Abstract
Thymopoiesis is essential and significant for development and maintenance of the robust and healthy immune system. The acute suppression of thymopoiesis induced by 5-Azacytidine (5-Aza) is an intractable clinical problem complicating chemotherapy. Interleukin 1 receptor antagonist (IL-1Ra) is a cytokine that competitively blocks binding of interleukin 1 (IL-1) to its receptor. This study aims to investigate the effects of the IL-1Ra on the thymus toxicity of 5-Aza in mouse. In this study, we treated the mice with the 5-Aza (100 mg/kg per mouse). The GeneChip methodology developed by Affymetrix was used to monitor global gene expression during mouse thymus regeneration induced by a single injection of 5-Aza. The total thymocytes were counted using a hemocytometer. Cell cycle of samples were analyzed on a Becton Dickinson FACScan. Cells surfaces were labeled with anti-CD4, anti-CD8 and anti-CD45RA antibodies, and detected by flow cytometry. BrdU incorporation was detected by flow cytometry. The results indicated that administering exogenous IL-1Ra to normal mice inhibited cell cycle progress of thymocytes in a dosage-dependent manner. Proliferation of immature CD4-CD8- double negative (DN) and CD4+CD8+ double positive (DP) thymocytes were both inhibited. The pretreatment of normal mice with exogenous IL-1Ra reduced acute toxicity on thymus and immune suppression induced by 5-Aza. Furthermore, thymus reconstitution after 5-Aza treatment was accelerated by IL-1Ra. In conclusion, interleukin 1 receptor antagonist could inhibit normal thymopoiesis and reduce thymus toxicity of 5-azacytidine in mouse. Pretreatment with IL-1Ra would offer a new and promising strategy to alleviate immunotoxicity of chemotherapy in clinical.
Keywords: 5-azacytidine, interleukin 1 receptor antagonist, thymopoiesis, chemotherapy
Introduction
Cancer chemotherapy attenuates thymus function and contributes to delayed immune reconstitution [1-4]. Immune suppression is one of the most significant dose-limiting toxic effects of intensive cancer treatment and is associated with adverse clinical and economic outcomes. Delayed immune reconstitution in adults has been correlated with increased mortality and morbidity caused by the infection [5,6], and tumor recurrence [7,8]. Peripheral T cell expansion helps to sustain secondary immune system, extensive studies have demonstrated the importance of thymus in T-cell development [9,10]. Immune-competence after chemotherapy requires T-cell regeneration via the thymic pathway. Therefore, we speculated that reducing the thymic immunotoxicity and promoting the T-cell regeneration in thymus-dependent pathways may be important in cancer chemotherapy. Unfortunately, there are no treatments available to deal with chemotherapy-induced acute thymic atrophy currently.
The interleukin 1 receptor antagonist (IL-1Ra) is a secreted protein with the molecular weight of 17 kDa [11]. It binds to IL-1 receptor as a competitor to the IL-1α and IL-1β, but such binding leads to no intracellular consequences. It competitively blocks the binding of IL-1 to the cell-surface receptors, thereby, inhibiting the biological actions of IL-1 [12]. The IL-1 receptors are present on human thymic epithelial cell surface [13]. IL-1 could activate T-cells in a co-stimulator assay [14-16], and it could increase thymic epithelial proliferation [17,18]. However, the role of IL-1Ra on normal human thymopoiesis remains elusive.
Our previous studies illustrated that the expression of IL-1Ra was temporarily increased in mice thymus following 5-Aza treatment. Exogenous IL-1Ra administered to normal mice inhibited the cell cycle progression of thymocytes and the proliferation of relatively immature thymocyte subsets, therefore, reducing the cytotoxicity on these cells by chemotherapy reagents. Pretreatment of IL-1Ra prior to 5-Aza chemotherapy attenuated the acute destruction of thymus cellularity, reduction of peripheral naïve T cells, and inhibition upon the proliferation of thymocyte subsets. IL-1Ra pretreatment also accelerated the post-chemotherapy recovery of thymocyte subsets. These results confirmed a suppressive role of IL-1Ra in normal thymopoiesis and its protective effect on thymus from chemotherapy, which suggests that the application of IL-1Ra before chemotherapy may provide a new clinical strategy for alleviating immunotoxicity of chemotherapy.
