Abstract
MicroRNA (miRNA) regulates gene expression in a post-transcriptional manner, which hybridizes the target mRNAs with complementary sequence and subsequently leads to translation repression or mRNA degradation. Online sequence alignment showed that there is a putative binding site of miR-223-3p on the 3’UTR of LIF, which is considered to be an important marker of endometrial receptivity. Thus, we hypothesized that miR-223-3p may affect embryo implantation by suppressing LIF expression. In this study, we found that miR-223-3p and LIF protein was inversely expressed in the endometrium of mice during implantation window. Then we proved that miR-223-3p directly binds to LIF 3’UTR with luciferase reporter assay and supresses the expression of LIF. To investigate whether miR-233-3p affects embryo implantation, miR-223-3p agonist was injected into the uteri of pregnant mice. The results demonstrated the suppressing effect of miR-223-3p on embryo implantation. Furthermore, over-expression of miR-223-3p was found to compromise pinopodes formation in the endometrium of mice. Taken all together, our findings revealed that miR-223-3p suppresses pinopodes formation and LIF protein expression, which may lead to diminished embryo implantation.
Keywords: MiR-223-3p, LIF, pinopodes, embryo implantation
Introduction
Infertility is a growing problem in China. Around 40 million Chinese people, 12.5% of the country’s reproductive population, suffer from infertility [1]. Great efforts have been made in assisted reproductive technology to improve this situation. However, the successful rate remains relatively low [2,3]. One of the main causes for these failures probably attributes to the non-receptive endometrium.
The implantation of embryo into the receptive endometrium is crucial for the establishment of pregnancy. Implantation occurs only within an optimal time frame, which is called implantation window. During implantation window, steroid hormones initiate genetic, molecular, and cellular interactions, and make the endometrium receptive to accept embryo implantation [4,5].
A number of complex molecular events are involved in the process of embryo implantation, including post-transcriptional modification by microRNAs (miRNAs). MiRNAs are single-stranded, non-coding RNAs consisting of 20-22 nucleotides. They are transcribed from specific genes with primary transcripts (pri-miRNAs). The pri-miRNAs undergo substantial processing and result in stem-loop precursor miRNA (pre-miRNA) in the nucleus. After transportation into the cytoplasm, the pre-miRNAs undergo a second cleavage by Dicer generating a double-stranded miRNA duplex [6]. The mature miRNAs incorporate into the RNA-induced silencing complex (RISC) and through complementary interaction with the mRNA of target genes. MiRNAs repression includes two distinct mechanisms: target gene mRNA cleavage or destabilization [7]. Through these mechanisms, miRNAs influence the outcome of various cellular activities under normal and disease conditions [8]. They participate in numerous biologic processes, such as cell proliferation, differentiation, apoptosis, and oncogenesis [9-11]. A number of miRNAs are also expressed in female reproductive system and associated with infertility [12-14]. Recent studies demonstrated that miR-223 participated in suppressing cell proliferation and angiogenesis, inflammation, virus infection and tumorigenesis [9,15,16]. However, its role in female reproduction has not been revealed.
Leukemia inhibitory factor (LIF) is a secreted glycoprotein with a molecular weight from 38-67 KD. LIF acts through the LIF cell-surface receptor, LIFR and gp130, and activates various cascades through different signaling pathways [17]. In female reproductive system, LIF mostly expresses in endometrial epithelial cells and show a menstruation cycle-dependent secretion pattern, which is higher in luteal phase and lower in proliferative phase [18,19]. It is also highly expressed in the peri-implantation endometrium of healthy women and decreased in women with recurrent implantation failure [20,21]. Moreover, a study has proved that maternal expression of LIF is required for blastocyst implantation in mice [22]. Thus, LIF obviously plays a key role in the process of embryo implantation. Online sequence alignment (http://www.targetscan.org/) showed that the sequence of miR-223-3p seed pairing is complementary to the 3’UTR of LIF (Table 1). Thus, we hypothesized that miR-223-3p may suppress the expression of LIF and compromise embryo implantation.
Table 1.
Predicted consequential pairing between miR-223-3p and LIF 3’-UTR

|
Materials and methods
Animal and tissue preparation
The presented work was performed in accordance with the guiding principles in the Care and Use of Research Animals, which was approved by the Ethics Committee of the Tongji Hospital and covered by Chinese Animal Husbandry Legislation. Adult mice of the Kunming white strain were supplied by the Center of Experimental Animals, Tongji Medical College (Wuhan, China).
