Abstract
Background: Pelvic oligo-recurrence is common in rectal cancer patients, and some could not achieve radical resection. Objective: The study was to analyze long-term outcomes and prognostic factors associated with survival in patients treated with intensity-modulated radiation therapy (IMRT). Methods: Study participants were identified from rectal patients with pelvic oligo-recurrence without distant metastases, who were not suitable for surgery (n=135). Patients were recommended to receive concurrent chemotherapy in the course of IMRT (median dose 64.5 Gy, range: 45-70 Gy). Additionally, 24.4% (33/135) of patients received radical surgery after preoperative radiotherapy. Median time to pelvic failure was 25.4 months (range: 1-144 months). With a median follow-up period of 45.5 months (range: 3-104 months), 5-year overall survival (OS) and disease-free survival (DFS) were 55.6% and 45.5%, respectively. Results: In univariate survival analysis, OS stratified by subsites indicated that 5-year OS for anastomotic recurrence (80.5%) was better than for anterior recurrence (57.7%) and other pelvic oligo-recurrences (44.5%) (P=0.005). Five-year DFS in the three groups was 60.3%, 49% and 36.6%, respectively (P=0.037). In multivariate survival analysis, pelvic oligo-recurrence and symptomatic recurrence patterns were independently associated with OS in recurrent rectal cancer after pelvic radiotherapy (RT). Conclusions: These results indicate that RT for rectal cancer patients with pelvic oligo-recurrence had favorable prognosis, especially for patients with anastomotic recurrence.
Keywords: Rectal cancer, pelvic oligo-recurrence, intensity-modulated radiation therapy
Introduction
Although preoperative radiotherapy (RT) plus total mesorectal excision (TME) for rectal cancer have all rapidly been developed in recent decades, pelvic recurrence continues to remain as a significant clinical problem after definitive treatment [1-3]. Approximately 70% of colorectal cancer recurrences are solitary [4]. The concept of oligo-recurrence as a disease stage is that there is a limited number of metastases and the primary tumor has been controlled [5]. Recurrence is associated with severe morbidity including severe pelvic pain, bleeding, bowel obstruction, fistula, chronic pelvic sepsis and eventual death [6]. It not only shortens life expectancy, but also decreases quality of life (QOL) [7].
Surgery remains as the mainstay of treatment, and offers the best hope for managing pelvic recurrence. However, surgery is not a widely accepted treatment, and only 20-30% of patients would have a potentially curative operation. Moreover, surgical resection for palliation is usually inappropriate due to its associated morbidity [8].
RT plays a definitive role in improving the clinical outcome of oligo-recurrence and is the primary approach for patients unsuitable for surgery, but the exact role and optimal strategy for RT remains to be established. In the present study, we aimed to assess clinical outcomes and prognostic factors in patients with oligo-recurrence treated with RT.
Patients and methods
Clinical data
Data from 135 rectal cancer patients with pelvic oligo-recurrence treated in Beijing Cancer Hospital from April 2006 to December 2011 were collected. Eligible patients were selected according to the following criteria: (1) patients with histologically identified rectal cancer, (2) patients who underwent transabdominal radical resection, (3) patients who underwent R0 resection, (4) patients diagnosed with pelvic oligo-recurrence, and (5) patients with no clinical evidence of distant metastases. Exclusion criteria were as follows: (1) patients who underwent palliative surgery, (2) patients with synchronous metastasis or history of metastasis, (3) patients who did not completed the planned RT, and (4) patients who died of complications or other non-cancer-related reasons.
Diagnosis of pelvic oligo-recurrence
Pelvic oligo-recurrence was diagnosed histologically, radiologically or clinically. The diagnosis of local recurrence met at least one of the following major criteria [9]: (1) histological confirmation, (2) palpable or evident disease with subsequent clinical progression, (3) clear evidence of bone destruction, and (4) confirmed positive by positron emission tomography (PET). Diagnosis was confirmed with the presence of at least one of the following minor criteria: progressive enlargement of the soft tissue mass on repeated computed tomography (CT) or magnetic resonance imaging (MRI), invasion of adjacent organs, subsequent increase in tumor markers, and typical appearance on endoscopic ultrasound, CT or MRI examination. In addition, the concept of oligo-recurrence was defined by Niibe et al. as a state in which cancer patients have ≤5 metastatic or recurrent lesions with controlled primary lesions [5].