Materials and methods
Mice
The pathogen-free, sex-matched, 4-week-old Balb/C mice (SLACCAS, Shanghai, China) were maintained in air-filtered units at 23 ± 5°C and 55% ± 5% relative humidity throughout the experiments. Mice were fed with sterile water and rodent food. Animal experiments were performed with the authorization of Animal Care and Use Committee of School of Pharmacy of Shanghai Jiao Tong University. This study was also approved by the ethics committee of Shanghai Jiao Tong University, Shanghai, China.
Treatment of mice
The 5-Aza (Sigma, USA) was dissolved in sterile saline at a concentration of 100 mg/ml, and then administered intraperitoneally with a dose of 100 mg/kg per mouse. Control mice received physiological saline alone.
Recombinant Human IL-1Ra (rHuIL-1Ra) with biological activity was produced using a strategy described by Di Xiang et al. (unpublished data of our team). The rHuIL-Ra or physiological saline was administered intraperitoneally. Variable concentrations of administration of the recombinant protein in each experiment were described in Section 3.
High-density oligonucleotide microarray
The GeneChip methodology developed by Affymetrix was used to monitor global gene expression during mouse thymus regeneration induced by a single injection of 5-Aza. Three RNA samples were extracted from thymocytes at each of the following time points: 0 day, 1.5, 3, 7, 11 and 14 days post 5-Aza treatment. Equal amount of poly (A) RNA from each sample was used to synthesize double-stranded cDNA. 3 cRNA probes were prepared by in vitro transcription using equal amount of cDNA of 3 samples. Equal amount of probes was used for hybridizations to mouse genome expression oligonucleotide arrays (GeneChip mouse expression set 430, Affymetrix, Santa Clara, CA) containing 34,323 well-substantiates mouse genes. The global scaling strategy was used for all arrays that set the average signal intensity of the array to a target signal of 500. Comparison analyses for expression data at each time point were calculated by using day 0-array base baseline.
Thymus cellularity
The mice were sacrificed by cervical dislocation. Fresh thymus was separated and thymus single cell suspension was prepared. The total thymocytes were counted using a hemocytometer after having red blood cells (RBCs) lysed with fresh 3% acetic acid in PBS. Number of thymocyte was counted using the automated Hematology Analyzer MEK-6318K (Nihon Kohden Co., Japan) according to the user’s manual.
Cell cycle
Staining with propidium iodide (PI) was conducted using a variation of method reported by Nicoletti et al. [19]. Briefly, no less than 106 thymocytes were suspended in PBS containing 0.5% fetal calf serum (FCS, Logan, Utah, USA) and fixed by the drop-wise addition of ice-cold 70% ethanol to a final concentration of 50%. The cells were then held on ice for at least 1 h. After extensive washing, the cells were suspended in PBS containing 50 mg/ml propidium iodide (Sigma, USA) and 50 mg/ml RNaseA (Sigma, USA) and were incubated for 1 h in the dark at room temperature. Samples were analyzed on a Becton Dickinson FACScan. Debris and doublets were eliminated from the analyses using pulse width/area discrimination. A minimum of 15,000 cells were analyzed.
Cell surface staining
Predominantly, cells were labeled with anti-CD4, anti-CD8 and anti-CD45RA antibodies, respectively. For analyzing the constituion of cell subsets in thymus, thymocytes were labeled with CD4-FITC, CD8-PE Cy5 monoclonal antibody (eBioscience, USA) at 4°C for 30 min, followed by washing and suspension with 0.5% FCS/PBS.
For naïve T cells analysis, peripheral blood samples were collected (0.5 ml of peripheral blood from orbital sinus) before sacrifice of mice. Cells were first treated with cold NH4Cl/PBS solution at 1:9 dilution for 10 min to lysis RBCs, followed by washing with 0.5% FCS/PBS. Three-color flow cytometry was performed using the following monoclonal antibodies, including FTIC anti-CD4, PE anti-CD8 (eBioscience, USA) and PE-Cy5 anti-CD45RA (BD Pharmingen, USA) monoclonal antibody at 4°C for 30 min, followed by washing and suspension with 0.5% FCS/PBS. Surface staining was detected by flow cytometry (Becton Dickinson dual laser FACSCalibur).
