Abstract
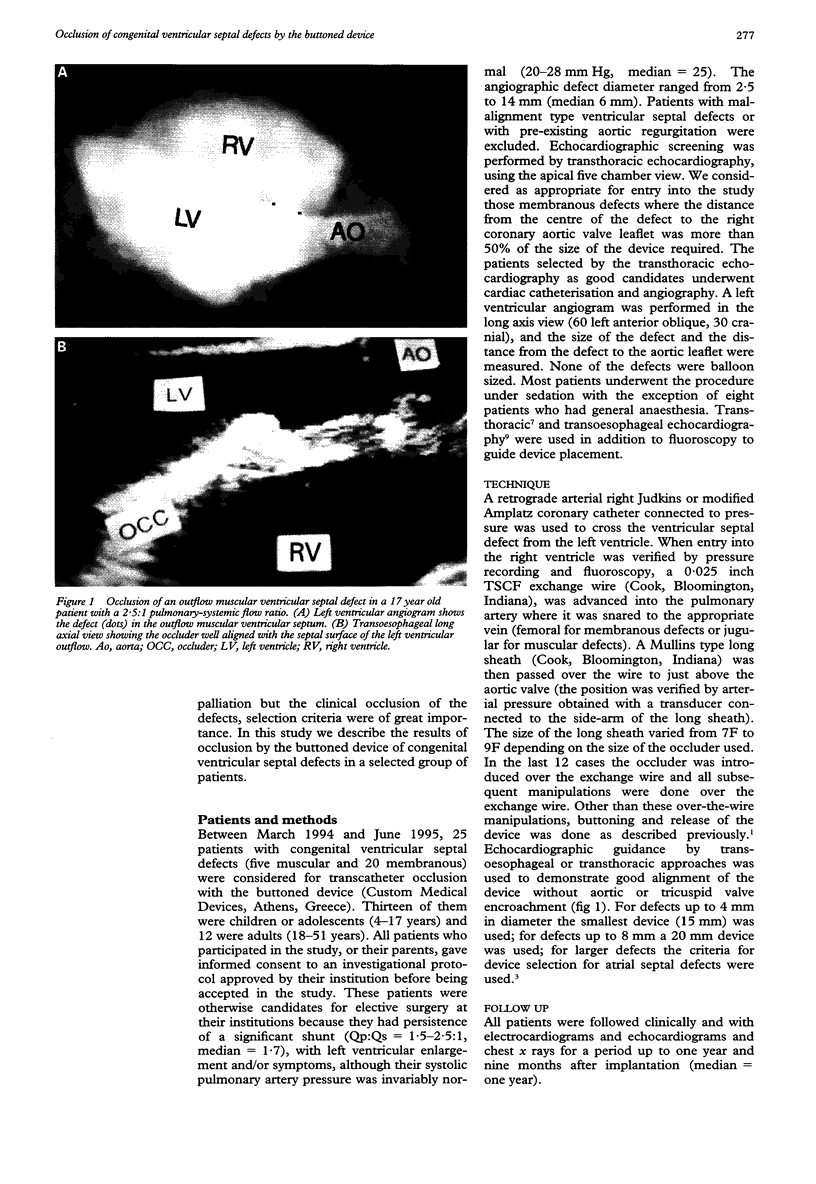
OBJECTIVES: To study the feasibility of congenital ventricular septal defect occlusion by the buttoned device and to establish guidelines for its safe and effective application. DESIGN: A descriptive study of all patients with a congenital ventricular septal defect undergoing transcatheter occlusion with the buttoned device, from March 1994 to May 1995. These patients were otherwise candidates for elective surgery at their institutions because they had persistence of a significant shunt (Qp:Qs = 1.5-2.1:1, median = 1.7), with left ventricular enlargement and/or symptoms, although their systolic pulmonary artery pressure was invariably normal (20-28 mm Hg, median = 25). The angiographic diameter of the defect ranged from 2.5 to 14 mm (median 6 mm). SETTING: A multi-institutional study. PATIENTS: Out of 25 cases attempted, 18 children and adults aged 4-35 years had devices implanted. Fifteen of these patients had membranous ventricular septal defects and three had muscular defects. All patients with a membranous ventricular septal defect had an associated aneurysm of the membranous septum. INTERVENTIONS: The buttoned device was introduced either directly or, in the last 12 cases, over a wire bridging the femoral artery and the femoral or jugular vein; the devices were delivered through 7-9 French (F) long sheaths. A membranous defect was regarded as suitable for device closure if the distance from the centre of the defect to the insertion of the right coronary aortic valve leaflet was more than 50% of the size of the required device. The device was guided by echocardiography and fluoroscopy. All muscular defects were corrected through the right jugular vein and all membranous ones through the femoral vein. RESULTS: All 18 patients underwent initial successful implantation of the device. In thirteen patients the shunts were completely occluded and in the remaining five there were trivial residual shunts. In two patients with membranous ventricular septal defects a change from the original position was noticed at two weeks; mild aortic regurgitation developed in one and the murmur recurred in the other; the devices had to be removed surgically. One patient developed transient third degree atrioventricular block during implantation; no tricuspid regurgitation was observed. CONCLUSION: Clinical occlusion of congenital ventricular septal defects was achieved in 16 out of the 18 attempted cases (13 full occlusions). Membranous ventricular septal defect occlusion can be effective and safe if patients and device sizes are carefully selected.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bridges N. D., Perry S. B., Keane J. F., Goldstein S. A., Mandell V., Mayer J. E., Jr, Jonas R. A., Casteneda A. R., Lock J. E. Preoperative transcatheter closure of congenital muscular ventricular septal defects. N Engl J Med. 1991 May 9;324(19):1312–1317. doi: 10.1056/NEJM199105093241903. [DOI] [PubMed] [Google Scholar]
