Abstract
Objectives: In the present study, we investigate the effects of Mas oncogene-related gene (Mrg) C receptors (MrgC) on the expression and activation of spinal Gi protein, N-methyl-D-aspartate receptor subunit 2B (NR2B), and neuronal nitric oxide synthase (nNOS) in mouse model of bone cancer pain. Methods: The number of spontaneous foot lift (NSF) and paw withdrawal mechanical threshold (PWMT) were measured after inoculation of tumor cells and intrathecal injection of MrgC agonist bovine adrenal medulla 8-22 (BAM8-22) or MrgC antagonist anti-MrgC for 14 days after operation. Expression of spinal MrgC, Gi protein, NR2B and nNOS and their phosphorylated forms after inoculation was examined by immunohistochemistry and Western blotting. Double labeling was used to identify the co-localization of NR2B or nNOS with MrgC in spinal cord dorsal horn (SCDH) neurons. The effects of intrathecal injection of BAM8-22 or anti-MrgC on nociceptive behaviors and the corresponding expression of spinal MrgC, Gi protein, NR2B and nNOS were also investigated. Results: The expression of spinal MrgC, Gi protein, NR2B, and nNOS was higher in tumor-bearing mice in comparison to sham mice or normal mice. Intrathecal injection of MrgC agonist BAM8-22 significantly alleviated bone cancer pain, up-regulated MrgC and Gi protein expression, and down-regulated the expression of spinal p-NR2B, t-nNOS and p-nNOS in SCDH on day 14 after operation, whereas administration of anti-MrgC produced the opposite effect. Meanwhile, MrgC-like immunoreactivity (IR) co-localizes with NR2B-IR or nNOS-IR in SCDH neurons. Conclusions: The present study demonstrates that MrgC-activated spinal Gi-NR2B-nNOS signaling pathway plays important roles in the development of bone cancer pain. These findings may provide a novel strategy for the treatment of bone cancer pain.
Keywords: MrgC, Gi protein, NR2B, nNOS, bone cancer pain, BAM8-22, anti-MrgC
Introduction
Bone cancer pain (BCP) that is characterized by persistent pain hypersensitivity, such as spontaneous pain and mechanical hyperalgesia, is a clinically challenging problem to be resolved [1]. As an agonist of Mas oncogene-related gene (Mrg) C receptor (MrgC), bovine adrenal medulla 8-22 (BAM8-22), a synthesized 15-amino acid peptide, has been demonstrated to relieve neuropathic pain and inflammatory pain by activating MrgC [2,3]. However, the effect of MrgC on bone cancer pain is still unclear.
MrgC belongs to the Mas oncogene-related gene (Mrg) G protein-coupled receptors, and is exclusively expressed in small-sized neurons in trigeminal (TG), spinal cord dorsal horn (SCDH) and dorsal root ganglia (DRG) in mammals [4]. After nociceptive stimulation, immunoreactive neuronal MrgC is reported to be increased in SCDH in adult male rats [5]. Activated MrgC may couple to different downstream targets (e.g., Gi and Gq), which can cause different cellular effects [6]. The precise mechanisms that underlie the analgesic effects of MrgC agonist remain unclear.
Abundant studies indicate that N-methyl-D-aspartate (NMDA) receptors containing a 2B subunit (NR2B) are restrictedly distributed in nociceptive transmission pathways such as SCDH, and are critical in various pain states [7,8]. Similarly, our previous study also shows that bone cancer pain hypersensitivity is associated with the up-regulation of spinal NR2B, and intrathecal administration of NR2B subunit-specific antagonist ifenprodil attenuates bone cancer pain in a mouse model [9].
Another response to neuronal activation in the sensory spinal cord areas is the expression of neuronal nitric oxide synthase (nNOS), which plays an important role in the development and maintenance of pain. Previous studies show that nNOS immunoreactive neurons in the lumbar enlargement of spinal cord are increased significantly, and induce pain-related behaviors [10,11]. In addition, intraperitoneal injection of nonspecific NOS antagonist significantly reduces thermal hyperalgesia caused by nerve damage [12]. Studies show that nNOS can be activated by NMDA receptor post-synaptically, and nitric oxide (NO)-producing neurons maintain and facilitate hyperalgesia [13,14]. NR2B integrates dynamically with cytoskeletal proteins and signal transduction factors such as nNOS and hence, participating in nociceptive signal transduction [15]. NR2B may be involved in nNOS-mediated synthesis of NO. Based on previous studies, we hypothesize that activation of spinal MrgC-Gi-NR2B-nNOS pathway mediates BCP in mouse model. In the present study, we investigate the effects of MrgC agonist BAM8-22 and antagonist anti-MrgC on the expression and activation of spinal MrgC, Gi protein, NR2B and nNOS in BCP.
Materials and methods
Animals
Adult male C3H/HeJ mice (4-6 weeks old; weighing 18-22 g) were obtained from Beijing Vital River Experimental Animal Center. Mice were habituated individually under a 12-h alternating light-dark cycle at constant room temperature (21 ± 1°C). The mice had free access to food and water. All experiments were approved by the Institutional Animal Care and Use Committee at the Medical School of Nanjing University and conformed to the guidelines for the use of laboratory animals [16]. All efforts were made to minimize animal suffering and to reduce the number of animals used in this study.
Cells
Osteosarcoma NCTC 2472 cells (2087787; American Type Culture Collection, Manassas, VA, USA) were incubated and subcultured in NCTC 135 medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% horse serum (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) at 37°C in an atmosphere of 5% CO2 and 95% air (Thermo Fisher Scientific, Waltham, MA, USA). The cells were passaged twice a week. The mouse model of BCP was constructed according to methods described by Schwei [17]. Briefly, the mice were anesthetized with intraperitoneal injection of 50 mg/kg pentobarbital sodium (1% in normal saline), and a superficial incision was made in the skin overlying the right articulatio genu with eye scissors. Gonarthrotomy was performed to expose the femur condyles. A light depression was made using a dental bur. A 30-gauge needle was used to perforate the cortex, and then a volume of 20 μl α-minimum essential medium containing no (sham group) or 2 × 105 NCTC 2472 cells (BCP group) were injected into the intramedullary space of the femur with a 25 μl microsyringe. Hereafter, dental amalgam was used to seal the injection hole, and normal saline was used for copious irrigation. The wound was closed at last.
