Abstract
Congenital heart disease (CHD) is the most common birth defect, affecting about 0.8% of live births. Advances in recent decades have allowed >85% of children with CHD to survive to adulthood, creating a growing population of adults with CHD. Little information exists regarding survival, demographics, late outcomes, and comorbidities in this emerging group, and multiple barriers impede research in adult CHD (ACHD). The National Heart, Lung, and Blood Institute and the Adult Congenital Heart Association convened a multidisciplinary Working Group to identify high-impact research questions in ACHD. This report summarizes the meeting discussions in the broad areas of CHD-related heart failure, vascular disease and multisystem complications. High-priority subtopics identified included heart failure in tetralogy of Fallot, mechanical circulatory support/transplantation, sudden cardiac death, vascular outcomes in coarctation of the aorta, late outcomes in single ventricle disease, cognitive and psychiatric issues, and pregnancy.
Keywords: single ventricle, death, sudden, cardiac, heart defects, congenital, outcomes, pregnancy, tetralogy of Fallot
Congenital heart disease (CHD) is the most common birth defect in the United States, affecting approximately 0.8% of live births (1). Improved treatment strategies and interventions have increased survival such that 85% to 90% of affected children are expected to live well into adulthood (2,3), thereby causing a demographic shift in which adults now outnumber children with CHD, and more people with complex CHD are living longer (4,5). Although significant resources have been dedicated to characterizing and improving neonatal and childhood survival, little information is available regarding survival, demographics, long-term outcomes, and comorbidities in the adult CHD (ACHD) population in the United States.
Challenges to developing evidenced-based care for ACHD include the heterogeneity of conditions, lack of infrastructure in the United States to track prevalence, fragmented care systems, loss to follow-up, and changes in treatment strategies over time. Heterogeneity in CHD conditions results in small numbers of adults with CHD from which to derive guidelines, and necessitates multicenter approaches to understanding their needs. As there are no longitudinal registries for CHD in the United States, population estimates of survival are based upon birth rates and estimated survival rates for various CHD conditions among children born in different eras (6,7). Although natural history studies were performed for common conditions, few have been conducted in the recent era of improved surgical and catheter-based interventions (8). Fragmented health care systems and high rates of loss to follow-up also pose challenges for following ACHD patients across changing geographic locations, and limit our understanding of the natural history of disease and emerging needs of the ACHD population.
Treatment strategies have changed over time; therefore, outcomes and survival may differ depending on the era of birth. For example, until the 1980s, the preferred surgical approach for d-loop transposition of the great arteries was the atrial switch operation (Mustard or Senning procedure); more recently, the arterial switch operation has become the preferred surgical technique. These 2 operations result in very different anatomy and sequelae. After the atrial switch, patients may experience heart failure (HF) due to systemic right ventricular and atrial arrhythmias (9,10), whereas after arterial switch, patients require monitoring for supravalvar pulmonary stenosis, aortic root dilation, and coronary abnormalities related to surgical manipulation (11,12). Another example of a temporal treatment change is the increasing use of catheter-based techniques for conditions that previously required surgery, including closure of secundum atrial septal defects, balloon dilation and stenting of coarctation of the aorta, and pulmonary valve replacement (PVR).
ACHD patients face not only the long-term sequelae of their cardiac procedures, but also the additive complexities of typical adult comorbidities, such as hypertension and diabetes. Moreover, psychosocial issues, including neurocognitive outcomes, reproductive health, social relationships, health insurability, and employment are understudied and likely impact health and quality of life.
Clinicians struggle to care for this evolving group of patients with few long-term outcomes studies. Since 1998, guidelines for “Care of the Adult CHD Patient” have been created in Canada, Europe, and the United States (13-17). Due to the fact that large cohort, mechanistic, and prospective ACHD research studies have been lacking, most recommendations remain level of evidence C, on the basis of expert opinion. Although the American College of Cardiology/American Heart Association valve disease and HF guidelines are based upon higher levels of evidence, their recommendations are often extrapolated to ACHD, and there are sparse data to suggest that acquired and congenital conditions would respond similarly to treatments. The paucity of high-quality evidence poses a real threat to the health and well-being of this growing population.
