was decreased by As2O3 treatment. (c) As2O3 markedly reduced the expression of stem cell biomarker CD133 and stem cell transcription factors such as Sox2 and Oct4. (d) As2O3 decreased the expression levels of Gli1 and its downstream genes such as N-myc and GAS1. In a word, As2O3 is a promising new approach to inhibit CSLCs in lung cancer, and the underlying mechanism may involve Gli1 blockade. As an FDA-approved drug, As2O3 has been widely used to treat patients with APL, and the security of the drug at therapeutic doses has been confirmed in human body. Our founding will provide a foundation for the application of As2O3 in the clinical treatment of lung cancer.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 81172227) and Research Foundation of Shanghai Municipal Education Commission (No. 12ZZ073). We sincerely thank Ping-Ping Zhang, from Department of Gastroenterology of Changhai Hospital, for her excellent support in our research.
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Alison MR, Lin WR, Lim SM, Nicholson LJ. Cancer stem cells: in the line of fire. Cancer Treat Rev. 2012;38:589–598. doi: 10.1016/j.ctrv.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 4.Sourisseau T, Hassan KA, Wistuba I, Penault-Llorca F, Adam J, Deutsch E, Soria JC. Lung Cancer Stem Cell Fancy Conceptual Model of Tumor Biology or Cornerstone of a Forthcoming Therapeutic Breakthrough? J Thorac Oncol. 2014;9:7–17. doi: 10.1097/JTO.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 5.Sun FF, Hu YH, Xiong LP, Tu XY, Zhao JH, Chen SS, Song J, Ye XQ. Enhanced expression of stem cell markers and drug resistance in sphere-forming non-small cell lung cancer cells. Int J Clin Exp Pathol. 2015;8:6287–6300. [PMC free article] [PubMed] [Google Scholar]
- 6.Qiu X, Wang Z, Li Y, Miao Y, Ren Y, Luan Y. Characterization of sphere-forming cells with stem-like properties from the small cell lung cancer cell line H446. Cancer Lett. 2012;323:161–170. doi: 10.1016/j.canlet.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Sarvi S, Mackinnon AC, Avlonitis N, Bradley M, Rintoul RC, Rassl DM, Wang W, Forbes SJ, Gregory CD, Sethi T. CD133+Cancer Stemlike Cells in Small Cell Lung Cancer Are Highly Tumorigenic and Chemoresistant but Sensitive to a Novel Neuropeptide Antagonist. Cancer Research. 2014;74:1554–1565. doi: 10.1158/0008-5472.CAN-13-1541. [DOI] [PubMed] [Google Scholar]
- 8.Hsu HS, Huang PI, Chang YL, Tzao C, Chen YW, Shih HC, Hung SC, Chen YC, Tseng LM, Chiou SH. Cucurbitacin I inhibits tumorigenic ability and enhances radiochemosensitivity in nonsmall cell lung cancer-derived CD133-positive cells. Cancer. 2011;117:2970–2985. doi: 10.1002/cncr.25869. [DOI] [PubMed] [Google Scholar]
- 9.Chen YC, Hsu HS, Chen YW, Tsai TH, How CK, Wang CY, Hung SC, Chang YL, Tsai ML, Lee YY, Ku HH, Chiou SH. Oct-4 expression maintained cancer stem-like properties in lung cancer-derived CD133-positive cells. PLoS One. 2008;3:e2637. doi: 10.1371/journal.pone.0002637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu K, Lin B, Zhao M, Yang X, Chen M, Gao A, Liu F, Que J, Lan X. The multiple roles for Sox2 in stem cell maintenance and tumorigenesis. Cell Signal. 2013;25:1264–1271. doi: 10.1016/j.cellsig.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao C, Chen A, Jamieson CH, Fereshteh M, Abrahamsson A, Blum J, Kwon HY, Kim J, Chute JP, Rizzieri D, Munchhof M, VanArsdale T, Beachy PA, Reya T. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 2009;458:776–779. doi: 10.1038/nature07737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peacock CD, Wang Q, Gesell GS, Corcoran-Schwartz IM, Jones E, Kim J, Devereux WL, Rhodes JT, Huff CA, Beachy PA, Watkins DN, Matsui W. Hedgehog signaling maintains a tumor stem cell compartment in multiple myeloma. Proc Natl Acad Sci U S A. 2007;104:4048–4053. doi: 10.1073/pnas.0611682104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie J, Murone M, Luoh SM, Ryan A, Gu Q, Zhang C, Bonifas JM, Lam CW, Hynes M, Goddard A, Rosenthal A, Epstein EH Jr, de Sauvage FJ. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391:90–92. doi: 10.1038/34201. [DOI] [PubMed] [Google Scholar]