Drug treatments
According to Jiang et al. [3], BAM8-22 (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in normal saline and then diluted to a final concentration of 8.0 nmol. Anti-MrgC (Biorbyt, San Francisco, CA, USA) was also dissolved in saline at a ratio of 1:20. Saline was used for vehicle treatment. The mice were divided into five groups: normal group (mice without any treatment, n = 12), sham group (mice injected with culture medium + vehicle, n = 20), BCP group (tumor-bearing mice + vehicle, n = 20), BCP + B group (tumor-bearing mice + BAM8-22, n = 20), and BCP + A group (tumor-bearing mice + anti-MrgC, n = 20). BAM8-22 solution (5 µl) or anti-MrgC solution (5 µl) were injected intrathecally (i.t.) on day 14 after operation according to Hylden and Wilcox [18]. The sham or BCP groups received 5 μl vehicle injection. The number of spontaneous foot lift (NSF) and paw withdrawal mechanical threshold (PWMT) were measured during a 2-week period: day 0 before operation and days 3, 5, 7, 10 and 14 after operation (including 0.5 h before intrathecal administration and 1 h, 2 h, 12 h, and 24 h after administration on day 14 after operation).
Behavioral tests
All tests were performed during the light phase. Mice were allowed to acclimatize for at least 30 min before each test. For spontaneous lifting behavioral test, the mice were housed in individual plexiglass compartments (10 cm × 10 cm × 15 cm) for 30 min and observed to count NSF of the right hind limb during 2 min. Every lift of the right hind limb not related to walking or grooming was considered to be one flinch. For mechanical allodynia test, von Frey filaments (Stoelting, Wood Dale, IL, USA) were used to assess mechanical allodynia as described by Chaplan et al. [19]. Mice were placed in individual transparent plexiglass compartments (10 cm × 10 cm × 15 cm) on a metal mesh floor (graticule: 0.5 cm graticule 0.5 cm). Mechanical threshold was measured using a set of von Frey filaments (0.16 g-2.0 g bending force). The filaments were poked vertically against the plantar surface with a sufficient force until causing slight bending against the paw. The force was held for 6-8 s with a 10-min interval between stimulations. Positive response was defined as brisk withdrawal or paw flinching. Sequentially increasing and decreasing stimulus strength (the “up-and-down” method) was used to determine PWMT. Each mouse hind paw was tested five times per stimulus strength. The cutoff force was 2.0 g. The lowest von Frey filaments that had three or more positive responses were regarded as PWMT.
Western blotting
Tissue samples were obtained from mice of each group at each time point. Mice were killed rapidly by decapitation after deep anesthesia. The spinal cord L3-L5 segments were removed rapidly and stored in liquid nitrogen. Tissue samples were homogenized in lysis buffer. The homogenate was centrifuged at 13,000 rpm for 10 minutes at 4°C, and the supernatant was removed. BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA) was used to determine protein concentrations, following the manufacturer’s instructions. Then, protein samples were separated on SDS-PAGE gel and transferred to polyvinylidene difluoride filters (Millipore, Billerica, MA, USA). The filters were blocked with 5% nonfat milk and then incubated with rabbit anti-MrgC primary antibody (1:500; Biorbyt, San Francisco, CA, USA), mouse monoclonal anti-NR2B antibody (1:1000; Abcam, Cambridge, UK) and mouse monoclonal anti-nNOS antibody (1:500; Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA), respectively. The membranes were washed with Tris-HCl buffer and incubated with secondary antibody of polyclonal goat anti-rabbit IgG (1:5000; Abcam, Cambridge, UK) or polyclonal goat anti-mouse IgG (1:5000; Abcam, Cambridge, UK). Immunoblots were visualized in electro-chemi-luminescence solution (Santa Cruz Biotechnology, Dallas, TX, USA) for 1 minute and exposed to hyper-films (Amersham Biosciences, Piscataway, NJ, USA) for 1 to 10 minutes. β-actin (1:1000; Abcam, Cambridge, UK) was used as a loading control for total protein. The gray value of each band was quantified with a computer-assisted imaging analysis system (IPLab software, Scanalytics, Fairfax, VA, USA).
Immunohistochemistry
The tissue samples were obtained from mice for immunohistochemistry. Under deep anesthesia with pentobarbital sodium (50 mg/kg, i.p.), the spinal cord L3-L5 segments were extracted and perfused transcardially with 4% paraformaldehyde in 0.1 M phosphate buffer at pH 7.4. The immunohistochemistry was performed according to previously published methods [20]. Serially frozen spinal cord sections were cut on a sliding microtome to a thickness of 25 mm, collected in phosphate-buffered saline, and processed as free-floating sections. After washing in phosphate-buffered saline, the sections were blocked for 60 min at room temperature with 10% (v/v) normal fetal bovine serum. The primary antibody of rabbit polyclonal anti-MrgC antibody (1:250; Biorbyt, San Francisco, CA, USA), mouse monoclonal anti-NR2B antibody (1:500; Abcam, Cambridge, UK) and mouse monoclonal anti-nNOS antibody (1:250; Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) were used, and samples were then incubated for 48 hours at 4°C. After washing, the secondary antibody Alexa Fluor 594 Goat anti-rabbit IgG (1:500; Abcam, Cambridge, UK) or/and Alexa Fluor 488 Goat anti-mouse IgG (1:500; Abcam, Cambridge, UK) were used, and samples were further incubated at 4°C overnight. Sections were mounted on glass slides, air-dried, and covered with coverslips by using Aquamount (Thermo Fisher Scientific, Waltham, MA, USA). Images were taken at 200× magnification by using the Leica TCS SP2 multiphoton confocal microscope (Leica Microsystems, Wetzlar, Germany). Images were randomly selected for further analysis. Image-Pro Plus analysis software (Media Cybernetics, Inc., Rockville, MD, USA) was adopted to analyze the fluorescence intensities of these images from different groups. For double immunostaining of MrgC with NR2B and nNOS, MrgC-IR shows red, whereas NR2B-IR and nNOS-IR show green. Images were captured using a laser scanning confocal microscope (TCS-SP2, Leica Microsystems, Wetzlar, Germany). If they co-localized with each other, yellow is shown.