Adult female mice (8-10 weeks) were mated with fertile male mice of the same strain to induce pregnancy. The morning when the vaginal sperm plug was observed was designated as Day 1 of pregnancy. Six pregnant or non-pregnant mice were sacrificed on Day 4 of pregnancy. The endometrial tissues were isolated from the uteri and then cryopreserved for real-time PCR. To investigate whether miR-223-3p affects embryo implantation, pregnant mice were randomly divided into sham, control and study groups. On Day 3 of pregnancy, the mice were anesthetized with 1% butaylone i.p. injection. A laparotomy was performed to expose the uterus. The left uterine horn of each mouse was respectively treated as below: Sham group: only puncture with a 27-gauge needle, study group: 10 nmol miRNA agonist (micrONTM mmu-miR-223-3p agomir, Ribobio, China) in 20 μl normal saline, control Group: 10 nmol miRNA negative control (micrONTM Negative Control #22 agomir, Ribobio, China) in 20 μl normal saline. On the morning of Day 4 of pregnancy, mice were sacrificed, and the uteri were excised and stored for quantitative real-time PCR, immunohistochemistry and transparent electron microscopy. On the morning of Day 9 of pregnancy, rest of mice was sacrificed and the number of implantation sites was counted.
RNA extraction and quantitative real-time PCR
Total RNA was extracted from endometrial tissues with TrIzol reagent (Ambion, USA) and reverse transcription reaction was performed by using a RT Kit (Takara, Japan) according to the manufacturer’s protocol. The RT products (cDNA) were amplified by real-time quantitative PCR with SYBR green Master Mix (Takara, Japan). The primers sequences are shown in Table 2. For sample analysis, the threshold was set based on the exponential phase of products, and the 2-ΔΔCT method was performed to analyze the data. The expression level of LIF was normalized to β-actin mRNA. All reactions were run in triplicate and all experiments were repeated 3 times independently.
Table 2.
Primer sequences (5’-3’) used in quantitative real-time PCR
MiRNA PCR
Mature miR-223-3p was detected with the All-in-One miRNA qRT–PCR Detection Kit (GeneCopoeia, USA). Briefly, total RNA was polyadenylated and reverse transcribed using a poly dT-adaptor primer. Quantitative RT-PCR was performed using a miRNA-specific forward primer and a universal reverse primer. The following forward primers were synthesized by GeneCopoeia: 5’-UGUCAGUUUGUCAAAUACCCCA-3’ for mmu-miR-223-3p and 5’-CAAATTCGTGAAGCGTTCCATAT-3’ for U6 small nuclear RNA (U6). The universal reverse primer was purchased from the same company. U6 small nuclear RNA was used as an internal control.
Immunohistochemistry
The expression of LIF protein was evaluated by immunohistochemistry using a rabbit anti-LIF antibody (1:100, Boster, China). The specimens were embedded in paraffin, after that serial 5 mm sections were prepared. The sections were deparaffinized in xylene and ethanol. Endogenous peroxidase was blocked with 3% H2O2 at room temperature for 10 min. After blocking for 45 min with PBS containing 1.5% normal horse serum, the sections were incubated with primary antibodies overnight at 4°C, and with secondary antibodies at 37°C for 30 min, also in PBS with 1.5% normal horse serum. After antiserum incubation the slides were washed 3 times for 10 min each in PBS. Subsequently, the sections were incubated with avidin and biotinylated peroxidase at room temperature for 45 min, and finally with DAB (400 mg/ml) at room temperature for 3-5 min. Antibody specificity was checked with isotype-specific antibodies as a control.
Luciferase reporter assay
HEK-293 cells (ATCC, USA) were seeded in 96-well plates. After 24 hour’s incubation, cells were cotransfected with either LIF 3’UTR clone or negative control clone and miR-223-3p lentivirus vector or scramble control (mole ratio=1:8), respectively (GeneCopoeia, USA). Forty-eight hours after transfection, the cells were assayed by both firefly and renilla luciferase using the dual luciferase assay system (GeneCopoeia, USA) according to manufacturer’s instructions. All transfection experiments were conducted in triplicate and repeated 3 times independently.