BrdU incorporation and measurement
Mice received two intraperitoneal injections of BrdU (Sigma, USA) at a dose of 100 mg/kg body weight in 100 μl of PBS, with an interval of 1.5 h. Control mice received physiological saline injections. 1.5 h after the second injection, the mice was killed and single thymocyte suspension were prepared as described above. Briefly, the cells were labeled with PE anti-CD4 and PE-Cy5 anti-CD8 antibodies (eBioscience, USA) and fixed in 2% paraformaldehyde/PBS for at least 12 h. Then, the fixed cells were washed, permeabilized with 0.1% Triton X-100/0.1% sodium citrate (pH 7.2) on ice for 2 min, and treated with 50 units of DNaseI (Takara, Japan) at 37°C for 20 min. Cells were then stained with FITC anti-BrdU antibody (BD Pharmingen, USA). BrdU incorporation was detected by flow cytometry. The data were collected by selectively gating acording to CD4 or CD8 populations, and analyses were performed with Cellquest software and FlowJo 7.2.5 (Becton Dickinson).
Statistical analysis
Results were presented as mean ± standard error mean (SEM). Statistically significant differences over time in the same treatment group, or among different treatment groups at a single time point were determined by two-tailed Student’s t-test. P values were also determined by nonparametric Wilcoxon test for paired samples on SPAW Statistics 18. Statistical significance was assumed for P-value ≤ 0.05.
Results
5-Aza treatment induces expression of IL-1Ra in thymocytes
Chemotherapy-induced immuno-suppression is followed by thymus regeneration. To identify candidate genetic signals contributing to the thymus damage and regeneration, 5-Aza was injected to normal mice to induce the thymus damage, and expressing level of IL-1Ra was detected. Total thymocytes reached quantitative nadir at day 7 after 5-Aza injection and then gradually increased (Figure 1A). The mRNA expression in total thymocytes during thymus suppression and regeneration phases was profiled using high density oligonucleotide microarray. The results showed that expression of IL-1Ra was highly induced from day 1.5 to day 7, decreased at day 11, and returned to pretreatment level at day 14 following 5-Aza injection (Figure 1B). The expression profiles of IL-1Ra inversely correlated to the thymus population after 5-Aza treatment, which suggested that IL-1Ra may play an important regulatory role in thymus homeostasis under stressed conditions.
Figure 1.
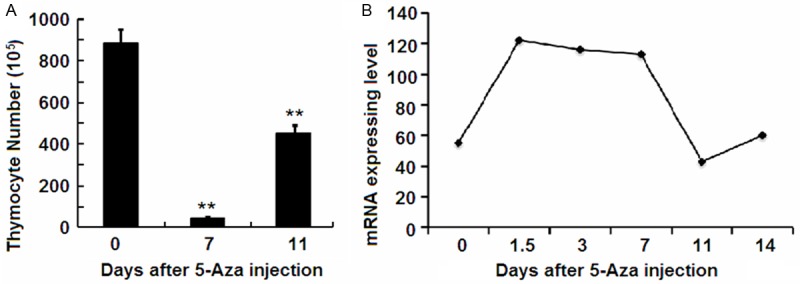
Observation of the expression of IL-1Ra in thymocytes. A. The thymocytes number was counted using a hemocytometer. B. Observation of the expression of IL-1Ra. **P<0.01 represents the thymocytes number in at 7 days or 11 days after 5-Azacytidine injection compared to the 0 day.
rHuIL-1Ra induces thymocytes suppression in normal mice by inhibition of cell cycle progression
Since level of IL-1Ra changes during the thymus damage and regeneration phases, we hypothesized that IL-1Ra may participate in the maintenance of thymus homeostasis in steady-state. To test our hypothesis and determine the dosage required for the reversible thymus suppression, normal mice were injected intraperitoneally with 0.3, 1 or 3 mg/kg rHuIL-1Ra daily for 5 days starting at day 0, control mice received physiological saline with the same volume. The thymocyte population was significantly reduced in rHuIL-1Ra treated mice in a dosage dependent manner (Figure 2A). When the protein was administered at 3 mg/kg for 5 days, the thymocyte population was reduced most significantly by 52.73% (P=0.0025).