Statistical analyses
All data were expressed as means ± standard deviations. Animals were assigned to different treatment groups in a randomized way. Repeated measures ANOVA was performed to determine overall differences at each time point in spontaneous lifting behavior and PWMT. One-way ANOVA was used to determine differences in the expression of proteins among all experiment groups. In both cases, when significant main effects were observed, LSD post hoc tests were performed to determine the source(s) of differences. P < 0.05 was considered to be statistically significant.
Results
Mice with BCP have prolonged NSF and decreased PWMT compared with normal and sham groups of mice
To assess pain behaviors following operation, spontaneous lifting behavior and mechanical allodynia experiments were performed. Before operation, there was no significant difference in NSF or PWMT between groups. On day 3 after operation, both BCP and sham groups showed spontaneous lifting behavior in the right hind limb. On day 5, NSF gradually returned to the level before operation. However, BCP mice showed spontaneous lifting behavior on day 7 and NSF was gradually increased over time. By contrast, only occasional lifting of the right hind limb was observed in normal or sham group from day 7 to day 14 (Figure 1A). Moreover, the ipsilateral hind limb in both BCP and sham groups showed significantly decreased PWMT in response to von Frey filaments stimulation on day 3, and PWMT was recovered to the level on day 0 on day 5. However, BCP mice showed a decrease in PWMT of the right hind limb on day 7, and PWMT was gradually decreased over time until day 14. In addition, no significant difference was observed in PWMT of normal or sham group (Figure 1B). These results suggest that mice with BCP have prolonged NSF and decreased PWMT compared with normal and sham groups of mice.
Figure 1.
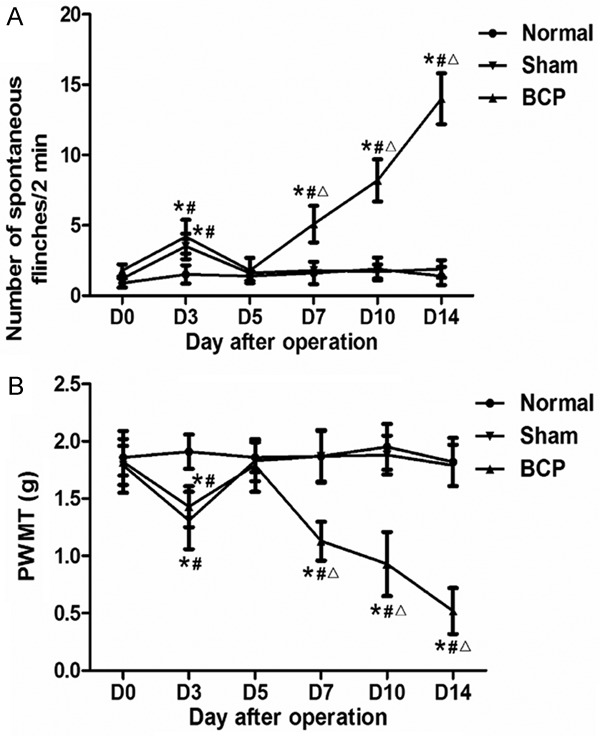
Changes in pain behaviors of the right hind limb over time in BCP mice, sham mice and normal mice (n = 8 each). A. NSF over 2 min in BCP, sham and normal mice. B. Paw withdraw mechanical threshold (PWMT) in BCP, sham and normal mice. NSF and PWMT were examined at six time points (day 0 before operation and days 3, 5, 7, 10 and 14 after operation). *, P < 0.05 compared with baseline; #, P < 0.05 compared with normal group; Δ, P < 0.05 compared with sham group.
Up-regulation of MrgC, Gi, t-nNOS, p-NR2B and p-nNOS proteins are involved in the development and maintenance of BCP
To determine the influence of BCP on MrgC-Gi-NR2B-nNOS pathway, Western blotting was performed to study the expression levels of spinal MrgC, Gi, NR2B and nNOS proteins and their phosphorylation state on days 3, 5, 7, 10 and 14 after operation. The data showed that inoculation of tumor cells in mice up-regulated the expression levels of MrgC, Gi, p-NR2B, t-nNOS and p-nNOS. The expression levels of spinal MrgC, Gi, p-NR2B, t-nNOS and p-nNOS of BCP mice began to increase on day 7 after operation, and gradually increased over time compared with normal and sham mice (P < 0.05). Meanwhile, there was no significant difference in the expression of total NR2B among groups (Figure 2). These results indicate that up-regulation of MrgC, Gi, t-nNOS and phosphorylation of NR2B and nNOS proteins are involved in the development and maintenance of BCP.
Figure 2.