Transmission electron microscopy
Individual uteri were fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4, and 2 mm-cross sections of each uterine horn were cut and incubated overnight at 4°C in 2.5% glutaraldehyde buffer. The buffer was removed, and the samples were rinsed three times, 15 minutes each in 0.1 M phosphate buffer. Samples were incubated in 1% osmium in 0.1 M phosphate buffer, rinsed and dehydrated in a series of ethanol (70%-100%) washes. Samples were incubated twice for 5 minutes in propylene oxide and then transferred to a rotor for 1 h at room temperature in a 1:1 mixture of propylene oxide and epon [47% Embed 812, 31% DDSA (dodenyl succinic anhydride), 19% NMA (nadic methyl anhydride), 3% BDMA (benzyldimethylamine)], followed by an overnight incubation in 1:2 propylene oxide-epon, and finally 100% epon for 2-3 hours. Individual uterine samples were embedded in 100% epon in silicon flat embedding molds, and capsules were polymerized in a 60°C oven for over 48 hours. Ultrathin transverse sections (70 nm) were prepared using a diamond knife (Diatome) on a MT 6000-XL ultramicrotome, captured on 300-mesh copper grids, and stained with 2% uranyl acetate. All reagents and materials were obtained from Electron Microscopy Sciences (Hatfield, PA, USA). The ultrathin sections were observed under a transmission electron microscope (Libra 120 Zeiss, Oberkochen, Germany).
Statistical analysis
SAS 9.2 (SAS, Inc., Cary, NC, USA) was used for the statistical analysis. The numerical data are presented as the mean ± SD and were compared with a t-test. A P value<0.05 was considered to be statistically significant.
Results
The expression of miR-223-3p and LIF in non-pregnant and pregnant mice
The expression of miR-223-3p and LIF in the endometrium of non-pregnant mice or pregnant mice on Day 4 of pregnancy was detected by using qRT-PCR, respectively. The results are shown in Figure 1. The expression of miR-223-3p was higher in the non-pregnant mice, compared with the pregnant mice (1.09±0.20 vs. 0.41±0.11, P<0.01). In contrast, the expression of LIF mRNA was lower in the non-pregnant mice, compared with the pregnant mice (1.04±0.34 vs. 6.03±0.42, P<0.001).
Figure 1.
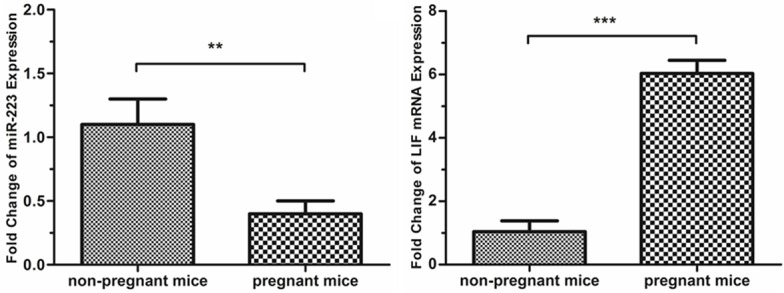
Analysis of miR-223-3p and LIF expression in the endometrium of pregnant and non-pregnant mice. The expression of miR-223-3p and LIF was measured on day 4 of pregnancy. The expression of miR-223-3p was higher in the non-pregnant mice, compared with the pregnant mice (1.09±0.20 vs. 0.41±0.11, P<0.01). The expression of LIF mRNA was lower in the non-pregnant mice, compared with the pregnant mice (1.04±0.34 vs. 6.03±0.42, P<0.001).
MiR-223-3p interacts with LIF 3’UTR directly
To confirm whether miR-223-3p could directly target LIF, we inspected the 3’-untranslated region (3’UTR) of LIF and found a site that could be recognized by miR-223-3p (Table 1). The luciferase reporter assays were performed by co-transfecting miR-223-3p or scramble control and luciferase constructs containing LIF 3’UTR or control clone into HEK293 cells, respectively. The results showed that the luciferase activity after 48 hours was decreased approximately 60% in miR-223-3p and LIF 3’UTR clone co-transfection group, compared with the other control groups (Figure 2).
Figure 2.
