Abstract
The mechanism of 5-HTT genetic polymorphisms related susceptibility of major depressive disorder (MDD) has not been fully understood. Two hundred MDD patients and 199 control subjects were included. A model of two binary logistical regressions with and without controlling for different psychosocial variables, was applied to investigate the possible mediation effects of psychosocial factors in contribution of 5-HTT polymorphisms in MDD development. These psychosocial variables included personality, trait coping style, life events and social support. Then, contribution of interactions between 5-HTT polymorphisms and psychosocial factors in MDD was investigated by a stepwise logistical approach. The results indicated a significant association of 5-HTT LPR with the MDD indicence, but not of VNTR genotype variances with the MDD incidence without counting effects of psychosocial factors. The ss genotype of LPR demonstrated 2.50 (95% CI: 1.11-5.62) times higher odds to develop MDD than ll genotype (p=0.026). After including psychosocial variables, the odds ratio of 5-HTT LPR ss to ll genotype dropped to 1.30 times (95% CI: 0.41-4.10) and became non-significant (p=0.658). While psychosocial variables all showed significant contributions to MDD susceptibility. Our data suggested an intermediator role of this psychosocial variable in LPR genetic pathogenesis of MDD. Whereas, 5-HTT VNTR could significantly affect MDD outcome by interacting with life events (p=0.043). In conclusion, 5-HTT LPR and VNTR polymorphisms could affect MDD susceptibility through mediation by trait coping styles and interaction with severe life events, respectively. The genetic information of 5-HTT can be potentially helpful for diagnosis and further therapy.
Keywords: Serotonin transporter, gene polymorphism, major depressive disorder, psychosocial factor
Introduction
Major depressive disorder (MDD) is one of the most common affective psychosocial disorders. This disease was less acknowledged and recognized in China till 2000. When the international diagnostic criteria and standardized interview instruments were adopted, the diagnosis rate has increased dramatically since then. The currently reported lifetime prevalence is about 3.5% in mainland China, which is similar to that in Japan (3%) and lower than the number in the United States (16.9%) [1,2].
Studies in the early 1980s [3-5] have already provided strong evidence for a salient role of serotonin transporter (5-HTT) in the patho-physiology of depression. In normal brain, after released into synaptic cleft, serotonin would be taken up, recycled and released again by presynaptic neurons via 5-HTT to eliminate excessive serotoninergic activity. Over-reactivated 5-HTT could result in a pathologically low extracellular level of serotonin, which could influence mood and further lead to psychological disorders including depression [6-8]. Nevertheless, the 5-HTT was soon proven to be an effective drug target for depressive disorders [9,10]. Widely prescript antidepressants, such as selective serotonin reuptake inhibitors (SSRIs) [11] and tricyclic antidepressants (TCAs) [12], are believed to restore or boost the serotoninergic neuron-transmission in the brain by suppressing the reuptake activity of 5-HTT.
Two genetic polymorphisms of 5-HTT were found associated to the susceptibility for MDD. In 1996, Ogilvie et al. [13] suggested that a 5-HTT polymorphism with different alleles was associated with susceptibility. The 5-HTT polymorphism had different numbers of variable-number-tandem-repeat (VNTR) region of 16-17 base-pair (bp) in the second in tron of 5-HTT gene. The most common frequent alleles of VNTR contain 10 (STin2.10) and 12 (STin2.12) repeats, which acts as transcriptional regulators with allele-dependent differential enhancer-like properties. STin2.12 allele showed a stronger transcriptional enhancing effect than STin2.10 both in vitro and in vivo level [14,15]. Another functional polymorphic region was identified at 1.2 kb upstream the start codon in the promoter of 5-HTT gene as linked polymorphic region (LPR). The LPR defines two types of allele, including short (s) with 14 and long (l) with 16 (or more) copies of a 22 to 23 bp repeat [16]. The LPR is also associated to the regulation of 5HTT activity. The previous studies showed that s allele reduced transcriptional efficiencies of the 5-HTT gene [17,18]. Lymphoblast with s allele exhibit decreased 5-HTT mRNA expression and less serotonin uptake compared with ll homozygotes [17], suggesting that the s variant may act as a dominant allele. The two polymorphisms of 5-HTT are potentially associated to various behaviors and psychiatric disorders including major depression. A few clinical studies reported significantly higher frequency of 5-HTT LPR s allele in MDD patients than in control subjects [19-21]. A recent meta analysis showed an interesting ethnic difference in the 5-HTT LPR epigenetic regulation. The meta results indicated that the ss genotype was associated with an increased risk of MDD among Caucasian populations, but not that clearly among Asians [22]. For VNTR variants, higher proportion of STin2.10 allele was found among MDD patients or suicidal subjects with MDD in comparison to control subjects in a few clinical studies [23,24]. However, the association between 5-HTT polymorphisms and MDD was not conclusive, due to non-statistical significance or unclear conclusion from other studies including meta-analysis [25-28]. The polymorphisms of 5-HTT seem to have rather a little predestined effect on the etiology of MDD. For the etiology of MDD, the genetic variation needs to react with or on other susceptive factors, such as environmental and/or other biological pathogenic influences. It was indeed demonstrated that gene by environment can significantly affect MDD by excessive number of studies [29-33]. These studies showed that the s allele of 5-HTT LPR was associated with an enhanced risk for developing depression under stress and challenges.
MDD is well known to be a complex multi-factorial disease. Besides the demographic influencers, the gender, education, history of mood, psychiatric disorders, physiological disease history, abuse history [34-36] could also contribute to the etiology of MDD. Furthermore, the other various psychosocial factors, including personality, trait coping styles, the important life events and social support, could also contribute to the etiology of MDD. However, except for the interaction between stressful life events and 5-HTT LPR polymorphisms mentioned above being excessively investigated, the other psychosocio-genetic relationships involved in the development of MDD are less clear. Other relevant psychosocial factors might also react with or link to specific genetic variants, and affect the development of MDD (Figure 1). The genetic influence of 5-HTT on depression could also be mediated by some of the psychosocial factors, which must be correlated to the genetic polymorphism itself. In this study, we would like to obtain a better insight of these complicated “psychosocial mechanisms” of the 5-HTT polymorphisms contribution in the development of MDD.
Figure 1.