In 2004, the National Heart, Lung and Blood Institute (NHLBI) convened a Working Group on ACHD research and outlined a 3-part research strategy: the development of a multicenter ACHD research network; the creation of data infrastructure necessary for a national ACHD patient registry; and better definition of high-impact research areas (18). Over the past decade, significant progress has been made toward these goals (19-21), due to the support of advocacy groups and federal agencies, increased training of ACHD clinician-scientists, and volunteerism of professionals and societies. The Alliance for Adult Research in Congenital Cardiology (22), a research network of investigators from 15 centers, was created in 2006 (19). The network has completed 2 National Institutes of Health-funded research studies and has 1 ongoing; the group has published multiple papers in peer-reviewed journals (23,24). Working with the Adult Congenital Heart Association (ACHA), the Alliance for Adult Research in Congenital Cardiology surveyed international ACHD providers to identify research priorities in the field. Although progress has been made in ACHD research, the field is still lacking high-quality, robust evidence to support clinical recommendations, and a sustainable infrastructure is not in place for large-scale comparative data collection and research.
Working Group
As a follow-up to the 2004 meeting, the NHLBI and ACHA convened a Working Group in June 2014 with a targeted focus on the science. The Working Group was designed to identify high-priority research topics, discuss methodological approaches for application to the unique challenges of studying ACHD, and foster collaborative relationships between ACHD and other complementary fields of research. It was not the mission of the group to promote a specific research project, but rather to stimulate ideas and innovative methodology by combining knowledge from multiple domains.
The Working Group comprised ACHD researchers together with researchers in the complementary fields of pediatric cardiology, adult HF, pulmonary vascular disease, congenital cardiac surgery, genomics, basic science, clinical trials, epidemiology, outcomes research, and population science. The group included experts from the United States and Canada, and incorporated representatives from the NHLBI's Pediatric Heart Network, Heart Failure Network, and Pediatric Cardiac Genomics Consortium, as well as the Centers for Disease Control and Prevention. The group also included a representative from the ACHA, recognizing the importance of the patient perspective in research efforts.
On the basis of expert opinion and published research priorities in CHD (20,21), 3 broad high-impact areas were identified: HF; vascular disease; and multisystem complications. The first interest area involved understanding the pathophysiology of and optimizing management strategies for HF in CHD, a common problem that will continue to grow as the ACHD population ages. Another area of interest was ventricular-vascular interactions and the long-term implications of abnormal loading on functional outcomes. A third area of interest was mechanisms of shared systemic responses between the abnormal heart/circulation and other organ systems, and how those responses contribute to outcomes.
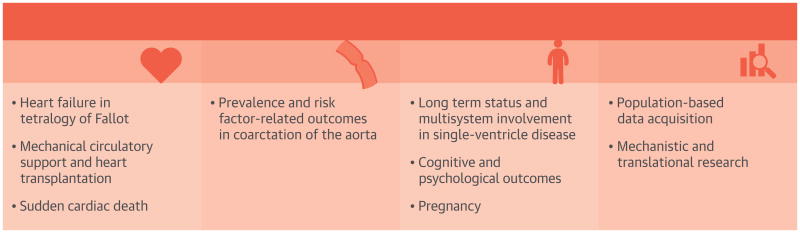
On the basis of these topic areas, participants were divided into 3 groups composed of ACHD researchers and researchers from related fields. Groups were challenged to narrow each broad topic to specific research subtopics and discuss methodologies to address each question (Central Illustration). The research areas discussed at the Working Group were not exhaustive, and a full list of high-priority research questions is beyond the scope of this document. The diverse expertise of the participants fostered dialogue about novel methods of patient engagement, existing resources to be leveraged, science with high translational potential, and lessons learned from related research fields that could be applied to ACHD.