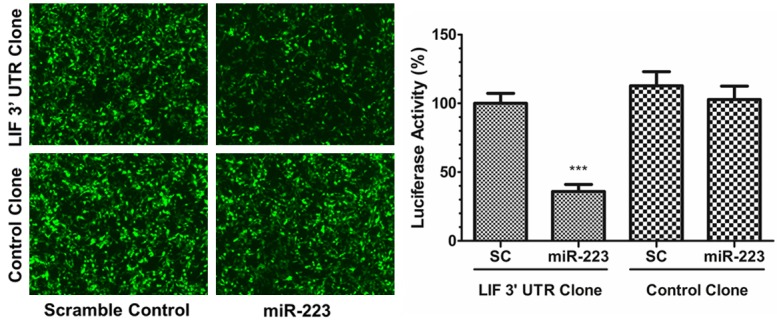
MiR-223-3p directly interacts with LIF 3’UTR. Scramble control or miR-223-3p lentiviral vector was co-transfected with LIF 3’UTR clone or control clone in HEK-293 cells, respectively. After 48 hours, luciferase activity was assayed. The luciferase activity was decreased approximately 64% in miR-223-3p and LIF 3’UTR clone co-transfection group, compared with the other control groups (P<0.001).
Administration with miR-223-3p down-regulates the expression of LIF
To investigate whether miR-223-3p regulates LIF in vivo, miR-223-3p agomir and negative control were injected into mouse uterine cavity, respectively. The expression of miR-223-3p increased approximately 2.5-fold in miR-223-3p agomir administration (1.11±0.5 vs. 2.71±0.5, P<0.05, Figure 3A). The LIF mRNA expression was repressed by approximately half (0.96±0.40 vs. 0.49±0.11, P=0.203, Figure 3B). The representative results of immunohistochemistry staining are displayed in Figure 3C, which showed that the expression of LIF protein was down-regulated after treatment with miR-223-3p agonist.
Figure 3.
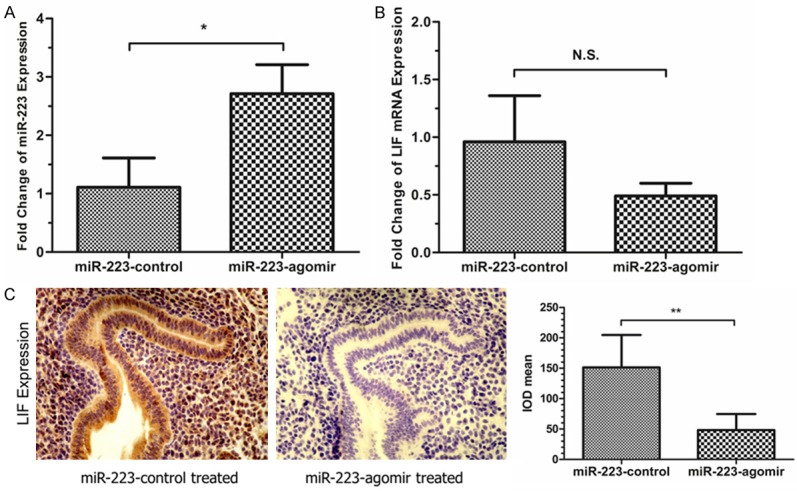
Administration with miR-223-3p agomir suppresses the expression of LIF in pregnant mice. MiR-223-3p agomir (miR-223-agomir) and negative control (miR-223-control) was administrated into the mouse uterine cavity on day 3 of pregnancy, respectively. A. The expression of miR-223-3p was increased approximately 2.5-fold in the miR-223-agomir treated group, compared with miR-223-control treated group (1.11±0.5 vs. 2.71±0.5, P<0.05) on day 4 of pregnancy. B. The expression of LIF mRNA was reduced approximately 50% in the miR-223-agomir treated group, compared with miR-223-control treated group (0.96±0.40 vs. 0.49±0.11, P=0.203) on day 4 of pregnancy. C. The expression of LIF protein in the endometrium of pregnant mice was detected by using immunohistochemistry. In miR-223-control treated group, the LIF protein was strongly expressed in endometrial epithelial cells and moderately expressed in stromal cells. In miR-223-agomir treated group, the LIF protein was weekly expressed in both endometrial epithelial cells and stromal cells.