Figure 2.
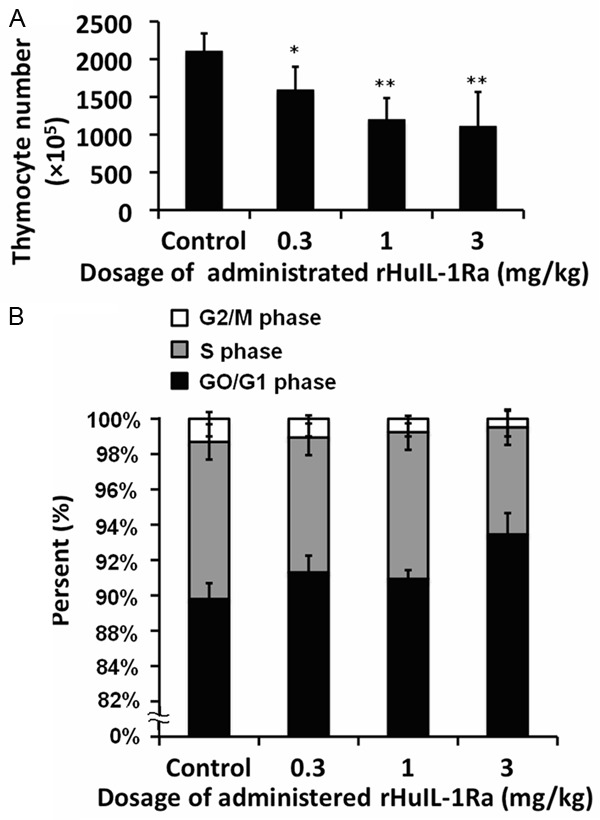
Examination of the thymocyte population and the cell cycle status of thymocytes when treated with rHuIL-1Ra. A. The thymocyte number was counted when administrated with different dosage of rHuIL-1Ra. B. Proportion of G2/M-phase, S phase and G0/G1 phase of thymocytes when administrated with different dosage of rHuIL-1Ra. **P<0.01 and *P<0.05 represents the thymocytes number in different dosage of rHuIL-1Ra administration compared to the control group.
To investigate the mechanisms of thymus suppressive role of IL-1Ra, the cell cycle status of thymocytes was analyzed at 24 h after the last administration of rHuIL-1Ra. The rHuIL-1Ra at 0.3 mg/kg or 1 mg/kg seemed to have no significant impact on the percentage of thymocytes in S-phase, although the percentage of them in G0/G1- and G2/M-phase was altered. When rHuIL-1Ra was injected at 3 mg/kg for 5 days, S-phase was reduced by 31.8% compared with the control group (Figure 2B, P=0.0021). Besides, there were significant changes in proportion of G2-phase and G1-phase cells. The results suggested that rHuIL-1Ra significantly reduced the percentages of thymocytes in S- and G2-phase and increased the proportion of cells in G0/G1-phase. Therefore, these results demonstrated that thymocyte cell cycle was arrested by rHuIL-1Ra.
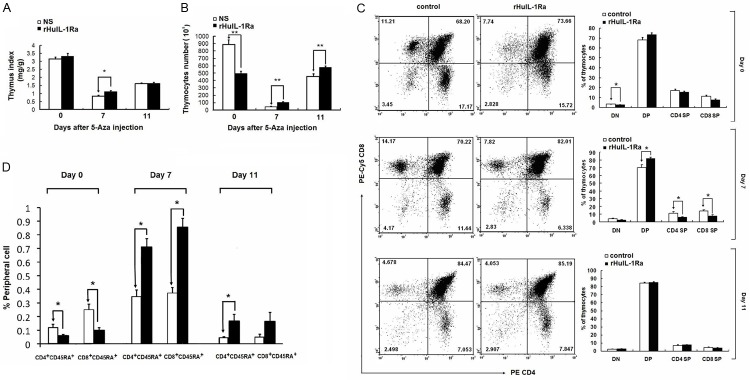
Pretreatment of rHuIL-1Ra reduces the damage of thymus induced by 5-Aza
The suppressive role of rHuIL-1Ra suggests that it might be applied to protect thymocytes from the cytotoxicity of chemotherapeutic agents. Administration of rHuIL-1Ra prior to 5-Aza treatment was carried out to examine the protective roles of rHuIL-1Ra. rHuIL-1Ra at 3 mg/kg or control physiological saline was administered daily for 5 days before 5-Aza injection. Previous studies suggested that the damage of thymus caused by 5-Aza was most serious at day 7 post chemotherapy, and that the thymocyte population began to recover. Therefore, we studied the damage and recover degree of thymus at 0, 7 and 11 days post chemotherapy.