Central Illustration. High-Priority ACHD Research Topics: NHLBI/ACHA Working Group.
The NHLBI/ACHA Working Group identified 3 broad topic areas for ACHD research and multiple high-priority research subtopics. The group also identified 2 major foundational gaps in ACHD research. ACHA = Adult Congenital Heart Association; ACHD = adult congenital heart disease; CHD = congenital heart disease; NHLBI = National Heart, Lung and Blood Institute.
High-Impact Research Areas
Heart Failure
HF is a clinical syndrome that occurs in patients who, because of an inherited or acquired abnormality of cardiac structure and/or function, develop a constellation of symptoms (dyspnea and fatigue) and signs (edema and rales) that lead to frequent hospitalizations, poor quality of life, and a shortened life expectancy. In acquired heart disease, HF often refers to systolic dysfunction of the systemic left ventricle (HF with reduced ejection fraction), although HF with preserved ejection fraction is increasingly recognized as a common cause of HF. In CHD, HF refers to signs and symptoms reflecting systolic dysfunction of the systemic ventricle, which can be a left or right ventricle in either a biventricular or single-ventricle circulation. HF is also used to describe subpulmonary ventricular dysfunction, similar to typical “right-sided HF.” The manifestations of CHD-associated HF may differ from typical HF, and treatment strategies may not be similarly effective. Although many HF topics, including resynchronization therapy, the systemic right ventricle, and medication efficacy, are of great interest in ACHD, time constraints did not allow the Working Group to address all of these issues. The Group identified 3 HF-related priority subtopics: HF in tetralogy of Fallot (TOF); mechanical circulatory support (MCS)/transplantation; and sudden cardiac death (SCD).
Heart Failure in Tetralogy of Fallot
TOF is the most common cyanotic congenital heart lesion. As treatments have been available since the 1940s, there are now a large number of adults with TOF. Surgical interventions frequently result in residual pulmonary regurgitation that, over time, can lead to right ventricular enlargement and dysfunction, and can predispose to HF, arrhythmias, and SCD (25,28). Pulmonary regurgitation can be ameliorated with PVR, and this has been shown to decrease right ventricular size and pulmonary regurgitation fraction, but has not yet been correlated with improved clinical outcomes (29,30). However, the studies addressing outcomes after PVR have been small and with inherent selection bias.
PVR can be performed surgically or as a catheter-based intervention, and the combined set of these procedures comprise one of the most common interventions performed in ACHD. However, the optimal timing of and selection criteria for PVR are yet to be determined. The group discussed potential ways to address this subtopic, including reviews of single or combined multicenter registries of TOF patients or via a prospective randomized controlled trial. Cohort approaches have inherent limitations, as retrospective registries may not contain the necessary data and prospective registries may require many years to assess outcomes. A randomized clinical trial would have the potential to answer clinically meaningful questions, but developing an appropriate study design that would achieve equipoise for doctors and patients to allow adequate enrollment is challenging.
Mechanical Circulatory Support and Heart Transplantation
Although the experience with advanced HF treatments in ACHD is limited, it is likely that different approaches from those used in acquired heart disease will be needed. Applying MCS to CHD presents anatomic challenges, including systemic right ventricles, single ventricles, dextrocardia, and vascular reconstruction of major arteries. Transplant and transplant candidacy can be affected by similar anatomic issues, as well as by antibodies from prior procedures, immune status, and fairly well-preserved New York Heart Association class and functional abilities, despite a failing heart. In the United States, CHD patients have a high waitlist mortality rate relative to other candidates, likely due, in part, to current limitations in applying MCS to CHD (31). MCS as destination therapy is emerging for the non-CHD population, and as devices become smaller, they may be better suited for CHD. MCS may be a way to improve outcomes or quality of life in ACHD patients when transplant is not an option (32,33).