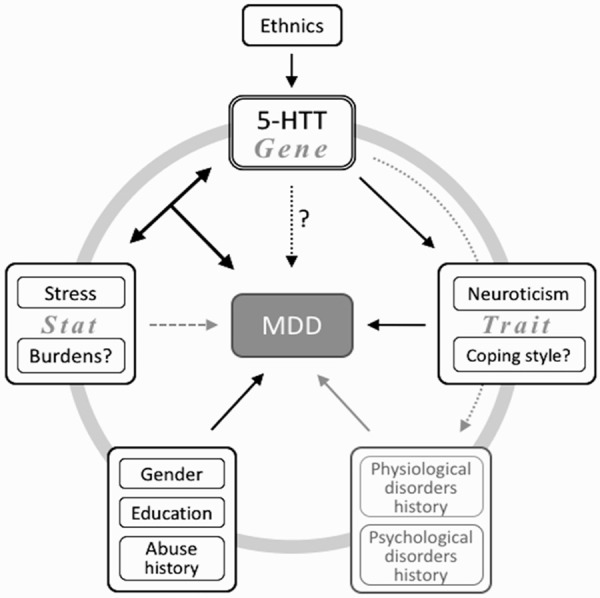
Schematic diagram of the contribution of 5-HTT gene interacting with different psychosocial factors to the etiology of MDD.
To investigate this question, we have detected 5-HTT LPR and VNTR genotypes and collected various psychosocial data. In this study, the psychosocial data, including personal trait, coping style, life events and social support were studied. All of these data were obtained from MDD patients and control subjects from ShanXi province in China under rigorous clinical process. We firstly used a model of two logistical regressions with and without controlling for different psychosocial variables, to compare the associations of 5-HTT polymorphisms to the susceptibility to MDD. Then the interactions between 5-HTT polymorphisms and psychosocial factors were investigated by a stepwise logistical approach.
Materials and methods
Subjects
This study was conducted in the Department of Psychiatry of the First Hospital of Shanxi Medical University and approved by the Ethical Committee for Medicine of First Hospital of Shanxi Medical University.
Total 314 Chinese Han participants were recruited from January 2008 to January 2010. The participants were categorized into two groups, including one group of patients with MDD and one control group. The patients with MDD were all aged 18 or over, from outpatients who were diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders Version IV (DSM-IV)MDD criteria [37]. All of the patients with MDD with a minimum Hamilton Depression Rating Scale (HAMD) score of 17. Patients with serious suicidal attempt, major psychiatric disorder history, severe physical disorder, organic disease or drug-induced secondary depression were excluded from the study. The final 200 patients with MDD consisted of 96 men and 104 women, aged from 14 to 52 years old with average age of 29.9 ± 8.9 (mean ± SD) years. The control subjects were selected among the healthy individuals visiting the same hospital for physical examination and matched by age. Subjectswere excluded from this study if they have significant depressive manifestations, serious organic disease, or families of psychiatric history. The selected 199 control subjects included 93 men and 106 women, aged 17 to 65 years old, with average age of 28.7 ± 9.0 years. The two groups all provided informed consent before participating in the study.
Clinical evaluation
The participants from both groups were assessed with the validated 17-item version of the HAMD in Chinese [38] by well trained psychiatrists with unified guidance language. The patients also received a Diagnostic Interview for Genetic Studies (DIGS) in Chinese version [39], which was specifically developed for the assessment of major mood and psychotic disorders [40]. The criteria for MDD in this study was set at a minimum score of 17 on the HAMD.
General demographic information, such as age and gender, was collected. Psychosocial variables assessed in this studies included personal trait, coping style, life events and social support. All of the above variables were evaluated by using highly reliable and efficient measurement tools that are widely used internationally and also adapted into a validated Chinese version.
Eysenck Personality Questionnaire (EPQ) Personality traits were evaluated with the Chinese version of EPQ revised by Gong [41], which was originally developed by Eysenck [42]. The questionnaire consisted of 88 entries of “Yes/No” questions within four dimensions, including Extraversion-introversion (with higher scores representing extraversion and sociability), Neuroticism (with higher scores suggesting anxiety and worry), Psychoticism (reflecting impulsiveness, solitude and stubbornness), and Lie scores (indicating pretense). The score was the sum of the answers within each dimension.
Life Event Scale (LES) was used to assess life events, to which participants were exposed in the past one year. This survey was developed by Yang and Zhang [43], and consisted of 48 items that described various life events. The life events include illness, housing problems, social difficulties, relationship breakdowns, unemployment, and financial crisis. All of the above life events were divided into three categories, including family life, working problems and social and other aspects. The occurrence, character (positive or negative), severity and influence of the life events were determined by the interviewers. The higher score means more sever and influential of the event. The total score of both positive and negative life events was used in the analysis.
Social Support Rating Scale (SSRS) was used to evaluate the social support available to the participants before the MD patients were clinical diagnosed and received medical therapy. Xiao [44] has developed the Chinese SSRS based on the measures and concepts of social support proposed by House and Kahn [45]. It has 10 items within 3 dimensions of objective support, subjective support and the use of social support. The items were assessed by a 4-point Likert scale (1-4). The total score was the sum of the results from every items, higher scores represent better social support.
Trait Coping Style Questionnaire (TCSQ), compiled by Jiang [46], was applied to assess regularly used coping strategies related to personality traits. The TCSQ comprised 20 items with two dimensions, including positive or negative coping strategies. Each item was rated by a 5-point Likert scale (1-5). The sum score of the results in each dimension represented the tendency of use.
Blood sampling and genotyping
After standard disinfection, 5 mL of venous blood was withdrawn from ante-cubital vein of each participant.
Genomic DNA was extracted and purified from the blood by standard phenol/chloroform method. The 5-HTT LPR and VNTR polymorphisms were amplified by using polymerase chain reaction (PCR) as described previously [47,48]. In brief, after cell-lysing and digestion with proteinase K, the DNA from blood lymphocytes was extracted with a phenol/chloroform/isoamyl alcohol (25:24:1) mixture and precipitated with 100% ethanol. The DNA pelleted was then washed with 70% ethanol and finally resuspended in ddH2O or buffer for further PRC experiments. 5-HTT LPR and VNTR genes were amplified by PCR, which was performed in a 25-μl reaction volume containing 2.5 μl of 10× GC buffer (Tiangen, Beijing, China), 1.0 U of Taq DNA polymerase (Tiangen, Beijing, China), 200 μM of dNTPs, 0.4 μM of each primer, and 60 ng of genomic DNA. The PCR amplification conditions and primers were listed in Table 1. The specific primers were designed using Primer 5.0 and verified with BLASTN against the human genome database (www.ncbi.nlm.nih.gov/BLAST) for their specificity.
Table 1.