Thymus damage induced by 5-Aza was most directly reflected in the decreased thymus index, which is the weight of thymus against the body weight. The pretreatment with rHuIL-1Ra significantly relieved the decrease of thymus index at day 7 (Figure 3A). Mice injected with rHuIL-1Ra also showed less reduction of thymocyte population compared with control mice at day 7 (Figure 3B). The constitution of thymic subsets, in other words, the thymus cellularity, was also altered. A small reduction of double negative (DN) thymocytes in rHuIL-1Ra treated mice was found at day 0. At day 7, mice treated with rHuIL-1Ra seemed to have higher proportion of CD4-CD8- double positive (DP) and lower proportion of the CD4+CD8- (CD4SP) and CD4-CD8+ (CD8SP) thymocytes (Figure 3C). At day 14, there was no significant difference between the cellularity of the rHuIL-1Ra treated mice and the control ones. These results indict rHuIL-1Ra exerts the protective roles in thymus damage induce the chemotherapy through inhibiting the differentiation of DP into single positive cells.
Figure 3.
Observation of the changes of thymous after pretreatment of rHuIL-1Ra. A. The thymus index statistics when injected with 5-Aza. B. The thymocytes number was counted when injected with 5-Aza. C. The CD4-CD8- double positive (DP), CD4+CD8- (CD4SP) and CD4-CD8+ (CD8SP) thymocytes were examined by using flow cytometry assay, and the proportion was analyzed by suing statistical analysis. D. Observation for the population of CD4+CD45RA+ T cells and proportion of CD8+CD45RA+ T cells. *P<0.05, **P<0.01 represent the values between the group marked with the arrow bar in graphs.
To test whether rHuIL-1Ra could play any in the peripheral immune system, blood samples were collected to analyze the peripheral naïve T cells. As progenies generated in a thymus-dependent manner, CD4+CD45RA+ and CD8+CD45RA+ peripheral T cells were analyzed to evaluate the level of T cells generation via a thymus-dependent manner [20-22]. After intensive treatment with rHuIL-1Ra, the population of CD4+CD45RA+ T cells decreased by 50.5%, similarly, the proportion of CD8+CD45RA+ T cells decreased by 61.2% (Figure 3D). 7 days after 5-Aza chemotherapy, the scale of CD4+CD45RA+ and CD8+45RA+ cells in both rHuIL-1Ra treated mice and control mice were enlarged. The proportion of CD4+CD45RA+ and CD8+CD45RA+ cells in rHuIL-1Ra treated mice were 2 to 3 times as large as controls. At day 11, scales of CD4+CD45RA+ and CD8+CD45RA+ decreased, and the proportion in rHuIL-1Ra treated mice was 1~2 folds higher than controls.
Analysis of thymocyte proliferation revealed that the percentage of BrdU+ thymocytes was substantially lower in rHuIL-1Ra treated mice at day 0, which was consistent with the effect of rHuIL-1Ra on cell cycle progress. At day 7, the percentage of BrdU+ thymocytes in rHuIL-1Ra treated mice was higher compared with control mice (Figure 4A, 4B), and these results were also consistent with that showed in Figure 3B. We then examined the thymic subsets. At day 0, for rHuIL-1Ra group, BrdU incorporation in DN and DP subsets was significantly reduced compared with control group. Since T cell differentiation occurs in the following sequence in DN, DP, and finally CD4SP or CD8SP subsets. The reduction of DN and DP subsets suggested that rHuIL-1Ra mainly inhibit the proliferation of relatively immature T cell subsets. Furthermore, proportion of BrdU+ cells in DN and DP (day 7), CD4SP (day 7), and CD8SP (day 7 and day 14) in rHuIL-1Ra treated mice was significantly higher than in control mice (Figure 4C). These results demonstrate that rHuIL-1Ra, when administered prior to chemotherapeutic agents, can reduce the acute toxicity on thymus. The above results demonstrated that the rHuIL-1Ra, when administered prior to chemotherapeutic agents, can reduce the acute toxicity on thymus.