- 14.Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007;17:165–172. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park KS, Martelotto LG, Peifer M, Sos ML, Karnezis AN, Mahjoub MR, Bernard K, Conklin JF, Szczepny A, Yuan J, Guo R, Ospina B, Falzon J, Bennett S, Brown TJ, Markovic A, Devereux WL, Ocasio CA, Chen JK, Stearns T, Thomas RK, Dorsch M, Buonamici S, Watkins DN, Peacock CD, Sage J. A crucial requirement for Hedgehog signaling in small cell lung cancer. Nat Med. 2011;17:1504–1508. doi: 10.1038/nm.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez-Blanco J, Schilling NS, Tokhunts R, Giambelli C, Long J, Liang Fei D, Singh S, Black KE, Wang Z, Galimberti F, Bejarano PA, Elliot S, Glassberg MK, Nguyen DM, Lockwood WW, Lam WL, Dmitrovsky E, Capobianco AJ, Robbins DJ. The hedgehog processing pathway is required for NSCLC growth and survival. Oncogene. 2013;32:2335–2345. doi: 10.1038/onc.2012.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu J, Rodova M, Roy SK, Sharma J, Singh KP, Srivastava RK, Shankar S. GANT-61 inhibits pancreatic cancer stem cell growth in vitro and in NOD/SCID/IL2R gamma null mice xenograft. Cancer Lett. 2013;330:22–32. doi: 10.1016/j.canlet.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong Z, Bi A, Chen D, Gao L, Yin Z, Luo L. Activation of hedgehog signaling pathway in human non-small cell lung cancers. Pathol Oncol Res. 2014;20:917–922. doi: 10.1007/s12253-014-9774-x. [DOI] [PubMed] [Google Scholar]
- 19.Bora-Singhal N, Perumal D, Nguyen J, Chellappan S. Gli1-Mediated Regulation of Sox2 Facilitates Self-Renewal of Stem-Like Cells and Confers Resistance to EGFR Inhibitors in Non-Small Cell Lung Cancer. Neoplasia. 2015;17:538–551. doi: 10.1016/j.neo.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antman KH. Introduction: the history of arsenic trioxide in cancer therapy. Oncologist. 2001;6(Suppl 2):1–2. doi: 10.1634/theoncologist.6-suppl_2-1. [DOI] [PubMed] [Google Scholar]
- 21.Mathews V, George B, Lakshmi KM, Viswabandya A, Bajel A, Balasubramanian P, Shaji RV, Srivastava VM, Srivastava A, Chandy M. Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: durable remissions with minimal toxicity. Blood. 2006;107:2627–2632. doi: 10.1182/blood-2005-08-3532. [DOI] [PubMed] [Google Scholar]
- 22.Zheng X, Seshire A, Rüster B, Bug G, Beissert T, Puccetti E, Hoelzer D, Henschler R, Ruthardt M. Arsenic but not all-trans retinoic acid overcomes the aberrant stem cell capacity of PML/RARα-positive leukemic stem cells. Haematologica. 2007;92:323–331. doi: 10.3324/haematol.10541. [DOI] [PubMed] [Google Scholar]
- 23.Hong Z, Xiao M, Yang Y, Han Z, Cao Y, Li C, Wu Y, Gong Q, Zhou X, Xu D, Meng L, Ma D, Zhou J. Arsenic disulfide synergizes with the phosphoinositide 3-kinase inhibitor PI-103 to eradicate acute myeloid leukemia stem cells by inducing differentiation. Carcinogenesis. 2011;32:1550–1558. doi: 10.1093/carcin/bgr176. [DOI] [PubMed] [Google Scholar]