Conditions of PCR
| Primers | Denaturation | 94°C for 5 min | |
|---|---|---|---|
| LPR | F: 5’-GGC GTT GCC GCT CTG AAT GC-3’ | 35 cycles | 94°C for 30 sec |
| R: 5’-GAG GGA CTG AGC TGG ACA ACC AC-3’ | 60°C for 30 sec | ||
| 72°C for 60 sec | |||
| VNTR | F: 5’-GTC AGT ATC ACA GGC TGC GAG-3’ | 30 cycles | 94°C for 30 sec |
| R: 5’-TGT TCC TAG TCT TAC GCC AGT G-3’ | 58°C for 40 sec | ||
| 72°C for 45 sec | |||
| Extension | 72°C for 10 min | ||
The PCR products of HTTLPR and HTTVNTR variants were separated on 12% polyacrylamide electrophoresis gels and visualized by using BIO IMAGING SYSTEM (Gene Genius, American). The purified PCR products were bi-directionally sequenced with an ABI 3700 DNA sequencer (Perkin-Elmer, Applied Biosystems, Foster City, CA, USA). Alleles and genotypes were defined by Chromas software (version 2.31) and four types alleles of the HTTLPR, including 14 repeats characterized as “s” allele, 16, 18 and 22 repeats as “l” allele, 10 and 12 repeats as and two types of HTTVNTR.
Statistical analysis
Difference in age and in measured psychosocial variables between the group of patients with MDD and control group was compared with independent t-test. Differences in sex were analyzed with the Pearson chi-square (χ2) test. The genotypic distribution of polymorphisms in both MDD and control groups was tested for Hardy-Weinberg equilibrium by χ2 test. The frequency of alleles and genotypes was compared between MDD group and control group with χ2 test. The association of 5-HTT LPR or VNTR with the probability of MDD was analyzed with two multivariable binary logistic regressions. Using 5-HTT genetic variance as the independent effect and MDD incidence as the dependent outcome and adjusted for demographic characteristics. The association between the psychosocial variables and 5-HTT LPR genotypes was checked with nonparametric Spearman correlation. The independent effect of the interactions between 5-HTT polymorphisms and various psychosocial factors on the MDD was investigated by a stepwise logistic regression using probability with cut-offs at 0.05 in and 0.10 out.
All of the statistical analyses were carried out with SPSS 22.0 software for Macintosh (IBM, USA). The significance level was set at a p value of 0.05 (2-tailed).
Results
Characteristics of participants
Participants were divided into two groups, including patients with MDD and controls, according to their diagnosis by professional psychiatrists. Characteristics of the two groups are presented in Table 2. The two groups had similar age and sex composition. Concerning 5-HTT polymorphisms, the distributions of LPR alleles were significantly different in the two groups (p=0.009). The MDD group had more patients carrying s allele (77.5%) compared with the control group (69.3%), and there were more patients of ss genotype in the MD group (60%) than in the control group (49.2%). No difference was found in VNTR distribution between the two groups. Our result suggested that the s allele of 5-HTT LPR was strongly related to the susceptibility to MDD, while the genotype difference of VNTR did not show any influence on the probability of the disease.
Table 2.
Characteristics of participants
| Control | MDD | t or x2 | df | p | ||
|---|---|---|---|---|---|---|
| n | 199 | 200 | ||||
| Demographic information | ||||||
| Gender (frequency) | M | 93 (46.7%) | 96 (48.0%) | 0.064 | 1 | 0.8 |
| F | 106 (53.3%) | 104 (52.0%) | ||||
| Age (years, mean ± SD) | 28.7 ± 9.0 | 29.9 ± 8.9 | -1.229 | 397 | 0.195 | |
| 5-HTT genetics | ||||||
| 5-HTT LPR Genotypes (frequency) | ll | 21 (10.6%) | 10 (5.0%) | 6.788* | 2 | 0.034 |
| ls | 80 (40.2%) | 70 (35.0%) | ||||
| ss | 98 (49.2%) | 120 (60.0%) | ||||
| 5-HTT LPR Alleles (frequency) | l | 122 (30.7%) | 90 (22.5%) | 6.789* | 1 | 0.009 |
| s | 276 (69.3%) | 310 (77.5%) | ||||
| 5-HTT VNTR Genotypes (frequency) | STin2.12/12 | 167 (83.9%) | 172 (86.0%) | 1.224 | 2 | 0.542 |
| STin2.12/10 | 31 (15.6%) | 28 (14.0%) | ||||
| STin2.10/10 | 1 (0.5%) | 0 (0.0%) | ||||
| 5-HTT VNTR Alleles (frequency) | STin2.12 | 365 (91.7%) | 372 (93.0%) | 0.471 | 1 | 0.492 |
| STin2.10 | 33 (8.3%) | 28 (7.0%) | ||||
| Psychosocial variables | ||||||
| EPQ (score, mean ± SD) | Total | 192.5 ± 16.4 | 199.9 ± 16.0 | -4.526* | 397 | 0.000 |
| Extraversion | 58.5 ± 10.2 | 43.9 ± 10.8 | 13.87* | 397 | 0.000 | |
| Neuroticism | 44.1 ± 10.6 | 61.5 ± 9.7 | -17.1* | 397 | 0.000 | |
| Psychoticism | 47.5 ± 8.7 | 51.4 ± 10.6 | -3.993* | 397 | 0.000 | |
| Lie | 42.4 ± 10.3 | 43.0 ± 10.2 | -0.664 | 397 | 0.507 | |
| LES (score, mean ± SD) | Total | 25.2 ± 41.0 | 46.8 ± 65.8 | -3.929* | 397 | 0.000 |
| Positive | 9.44 ± 18.4 | 7.9 ± 18.8 | 0.845 | 397 | 0.399 | |
| Negative | 15.8 ± 29.3 | 38.9 ± 52.7 | -5.423* | 397 | 0.000 | |
| SSRS (score, mean ± SD) | Total | 38.1 ± 7.5 | 33.8 ± 7.4 | 5.747* | 397 | 0.000 |
| Objective | 9.1 ± 2.8 | 8.6 ± 2.6 | 1.865 | 397 | 0.063 | |
| Subjective | 21.0 ± 5.1 | 18.67 ± 5.3 | 4.541* | 397 | 0.000 | |
| Use | 8.1 ± 1.8 | 6.6 ± 2.1 | 7.697* | 397 | 0.000 | |
| TCSQ (score, mean ± SD) | Positive | 35.9 ± 6.2 | 25.7 ± 8.1 | 14.151* | 397 | 0.000 |
| Negative | 25.5 ± 7.1 | 35.2 ± 7.6 | -13.42* | 397 | 0.000 |
Frequency: frequencies of each subtype among their may category; t: t value in independent t-test; x2: x2 value in Pearson chi-square test; df: degrees of freedom; SD: standard deviation; 5-HTT: serotonin transporter; LPR: linked polymorphic region in the promoter; VNTR: variable-number-tandem-repeat region in the second intron; EPQ: Eysenck Personality Questionnaire; LES: Life Event Scale; SSRS: Social Support Rating Scale; TCSQ: Trait Coping Style Questionnaire;
p<0.05, considered statistically significant.