Figure 4.
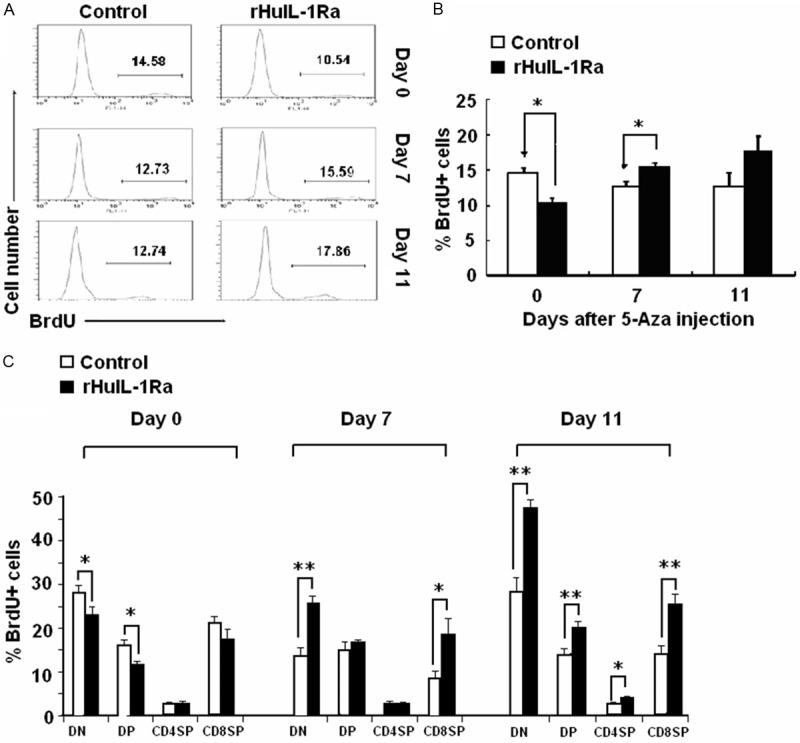
BrdU+ thymocytes amounts and BrdU+ cells proportion in DN, DP, CD4SP and CD4SP in rHuIL-1Ra treated mice. The percentage of BrdU+ thymocytes in rHuIL-1Ra treated mice was examined by using flow cytometry assay (A), and was analyzed (B). (C) Examination for the proportion of BrdU+ cells in DN, DP, CD4SP and CD8SP in rHuIL-1Ra treated mice and in control mice. *P<0.05, **P<0.01 represent the values between the group marked with the arrow bar in graphs.
Discussion
Our results showed that the mRNA level of murine IL-1Ra in thymocytes was transiently increased after 5-Aza chemotherapy. Activation of IL-1Ra expression has been reported in other stress states that the circulating IL-1Ra was significantly elevated in health volunteers injected with low-dose of E.coli endotoxin [23], and in patients with septic shock, juvenile rheumatoid arthritis, or inflammatory bowel disease [24]; levels of IL-1Ra correlated with the burn surface area in patients with thermal burns [25]; high levels of circulating IL-1Ra have been observed after myocardial infarction and general surgery, or in asymptomatic patients infected with HIV-1 [26].
Our results demonstrated certain roles of IL-1Ra in normal thymopoiesis. First of all, injection of rHuIL-1Ra could induce the suppression of thymopoiesis. The progression of thymocytes cycle and proliferation of relatively immature DN and DP thymocyte subsets in normal mice were inhibited. As a consequence of its suppressive roles on cell cycle and cell proliferation, pretreatment of IL-1Ra before 5-Aza injection could significantly alleviate the thymic toxicity and accelerates the reconstitution of thymopoiesis after chemotherapy.