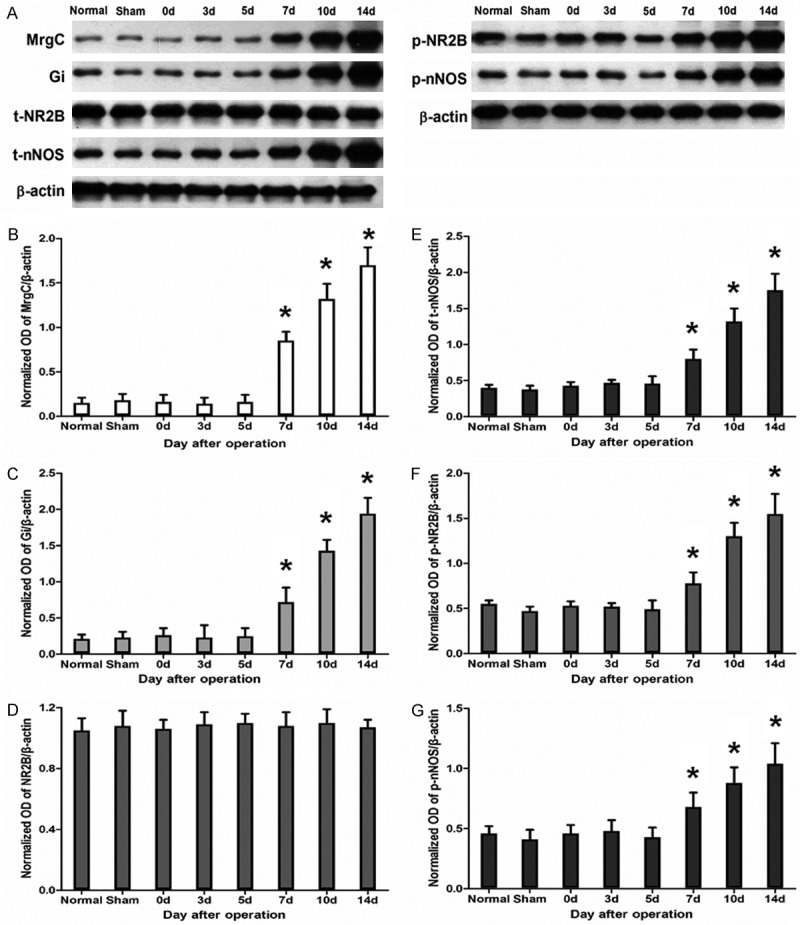
Changes in spinal MrgC, Gi, NR2B, p-NR2B, nNOS and p-nNOS protein expression after tumor cell inoculation (n = 6 each). (A) Protein expression of MrgC, Gi, t-NR2B, p-NR2B, t-nNOS and p-nNOS assessed using Western blotting. β-actin was used as a loading control. Quantification of the normalized OD of (B) MrgC/β-actin, (C) Gi/β-actin, (D) t-NR2B/β-actin, (E) t-nNOS/β-actin, (F) p-NR2B/β-actin, and (G) p-nNOS/β-actin. *, P < 0.05 compared with sham group.
Intrathecal administration of MrgC agonist or antagonist changes pain behaviors induced by BCP
To study the effect of MrgC on cancer pain, we measured NSF and PWMT on day 14 after operation. Compared with the baseline before injection or vehicle-injected BCP mice at the same time point, intrathecal injection of BAM8-22 reduced NSF and increased PWMT in the ipsilateral hind paw of BCP mice (P < 0.05). By contrast, NSF was dramatically increased and PWMT was dramatically decreased after injection of anti-MrgC (P < 0.05). Of note, the efficacy of BAM8-22 and anti-MrgC reached maximum at 2 h, but declined at 12 h and vanished at 24 h. At all time points, NSF and PWMT never reached the level of sham group (Figure 3). These results suggest that intrathecal administration of MrgC agonist or antagonist changes pain behaviors induced by BCP.
Figure 3.
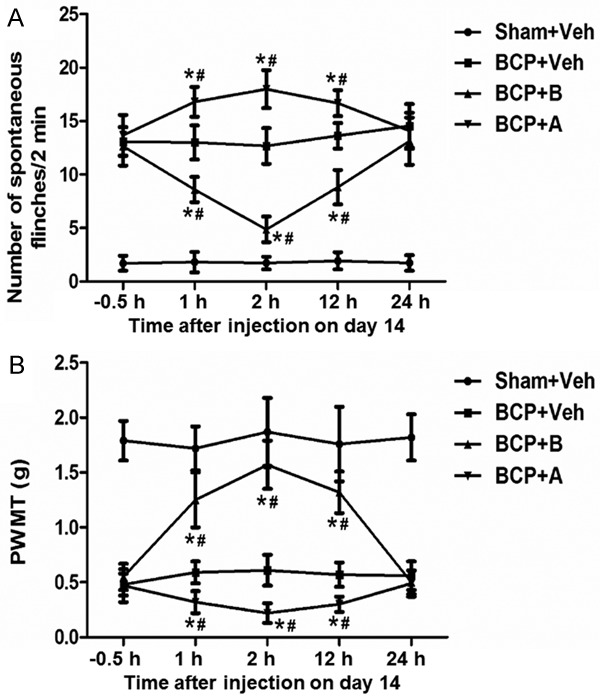
The effect of intrathecal injection of BAM8-22 and anti-MrgC on BCP-related behaviors on day 14 (n = 8 each). (A) The number of spontaneous flinches (NSF) over 2 min and (B) paw withdraw mechanical threshold (PWMT) were tested at 0.5 h pre-injection and 1 h, 2 h, 12 h, and 24 h after injection. *, P < 0.05 compared with BCP + vehicle group; #, P < 0.05 compared with baseline before injection.
MrgC activity regulates the expression of spinal Gi, NR2B and nNOS proteins in BCP
To investigate the effect of activated MrgC on Gi-NR2B-nNOS pathway in spinal dorsal horn, we measured the expression of MrgC, Gi, t-NR2B, p-NR2B, t-nNOS and p-nNOS using Western blotting. Compared with the BCP group of mice that received vehicle by intrathecal injection on day 14 after operation, MrgC agonist BAM8-22 up-regulated the expression of spinal MrgC and Gi proteins, but down-regulated p-NR2B, t-nNOS and p-nNOS proteins on day 14 after operation. Moreover, MrgC antagonist anti-MrgC down-regulated the expression of spinal MrgC and Gi proteins, but up-regulated p-NR2B, nNOS and p-nNOS proteins compared with BCP mice that received intrathecal injection of vehicle on day 14 after operation. The effect of BAM8-22 on MrgC, Gi, p-NR2B, t-nNOS and p-nNOS expression began at 1 h after administration, reached maximum at 2 h, attenuated at 12 h and disappeared at 24 h (Figure 4). To identify the co-localization of MrgC receptors with NR2B or nNOS, immunohistochemical staining was performed. Staining for MrgC receptors was diffusely distributed throughout the cytoplasm except for the nucleus. In addition, NR2B or nNOS was localized in the soma of small- and medium-sized SCDH neurons. Co-localization of MrgC receptors with NR2B or nNOS was indicated by yellow staining (Figure 5). These results suggest that activation of MrgC function up-regulates the expression of Gi protein and down-regulates the expression of proteins in the NR2B/nNOS pathway, while inhibition of MrgC activity produces the opposite effect.
Figure 4.