- Laussen P. C., Hansen D. D., Perry S. B., Fox M. L., Javorski J. J., Burrows F. A., Lock J. E., Hickey P. R. Transcatheter closure of ventricular septal defects: hemodynamic instability and anesthetic management. Anesth Analg. 1995 Jun;80(6):1076–1082. doi: 10.1097/00000539-199506000-00002. [DOI] [PubMed] [Google Scholar]
- Lloyd T. R., Rao P. S., Beekman R. H., 3rd, Mendelsohn A. M., Sideris E. B. Atrial septal defect occlusion with the buttoned device (a multi-institutional U.S. trial). Am J Cardiol. 1994 Feb 1;73(4):286–291. doi: 10.1016/0002-9149(94)90235-6. [DOI] [PubMed] [Google Scholar]
- Lock J. E., Block P. C., McKay R. G., Baim D. S., Keane J. F. Transcatheter closure of ventricular septal defects. Circulation. 1988 Aug;78(2):361–368. doi: 10.1161/01.cir.78.2.361. [DOI] [PubMed] [Google Scholar]
- O'Laughlin M. P., Mullins C. E. Transcatheter occlusion of ventricular septal defect. Cathet Cardiovasc Diagn. 1989 Jul;17(3):175–179. doi: 10.1002/ccd.1810170311. [DOI] [PubMed] [Google Scholar]
- Rao P. S., Langhough R., Beekman R. H., Lloyd T. R., Sideris E. B. Echocardiographic estimation of balloon-stretched diameter of secundum atrial septal defect for transcatheter occlusion. Am Heart J. 1992 Jul;124(1):172–175. doi: 10.1016/0002-8703(92)90937-q. [DOI] [PubMed] [Google Scholar]
- Rao P. S., Sideris E. B., Haddad J., Rey C., Hausdorf G., Wilson A. D., Smith P. A., Chopra P. S. Transcatheter occlusion of patent ductus arteriosus with adjustable buttoned device. Initial clinical experience. Circulation. 1993 Sep;88(3):1119–1126. doi: 10.1161/01.cir.88.3.1119. [DOI] [PubMed] [Google Scholar]
- Rao P. S., Sideris E. B., Hausdorf G., Rey C., Lloyd T. R., Beekman R. H., Worms A. M., Bourlon F., Onorato E., Khalilullah M. International experience with secundum atrial septal defect occlusion by the buttoned device. Am Heart J. 1994 Nov;128(5):1022–1035. doi: 10.1016/0002-8703(94)90602-5. [DOI] [PubMed] [Google Scholar]
- Rigby M. L., Redington A. N. Primary transcatheter umbrella closure of perimembranous ventricular septal defect. Br Heart J. 1994 Oct;72(4):368–371. doi: 10.1136/hrt.72.4.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sideris E. B., Sideris S. E., Thanopoulos B. D., Ehly R. L., Fowlkes J. P. Transvenous atrial septal defect occlusion by the buttoned device. Am J Cardiol. 1990 Dec 15;66(20):1524–1526. doi: 10.1016/0002-9149(90)90552-c. [DOI] [PubMed] [Google Scholar]