- 24.Zhen Y, Zhao S, Li Q, Li Y, Kawamoto K. Arsenic trioxide-mediated Notch pathway inhibition depletes the cancer stem-like cell population in gliomas. Cancer Lett. 2010;292:64–72. doi: 10.1016/j.canlet.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Zhang KZ, Zhang QB, Zhang QB, Sun HC, Ao JY, Chai ZT, Zhu XD, Lu L, Zhang YY, Bu Y, Kong LQ, Tang ZY. Arsenic trioxide induces differentiation of CD133+ hepatocellular carcinoma cells and prolongs posthepatectomy survival by targeting GLI1 expression in a mouse model. J Hematol Oncol. 2014;7:28. doi: 10.1186/1756-8722-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qu GP, Xiu QY, Li B, Liu YA, Zhang LZ. Arsenic trioxide inhibits the growth of human lung cancer cell lines via cell cycle arrest and induction of apoptosis at both normoxia and hypoxia. Toxicol Ind Health. 2009;25:505–515. doi: 10.1177/0748233709345936. [DOI] [PubMed] [Google Scholar]
- 27.Yang MH, Zang YS, Huang H, Chen K, Li B, Sun GY, Zhao XW. Arsenic trioxide exerts antilung cancer activity by inhibiting angiogenesis. Curr Cancer Drug Targets. 2014;14:557–566. doi: 10.2174/1568009614666140725090000. [DOI] [PubMed] [Google Scholar]
- 28.Xie SL, Yang MH, Chen K, Huang H, Zhao XW, Zang YS, Li B. Efficacy of Arsenic Trioxide in the Treatment of Malignant Pleural Effusion Caused by Pleural Metastasis of Lung Cancer. Cell Biochem Biophys. 2015;71:1325–33. doi: 10.1007/s12013-014-0352-3. [DOI] [PubMed] [Google Scholar]
- 29.Maitah MY, Ali S, Ahmad A, Gadgeel S, Sarkar FH. Up-regulation of sonic hedgehog contributes to TGF-beta1-induced epithelial to mesenchymal transition in NSCLC cells. PLoS One. 2011;6:e16068. doi: 10.1371/journal.pone.0016068. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Velcheti V, Govindan R. Hedgehog signaling pathway and lung cancer. J Thorac Oncol. 2007;2:7–10. doi: 10.1097/JTO.0b013e31802c0276. [DOI] [PubMed] [Google Scholar]
- 31.Watkins DN, Berman DM, Burkholder SG, Wang B, Beachy PA, Baylin SB. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature. 2003;422:313–317. doi: 10.1038/nature01493. [DOI] [PubMed] [Google Scholar]
- 32.Cochrane CR, Szczepny A, Watkins DN, Cain JE. Hedgehog Signaling in the Maintenance of Cancer Stem Cells. Cancers (Basel) 2015;7:1554–1585. doi: 10.3390/cancers7030851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian F, Mysliwietz J, Ellwart J, Gamarra F, Huber RM, Bergner A. Effects of the Hedgehog pathway inhibitor GDC-0449 on lung cancer cell lines are mediated by side populations. Clin Exp Med. 2012;12:25–30. doi: 10.1007/s10238-011-0135-8. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida GJ, Saya H. Therapeutic strategies targeting cancer stem cells. Cancer Sci. 2016;107:5–11. doi: 10.1111/cas.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–699. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- 36.Wang J, Li ZH, White J, Zhang LB. Lung cancer stem cells and implications for future therapeutics. Cell Biochem Biophys. 2014;69:389–398. doi: 10.1007/s12013-014-9844-4. [DOI] [PubMed] [Google Scholar]
- 37.Chen GQ, Shi XG, Tang W, Xiong SM, Zhu J, Cai X, Han ZG, Ni JH, Shi GY, Jia PM, Liu MM, He KL, Niu C, Ma J, Zhang P, Zhang TD, Paul P, Naoe T, Kitamura K, Miller W, Waxman S, Wang ZY, de The H, Chen SJ, Chen Z. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells. Blood. 1997;89:3345–3353. [PubMed] [Google Scholar]
- 38.Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qiu QY, Zhu J, Tang W, Sun GL, Yang KQ, Chen Y, Zhou L, Fang ZW, Wang YT, Ma J, Zhang P, Zhang TD, Chen SJ, Chen Z, Wang ZY. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89:3354–3360. [PubMed] [Google Scholar]
- 39.Wu J, Ji Z, Liu H, Liu Y, Han D, Shi C, Shi C, Wang C, Yang G, Chen X, Shen C, Li H, Bi Y, Zhang D, Zhao S. Arsenic trioxide depletes cancer stem-like cells and inhibits repopulation of neurosphere derived from glioblastoma by downregulation of Notch pathway. Toxicol Lett. 2013;220:61–69. doi: 10.1016/j.toxlet.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 40.Sun H, Zhang S. Arsenic trioxide regulates the apoptosis of glioma cell and glioma stem cell via down-regulation of stem cell marker Sox2. Biochem Biophys Res Commun. 2011;410:692–697. doi: 10.1016/j.bbrc.2011.06.060. [DOI] [PubMed] [Google Scholar]