Sixty-seven of 135 patients were histologically confirmed, and the remaining 68 patients met at least one of the three major criteria including 42 patients with evident disease progression, eight patients with severe bone destruction and 18 patients confirmed positive by PET.
Classification of LR
In an attempt to classify subsites of pelvic recurrence more accurately, we defined these pelvic areas as follows [10-14]: Pre-sacrum: involving the sacrum and pre-sacral fascia (Figure 1A); Genitourinary: involving the genitourinary tract including the bladder, vagina, uterus, seminal vesicles and prostate (Figure 1B); Anastomosis and perineum: involving anastomotic recurrence (Figure 1C) after LAR and perineal recurrence (Figure 1D) after APR that involve the anal triangle comprising the perineum, anal sphincter complex and surrounding perianal and ischiorectal space; Lateral: area around the lateral aspect of the mesorectal fascia involving soft tissues of the pelvic sidewall, lateral bony pelvis, pelvic autonomic nerves, pelvic ureters, the greater sciatic foramen and the obturator (Figure 1E); Lymph nodes: along the internal (Figure 1F) and external (Figure 1G) iliac vessels and superficial inguinal lymph nodes (Figure 1H).
Figure 1.
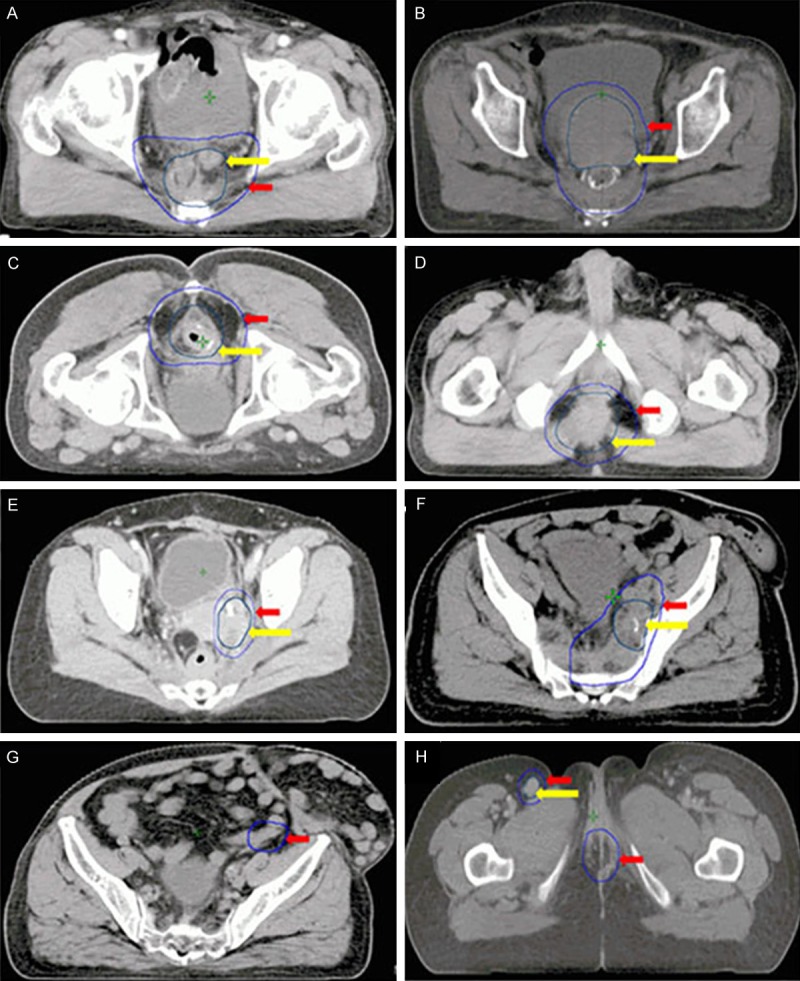
Transverse computed tomographic slices of the different recurrence pelvic subsites and treatment regions. Yellow arrow: GTV; Red arrow: CTV. A. Pre-sacral recurrence. B. Genitourinary recurrence. C. Anastomatic recurrence. D. Perineal recurrence. E. Lateral recurrence. F. Lymph nodes along the internal iliac vessels. G. Lymph nodes along the external iliac vessels. H. Superficial inguinal lymph nodes.
Data for all recurrences, CT, MRI, PET/CT, or diagnostic report recurrences (e.g., sigmoidoscopy for anastomotic recurrences) were reviewed by at least three radiation oncologists to verify the exact location of the recurrence and classify these into one of the subsites.