Administration with miR-223-3p impedes embryo implantation in vivo
The images of implantation sites after treatment with MiR-223-3p agomir, Negative Control and sham are presented in Figure 4. The number of implanted embryos in the MiR-223-3p agomir treated group was dramatically lower compared with those in the sham group (2.3±1.7 vs. 6.8±1.0, P<0.001) and negative control group (2.3±1.7 vs. 6.9±2.4, P<0.001). There is no difference in the number of implanted embryos between the sham and negative control group, which certified that the injecting operation did not affect the embryo implantation.
Figure 4.
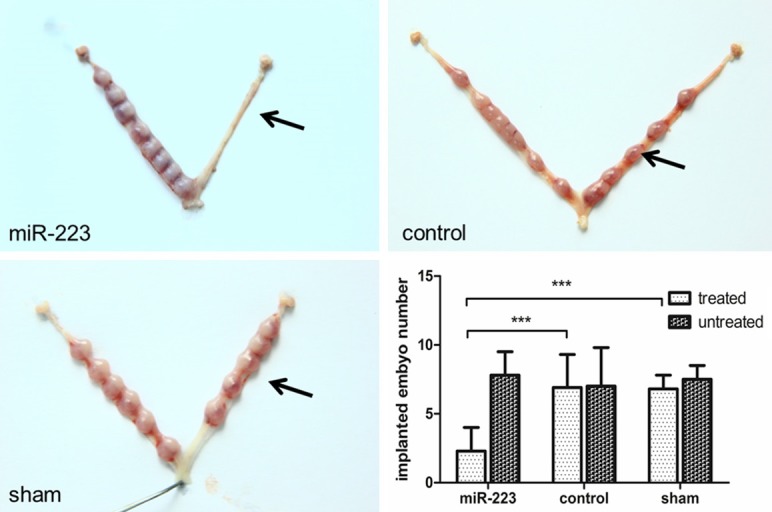
Administration with miR-223-3p agomir decreases the number of implanted embryo in mice. Pregnant mice were divided into three groups randomly and treated as described in section Materials and Methods. The black arrow denotes the side of uterus that underwent the treatment. The other side (untreated side) was considered as self-control. The number of implanted embryo in miR-223-agomir treated group (2.3±1.7) was lower than that in either miR-223-control group (6.9±2.4) or sham group (6.8±1.0). The number of implanted embryo in sham group was similar to its self-control, which proved that the uterine puncture did not affect embryo implantation.
MiR-223-3p impedes pinopodes formation
To confirm whether miR-223-3p affects pinopode formation, we observed the apical membrane of mouse endometrial epithelium by using a transmission electron microscopy (TEM). The photomicrographs showed that there were amount of pinopodes on the apical membrane of endometrial epithelial cells in the mice of negative control group (Figure 5A). Administration with miR-223-3p agomir resulted in lower expression of pinopodes, and most of the apical membrane was covered by microvilli (Figure 5B).
Figure 5.
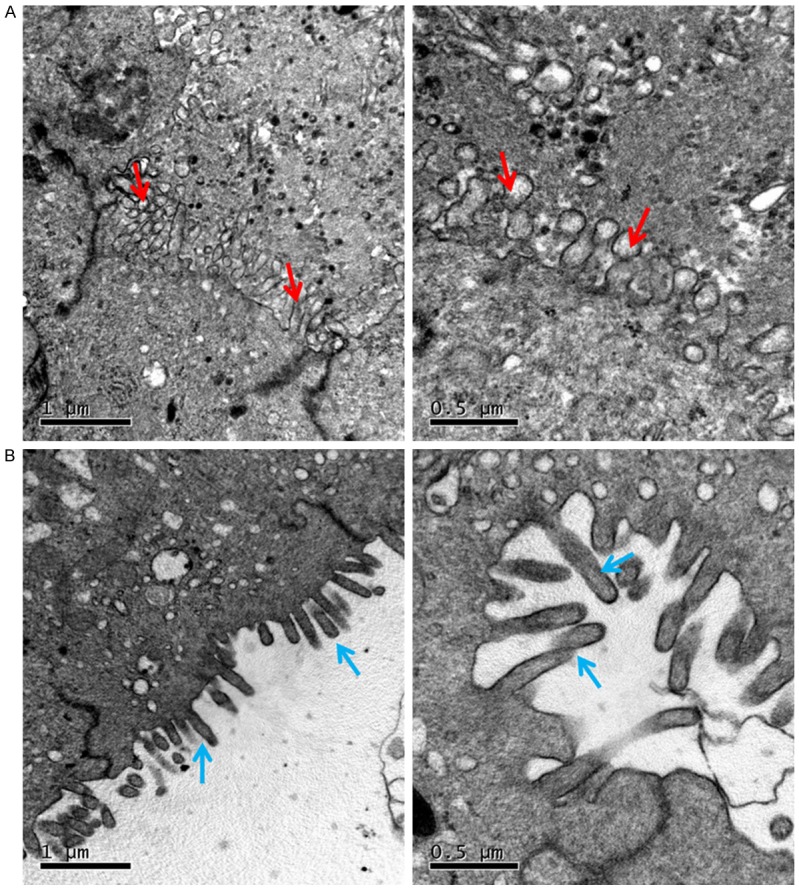
Photomicrographs of transmission electron microscopy (TEM) from horizon section of pregnant mouse endometrium on day 4 of pregnancy show the apical surface of some luminal epithelial cells. In miR-223-control treated group (A), the pinopodes (red arrows) was expressed on the apical surface of luminal epithelial cells. However, in miR-223-agomir treated group, the apical surface of luminal epithelial cells were covered by microvilli (blue arrows) instead (B). Scale bars=0.5 µm, 1 µm.