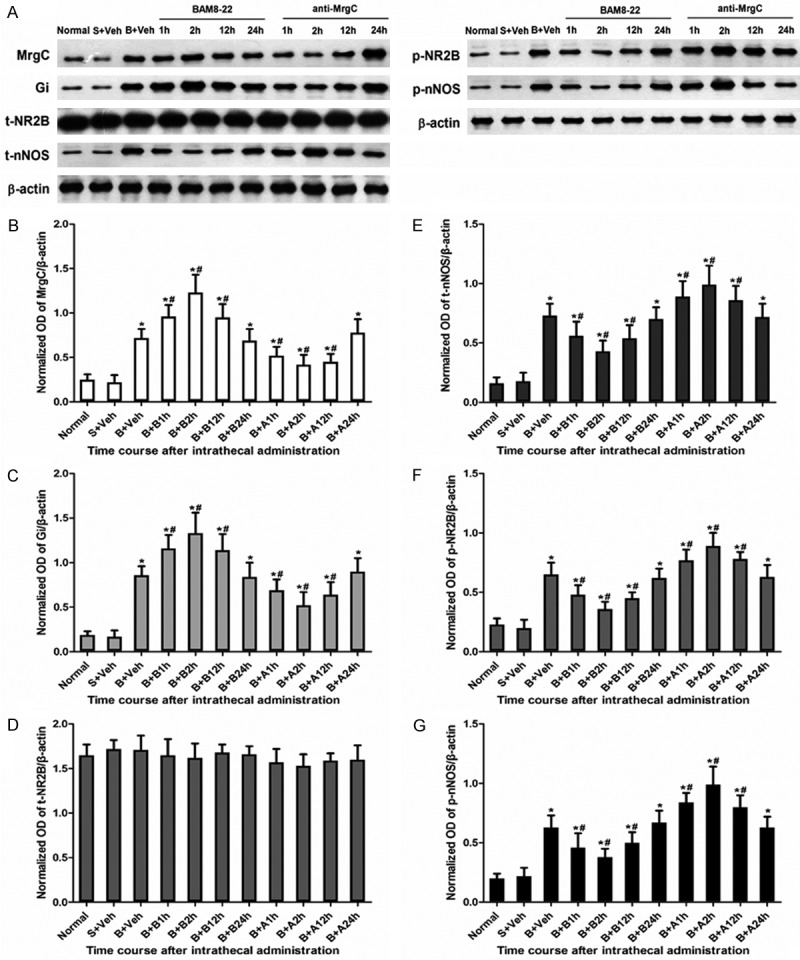
Changes in spinal MrgC, Gi, NR2B, p-NR2B, t-nNOS and p-nNOS protein expression at different time points after intrathecal injection of BAM8-22 and anti-MrgC on day 14 after operation (n = 6 each). (A) Protein expression of MrgC, Gi, t-NR2B, p-NR2B, t-nNOS and p-nNOS assessed using Western blotting. β-actin was used as a loading control. Quantification of the normalized OD of (B) MrgC/β-actin, (C) Gi/β-actin, (D) t-NR2B/β-actin, (E) nNOS/β-actin, (F) p-NR2B/β-actin, and (G) p-nNOS/β-actin. *, P < 0.05 compared with sham + vehicle group; #, P < 0.05 compared with BCP + vehicle group.
Figure 5.
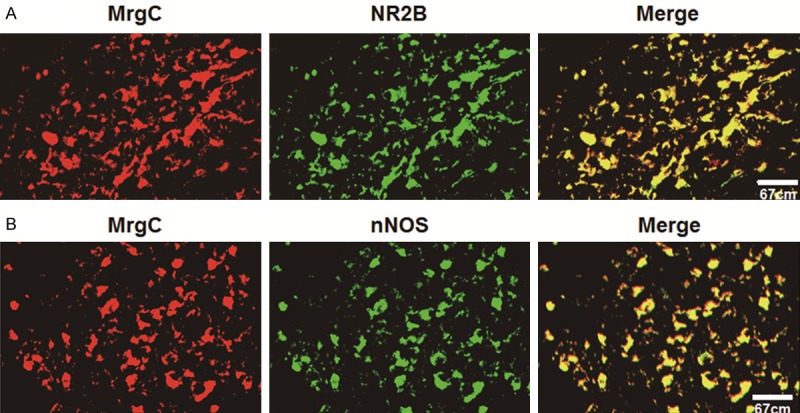
Confocal images showing the localization of (A) NR2B-IR or (B) nNOS-IR with MrgC-IR in SCDH neurons. (A) NR2B-IR neurons are identified by Alexa Fluor 488 fluorescence (green), whereas MrgC-IR-positive neurons by Alexa Fluor 594 (red). Co-localization of NR2B-IR with MrgC-IR in DRG neurons is identified by yellow. (B) nNOS-IR neurons are identified by Alexa Fluor 488 fluorescence (green) whereas MrgC-IR-positive neurons by Alexa Fluor 594 (red). Co-localization of nNOS-IR with MrgC-IR in DRG neurons is identified by yellow. Scale bar = 50 μm.
Discussion
The present study and our previous studies demonstrate that inoculation of sarcoma cells in the femur of male C3H/HeJ mice produces progressive spontaneous flinches and mechanical allodynia, and tumor infiltration and bone destruction induced by sarcoma cells have been demonstrated by hematoxylin-eosin staining and X-ray imaging [20,21]. BCP-related behaviors tend to be stable on day 14 after operation, so we chose the time point to study the analgesic effect of activated MrgC on Gi-NR2B-nNOS pathway in BCP mice model.
Recent studies suggest that spinal sensitization may be one of the underlying mechanisms for the development of chronic cancer pain, including BCP [22]. Our results show that hyperalgesia and allodynia induced by BCP are associated with the up-regulation of MrgC, Gi, and p-NR2B and nNOS in the spinal cord on day 14 after operation compared with that of normal group or sham group, indicating that spinal MrgC, Gi, NR2B and nNOS play an important role in the development of BCP. NMDA receptor, as an ionotropic glutamatergic, and voltage- and ligand-gated receptor, demonstrates high permeability to Ca2+ and is responsible for pain signal transduction and regulation induced by various kinds of tissue injury. Specifically, NR2B subunit-dependent synaptic plasticity in the pain pathway may contribute to central sensitization [23] and may be involved in the activation of nNOS and the synthesis of NO, referring to an increased synaptic excitability based on somatosensory neurons in the spinal cord and underlying the central mechanisms of BCP [24,25]. In addition, intrathecal administration of NR2B-selective NMDA receptor antagonists ifenprodil or Ro25-6981, produces great analgesic effects in various pain models, including BCP [9]. Furthermore, phosphorylation of NR2B at Tyr1472, which is important for stable surface expression of NMDA receptor, regulates NMDA receptor activity and plays a key role in synaptic plasticity [26]. Therefore, a series of studies are carried out, focusing on NR2B and p-NR2B, the key modulators in pain signal transduction. However, NR2B antagonists have limited utility in clinical patients because activated NMDA receptor is essential for many key physiological functions, and numerous intolerable side effects, such as memory impairment and psychotomimetic effects. Therefore, exploring novel ways to modulate NMDA receptor without affecting basal receptor activity may be a better analgesic strategy.