Treatment
Intensity-modulated radiation therapy (IMRT) was used for all 135 patients. The gross tumor volume (GTV), which consisted of all detectable tumors, was determined from the CT, PET/CT or MRI database. A safety margin of 5-20 mm was added to the clinical target volume (CTV) that covered the GTV depending on clinical circumstances, and the location of the lesion was taken into account for potential microscopic spreading. Organs at risk such as the bladder, femoral head and small bowel were delineated. Dose constraints of normal tissues were respected according to the study of Emami et al. [15].
GTV was delivered a total dose of 45-70 Gy in 22-35 fractions over 4.5-7 weeks with a 6-10 MV-X beam. Median RT dose was 64.5 Gy, and individual radiation dose was adjusted according to the status of the residual tumor, radiation history and proximity to the small bowel. These concurrent doses were lower in patients with prior RT compared to patients without history of RT (median total dose, 58.4 Gy vs. 66.5 Gy). Thus, adverse effects were reduced as far as possible. Patients with anastomotic relapse generally received pre-operative salvage RT at a dose of 45-50 Gy in 22 fractions, and were evaluated to determine whether surgery was appropriate. Thirty-one patients with anastomotic recurrence underwent surgery after pre-operative salvage RT. Two patients with anterior failure received R0 resection after pre-operative salvage radiation at a dose of 50 Gy.
Concurrent chemoradiotherapy (CRT), which was mostly fluorouracil-based, was recommended for patients with pelvic oligo-recurrence. Thus, most of the patients underwent concurrent chemotherapy. Thirty-two patients were unable to receive concurrent chemotherapy due to myelosuppression, hepatitis or poor performance status (PS). Maintenance chemotherapy was administered for four months after the completion of RT or CRT, if possible.
Follow-up and endpoints
Patients were assessed weekly during therapy for the evaluation of adverse events. Thereafter, they were evaluated at 3-month intervals for the first year, every six months for the next two years, and annually thereafter. Post-treatment follow-up included medical history, physical examination, measurement of hematology, liver and kidney function, and gastrointestinal tumor markers. Chest radiography, abdominal ultrasound and pelvic CT scans were also performed. Toxicity was analyzed according to the National Cancer Institute Common Toxicity Criteria (NCI-CTC), version 3.0. Endpoints of this study were 5-year overall survival (OS) and 5-year disease-free survival (DFS).
Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS, version 18.0; SPSS Inc., Chicago, IL, USA). Categorical variables were analyzed with Pearson X2 or Fisher’s exact test. Survival curves were plotted using the Kaplan-Meier method. Differences were assessed using log-rank tests. Two-sided tests of significance were used, and P values <0.05 were considered statistically significant.
Results
Patient characteristics
A summary of the clinical and pathological characteristics of patients is listed in Table 1. A total of 135 rectal cancer patients (73 male and 62 female patients) with pelvic oligo-recurrence treated with RT were included in this study. Patients had a median age was 56 years (range: 37-75 years), and majority of these patients (87.4%, 118/135) were classified as pT3-4/N+ during the initial diagnosis. Fifty-seven (42.2%) patients experienced pelvic oligo-recurrence after low anterior resection (LAR) and 78 (57.8%) patients experienced recurrence after abdominoperineal resection (APR). Forty patients (29.6%) underwent surgery alone and 95 patients (70.4%) underwent adjuvant chemotherapy or RT. Furthermore, 25 (18.5%) patients initially received pelvic RT including 19 pre-operative RT cases and six post-operative RT cases. Symptoms related to pelvic oligo-recurrence were pelvic pain (29 patients), bowel habit changes (21 patients), incomplete intestinal obstruction (three patients), and ureteral obstruction (one patient). In the diagnosis of pelvic oligo-recurrence, 60% of the patients (81/135) were asymptomatic. Pre-sacral recurrences occurred more often (33 patients, 24.4%). Among these 33 patients with pre-sacral recurrence, 25 patients underwent APR during initial treatment. Lateral recurrences comprised of 22.2% (30/135) of all pelvic recurrences. Thirty-one patients were involved with anastomosis, which accounted for 23% of the patients.
Table 1.