To investigate the involvement of different psychosocial variables in the development of MDD, personal trait, coping style, life events and social support were evaluated. Interestingly, all of these psychosocial variables showed significantly difference (p<0.001) between the two groups. When looked into the subcategories, in the MD group, the participants tended to be more introvert (p<0.001) and showed higher scores in neuroticism (p<0.001) and psychoticism (p<0.001). Furthermore, they were also inclined to apply more negative coping strategies (p<0.001) and less positive coping strategies (p<0.001). The participants from the MD group suffered a higher degree from important life events, particularly from the negative ones (p<0.001). Meanwhile, they also used less social support than the control group (p<0.001). These results confirmed that MD is quite a psychosocial disorder, various psychosocial factors could affect the development the disease.
Psychosocial intermediation in the association of 5-HTT genetic polymorphisms with MDD incidence
To investigate the possible intermediation of psychosocial variables in the genetic responsibility of MD, two binary logistic regressions were performed with and without controlling for psychosocial variables. In this logistic regression analysis we used the 5HTT LPR or VNTR genotype set as independent variable and the MDD incidence as dependent. The first regression only included age and gender as covariates, while the second regression also included the psychosocial variables as covariates. The odds ratio (OR) for the 5HTT LPR ss genotype, compared with ll genotype, in the first regression without counting the effects of psychosocial variables, was 2.50 (95% CI: 1.12-5.62) with a p value of 0.026, which indicated a significantly higher possibility for 5HTT LPR ss genotype to develop MDD. The OR for 5-HTT VNTR genotype variants in their first regression did not show significant influence on the odds of MDD. All of these results corresponded well to the comparison outcomes between the two groups. The second regression included psychosocial variables, which were the total scores of personality test (EPQ), life events scale (LES), social support measure (SSRS) and separate positive and negative coping styles questionnaires (TCSQ). The OR of 5-HTT LPR ss to ll was reduced to 1.30 (95% CI: 0.41-4.10), and became non-significant (p=0.658) to the outcome of MDD. The association of the 5-HTT VNTR genotypes to the outcome of MDD still maintained non-significant in the second regression model (Table 3). The result suggested that the effect of 5-HTT LPR genetic variance on the development of MDD was probably medicated by the psychosocial variables. It is worth noting that, in the second regression model, the ORs of all the psychosocial factors had significant contributions to the odds for MDD, which reflects the importance of psychosocial influence on the development of MDD.
Table 3.
The contribution of 5-HTT LPR or VNTR genotype variance to the odds ratio for MDD, with or without controlling for psychosocial variables (PVs)
| OR (95% CI) | w/o PVs | p w/o PVs | OR’ (95% CI) | w PVs | p’ w PVs | ||
|---|---|---|---|---|---|---|---|
| Gender | F to M | 0.929 (0.623, 1.386) | 0.719 | 0.940 (0.533, 1.656) | 0.830 | ||
| Age | 1.010 (0.987, 1.033) | 0.402 | 1.014 (0.976, 1.054) | 0.477 | |||
| 5-HTT LPR Genotypes | ls to ll | 1.852 (0.813, 4.221) | 0.142 | 1.018 (0.314, 3.296) | 0.976 | ||
| ss to ll | 2.504* (1.116, 5.619) | 0.026 | 1.297 (0.410, 4.096) | 0.658 | |||
| EPQ | 1.029* (1.011, 1.048) | 0.001 | |||||
| LES | 1.008* (1.002, 1.015) | 0.012 | |||||
| SSRS | 0.922* (0.880, 0.966) | 0.001 | |||||
| TCSQ | Positive | 0.862* (0.827, 0.899) | 0.000 | ||||
| Negative | 1.104* (1.059, 1.151) | 0.000 | |||||
| Gender | F to M | 0.954 (0.642, 1.417) | 0.815 | 0.931 (0.530, 1.634) | 0.803 | ||
| Age | 1.014 (0.992, 1.037) | 0.205 | 1.017 (0.979, 1.056) | 0.393 | |||
| 5-HTT VNTR Genotypes | STin2.12/10 | 0.876 (0.503, 1.525) | 0.639 | 1.072 (0.511, 2.247) | 0.854 | ||
| STin2.10/10 | n.a. | n.a. | n.a. | n.a. | |||
| EPQ | 1.029* (1.012, 1.048) | 0.001 | |||||
| LES | 1.008* (1.002, 1.015) | 0.010 | |||||
| SSRS | 0.921* (0.879, 0.965) | 0.001 | |||||
| TCSQ | Positive | 0.861* (0.826, 0.897) | 0.000 | ||||
| Negative | 1.103* (1.058, 1.150) | 0.000 | |||||
OR: odds ratio of independent factors without PVs; OR’: odds ratio of independent factors including PVs; CI: confidence intervals;
p<0.05, considered statistically significant.
To further identify the mediation psychosocial factors in the contribution of 5-HTT LPR to the MDD, the correlations between the genotype of 5-HTT LPR and the psychosocial factors including their sub-categories were investigated. As shown in the Table 4, the positive trait coping styles was significantly correlated to the 5-HTT LPR genotypes (p=0.02), participants carrying 5-HTT LPR s allele demonstrated lower scores in positive coping styles than those of ll genotype, which were 30.6 ± 8.8 and 33.6 ± 8.6, respectively. Potential correlation was also found between the gene and the negative trait coping styles (p=0.065), with s allele carriers showing higher sores than ll genotype people, 30.6 ± 8.6 and 27.1 ± 8.4, respectively. These trait coping styles are stable over time and generally believed inner sourced-meaning different for each person. According to this result, the 5-HTT LPR polymorphism could strongly influence the differentiation of individual trait coping styles, which then would result in different susceptibility to MDD.
Table 4.