There are approximately 1,800 CHD patients in the International Society for Heart and Lung Transplant registry, and the relative number of patients with CHD continues to increase (33). As patients with CHD survive longer, we can expect larger numbers with HF and an increasing need for MCS and/or transplantation. Available data suggests that patients with CHD have a higher 1-year post-transplant mortality than non-CHD patients, but survival after 1 year is excellent (median survival >20 years) (34). Exploring the causes of increased 1-year mortality, but greater longer-term survival in CHD will be crucial to improving outcomes and evaluating types and timing of interventions. Additionally, the United Network for Organ Sharing listing criteria may leave some ACHD patients at a disadvantage, as the current system is on the basis of the need for hemodynamic support or MCS, which may not be beneficial or possible in some ACHD patients. Therefore, clarifying how ACHD patients fit into the listing criteria is also of critical importance. The Interagency Registry for Mechanically Assisted Circulatory Support and the International Society for Heart and Lung Transplant registry could be leveraged to address these questions.
Sudden Cardiac Death
SCD accounts for approximately one-quarter of ACHD deaths identified from limited individual datasets (35). Specific lesions are thought to predispose to SCD, including TOF and d-loop transposition with an atrial switch procedure; however, SCD can occur across diverse defects, including left-sided obstructive lesions and septal defects (36). Despite the fact that implantable cardioverter defibrillator indications in adult HF are on the basis of evidence from randomized clinical trials, equivalent information is not available to guide primary prevention therapy in ACHD (37). Although many deaths in CHD are ascribed to SCD, the diversity of underlying conditions and lack of longitudinal CHD registries makes studying potential risk factors difficult. Some CHD lesions, including TOF, have been evaluated for risk of SCD or defibrillator benefit using single-center and retrospective multicenter cohorts (12,28,38). Emerging registries for electrophysiology and defibrillators could be leveraged to explore factors associated with SCD, refine risk stratification schemes, and prospectively assess the use of defibrillators.
Vascular Disease
The Working Group identified a knowledge gap related to ventricular-vascular interactions. In the ACHD population, coarctation of the aorta is 1 example of a lesion that provides a relevant model for improving our understanding of such interactions.
Prevalence and Risk Factor-Related Outcomes in Coarctation of the Aorta
Coarctation of the aorta is a relatively common congenital anomaly, comprising about 7% of CHD (39,40). Current treatments allow for early assessment and intervention, resulting in excellent survival to adulthood for most patients. Prior long-term studies in this population revealed early morbidity and mortality in adulthood related to hypertension, atherosclerosis, and HF (41). In these cohorts, older age at intervention was a risk factor for worse outcomes. One study on contemporary outcomes shows much improved survival, but continued morbidity related to aortic complications and hypertension (42). Additional contemporary studies on long-term outcomes in patients with early repairs are lacking.
In the general population, there are well-established prevention and intervention strategies for hypertension and atherosclerotic disease. These conditions have also been identified in adults with coarctation, but the true rates and burden in the larger ACHD population are unknown. Moreover, intervention strategies for hypertension and hyperlipidemia have been adapted from the general adult population, but have not been validated in patients with coarctation. It is possible that coarctation accompanies an underlying vasculopathy that may predispose to hypertension and require different prevention/treatment methods. For example, patients with coarctation are often normotensive at rest, but have exaggerated responses to exercise (43,44). It remains unclear if there is any benefit to treating isolated exercise-induced hypertension in this group. There may also be genetic associations in coarctation that warrant exploration, including bicuspid aortic valve, male sex, and predispositions for acquired cardiovascular risk factors (41,45).
Multisystem Complications
The majority of care for children with CHD is focused on the heart and attempts to correct or palliate underlying anatomic abnormalities. Over years of growth and development, other cardiac issues may develop, including arrhythmias and HF. Through adulthood, the focus changes to also include noncardiac comorbidities and adult-onset conditions that interact with the underlying CHD. This concept applies to multiple organ systems, including the hepatic, renal, pulmonary, gastrointestinal, neurological, and reproductive systems. The multisystem group identified 3 high-priority research subtopics: understanding long-term status and other organ involvement in single-ventricle anatomy with Fontan physiology; cognitive and psychiatric issues in ACHD; and pregnancy physiology and outcomes for mother and offspring.