It is likely that the thymopoiesis suppression by IL-1Ra is an indirect effect mediated by blocking the IL-1 signaling. The IL-1 signaling pathway affects thymopoiesis through various mechanisms. One of the major roles of IL-1 in thymus is T-cell activation and increasing thymic epithelial proliferation. We postulated that IL-1Ra could inhibit thymopoiesis by blocking the binding of IL-1 to cell-surface IL-1R. In our mouse model, thymus damage occurred as a consequence of treatment with chemotherapeutic agents such as 5-Aza, and the elevation of IL-1Ra level could temporarily drive the rapidly proliferating immature thymocyte subsets out of active cell cycle in order to escape from the cytotoxic insults. The conserved cells would form a resource for the regeneration of injured thymus post chemotherapy. This explains the elevated thymocyte proliferation after chemotherapy. Moreover, these cells are later differentiated into mature T cells and exported to peripheral blood acting as naïve T cells. Therefore, the suppressive role of IL-1Ra may protect thymus from the cytotoxicity of chemotherapy drugs. IL-1Ra should no longer be viewed as a simple IL-1 antagonist, but rather as a dynamic participant that adapts to pathological requirements to protect the thymus in cancer therapy.
Acknowledgements
This work was supported in part by the Science and Technology Commission of Shanghai Municipality (grand 075407071, 09540700600).
Disclosure of conflict of interest
None.
References
- 1.Xu Z, Chen Y, Gu D, Lee NP, Sun S, Gong W, Tan Y, Luk JM, Chen J. SOD2 rs4880 CT/CC genotype predicts poor survival for Chinese gastric cancer patients received platinum and fluorouracil based adjuvant chemotherapy. Am J Transl Res. 2015;7:401–410. [PMC free article] [PubMed] [Google Scholar]
- 2.Hendrickx P, Döhring W. Thymic atrophy and rebound enlargement following chemotherapy for testicular cancer. Acta Radiol. 1989;30:263–267. [PubMed] [Google Scholar]
- 3.Choyke PL, Zeman RK, Gootenberg JE, Greenberg JN, Hoffer F, Frank JA. Thymic atrophy and regrowth in response to chemotherapy: CT evaluation. Am J Roentgenol. 1987;149:269–272. doi: 10.2214/ajr.149.2.269. [DOI] [PubMed] [Google Scholar]
- 4.Csordas A, Schauenstein K. Thymus Involution induced by 5-Azacytidine. Biosci Rep. 1986;6:603–612. doi: 10.1007/BF01114754. [DOI] [PubMed] [Google Scholar]
- 5.Avigan D, Pirofski LA, Lazarus HM. Vaccination against infectious disease following hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2001;7:171–183. doi: 10.1053/bbmt.2001.v7.pm11302551. [DOI] [PubMed] [Google Scholar]
- 6.Whimbey E, Champlin RE, Couch RB, Englund JA, Goodrich JM, Raad I, Przepiorka D, Lewis VA, Mirza N, Yousuf H, Tarrand JJ, Bodey GP. Community respiratory virus infections among hospitalized adult bone marrow transplant recipients. Clin Infect Dis. 1996;22:778–782. doi: 10.1093/clinids/22.5.778. [DOI] [PubMed] [Google Scholar]
- 7.Parkman R, Cohen G, Carter SL, Weinberg KI, Masinsin B, Guinan E, Kurtzberg J, Wagner JE, Kernan NA. Successful immune reconstitution decreases leukemic relapse and improves survival in recipients of unrelated cord blood transplantation. Biol Blood Marrow Transplant. 2006;12:919–927. doi: 10.1016/j.bbmt.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Savani BN, Rezvani K, Mielke S, Monter A, Kurlander R, Carter CS, Leitman S, Read EJ, Childs R, Barrett AJ. Factors associated with early molecular remission after T cell-depleted allogeneic stem cell transplantation for chronic myelogenous leukemia. Blood. 2006;107:1688–1695. doi: 10.1182/blood-2005-05-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joao C, Porrata LF, Inwards DJ, Ansell SM, Micallef IN, Johnston PB, Gastineau DA, Markovic SN. Early lymphocyte recovery after autologous stem cell transplantation predicts superior survival in mantle-cell lymphoma. Bone Marrow Transplant. 2006;37:865–871. doi: 10.1038/sj.bmt.1705342. [DOI] [PubMed] [Google Scholar]