Some studies show that MrgC plays an important role in pain sensation [2,27]. Activation of MrgC produces analgesia in neuropathic pain and inflammatory pain. As MrgC are highly restrictedly distributed in DRG, activation of MrgC only exhibits analgesic activity instead of adverse effects [28]. Therefore, MrgC may be the potential target of persistent pain conditions, leading to the discovery of effective therapeutic agents. In the present study, the expression of spinal MrgC is up-regulated on day 14 after operation, and MrgC agonist BAM8-22 significantly inhibits NSF and PWMT induced by BCP in mice at 2 h after intrathecal administration. The antihyperalgesic efficacy reaches maximum, but pain behaviors are still not restored to the level of sham mice. Relief of BCP behaviors by BAM8-22 attenuates at 12 h and disappears at 24 h. However, spinal NR2B and nNOS are decreased after BAM8-22 injection. Therefore, our results indicate that changes of MrgC activity affect the expression of MrgC, Gi, p-NR2B, t-nNOS and p-nNOS. Furthermore, activation of MrgC attenuates BCP-induced NR2B and nNOS expression, and MrgC co-localizes with NR2B or nNOS in SCDH neurons. First, MrgC is located specifically in small-diameter DRG neurons, which is involved in pain signal transmission. After peripheral nerve injury, the expression of MrgC in SCDH is increased, being similar to pain behaviors [2]. Our research has confirmed that the expression of MrgC is increased during BCP, and intrathecal injection of BAM8-22 inhibits pain behaviors. In addition, enhancement of MrgC activity reduces NSF and increases PWMT. Second, a recent study suggests that MrgC may couple to Gi pathways and thus cause analgesia effects [29]. The inhibition of high-voltage-activated Ca2+ channels by BAM8-22 is pertussis toxin-sensitive, indicating the involvement of Gi pathway [30]. Activation of MrgC inhibits HVA Ca2+ currents in native rodent DRG neurons. MrgC agonists reduce presynaptic neurotransmitter release into dorsal horn, thereby inhibiting pain. In addition, MrgC have no effect in pain processing under normal conditions, because activation of MrgC does not alter the basal nociceptive thresholds [31]. Therefore, activated MrgC/Gi signaling pathway may play a crucial role in peripheral analgesia.
The close correlation between nNOS and pain has been reported. NO-producing neurons maintain and facilitate hyperalgesia [32]. Peripheral nerve injury may result in increased excitability of spinal cord neurons through the activation of nociceptive afferents, leading to spinal production of NO. Therefore, an altered expression of spinal nNOS and its enhanced catalytic activity may contribute to neuroplasticity after nerve injury and may play an important role in central sensitization. In our previous study, BCP also produces a unilateral increase in the level of nNOS positive neurons in the superficial layers within laminae I-III [33]. Marked increase of nNOS expression in DRG and spinal cord contributes to spinal sensory processing in neuropathic pain model [34]. Intrathecal injection of selective nNOS inhibitor 7-nitroindole in NOS-deficient mice reveals that nNOS is the most important NO-producing enzyme in the spinal cord during the development and maintenance of neuropathic pain [35]. As a downstream target of NMDA receptor, nNOS contributes greatly to the incidence of pain [15]. The selective NR2B antagonist can inhibit the increase of nNOS activity in the spinal cord. Reuss and Reuss report that NMDA receptor activates NOS postsynaptically to produce NO release to pre-synaptically membrane and up-regulates the function of NMDA receptor [36]. Therefore, NMDA receptor and NOS comprise a local circuit that amplifies the signals of nociceptive transmission. Our data show that the activation of NMDA-nNOS pathway contributes to the development of BCP, and that the phosphorylation of both NR2B and nNOS is inhibited by BAM8-22. It is reported that BAM8-22 reduces inflammatory pain and neuropathic pain, which may be associated with the inhibition of the up-regulation of nNOS, transient receptor potential vanilloid subfamily member 1 and calcitonin gene related peptide as well as c-Fos expression in SCDH or DRG [3,37]. Intrathecal BAM8-22 dose-dependently diminishes NMDA-evoked nocifensive behaviors [38]. No system or NMDA receptor system, as important pathway to modulate nociceptive transmission, may be involved in these nociceptive effects. Our results show that MrgC agonist BAM8-22 administered intrathecally inhibits hyperalgesic effect, suggesting that NR2B and nNOS may be involved in hyperalgesia elicited by BCP. Therefore, our results indicate that analgesic effect induced by BAM8-22 may be related with the activation of spinal MrgC-Gi-NR2B/nNOS signal pathway in BCP. However, the mechanism of this regulation needs further investigation. In conclusion, the present study demonstrates that the expression of MrgC, Gi, NR2B and nNOS in spinal cord is increased by BCP in mice, and intrathecal MrgC agonist BAM8-22 reduces hyperalgesic effect and nociceptive behavioral responses. MrgC antagonist anti-MrgC induces pain behaviors and inhibits the activity of MrgC-Gi-NR2B-nNOS signaling pathway, which may be mediated by Gi-NR2B system-nNOS system in the spinal cord. These results potentially help understand the roles of MrgC in pain transmission. The preclinical findings of BAM8-22 in the present study have positive effects on the clinical applications of this drug in the treatment of BCP-related behaviors.
Acknowledgements
This research was supported by National Natural Science Foundation of China (Nos. 81400914, 81171048, 81371207, 81300950, and 81171047), Natural Science Foundation of Jiangsu Province, China (Nos. RC2011006, and XK201140), and Medical Technology Development Project of Nanjing (Nos. YKK13068, and YKK13070).