Long-Term Status and Multisystem Involvement in Single-Ventricle Disease
The adult single-ventricle population accounts for approximately 1.5% of adults with CHD or approximately 20,000 people in the United States (5). Compared with other adults with CHD, the single-ventricle population has increased frequency of inpatient and outpatient visits, earlier mortality, and a relatively higher percentage of health resource use (46,47). HF or “Fontan circulatory failure” manifests differently than typical adult HF, is increasingly recognized by the second decade of life, and is often accompanied by diastolic hemodynamic abnormalities. This is generally manifested by decreased functional capacity and increased resource use and hospitalizations. It is also accompanied by changes in other organ systems that affect and are affected by heart function; including the pulmonary, renal, hepatic, and peripheral vascular systems (48-50).
Interactions among the noncardiac organ systems in single-ventricle physiology remain unclear. Available clinical and outcomes data come mostly from single-center studies with small numbers and often focus on a single organ system. Some small biological studies have explored organ physiology, such as the gastrointestinal system in protein-losing enteropathy or the respiratory system and mechanics related to functional capacity. There are also case reports, small series, and a few trials assessing outcomes with medications used to improve physiology or hemodynamics, such as anti-inflammatories or pulmonary vasodilators (51,52). Gathering clinical and biological data from a multicenter longitudinal cohort would be beneficial in understanding the multiorgan interactions; however, data may be more quickly available from a diverse cross-sectional cohort of patients of different ages. A challenge in this cohort would be the diversity of Fontan modifications that have been performed surgically in different eras and at different patient ages, resulting in different sequelae.
Currently, the Fontan procedure for single-ventricle physiology is the only available treatment pathway to avoid cyanosis for the majority of patients, and it appears to have significant long-term, multiorgan physiological consequences. The adult population of single-ventricle patients will continue to grow, and it is crucial to begin to understand the underlying mechanisms of complications to initiate early treatment and surveillance, or potentially change early treatment pathways to avoid later adverse physiological effects.
Cognitive and Psychiatric Outcomes
Individuals with CHD are at risk of neurodevelopmental and behavioral abnormalities; therefore, screening and early intervention have been recommended by the American Heart Association and American Academy of Pediatrics (53). The CHD population has an increased incidence of executive function impairments and attention-deficit disorder, among other conditions (53,54). As neurodevelopmental screening is relatively new for children with CHD, most current adults with CHD were not evaluated and many conditions may be unrecognized. Post-traumatic stress disorder (PTSD) has also been described in children following cardiac surgery or intensive care unit admissions, and may persist into adulthood (55,56).
Multiple small studies in ACHD have described an increased risk for psychiatric conditions, such as loss of executive function and attention, anxiety, depression, and PTSD (56-59). These conditions were identified in a variety of CHD lesions, regardless of anatomic complexity. Anxiety, depression, and PTSD are also known to be associated with acquired heart disease and have been related to worse outcomes, including increased hospital readmissions and medication noncompliance (60,61).
Pharmacological and nonpharmacological interventions have shown benefit in medical and quality of life outcomes for anxiety, depression, and PTSD in other medical populations and warrant further study in ACHD. The effect of cognitive and psychiatric conditions and structured interventions on outcomes in ACHD has not been reported; thus, it remains unknown if, similar to acquired heart disease, these conditions affect hospital admission rates, adherence to care, or mortality. It is also unknown if cognitive and psychiatric issues identified in childhood will persist into adulthood or cause additional problems, such as social, educational, or employment challenges.
Understanding the prevalence of cognitive function and mental health issues in ACHD patients is a critical first step to improving their functionality, quality of life, and medical care. The development and testing of treatments in a group with a lifelong chronic condition may be different than devising strategies for acquired conditions. With advances in congenital cardiac care in childhood producing an ever-increasing number of survivors to adulthood, optimizing the functionality and quality of life in the ACHD population is of paramount importance.