- 10.Miller JF. Immunological function of the thymus. Lancet. 1961;2:748–749. doi: 10.1016/s0140-6736(61)90693-6. [DOI] [PubMed] [Google Scholar]
- 11.Hannum CH, Wilcox CJ, Arend WP, Joslin FG, Dripps DJ, Heimdal PL, Armes LG, Sommer A, Eisenberg SP, Thompson RC. Interleukin-1 receptor antagonist activity of a human interleukin-1 inhibitor. Nature. 1990;343:336–340. doi: 10.1038/343336a0. [DOI] [PubMed] [Google Scholar]
- 12.Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13:323–340. doi: 10.1016/s1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 13.Le PT, Tuck DT, Dinarello CA, Haynes BF, Singer KH. Human thymic epithelial cells produce interleukin 1. J Immunol. 1987;138:2520–2526. [PubMed] [Google Scholar]
- 14.Simic MM, Stosić-Grujicic S. The dual role of interleukin 1 in lectin-induced proliferation of T cells. Folia Biol (Praha) 1985;31:410–424. [PubMed] [Google Scholar]
- 15.Rothenberg EV, Diamond RA, Pepper KA, Yang JA. IL-2 gene inducibility in T cells before T cell receptor expression. J Immunol. 1990;144:1614–1624. [PubMed] [Google Scholar]
- 16.Alter BJ, Bach FH. Cellular basis of the proliferative response of human T cells to mouse xenoantigens. J Exp Med. 1990;171:333–338. doi: 10.1084/jem.171.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galy AH, Dinarello CA, Kupper TS, Kameda A, Hadden JW. Effects of cytokines on human thymic epithelial cells in culture. II. Recombinant IL 1 stimulates thymic epithelial cells to produce IL6 and GM-CSF. Cell Immunol. 1990;129:161–175. doi: 10.1016/0008-8749(90)90195-w. [DOI] [PubMed] [Google Scholar]
- 18.McConkey DJ, Hartzell P, Chow SC, Orrenius S, Jondal M. Interleukin-1 inhibits T cell receptor mediated apoptosis in immature thymocytes. J Biol Chem. 1990;265:3009–3011. [PubMed] [Google Scholar]
- 19.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi CA. rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 20.Pilarski LM, Gillitzer R, Zola H, Shortman K, Scollay R. Definition of the thymic generative lineage by selective expression of high molecular weight isoforms of CD45 (T200) Eur J Immunol. 1989;19:589–597. doi: 10.1002/eji.1830190403. [DOI] [PubMed] [Google Scholar]
- 21.Yang W, Jia X, Su Y, Li Q. Immunophenotypic characterization of CD45RO+ and CD45RA+ T cells in peripheral blood of peripheral T cell lymphoma patients. Cell Biochem Biophys. 2014;70:993–997. doi: 10.1007/s12013-014-0008-3. [DOI] [PubMed] [Google Scholar]
- 22.Prelog M, Kipp S, Kem H, Neu N. Accumulation of CD8+CD45RA+CD62L T cells in acute chylothorax in neonates. Neonatology. 2009;95:86–90. doi: 10.1159/000151760. [DOI] [PubMed] [Google Scholar]
- 23.Machura E, Mazur B, Pieniazek W, Karczewska K. Expression of naïve/memory (CD45RA/CD45RO) markers by peripheral blood CD4+ and CD8+ T cells in children with asthma. Arch Immunol Ther Exp (Warsz) 2008;56:55–62. doi: 10.1007/s00005-008-0005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischer E, Van Zee KJ, Marano MA, Rock CS, Kenney JS, Poutsiaka DD, Dinarello CA, Lowry SF, Moldawer LL. Interleukin-1 receptor antagonist circulates in experimental inflammation and in human disease. Blood. 1992;79:2196–2200. [PubMed] [Google Scholar]
- 25.Mandrup-Poulsen T, Wogensen LD, Jensen M, Svensson P, Nilsson P, Emdal T, Mølvig J, Dinarello CA, Nerup J. Circulating interleukin-1 receptor antagonist concentrations are increased in adult patients with thermal injury. Crit Care Med. 1995;23:26–33. doi: 10.1097/00003246-199501000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]