Correlations between psychosocial variables and 5-HTT LRP genotypes
| Genotypes | ss | ls | ll | Correlation coefficient | p | |
|---|---|---|---|---|---|---|
| n | 218 | 150 | 31 | |||
| EPQ (score, mean ± SD) | Total | 196.9 ± 16.9 | 196.2 ± 15.3 | 191.5 ± 19.9 | -0.046 | 0.361 |
| Extraversion | 50.5 ± 12.5 | 51.9 ± 13.0 | 52.5 ± 10.9 | 0.057 | 0.253 | |
| Neuroticism | 53.0 ± 14.0 | 53.4 ± 12.2 | 48.7 ± 13.5 | -0.042 | 0.401 | |
| Psychoticism | 50.1 ± 9.7 | 48.6 ± 10.2 | 49.3 ± 9.9 | -0.073 | 0.145 | |
| Lie | 43.3 ± 10.6 | 42.2 ± 9.9 | 41.1 ± 9.5 | -0.062 | 0.214 | |
| LES (score, mean ± SD) | Total | 41.2 ± 64.5 | 30.0 ± 41.9 | 28.5 ± 46.6 | -0.071 | 0.158 |
| Positive | 10.0 ± 20.7 | 6.9 ± 15.4 | 8.1 ± 17.0 | -0.074 | 0.143 | |
| Negative | 31.3 ± 51.2 | 23.1 ± 32.7 | 20.4 ± 36.1 | -0.048 | 0.339 | |
| SSRS (score, mean ± SD) | Total | 36.6 ± 7.8 | 34.9 ± 7.3 | 36.8 ± 9.0 | -0.067 | 0.184 |
| Objective | 8.9 ± 2.9 | 8.7 ± 2.4 | 8.8 ± 2.9 | -0.028 | 0.580 | |
| Subjective | 20.2 ± 5.3 | 19.1 ± 5.1 | 20.7 ± 6.0 | -0.068 | 0.177 | |
| Use | 7.4 ± 2.2 | 7.2 ± 1.9 | 7.4 ± 2.1 | -0.038 | 0.449 | |
| TCSQ (score, mean ± SD) | Positive | 29.9 ± 8.9 | 31.5 ± 8.7 | 33.6 ± 8.6 | 0.117* | 0.020 |
| Negative | 30.9 ± 8.6 | 30.2 ± 8.6 | 27.1 ± 8.4 | -0.092 | 0.065 |
p<0.05, considered statistically significant.
Psycho-socio-genetic interaction in the association of 5-HTT genetic polymorphisms with MDD incidence
Our result did not show a direct associated between 5-HTT VNTR polymorphism with the MDD, however, the genetic variance may well affect individual vulnerability for the disease under stressful and/or unfriendly environment. We therefore investigated the involvement of the interactions between the genetic polymorphisms, including both 5-HTT LPR and VNTR, and the psychosocial factors in the MDD development by using a stepwise binary logistic regression. In this model, the MDD outcome was set as a dependent factor. Both 5-HTT LPR and VNTR genotype variances and psychosocial variables were entered as covariates, and the psycho-socio-genetic interactions were statistically selected as independent factors in a forward stepwise process for significant contribution. Among all the tested interactions, the life events by 5-HTT VNTR showed a significant contribution (p=0.043) in the MDD development. And the OR of life events by STin2.12/10 to life events by STin2.12/12 was 1.03 with 95% confidence intervals between 1.00 and 1.05, The contributions from other covariates were shown in the Table 5.
Table 5.
The contribution of 5-HTT gene by psychosocial variables to the odds ratio for MDD
| OR (95% CI) | p | ||
|---|---|---|---|
| Gender | F to M | 0.864 (0.487, 1.534) | 0.617 |
| Age | 1.017 (0.978, 1.058) | 0.391 | |
| 5-HTT LPR Genotypes | ls to ll | 1.036 (0.318, 3.375) | 0.953 |
| ss to ll | 1.274 (0.401, 4.041) | 0.681 | |
| 5-HTT VNTR Genotypes | STin2.12/10 | 0.575 (0.217, 1.524) | 0.266 |
| STin2.10/10 | n.a. | n.a. | |
| EPQ | 1.029* (1.011, 1.048) | 0.002 | |
| LES | 1.005 (0.998, 1.013) | 0.139 | |
| SSRS | 0.919* (0.876, 0.964) | 0.001 | |
| TCSQ | Positive | 0.858* (0.822, 0.896) | 0.000 |
| Negative | 1.103* (1.057, 1.151) | 0.000 | |
| LES x VNTR | LES x STin2.12/10 | 1.025* (1.001, 1.050) | 0.043 |
| LES x STin2.10/10 | n.a. | n.a. | |
p<0.05, considered statistically significant.
Discussion
Previous studies have shown that specific psychosocial predictors [49] of MDD include, but not limited to stressful life events [50,51], neuroticism of personality [52,53], self-criticism and interpersonal dependency [52], work stress [54], social support [55] and coping style [36,56]. Anxiety-related personality trait, such as neuroticism, is closely related to the genetic difference itself. The higher neuroticism score was observed in ss genotype group of 5-HTT LPR compared with the l allele containing groups. It has been demonstrated that the association of 5-HTT gene with MDD is actually mediated by neuroticism [57,58]. Similar as the previous studies, our results suggested that the contribution of 5-HTT LPR genetic polymorphism to the MDD was mediated by trait coping styles. We also found a tendency of increased neuroticism scores in s allele carrier (53.2 ± 13.3) than in ll genotype (48.7 ± 13.5), however the correlation between neuroticism and 5-HTT LPR genotypes was not significant in our study (p=0.401; Table 4). As mentioned before, the contribution of the interaction between stressful life events and genetic variance to the vulnerability of MDD has been well documented and reviewed. The findings showed that 5-HTT LPR allele and/or other polymorphism-dependent stress sensitivity to severe life events, namely childhood maltreatment or major medical conditions, can predispose someone to develop depression [59,60]. We used a stepwise logistic regression to identify significant contribution(s) from the psycho-socio-genetic interactions for the susceptibility to MDD, while counting the independent main effects from these genetic and psychosocial factors themselves. The interaction between life events and 5-HTT VNTR was showed up in our analysis result. Interestingly, the odds ratio of life events (1.005 with 95% CI of 0.998 to 1.013; Table 5) became non-significant (p=0.139) for the MDD. This would also indicate that the life events affect the development of MDD mainly through the interaction with 5-HTT gene. To our best knowledge, this is the first time to show both mediation and interaction psychosocial mechanisms of the 5-HTT genetic effects (with different polymorphisms) on the psychosocial disorder. At the same time, MDD is helpful to understand a more complete picture of how the 5-HTT gene affect the development of MDD.
Our results have shown a significant overall influence of 5-HTT LPR polymorphism on the susceptibility to MDD, and the chance to develop MDD increased about two times in ss genotype compared with ll genotype. It is shown by previous studies that Asians tend to have higher frequency of ss and lower frequency of ll genotype of LPR compared with western population [61]. In our study, there was 54.6% participant of ss genotype, which was in line with the previous report. This probably explains the significant raw effect of 5-HTT LPR on the MDD development without controlling any psychosocial variables in our study. We did not find direct significant contribution of 5-HTT VNTR polymorphism. This may be because of the predominated STin2.12 allele distribution (92.4%) in our samples. There were only 1 participant was STin2.10/10 genotype who was in the control group, and the STin2.12/12 genotype occupied 85% of the participants, which was also found in previous research [62]. Due to the limited size of sample, the conclusion needs to be confirmed by a bigger size of sample, even better in different areas of China.