Clinical and pathological features
| Variables | n (%) |
|---|---|
| Age, Median (range), years | 56 (37-75) |
| Male | 73 (54.1) |
| Female | 62 (45.9) |
| Distance from anus | |
| ≤5 cm | 71 (52.6) |
| >5 cm to ≤10 cm | 60 (44.4) |
| >10 cm | 4 (3) |
| Resection type | |
| APR | 78 (57.8) |
| LAR | 57 (42.2) |
| pT-stage | |
| T1 | 2 (1.5) |
| T2 | 35 (25.9) |
| T3 | 68 (50.4) |
| T4 | 30 (22.2) |
| pN-stage | |
| N0 | 43 (31.9) |
| N1 | 62 (45.9) |
| N2 | 30 (22.2) |
| CEA | |
| Normal | 42 (31.1) |
| Abnormal | 93 (68.9) |
| Symptoms related to oligo-recurrence | |
| Yes | 54 (40) |
| No | 81 (60) |
| Subsites of pelvic oligo-recurrence | |
| Pre-sacral | 33 (24.4) |
| Lateral | 30 (22.2) |
| Anterior | 26 (19.3) |
| Anastomosis | 31 (23) |
| Perineum | 7 (5.2) |
| Internal iliac LN | 8 (5.9) |
Disease recurrence
Fifteen patients (11.1%) had second pelvic recurrence, 70 (51.9%) had distant recurrence, and nine (6.7%) had mixed recurrence. Liver metastasis accounted for 65.7% (46/70) of patients with distant failure, followed by lung metastasis (52.9%, 37/51).
Survival analysis
Median time to pelvic failure was 25.4 months (range: 1-144 months), which ranged from 20.1 months (range: 5-47 months) in T4 or N2 cases to 52.1 months (range: 1-144 months) in T1-2 or N0 cases. Median follow-up period was 45.5 months (range: 3-104 months). Five-year OS and DFS was 55.6% and 45.5%, respectively (Figure 2). OS stratified by subsites (Figure 3A) indicated that 5-year OS for anastomotic recurrence (80.5%) was better than for anterior recurrence (57.7%) and other pelvic oligo-recurrences (44.5%) including pre-sacral, lateral, perineal and internal iliac lymph node recurrence (P=0.005). Five-year DFS in the three groups was 60.3%, 49% and 36.6%, respectively (P=0.037, Figure 3B).
Figure 2.
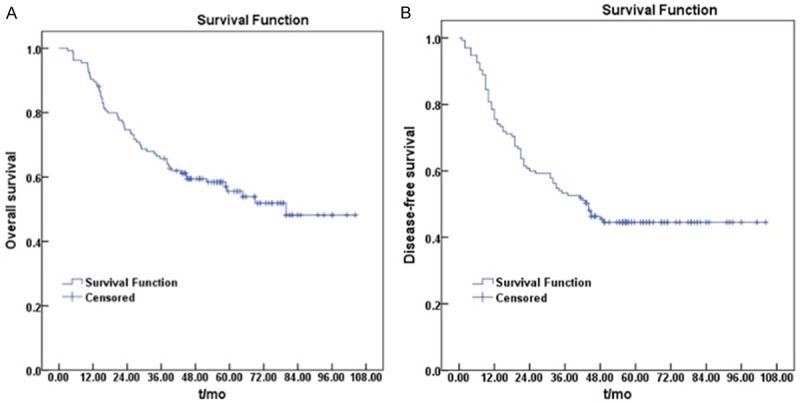
Kaplan-Meier curves of overall survival (A) and disease-free survival (B) in rectal cancer patients with pelvic oligo-recurrence after radiotherapy.
Figure 3.
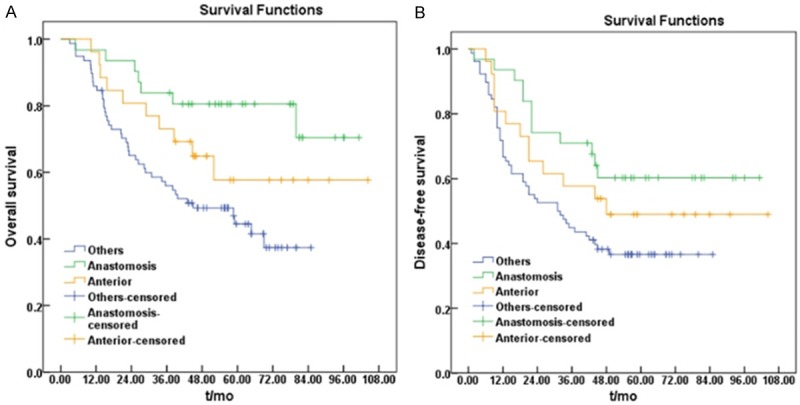
Kaplan-Meier curves of overall survival (A) and disease-free survival (B) in three groups classified as different subsites of pelvic oligo-recurrence. Others: Pre-sacral, lateral, perineal and internal iliac lymph nodes recurrences; Anastomosis: recurrences in anastomosis; Anterior: recurrences in anterior of rectum including bladder, vagina, uterus, seminal vesicles and prostate.