Discussion
Embryo implantation is a crucial step of pregnancy, which depends on synchronous crosstalk between embryo and receptive endometrium. A number of miRNAs from different families possibly play a pivot role in regulating endometrial receptivity [12,23]. This study investigated the regulatory role of miR-223-3p during embryo implantation in mice. The results showed that miR-223-3p down-regulated the expression of LIF, decreased pinopodes formation and affected embryo implantation in mice.
Previous studies have demonstrated the expression and localization of LIF in endometrium, and its importance in embryo implantation and early pregnancy [19,20]. In human endometrium, LIF expression is relatively low in the proliferative phase, rises after ovulation, and remains high during the mid-luteal phase [18,24]. LIF is expressed mainly in glandular and luminal epithelium [18]. Our results also showed dramatically higher expression of LIF in the endometrium of mice on day 4 of pregnancy. In contrast, the expression of miR-223-3p during that period was lowered by more than half, which suggests that low level of miR-223-3p may be a negative factor for embryo implantation. We further proved that miR-223-3p directly targets LIF 3’UTR, suppressed LIF expression and the number of implanted embryo. Taken together, these data indicates that low expression of miR-223-3p during implantation window may relieve its inhibitory effect on LIF, subsequently increase the expression of LIF and finally facilitate embryo implantation.
Pinopodes, also called uterodomes, are mushroom-like projections that appear on the apical surface of endometrial luminal epithelial cells [25,26]. Pinopodes are formed at the beginning of implantation window, by fusing several adjacent microvilli together [27]. The clinical usefulness of pinopodes on predicting endometrial receptivity as a morphological marker is still under debates [28]. However, their appearance is strongly synchronized with several endometrial receptivity associated factors, such as LIF, integrins, mucin-1 and glycodelin [20,29,30]. They were also proved to secrete LIF [31]. These studies suggest that the presence of pinopodes is crucial for embryo implantation. In our study, we found that miR-223-3p impeded the formation of pinopodes. The apical surface of luminal epithelial cells of miR-223-3p treated mice remained to be covered with microvilli. These results suggest that miR-223-3p may also affect embryo implantation by hindering pinopodes formation. However, our data did not show the mechanism how miR-223-3p impaired pinopodes formation. Existing data only proved that pinopodes formation is strongly progesterone dependent [32,33]. Thus, further studies are required to reveal the mechanism.
This study contributed to understand the mechanism how endometrial miR-223-3p targets LIF and regulates endometrial receptivity. Aberrant expression of miR-223-3p during implantation window may lead to the establishment and progression of certain infertility. This study may also have clinical implications. MiR-223-3p may serve as a potential biomarker of evaluating endometrial receptivity. Preventive and therapeutic strategies targeting miR-223-3p may improve the pregnancy outcomes.
In conclusion, this study validated the interaction between miR-223-3p and LIF. The results of animal experiments suggest that miR-223-3p may affect the embryo implantation via suppressing the expression of LIF and pinopodes in the endometrium of pregnant mice. However, further studies on human cells and tissues are required to confirm our findings.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (81170619 and 81471459).
Disclosure of conflict of interest
None.