Recent research indicated that strict measures and clear divisions of environmental risks should be taken into account to study the contribution of the gene-environment relationship in psychiatric disorders [63]. Because different types and duration of environmental risks could confuse their impacts and interactions with genetic variants to develop further complications and dilute the statistical power. Therefore, in this study, two well trained psychiatrists with M.D. degree have used those internationally standardized questionnaires with scale measures for precisely divided categories. The psychosocial factors investigated in this study could be divided into two types, including state variables (such as life events and social supports) and trait variables (such as personality and trait coping style). Trait variables tend to represent long-last characteristics thatare highly heritable, which means that they could well be associated with genetic variance, and mediate the genetic contribution to certain psychiatric disorders [64,65]. Since state variables are to describe temporary emotional perception and response, they could be affected by trait variables, such as coping could mediate the correlation effect of stressful life events on anxiety and depression [66]. The state and trait variables are entangled under different circumstances, which may lead to various degrees of psychiatric disorders. With the fast development of molecular science and biostatistics, it is suspected that certain genes such as 5-HTT may play a central role in these complicated interactions between the trait and the stat psychosocial factors. In our study, the results showed a significant association between the positive trait coping styles with 5-HTT LPR genotypes and alleles. The potential correlations were also found with the negative coping styles. S-allele carriers showed higher potential to engage less positive and more negative coping styles. The results also suggested that different genotypes of 5-HTT VNTR tend to lead different vulnerability to MDD when encountering severe life events. People with the STin2.10 alleles might have a higher chance to develop MDD then. These results highlight the importance of 5-HTT in the MDD development, which brings valuable information to potentially refine the diagnosis and even the therapeutic strategies for MDD.
In conclusion, our results indicated that 5-HTT polymorphisms could affect the susceptibility to MDD through different psychosocial mechanisms, such as mediation by trait psychosocial factors (including coping styles) and through interacting with stat psychosocial factors (such as severe life events). Diagnosis and even therapeutic strategies for MDD can therefore be potentially improved by using the genetic information of 5-HTT from the patients.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant No. 81171290, 81471379) and Scientific and Technological Project of Shanxi Province (Grant No. 20130313023-2). We are very grateful to all the participants involved in this study, and thankful for the support from their families.
Disclosure of conflict of interest
None.
References
- 1.Gu L, Xie J, Long J, Chen Q, Chen Q, Pan R, Yan Y, Wu G, Liang B, Tan J, Xie X, Wei B, Su L. Epidemiology of Major Depressive Disorder in Mainland China: A Systematic Review. PLoS One. 2013;8:e65356. doi: 10.1371/journal.pone.0065356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu J, Yan F, Ma X, Guo HL, Tang YL, Rakofsky JJ, Wu XM, Li XQ, Zhu H, Guo XB, Yang Y, Li P, Cao XD, Li HY, Li ZB, Wang P, Wu QY. Prevalence of major depressive disorder and sociodemographic correlates: Results of a representative household epidemiological survey in Beijing, China. J Affect Disord. 2015;179:74–81. doi: 10.1016/j.jad.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meltzer HY, Arora RC, Baber R, Tricou BJ. Serotonin uptake in blood platelets of psychiatric patients. Arch Gen Psychiatry. 1981;38:1322–1326. doi: 10.1001/archpsyc.1981.01780370024002. [DOI] [PubMed] [Google Scholar]
- 4.Owens MJ, Nemeroff CB. Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clin Chem. 1994;40:288–295. [PubMed] [Google Scholar]
- 5.Paul SM, Rehavi M, Skolnick P, Ballenger JC, Goodwin FK. Depressed patients have decreased binding of tritiated imipramine to platelet serotonin “transporter”. Arch Gen Psychiatry. 1981;38:1315–1317. doi: 10.1001/archpsyc.1981.01780370017001. [DOI] [PubMed] [Google Scholar]
- 6.Lanfumey L, Mannoury La Cour C, Froger N, Hamon M. 5-HT-HPA interactions in two models of transgenic mice relevant to major depression. Neurochem Res. 1999;25:1199–1206. doi: 10.1023/a:1007683810230. [DOI] [PubMed] [Google Scholar]
- 7.McAllister-Williams RH, Ferrier IN, Young AH. Mood and neuropsychological function in depression: the role of corticosteroids and serotonin. Psychol Med. 1998;28:573–584. doi: 10.1017/s0033291798006680. [DOI] [PubMed] [Google Scholar]
- 8.Delgado PL. Depression: the case for a monoamine deficiency. J Clin Psychiatry. 2000;61(Suppl 6):7–11. [PubMed] [Google Scholar]
- 9.Holmes A, Yang RJ, Murphy DL, Crawley JN. Evaluation of Antidepressant-related Behavioral Responses in Mice Lacking the Serotonin Transporter. Neuropsychopharmacology. 2002;27:914–923. doi: 10.1016/S0893-133X(02)00374-3. [DOI] [PubMed] [Google Scholar]
- 10.Schloss P, Williams DC. The serotonin transporter: a primary target for antidepressant drugs. J Psychopharmacol. 1998;12:115–121. doi: 10.1177/026988119801200201. [DOI] [PubMed] [Google Scholar]
- 11.Hyttel J. Pharmacological characterization of selective serotonin reuptake inhibitors (SSRIs) Int Clin Psychopharmacol. 1994;9(Suppl 1):19–26. doi: 10.1097/00004850-199403001-00004. [DOI] [PubMed] [Google Scholar]
- 12.de Montigny C, Aghajanian GK. Tricyclic antidepressants: long-term treatment increases responsivity of rat forebrain neurons to serotonin. Science. 1978;202:1303–1306. doi: 10.1126/science.725608. [DOI] [PubMed] [Google Scholar]
- 13.Ogilvie AD, Battersby S, Fink G, Harmar AJ, Goodwin GM, Bubb VJ. Polymorphism in serotonin transporter gene associated with susceptibility to major depression. Lancet. 1998;347:731–733. doi: 10.1016/s0140-6736(96)90079-3. [DOI] [PubMed] [Google Scholar]