Disclosure of conflict of interest
None.
References
- 1.Pan HL, Zhang YQ, Zhao ZQ. Involvement of lysophosphatidic acid in bone cancer pain by potentiation of TRPV1 via PKC pathway in dorsal root ganglion neurons. Mol Pain. 2010;6:85. doi: 10.1186/1744-8069-6-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He SQ, Li Z, Chu YX, Han L, Xu Q, Li M, Yang F, Liu Q, Tang Z, Wang Y, Hin N, Tsukamoto T, Slusher B, Tiwari V, Shechter R, Wei F, Raja SN, Dong X, Guan Y. MrgC agonism at central terminals of primary sensory neurons inhibits neuropathic pain. Pain. 2014;155:534–44. doi: 10.1016/j.pain.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang J, Wang D, Zhou X, Huo Y, Chen T, Hu F, Quirion R, Hong Y. Effect of Mas-related gene (Mrg) receptors on hyperalgesia in rats with CFA-induced inflammation via direct and indirect mechanisms. Br J Pharmacol. 2013;170:1027–1040. doi: 10.1111/bph.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lembo PM, Grazzini E, Groblewski T, O’Donnell D, Roy MO, Zhang J, Hoffert C, Cao J, Schmidt R, Pelletier M, Labarre M, Gosselin M, Fortin Y, Banville D, Shen SH, Strom P, Payza K, Dray A, Walker P, Ahmad S. Proenkephalin A gene products activate a new family of sensory neuron--specific GPCRs. Nat Neurosci. 2002;5:201–209. doi: 10.1038/nn815. [DOI] [PubMed] [Google Scholar]
- 5.Hager UA, Hein A, Lennerz J, Zimmermann K, Neuhuber W, Reeh P. Morphological characterization of rat Mas-related G-protein-coupled receptor C and functional analysis of agonists. Neuroscience. 2008;151:242–254. doi: 10.1016/j.neuroscience.2007.09.085. [DOI] [PubMed] [Google Scholar]
- 6.Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Bautista DM. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci. 2011;14:595–602. doi: 10.1038/nn.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swartjes M, Morariu A, Niesters M, Aarts L, Dahan A. Nonselective and NR2B-selective N-methyl-D-aspartic acid receptor antagonists produce antinociception and long-term relief of allodynia in acute and neuropathic pain. Anesthesiology. 2011;115:165–174. doi: 10.1097/ALN.0b013e31821bdb9b. [DOI] [PubMed] [Google Scholar]
- 8.Xu L, Pan Y, Zhu Q, Gong S, Tao J, Xu GY, Jiang X. Arcuate Src activation-induced phosphorylation of NR2B NMDA subunit contributes to inflammatory pain in rats. J Neurophysiol. 2012;108:3024–3033. doi: 10.1152/jn.01047.2011. [DOI] [PubMed] [Google Scholar]
- 9.Gu X, Zhang J, Ma Z, Wang J, Zhou X, Jin Y, Xia X, Gao Q, Mei F. The role of N-methyl-D-aspartate receptor subunit NR2B in spinal cord in cancer pain. Eur J Pain. 2010;14:496–502. doi: 10.1016/j.ejpain.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Wang HY, Tsai YJ, Chen SH, Lin CT, Lue JH. Lysophosphatidylcholine causes neuropathic pain via the increase of neuronal nitric oxide synthase in the dorsal root ganglion and cuneate nucleus. Pharmacol Biochem Behav. 2013;106:47–56. doi: 10.1016/j.pbb.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Čížková D, Lukáčová N, Maršala M, Maršala J. Neuropathic pain is associated with alterations of nitric oxide synthase immunoreactivity and catalytic activity in dorsal root ganglia and spinal dorsal horn. Brain Res Bull. 2002;58:161–171. doi: 10.1016/s0361-9230(02)00761-x. [DOI] [PubMed] [Google Scholar]
- 12.Mukherjee P, Cinelli MA, Kang S, Silverman RB. Development of nitric oxide synthase inhibitors for neurodegeneration and neuropathic pain. Chem Soc Rev. 2014;43:6814–6838. doi: 10.1039/c3cs60467e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coleman CG, Wang G, Park L, Anrather J, Delagrammatikas GJ, Chan J, Zhou J, Iadecola C, Pickel VM. Chronic intermittent hypoxia induces NMDA receptor-dependent plasticity and suppresses nitric oxide signaling in the mouse hypothalamic paraventricular nucleus. J Neurosci. 2010;30:12103–12112. doi: 10.1523/JNEUROSCI.3367-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma ZL, Zhang W, Gu XP, Yang WS, Zeng YM. Effects of intrathecal injection of prednisolone acetate on expression of NR2B subunit and nNOS in spinal cord of rats after chronic compression of dorsal root ganglia. Ann Clin Lab Sci. 2007;37:349–355. [PubMed] [Google Scholar]
- 15.Mihara Y, Egashira N, Sada H, Kawashiri T, Ushio S, Yano T, Ikesue H, Oishi R. Involvement of spinal NR2B-containing NMDA receptors in oxaliplatin-induced mechanical allodynia in rats. Mol Pain. 2011;7:8. doi: 10.1186/1744-8069-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- 17.Schwei MJ, Honore P, Rogers SD, Salak-Johnson JL, Finke MP, Ramnaraine ML, Clohisy DR, Mantyh PW. Neurochemical and cellular reorganization of the spinal cord in a murine model of bone cancer pain. J Neurosci. 1999;19:10886–10897. doi: 10.1523/JNEUROSCI.19-24-10886.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- 19.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 20.Ren BX, Gu XP, Zheng YG, Liu CL, Wang D, Sun YE, Ma ZL. Intrathecal injection of metabotropic glutamate receptor subtype 3 and 5 agonist/antagonist attenuates bone cancer pain by inhibition of spinal astrocyte activation in a mouse model. Anesthesiology. 2012;116:122–132. doi: 10.1097/ALN.0b013e31823de68d. [DOI] [PubMed] [Google Scholar]