In the multivariate COX regression model, pelvic oligo-recurrence (HR=1.671, 95% CI: 1.303-5.474, P=0.037) and symptomatic or non-symptomatic diagnosed recurrence (HR=1.807, 95% CI: 1.314-8.343, P=0.025) patterns were independently associated with OS in recurrent rectal cancer after pelvic RT.
Toxic analysis
There were no treatment-related deaths. Eighteen patients (13.3%) presented with nausea/vomiting (Grade 2), 41 patients (31.1%) presented with diarrhea (Grade 1-2), three patients (2.2%) presented with abnormal liver function (Grade 1-2), and seven patients (5.2%) presented with edema on the ipsilateral leg (Grade 2). Moreover, two patients (1.5%) suffered from Grade 3 radiation dermatitis, while seven patients (5.2%) experienced Grade 3 myelosuppression.
During the follow-up period, five patients (3.7%) presented with anorexia (Grade 2), and three patients (2.2%) had persisting myelosuppression (Grade 1-2). At the last follow-up, there were no severe complications associated with RT.
Discussion
This study assessed clinical outcomes in rectal cancer patients with pelvic oligo-recurrence after RT. There was a high degree of correlation between pelvic oligo-recurrence patterns and prognosis. R0 resection of local recurrence is the most significant predictor of improved survival [16]. It has been proposed that higher resectability (77%) in anastomotic relapse might result in better survival rates (60% 5-year survival) [17]. Likewise, in this study, the prognosis for anastomotic recurrence was much better than for other recurrences. Some anastomotic relapses were curably resected after pre-CRT, and 5-year OS reached 80%. However, a Dutch trial revealed that pre-sacral and lateral LR resulted in poor prognosis in contrast to anastomotic or anterior recurrence, which was associated with a relatively good prognosis [10]. The main reason was that traditionally, invasion of the sacrum, lateral pelvic wall and/or envelopment of the iliac vessels were contraindications to a local radical procedure.
Clinical outcomes were better in our patients than in other studies [13,17,18]. One possible cause was the high proportion of anastomotic relapses in our study, which were generally associated with a satisfactory outcome. Five-year survival rate from the time of recurrence in the anastomotic relapse group was 80%, and this was also notably better compared with other studies [16,17]. After pre-operative concurrent CRT for anastomotic recurrences, most patients achieved R0 resection status; enabling the possibility of cure and long-term survival. In addition, all recurrent patients in this study were patients with oligo-recurrence without metastasis. Moreover, median RT dose was 66.5 Gy except for anastomotic recurrences. Some studies have demonstrated that higher radiation doses for patients with LRRC are correlated with better clinical outcomes [19,20]. Even though the dose of salvage RT in this study had no statistically significant effect on OS after LR, the main reason was that most patients with salvage doses less than 60 Gy recurred in anastomosis, making a large impact in survival after LR. This study revealed that the purpose of RT should not be just palliative, but an aggressive curative local control similar to surgery. Especially for patients with anastomotic failure, this could be aimed at increasing the resectable rate after pre-RT or CRT to achieve a curative effect.
Pre-operative CRT has become a standard component of multimodal treatment for locally advanced rectal cancer (T3-4 or node positive). After pre-operative RT or CRT, many locally advanced lesions could be rendered resectable, and local failure rate declined to 5-10% [21-24]. In our study, stage T3-4 tumors were initially present in 72.6% of the 135 patients, and lymph nodes were metastatic in 68.1% cases. Patients with T3-4/N+ accounted for 87.4% of patients. However, only 16.1% (19/118) of patients received pre-RT or CRT, in which most of whom were from other hospitals in China. Nonetheless, for various reasons, a part of patients with T3-4/N+ did not received adjunctive RT. These were probably the reasons why patients underwent pelvic recurrence in the end.