References
- 1.Cai QF, Wan F, Dong XY, Liao XH, Zheng J, Wang R, Wang L, Ji LC, Zhang HW. Fertility clinicians and infertile patients in China have different preferences in fertility care. Hum Reprod. 2014;29:712–719. doi: 10.1093/humrep/deu023. [DOI] [PubMed] [Google Scholar]
- 2.Zheng Y, Dong X, Wang R, Yang W, Zhang H, Zhu G, Ai J. The criteria for optimal down-regulation with gonadotropin-releasing hormone-agonist: a retrospective cohort study. Gynecol Endocrinol. 2015;31:959–965. doi: 10.3109/09513590.2015.1101437. [DOI] [PubMed] [Google Scholar]
- 3.Zheng Y, Dong X, Huang B, Zhang H, Ai J. The artificial cycle method improves the pregnancy outcome in frozen-thawed embryo transfer: a retrospective cohort study. Gynecol Endocrinol. 2015;31:70–74. doi: 10.3109/09513590.2014.958988. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7:185–199. doi: 10.1038/nrg1808. [DOI] [PubMed] [Google Scholar]
- 5.Lee KY, Jeong JW, Tsai SY, Lydon JP, DeMayo FJ. Mouse models of implantation. Trends Endocrinol Metab. 2007;18:234–239. doi: 10.1016/j.tem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 7.Zamore PD, Haley B. Ribo-gnome: the big world of small RNAs. Science. 2005;309:1519–1524. doi: 10.1126/science.1111444. [DOI] [PubMed] [Google Scholar]
- 8.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 9.Huang K, Dong X, Sui C, Hu D, Xiong T, Liao S, Zhang H. MiR-223 suppresses endometrial carcinoma cells proliferation by targeting IGF-1R. Am J Transl Res. 2014;6:841–849. [PMC free article] [PubMed] [Google Scholar]
- 10.Li Z, Gou J, Jia J, Zhao X. MicroRNA-429 functions as a regulator of epithelial-mesenchymal transition by targeting Pcdh8 during murine embryo implantation. Hum Reprod. 2015;30:507–518. doi: 10.1093/humrep/dev001. [DOI] [PubMed] [Google Scholar]
- 11.Li Z, Jia J, Gou J, Zhao X, Yi T. MicroRNA-451 plays a role in murine embryo implantation through targeting Ankrd46, as implicated by a microarray-based analysis. Fertil Steril. 2015;103:834–844. e834. doi: 10.1016/j.fertnstert.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 12.Pan Q, Chegini N. MicroRNA signature and regulatory functions in the endometrium during normal and disease states. Semin Reprod Med. 2008;26:479–493. doi: 10.1055/s-0028-1096128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inyawilert W, Fu TY, Lin CT, Tang PC. Let-7-mediated suppression of mucin 1 expression in the mouse uterus during embryo implantation. J Reprod Dev. 2015;61:138–144. doi: 10.1262/jrd.2014-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Z, Jia J, Gou J, Tong A, Liu X, Zhao X, Yi T. Mmu-miR-126a-3p plays a role in murine embryo implantation by regulating Itga11. Reprod Biomed Online. 2015;31:384–393. doi: 10.1016/j.rbmo.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Dai GH, Ma PZ, Song XB, Liu N, Zhang T, Wu B. MicroRNA-223-3p inhibits the angiogenesis of ischemic cardiac microvascular endothelial cells via affecting RPS6KB1/hif-1a signal pathway. PLoS One. 2014;9:e108468. doi: 10.1371/journal.pone.0108468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haneklaus M, Gerlic M, O’Neill LA, Masters SL. miR-223: infection, inflammation and cancer. J Intern Med. 2013;274:215–226. doi: 10.1111/joim.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicola NA, Babon JJ. Leukemia inhibitory factor (LIF) Cytokine Growth Factor Rev. 2015;26:533–544. doi: 10.1016/j.cytogfr.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yue ZP, Yang ZM, Wei P, Li SJ, Wang HB, Tan JH, Harper MJ. Leukemia inhibitory factor, leukemia inhibitory factor receptor, and glycoprotein 130 in rhesus monkey uterus during menstrual cycle and early pregnancy. Biol Reprod. 2000;63:508–512. doi: 10.1095/biolreprod63.2.508. [DOI] [PubMed] [Google Scholar]