- 41.Han JB, Sang F, Chang JJ, Hua YQ, Shi WD, Tang LH, Liu LM. Arsenic trioxide inhibits viability of pancreatic cancer stem cells in culture and in a xenograft model via binding to SHH-Gli. Onco Targets Ther. 2013;6:1129–1138. doi: 10.2147/OTT.S49148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C, De Maria R. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–514. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- 43.Wu S, Yu L, Wang D, Zhou L, Cheng Z, Chai D, Ma L, Tao Y. Aberrant expression of CD133 in non-small cell lung cancer and its relationship to vasculogenic mimicry. BMC Cancer. 2012;12:535. doi: 10.1186/1471-2407-12-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mizugaki H, Sakakibara-Konishi J, Kikuchi J, Moriya J, Hatanaka KC, Kikuchi E, Kinoshita I, Oizumi S, Dosaka-Akita H, Matsuno Y, Nishimura M. CD133 expression: a potential prognostic marker for non-small cell lung cancers. Int J Clin Oncol. 2014;19:254–259. doi: 10.1007/s10147-013-0541-x. [DOI] [PubMed] [Google Scholar]
- 45.Li F, Zeng H, Ying K. The combination of stem cell markers CD133 and ABCG2 predicts relapse in stage I non-small cell lung carcinomas. Med Oncol. 2011;28:1458–1462. doi: 10.1007/s12032-010-9646-5. [DOI] [PubMed] [Google Scholar]
- 46.Chen S, Xu Y, Chen Y, Li X, Mou W, Wang L, Liu Y, Reisfeld RA, Xiang R, Lv D, Li N. SOX2 gene regulates the transcriptional network of oncogenes and affects tumorigenesis of human lung cancer cells. PLoS One. 2012;7:e36326. doi: 10.1371/journal.pone.0036326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 48.Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev. 2008;22:2454–2472. doi: 10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- 49.Robbins DJ, Fei DL, Riobo NA. The Hedgehog signal transduction network. Sci Signal. 2012;5:re6. doi: 10.1126/scisignal.2002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee Y, Kawagoe R, Sasai K, Li Y, Russell HR, Curran T, McKinnon PJ. Loss of suppressor-of-fused function promotes tumorigenesis. Oncogene. 2007;26:6442–6447. doi: 10.1038/sj.onc.1210467. [DOI] [PubMed] [Google Scholar]
- 51.Nolan-Stevaux O, Lau J, Truitt ML, Chu GC, Hebrok M, Fernandez-Zapico ME, Hanahan D. GLI1 is regulated through Smoothenedindependent mechanisms in neoplastic pancreatic ducts and mediates PDAC cell survival and transformation. Genes Dev. 2009;23:24–36. doi: 10.1101/gad.1753809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stein U, Eder C, Karsten U, Haensch W, Walther W, Schlag PM. GLI gene expression in bone and soft tissue sarcomas of adult patients correlates with tumor grade. Cancer Res. 1999;59:1890–1895. [PubMed] [Google Scholar]
- 53.Beauchamp EM, Ringer L, Bulut G, Sajwan KP, Hall MD, Lee YC, Peaceman D, Ozdemirli M, Rodriguez O, Macdonald TJ, Albanese C, Toretsky JA, Uren A. Arsenic trioxide inhibits human cancer cell growth and tumor development in mice by blocking Hedgehog/GLI pathway. J Clin Invest. 2011;121:148–160. doi: 10.1172/JCI42874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang XW, Yan XJ, Zhou ZR, Yang FF, Wu ZY, Sun HB, Liang WX, Song AX, Lallemand-Breitenbach V, Jeanne M, Zhang QY, Yang HY, Huang QH, Zhou GB, Tong JH, Zhang Y, Wu JH, Hu HY, de The H, Chen SJ, Chen Z. Arsenic trioxide controls the fate of the PML-RARalpha oncoprotein by directly binding PML. Science. 2010;328:240–243. doi: 10.1126/science.1183424. [DOI] [PubMed] [Google Scholar]
- 55.Batsaikhan BE, Yoshikawa K, Kurita N, Iwata T, Takasu C, Kashihara H, Shimada M. Cyclopamine decreased the expression of Sonic Hedgehog and its downstream genes in colon cancer stem cells. Anticancer Res. 2014;34:6339–6344. [PubMed] [Google Scholar]