PS, AJCC stage, chemotherapy, surgery, extent of resection and histologic grading were among the many predictors for outcome in patients who received RT for local failure [25]. In our study, symptoms related to pelvic oligo-recurrence had a significant effect on OS. QOL may also be related to clinical outcome, and was perhaps considered a potential prognostic factor. Generally, local recurrence has a dramatic impact on QOL, as it is usually accompanied by severe pain and disabilities. Without treatment, patients would have a short life expectancy and QOL would be extremely poor. Surgery, if performed for recurrence, might be the most significant predictor for survival after recurrence. In this study, most patients who received surgery after pre-RT or CRT were limited to anastomotic relapses, which is a prognostic predictor.
Although salvage RT could not substitute for radical surgery, it is an option for achieving long-term survival. Our analysis revealed that improved local control resulted in improved OS, while patients who received appropriate treatment had a long-term survival benefit with improved QOL; indicating that it was not reasonable to stop treatment due to advanced disease stage.
Our study had some limitations. First, this study was retrospective in nature; thus, there may be selection bias. Second, some locations of recurrent tumors spread across two or more subsites; but these were classified according to major subsites, regardless of the minor ones. Therefore, we could not conclude whether these affected our results when treatment modalities and prognosis were analyzed. Third, the case number in some subgroups was small and initial treatment modalities were different, which might have influenced the reliability of the statistics.
In summary, our study demonstrates that RT is a significant approach for rectal cancer patients with pelvic oligo-recurrence, who are unsuitable for surgery. More intensive pre-RT or CRT regimens, improved surgical techniques, and accurate diagnosis and therapeutic evaluation methods should be recommended for pelvic oligo-recurrence from rectal cancer in the future.
References
- 1.Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R German Rectal Cancer Study Group. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–40. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 2.Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, Quirke P, Couture J, de Metz C, Myint AS, Bessell E, Griffiths G, Thompson LC, Parmar M. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373:811–20. doi: 10.1016/S0140-6736(09)60484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH, Leer JW, van de Velde CJ Dutch Colorectal Cancer Group. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–46. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 4.Tepper JE, O’Connell M, Hollis D, Niedzwiecki D, Cooke E, Mayer RJ Intergroup Study 0114. Analysis of surgical salvage after failure of primary therapy in rectal cancer: results from Intergroup Study 0114. J. Clin. Oncol. 2003;21:3623–8. doi: 10.1200/JCO.2003.03.018. [DOI] [PubMed] [Google Scholar]
- 5.Niibe Y, Hayakawa K. Oligometastases and oligo-recurrence: the new era of cancer therapy. Jpn J Clin Oncol. 2010;40:107–11. doi: 10.1093/jjco/hyp167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Chaisemartin C, Penna C, Goere D, Benoist S, Beauchet A, Julie C, Nordlinger B. Presentation and prognosis of local recurrence after total mesorectal excision. Colorectal Dis. 2009;11:60–6. doi: 10.1111/j.1463-1318.2008.01537.x. [DOI] [PubMed] [Google Scholar]
- 7.Camilleri-Brennan J, Steele RJ. The impact of recurrent rectal cancer on quality of life. Eur J Surg Oncol. 2001;27:349–353. doi: 10.1053/ejso.2001.1115. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen MB, Laurberg S, Holm T. Current management of locally recurrent rectal cancer. Colorectal Dis. 2011;13:732–742. doi: 10.1111/j.1463-1318.2009.02167.x. [DOI] [PubMed] [Google Scholar]