- 14.Fiskerstrand CE, Lovejoy EA, Quinn JP. An intronic polymorphic domain often associated with susceptibility to affective disorders has allele dependent differential enhancer activity in embryonic stem cells. FEBS Lett. 1999;458:171–174. doi: 10.1016/s0014-5793(99)01150-3. [DOI] [PubMed] [Google Scholar]
- 15.MacKenzie A, Quinn J. A serotonin transporter gene intron 2 polymorphic region, correlated with affective disorders, has allele-dependent differential enhancer-like properties in the mouse embryo. Proc Natl Acad Sci U S A. 1999;96:15251–15255. doi: 10.1073/pnas.96.26.15251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- 17.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 18.Mortensen OV, Thomassen M, Larsen MB, Whittemore SR, Wiborg O. Functional analysis of a novel human serotonin transporter gene promoter in immortalized raphe cells. Brain Res Mol Brain Res. 1999;68:141–148. doi: 10.1016/s0169-328x(99)00083-2. [DOI] [PubMed] [Google Scholar]
- 19.Cervilla JA, Rivera M, Molina E, Torres-Gonzalez F, Bellon JA, Moreno B. The 5-HTTLPR s/s genotype at the serotonin transporter gene (SLC6A4) increases the risk for depression in a large cohort of primary care attendees: the PREDICT-gene study. Am J Med Genet B Neuropsychiatr Genet. 2006;141b:912–917. doi: 10.1002/ajmg.b.30455. [DOI] [PubMed] [Google Scholar]
- 20.Hoefgen B, Schulze TG, Ohlraun S, von Widdern O, Hofels S, Gross M. The power of sample size and homogenous sampling: association between the 5-HTTLPR serotonin transporter polymorphism and major depressive disorder. Biol Psychiatry. 2005;57:247–251. doi: 10.1016/j.biopsych.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 21.Ramasubbu R, Tobias R, Buchan AM, Bech-Hansen NT. Serotonin transporter gene promoter region polymorphism associated with poststroke major depression. J Neuropsychiatry Clin Neurosci. 2006;18:96–99. doi: 10.1176/jnp.18.1.96. [DOI] [PubMed] [Google Scholar]
- 22.Kiyohara C, Yoshimasu K. Association between major depressive disorder and a functional polymorphism of the 5-hydroxytryptamine (serotonin) transporter gene: a meta-analysis. Psychiatr Genet. 2010;20:49–58. doi: 10.1097/YPG.0b013e328335112b. [DOI] [PubMed] [Google Scholar]
- 23.Lee HY, Hong JP, Hwang JA, Lee HJ, Yoon HK, Lee BH. Possible Association between Serotonin Transporter Gene Polymorphism and Suicide Behavior in Major Depressive Disorder. Psychiatry Investig. 2015;12:136–141. doi: 10.4306/pi.2015.12.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarosi A, Gonda X, Balogh G, Domotor E, Szekely A, Hejjas K. Association of the STin2 polymorphism of the serotonin transporter gene with a neurocognitive endophenotype in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1667–1672. doi: 10.1016/j.pnpbp.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 25.Cajal AR, Redal MA, Costa LD, Lesik LA, Faccioli JL, Finkelsztein CA. Influence of 5-HTTLPR and 5-HTTVNTR polymorphisms of the serotonin transporter gene (SLC6A4) on major depressive disorder in a sample of Argentinean population. Psychiatr Genet. 2012;22:103–104. doi: 10.1097/YPG.0b013e32834acc9b. [DOI] [PubMed] [Google Scholar]
- 26.Mann JJ, Huang YY, Underwood MD, Kassir SA, Oppenheim S, Kelly TM. A serotonin transporter gene promoter polymorphism (5-HTTLPR) and prefrontal cortical binding in major depression and suicide. Arch Gen Psychiatry. 2000;57:729–738. doi: 10.1001/archpsyc.57.8.729. [DOI] [PubMed] [Google Scholar]
- 27.Risch N, Herrell R, Lehner T. Interaction between the serotonin transporter gene (5-httlpr), stressful life events, and risk of depression: A meta-analysis. JAMA. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zaboli G, Jonsson EG, Gizatullin R, De Franciscis A, Asberg M, Leopardi R. Haplotype analysis confirms association of the serotonin transporter (5-HTT) gene with schizophrenia but not with major depression. Am J Med Genet B Neuropsychiatr Genet. 2008;147:301–307. doi: 10.1002/ajmg.b.30597. [DOI] [PubMed] [Google Scholar]
- 29.Brown GW, Harris TO. Depression and the serotonin transporter 5-HTTLPR polymorphism: a review and a hypothesis concerning gene-environment interaction. J Affect Disord. 2008;111:1–12. doi: 10.1016/j.jad.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 30.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 31.Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication. Arch Gen Psychiatry. 2005;62:529–535. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- 32.Ming Q, Zhang Y, Chai Q, Chen H, Hou C, Wang M. Interaction between a serotonin transporter gene promoter region polymorphism and stress predicts depressive symptoms in Chinese adolescents: a multi-wave longitudinal study. BMC Psychiatry. 2013;13:142–142. doi: 10.1186/1471-244X-13-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharpley CF, Palanisamy SK, Glyde NS, Dillingham PW, Agnew LL. An update on the interaction between the serotonin transporter promoter variant (5-HTTLPR), stress and depression, plus an exploration of non-confirming findings. Behav Brain Res. 2014;273:89–105. doi: 10.1016/j.bbr.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 34.Kendler KS, Gardner CO, Prescott CA. Toward a comprehensive developmental model for major depression in women. Am J Psychiatry. 2012;159:1133–1145. doi: 10.1176/appi.ajp.159.7.1133. [DOI] [PubMed] [Google Scholar]
- 35.Klein DN, Glenn CR, Kosty DB, Seeley JR, Rohde P, Lewinsohn PM. Predictors of first lifetime onset of major depressive disorder in young adulthood. J Abnorm Psychol. 2013;122:1–6. doi: 10.1037/a0029567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mugisha J, Muyinda H, Malamba S, Kinyanda E. Major depressive disorder seven years after the conflict in northern Uganda: burden, risk factors and impact on outcomes (The Wayo-Nero Study) BMC Psychiatry. 2015;15:48. doi: 10.1186/s12888-015-0423-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM): DSM-IV. 4th ed. Washinton (DC): American Psychiatric Association; 1994. [Google Scholar]
- 38.Zheng JP. Validity and reliability of the Chinese Hamilton Depression Rating Scale. Br J Psychiatry J Ment Sci. 1988;152:660–664. doi: 10.1192/bjp.152.5.660. [DOI] [PubMed] [Google Scholar]