- 19.Chen DB, Hilsenrath R, Yang ZM, Le SP, Kim SR, Chuong CJ, Poindexter AN 3rd, Harper MJ. Leukaemia inhibitory factor in human endometrium during the menstrual cycle: cellular origin and action on production of glandular epithelial cell prostaglandin in vitro. Hum Reprod. 1995;10:911–918. doi: 10.1093/oxfordjournals.humrep.a136060. [DOI] [PubMed] [Google Scholar]
- 20.Xu B, Sun X, Li L, Wu L, Zhang A, Feng Y. Pinopodes, leukemia inhibitory factor, integrin-beta3, and mucin-1 expression in the peri-implantation endometrium of women with unexplained recurrent pregnancy loss. Fertil Steril. 2012;98:389–395. doi: 10.1016/j.fertnstert.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 21.Karaer A, Cigremis Y, Celik E, Urhan Gonullu R. Prokineticin 1 and leukemia inhibitory factor mRNA expression in the endometrium of women with idiopathic recurrent pregnancy loss. Fertil Steril. 2014;102:1091–1095. e1091. doi: 10.1016/j.fertnstert.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, Abbondanzo SJ. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 23.Chu B, Zhong L, Dou S, Wang J, Li J, Wang M, Shi Q, Mei Y, Wu M. miRNA-181 regulates embryo implantation in mice through targeting leukemia inhibitory factor. J Mol Cell Biol. 2015;7:12–22. doi: 10.1093/jmcb/mjv006. [DOI] [PubMed] [Google Scholar]
- 24.Senturk LM, Arici A. Leukemia inhibitory factor in human reproduction. Am J Reprod Immunol. 1998;39:144–151. doi: 10.1111/j.1600-0897.1998.tb00346.x. [DOI] [PubMed] [Google Scholar]
- 25.Johannisson E, Nilsson L. Scanning electron microscopic study of the human endometrium. Fertil Steril. 1972;23:613–625. [PubMed] [Google Scholar]
- 26.Murphy CR. Understanding the apical surface markers of uterine receptivity: pinopods-or uterodomes? Hum Reprod. 2000;15:2451–2454. doi: 10.1093/humrep/15.12.2451. [DOI] [PubMed] [Google Scholar]
- 27.Nikas G, Makrigiannakis A. Endometrial pinopodes and uterine receptivity. Ann N Y Acad Sci. 2003;997:120–123. doi: 10.1196/annals.1290.042. [DOI] [PubMed] [Google Scholar]
- 28.Quinn CE, Casper RF. Pinopodes: a questionable role in endometrial receptivity. Hum Reprod Update. 2009;15:229–236. doi: 10.1093/humupd/dmn052. [DOI] [PubMed] [Google Scholar]
- 29.Nardo LG, Nikas G, Makrigiannakis A, Sinatra F, Nardo F. Synchronous expression of pinopodes and alpha v beta 3 and alpha 4 beta 1 integrins in the endometrial surface epithelium of normally menstruating women during the implantation window. J Reprod Med. 2003;48:355–361. [PubMed] [Google Scholar]
- 30.Stavreus-Evers A, Mandelin E, Koistinen R, Aghajanova L, Hovatta O, Seppala M. Glycodelin is present in pinopodes of receptive-phase human endometrium and is associated with down-regulation of progesterone receptor B. Fertil Steril. 2006;85:1803–1811. doi: 10.1016/j.fertnstert.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 31.Kabir-Salmani M, Nikzad H, Shiokawa S, Akimoto Y, Iwashita M. Secretory role for human uterodomes (pinopods): secretion of LIF. Mol Hum Reprod. 2005;11:553–559. doi: 10.1093/molehr/gah218. [DOI] [PubMed] [Google Scholar]
- 32.Martel D, Monier MN, Roche D, Psychoyos A. Hormonal dependence of pinopode formation at the uterine luminal surface. Hum Reprod. 1991;6:597–603. doi: 10.1093/oxfordjournals.humrep.a137386. [DOI] [PubMed] [Google Scholar]
- 33.Stavreus-Evers A, Nikas G, Sahlin L, Eriksson H, Landgren BM. Formation of pinopodes in human endometrium is associated with the concentrations of progesterone and progesterone receptors. Fertil Steril. 2001;76:782–791. doi: 10.1016/s0015-0282(01)01993-8. [DOI] [PubMed] [Google Scholar]