- 9.Enríquez-Navascués JM, Borda N, Lizerazu A, Placer C, Elosegui JL, Ciria JP, Lacasta A, Bujanda L. Patterns of local recurrence in rectal cancer after a multidisciplinary approach. World J Gastroenterol. 2011;17:1674–84. doi: 10.3748/wjg.v17.i13.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kusters M, Marijnen CA, van de Velde CJ, Rutten HJ, Lahaye MJ, Kim JH, Beets-Tan RG, Beets GL. Patterns of local recurrence in rectal cancer; a study of the Dutch TME trial. Eur J Surg Oncol. 2010;36:470–6. doi: 10.1016/j.ejso.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Roels S, Duthoy W, Haustermans K, Penninckx F, Vandecaveye V, Boterberg T, De Neve W. Definition and delineation of the clinical target volume for rectal cancer. Int J Radiat Oncol Biol Phys. 2006;65:1129–1142. doi: 10.1016/j.ijrobp.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 12.Yamada K, Ishizawa T, Niwa K, Chuman Y, Akiba S, Aikou T. Patterns of pelvic invasion are prognostic in the treatment of locally recurrent rectal cancer. Br J Surg. 2001;88:988–993. doi: 10.1046/j.0007-1323.2001.01811.x. [DOI] [PubMed] [Google Scholar]
- 13.Bouchard P, Efron J. Management of recurrent rectal cancer. Ann Surg Oncol. 2010;17:1343–1356. doi: 10.1245/s10434-009-0861-2. [DOI] [PubMed] [Google Scholar]
- 14.Mirnezami AH, Sagar PM. Surgery for recurrent rectal cancer: technical notes and management of complications. Tech Coloproctol. 2010;14:209–216. doi: 10.1007/s10151-010-0585-0. [DOI] [PubMed] [Google Scholar]
- 15.Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, Shank B, Solin LJ, Wesson M. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 16.Bedrosian I, Giacco G, Pederson L, Rodriguez-Bigas MA, Feig B, Hunt KK, Ellis L, Curley SA, Vauthey JN, Delclos M, Crane CH, Janjan N, Skibber JM. Outcome after curative resection for locally recurrent rectal cancer. Dis Colon Rectum. 2006;49:175–82. doi: 10.1007/s10350-005-0276-5. [DOI] [PubMed] [Google Scholar]
- 17.Kusters M, Dresen RC, Martijn H, Nieuwenhuijzen GA, van de Velde CJ, van den Berg HA, Beets-Tan RG, Rutten HJ. Radicality of resection and survival after multimodality treatment is influenced by subsite of locally recurrent rectal cancer. Int J Radiat Oncol Biol Phys. 2009;75:1444–9. doi: 10.1016/j.ijrobp.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Tepper JE, O’Connell M, Niedzwiecki D, Hollis DR, Benson AB 3rd, Cummings B, Gunderson LL, Macdonald JS, Martenson JA, Mayer RJ. Adjuvant therapy in rectal cancer: analysis of stage, sex, and local control--final report of intergroup 0114. J. Clin. Oncol. 2002;20:1744–50. doi: 10.1200/JCO.2002.07.132. [DOI] [PubMed] [Google Scholar]
- 19.Dresen RC, Gosens MJ, Martijn H, Nieuwenhuijzen GA, Creemers GJ, Daniels-Gooszen AW, van den Brule AJ, van den Berg HA, Rutten HJ. Radical resection after IORT-containing multimodality treatment is the most important determinant for outcome in patients treated for locally recurrent rectal cancer. Ann Surg Oncol. 2008;15:1937–47. doi: 10.1245/s10434-008-9896-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schurr P, Lentz E, Block S, Kaifi J, Kleinhans H, Cataldegirmen G, Kutup A, Schneider C, Strate T, Yekebas E, Izbicki J. Radical redo surgery for local rectal cancer recurrence improves overall survival: a single center experience. J Gastrointest Surg. 2008;12:1232–1238. doi: 10.1007/s11605-008-0517-8. [DOI] [PubMed] [Google Scholar]
- 21.Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC EORTC Radiotherapy Group Trial 22921. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–1123. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 22.Bujko K, Nowacki MP, Nasierowska-Guttmejer A, Michalski W, Bebenek M, Kryj M. Longterm results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006;93:1215–1223. doi: 10.1002/bjs.5506. [DOI] [PubMed] [Google Scholar]
- 23.Peeters KC, Marijnen CA, Nagtegaal ID, Kranenbarg EK, Putter H, Wiggers T, Rutten H, Pahlman L, Glimelius B, Leer JW, van de Velde CJ Dutch Colorectal Cancer Group. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007;246:693–701. doi: 10.1097/01.sla.0000257358.56863.ce. [DOI] [PubMed] [Google Scholar]
- 24.van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, Rutten HJ, Påhlman L, Glimelius B, van de Velde CJ Dutch Colorectal Cancer Group. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575–582. doi: 10.1016/S1470-2045(11)70097-3. [DOI] [PubMed] [Google Scholar]
- 25.Rades D, Kuhn H, Schultze J, Homann N, Brandenburg B, Schulte R, Krull A, Schild SE, Dunst J. Prognostic factors affecting locally recurrent rectal cancer and clinical significance of hemoglobin. Int J Radiat Oncol Biol Phys. 2008;70:1087–1093. doi: 10.1016/j.ijrobp.2007.07.2364. [DOI] [PubMed] [Google Scholar]