- 21.Ni K, Zhou Y, Sun YE, Liu Y, Gu XP, Ma ZL. Intrathecal injection of selected peptide Myr-RC-13 attenuates bone cancer pain by inhibiting KIF17 and NR2B expression. Pharmacol Biochem Behav. 2014;122:228–233. doi: 10.1016/j.pbb.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 22.Yanagisawa Y, Furue H, Kawamata T, Uta D, Yamamoto J, Furuse S, Katafuchi T, Imoto K, Iwamoto Y, Yoshimura M. Bone cancer induces a unique central sensitization through synaptic changes in a wide area of the spinal cord. Mol Pain. 2010;6:38. doi: 10.1186/1744-8069-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qu XX, Cai J, Li MJ, Chi YN, Liao FF, Liu FY, Wan Y, Han JS, Xing GG. Role of the spinal cord NR2B-containing NMDA receptors in the development of neuropathic pain. Exp Neurol. 2009;215:298–307. doi: 10.1016/j.expneurol.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 24.Ren F, Jiao H, Cai H. Analgesic Effect of Intrathecal Administration of Chemokine Receptor CCR2 Antagonist is Related to Change in Spinal NR2B, nNOS, and SIGIRR Expression in Rat with Bone Cancer Pain. Cell Biochem Biophys. 2015 doi: 10.1007/s12013-014-0510-7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Abe T, Matsumura S, Katano T, Mabuchi T, Takagi K, Xu L, Yamamoto A, Hattori K, Yagi T, Watanabe M. Fyn kinase-mediated phosphorylation of NMDA receptor NR2B subunit at Tyr1472 is essential for maintenance of neuropathic pain. Eur J Neurosci. 2005;22:1445–54. doi: 10.1111/j.1460-9568.2005.04340.x. [DOI] [PubMed] [Google Scholar]
- 26.Guo W, Wei F, Zou S, Robbins MT, Sugiyo S, Ikeda T, Tu JC, Worley PF, Dubner R, Ren K. Group I metabotropic glutamate receptor NMDA receptor coupling and signaling cascade mediate spinal dorsal horn NMDA receptor 2B tyrosine phosphorylation associated with inflammatory hyperalgesia. J Neurosci. 2004;24:9161–9173. doi: 10.1523/JNEUROSCI.3422-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang D, Chen T, Zhou X, Couture R, Hong Y. Activation of Mas oncogene-related gene (Mrg) C receptors enhances morphine-induced analgesia through modulation of coupling of μ-opioid receptor to Gi-protein in rat spinal dorsal horn. Neuroscience. 2013;253:455–464. doi: 10.1016/j.neuroscience.2013.08.069. [DOI] [PubMed] [Google Scholar]
- 28.Hager UA, Hein A, Lennerz JK, Zimmermann K, Neuhuber WL, Reeh PW. Morphological characterization of rat Mas-related G-proteincoupled receptor C and functional analysis of agonists. Neuroscience. 2008;151:242–254. doi: 10.1016/j.neuroscience.2007.09.085. [DOI] [PubMed] [Google Scholar]
- 29.Han SK, Dong X, Hwang JI, Zylka MJ, Anderson DJ, Simon MI. Orphan G protein-coupled receptors MrgA1 and MrgC11 are distinctively activated by RF-amide-related peptides through the Gαq/11 pathway. Proc Natl Acad Sci U S A. 2002;99:14740–5. doi: 10.1073/pnas.192565799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen H, Ikeda SR. Modulation of ion channels and synaptic transmission by a human sensory neuron-specific G-protein-coupled receptor, SNSR4/mrgX1, heterologously expressed in cultured rat neurons. J Neurosci. 2004;24:5044–5053. doi: 10.1523/JNEUROSCI.0990-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai Q, Jiang J, Chen T, Hong Y. Sensory neuron-specific receptor agonist BAM8-22 inhibits the development and expression of tolerance to morphine in rats. Behav Brain Res. 2007;178:154–159. doi: 10.1016/j.bbr.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 32.Gong L, Gao F, Li J, Li J, Yu X, Ma X, Zheng W, Cui S, Liu K, Zhang M, Kunze W, Liu CY. Oxytocin-induced membrane hyperpolarization in pain-sensitive dorsal root ganglia neurons mediated by Ca(2+)/nNOS/NO/KATP pathway. Neuroscience. 2015;289:417–428. doi: 10.1016/j.neuroscience.2014.12.058. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Cui X, Sun YE, Yang X, Ni K, Zhou Y, Ma Z, Gu X. Intrathecal injection of the peptide myr-NR2B9c attenuates bone cancer pain via perturbing N-methyl-D-aspartate receptor-PSD-95 protein interactions in mice. Anesth Analg. 2014;118:1345–1354. doi: 10.1213/ANE.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 34.Ahlawat A, Rana A, Goyal N, Sharma S. Potential role of nitric oxide synthase isoforms in pathophysiology of neuropathic pain. Inflammopharmacology. 2014;22:269–278. doi: 10.1007/s10787-014-0213-0. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Zhang W, Sun Y, Liu Y, Song L, Ma Z, Gu X. Activation of GRs-Akt-nNOs-NR2B signaling pathway by second dose GR agonist contributes to exacerbated hyperalgesia in a rat model of radicular pain. Mol Biol Rep. 2014;41:4053–4061. doi: 10.1007/s11033-014-3274-7. [DOI] [PubMed] [Google Scholar]
- 36.Reuss MH, Reuss S. Nitric oxide synthase neurons in the rodent spinal cord: distribution, relation to Substance P fibers, and effects of dorsal rhizotomy. J Chem Neuroanat. 2001;21:181–196. doi: 10.1016/s0891-0618(01)00091-6. [DOI] [PubMed] [Google Scholar]
- 37.Huang H, Li Q, Hong YG, Wang DM. MrgC receptor activation reverses chronic morphineevoked alterations of glutamate transporters and nNOS in rats. Sheng Li Xue Bao. 2014;66:449–456. [PubMed] [Google Scholar]
- 38.Chen T, Hu Z, Quirion R, Hong Y. Modulation of NMDA receptors by intrathecal administration of the sensory neuron-specific receptor agonist BAM8-22. Neuropharmacology. 2008;54:796–803. doi: 10.1016/j.neuropharm.2007.12.010. [DOI] [PubMed] [Google Scholar]