- 39.Chen WJ, Liu SK, Chang CJ, Lien YJ, Chang YH, Hwu HG. Sustained Attention Deficit and Schizotypal Personality Features in Nonpsychotic Relatives of Schizophrenic Patients. Am J Psychiatry. 1998;155:1214–1220. doi: 10.1176/ajp.155.9.1214. [DOI] [PubMed] [Google Scholar]
- 40.Nurnberger JI Jr, Blehar MC, Kaufmann CA. Diagnostic interview for genetic studies: Rationale, unique features, and training. Arch Gen Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- 41.Gong Y. Use of the Eysenck Personality Questionnaire in China. Personal Individ Differ. 1984;5:431–438. [Google Scholar]
- 42.Eysenck HJ, Eysenck SBG. Manual of the Eysenck Personality Questionnaire. London: Hodder & Stoughton; 1975. [Google Scholar]
- 43.Yang DS, Zhang YL. Life Event Scale (LES) In: Wang XD, Wang XL, Ma H, editors. Rat. Scales Ment. Health. 2nd ed. Beijing: Chinese Mental Health Journal Press; 1999. pp. 101–6. [Google Scholar]
- 44.Xiao SY. Chinese social support rate scale (SSRS) In: Wang XD, Wang XL, Ma H, editors. Rat. Scales Ment. Health. 2nd ed. Beijing: Chinese Mental Health Journal Press; 1999. pp. 127–131. [Google Scholar]
- 45.House JS, Kahn RA. Measures and concepts of social support. In: Cohen Syme SL, editor. Soc. Support Health. Orlando: Academic Press; 1985. pp. 83–108. [Google Scholar]
- 46.Jiang QJ. The Chinese Trait Coping Style Questionnaire. In: Wang XD, Wang XL, Ma H, editors. Rat. Scales Ment. Health. 2nd ed. Beijing: Chinese Mental Health Journal Press; 1999. pp. 120–122. [Google Scholar]
- 47.Wang S, Zhang K, Xu Y, Sun N, Shen Y, Xu Q. An association study of the serotonin transporter and receptor genes with the suicidal ideation of major depression in a Chinese Han population. Psychiatry Res. 2009;170:204–207. doi: 10.1016/j.psychres.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 48.Zhang K, Xu Q, Xu Y, Yang H, Luo J, Sun Y. The combined effects of the 5-HTTLPR and 5-HTR1A genes modulates the relationship between negative life events and major depressive disorder in a Chinese population. J Affect Disord. 2009;114:224–231. doi: 10.1016/j.jad.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 49.Kupfer DJ, Frank E. Role of psychosocial factors in the onset of major depression. Ann N Y Acad Sci. 1997;807:429–439. doi: 10.1111/j.1749-6632.1997.tb51937.x. [DOI] [PubMed] [Google Scholar]
- 50.Bromberger JT, Kravitz HM, Matthews K, Youk A, Brown C, Feng W. Predictors of first lifetime episodes of major depression in midlife women. Psychol Med. 2009;39:55–64. doi: 10.1017/S0033291708003218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tao M, Li Y, Xie D, Wang Z, Qiu J, Wu W. Examining the relationship between lifetime stressful life events and the onset of major depression in Chinese women. J Affect Disord. 2011;135:95–99. doi: 10.1016/j.jad.2011.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cox BJ, McWilliams LA, Enns MW, Clara IP. Broad and specific personality dimensions associated with major depression in a nationally representative sample. Compr Psychiatry. 2004;45:246–253. doi: 10.1016/j.comppsych.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 53.Kendler KS, Kuhn J, Prescott CA. The interrelationship of neuroticism, sex, and stressful life events in the prediction of episodes of major depression. Am J Psychiatry. 2004;161:631–636. doi: 10.1176/appi.ajp.161.4.631. [DOI] [PubMed] [Google Scholar]
- 54.Wang J. Work stress as a risk factor for major depressive episode(s) Psychol Med. 2005;35:865–871. doi: 10.1017/s0033291704003241. [DOI] [PubMed] [Google Scholar]
- 55.Boyce P, Hickey A. Psychosocial risk factors to major depression after childbirth. Soc Psychiatry Psychiatr Epidemiol. 2005;40:605–612. doi: 10.1007/s00127-005-0931-0. [DOI] [PubMed] [Google Scholar]
- 56.Enns MW, Cox BJ. Psychosocial and clinical predictors of symptom persistence vs remission in major depressive disorder. Can J Psychiatry. 2005;50:769–777. doi: 10.1177/070674370505001206. [DOI] [PubMed] [Google Scholar]
- 57.Middeldorp CM, de Moor MH, McGrath LM, Gordon SD, Blackwood DH, Costa PT. The genetic association between personality and major depression or bipolar disorder. A polygenic score analysis using genome-wide association data. Transl Psychiatry. 2011;1:e50. doi: 10.1038/tp.2011.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Munafò MR, Clark TG, Roberts KH, Johnstone EC. Neuroticism mediates the association of the serotonin transporter gene with lifetime major depression. Neuropsychobiology. 2006;53:1–8. doi: 10.1159/000089915. [DOI] [PubMed] [Google Scholar]
- 59.Klengel T, Binder EB. Gene-environment interactions in major depressive disorder. Can J Psychiatry. 2013;58:76–83. doi: 10.1177/070674371305800203. [DOI] [PubMed] [Google Scholar]
- 60.Lopizzo N, Bocchio Chiavetto L, Cattane N, Plazzotta G, Tarazi FI, Pariante CM. Gene-Environment Interaction in Major Depression: Focus on Experience-Dependent Biological Systems. Front Psychiatry. 2015;6:68. doi: 10.3389/fpsyt.2015.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goldman N, Glei DA, Lin YH, Weinstein M. The Serotonin Transporter Polymorphism (5-HTTLPR): Allelic Variation and Links with Depressive Symptoms. Depress Anxiety. 2010;27:260–269. doi: 10.1002/da.20660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shen Y, Li H, Gu N, Tan Z, Tang J, Fan J. Relationship between suicidal behavior of psychotic inpatients and serotonin transporter gene in Han Chinese. Neurosci Lett. 2004;372:94–98. doi: 10.1016/j.neulet.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 63.Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Griffiths AJ, Miller JH, Suzuki DT, Lewontin RC, Gelbart WM. Heritability of a trait. 2000 [Google Scholar]
- 65.Kendler KS. What psychiatric genetics has taught us about the nature of psychiatric illness and what is left to learn. Mol Psychiatry. 2013;18:1058–1066. doi: 10.1038/mp.2013.50. [DOI] [PubMed] [Google Scholar]
- 66.Meng XH, Tao FB, Wan YH, Hu Y, Wang RX. Coping as a mechanism linking stressful life events and mental health problems in adolescents. Biomed Environ Sci BES. 2011;24:649–655. doi: 10.3967/0895-3988.2011.06.009. [DOI] [PubMed] [Google Scholar]