Pregnancy
The majority of girls born with CHD will survive to childbearing age, and many desire information on the potential impact of pregnancy on their health. Pregnancy is an important issue for the CHD population, as there can be additional risks to the mother and fetus due to the underlying CHD. Although maternal mortality is rare in developed countries, the leading cause is heart disease and the most common etiology is CHD (62). Mortality is rare; however, complications affecting the mother and/or fetus are common. Complications for pregnant women with CHD include hypertension, HF, and arrhythmias, but their prevalence in different forms of CHD is not well understood. Common risks to the fetus include premature birth and low birth weight, but how to identify which women and fetuses are at higher risk remains unclear (63). The majority of clinical data on pregnant women with CHD come from a small number of ACHD centers in the United States and Canada, and European registries (62-65). A few studies use administrative data to evaluate this population, but available clinical and anatomic information is limited. As only a minority of ACHD patients receive care in ACHD specialty centers, little is actually known about pregnancy experience and outcomes for most adults with CHD (66).
In addition to characterizing outcomes and complications, it is important to understand the physiology of pregnancy in CHD, including not only how the CHD physiology affects the pregnant woman and the fetus, but also how the hemodynamics of pregnancy influence long-term cardiac status in these women. Beyond the physiological concerns of pregnancy, there is also an underlying increased risk of the child being born with CHD due to genetic risk factors.
A large and diverse longitudinal cohort study including biological and genetic information may address short-term questions regarding mother and offspring as well as potentially characterize hemodynamics of the mother and fetus during pregnancy. A longitudinal cohort study design would optimally also recognize the population of women with CHD not cared for in ACHD centers. With electronic medical records, existing national registries, and methods, such as crowd sourcing, it may be possible to reach a patient population outside of ACHD centers in order to gather information about pregnancy, delivery, and maternal and fetal outcomes; however, it will be important to consider the impact of potential selection bias on the basis of who is recruited in such an effort.
Foundational Gaps and Future Directions
One barrier that has hindered research in CHD, and particularly ACHD, was evident to the Working Group: the lack of a basic epidemiological understanding of the CHD population. There are no population-based U.S. data regarding prevalence, demographics, or long-term outcomes of even the most common CHD conditions. For clinical or translational research in CHD, this is a basic and critical need. ACHD research has been limited by studies done in individual centers, small consortia, countries with smaller populations, or by imprecise estimates from larger datasets. Knowledge of clinical outcomes in ACHD consists almost entirely of case series from a limited number of referral-based institutions. For this reason, the generalizability in the United States of any study can be questioned, there is limited ability to design studies using background knowledge of outcomes and event rates, and enrollment in studies will lack adequate power to provide definitive knowledge for practice. Gathering basic information, including anatomic diagnosis, survival, and late comorbidities, and devising a true population-based longitudinal registry may become easier with electronic data resource networks and involvement of advocacy groups to promote patient participation in research. These types of networks may also be able to identify more diverse cohorts to study, rather than those from single referral centers or small consortia. Until these resources are available, understanding the ACHD population in the United States will rely on collaboration with other countries, such as Canada or Denmark, where robust national healthcare information is more easily available.
An additional large research gap in ACHD is biological mechanistic and translational work. Although the NHLBI's Pediatric Cardiac Genomics Consortium and other investigators have been exploring the complex genomics of CHD and its impact on outcomes, there is much more work to be done. There is also a gap in research regarding the biological mechanisms underlying later sequelae of CHD, including HF and vascular biology as they relate to CHD, rather than the typical adult heart. Research in these areas and support of broad-based biological banking in ACHD might greatly improve the understanding of the conditions and complications, as well as identify potential therapeutic targets or preventive strategies.
Many of the topic areas in ACHD research are likely to benefit from a multidisciplinary approach, including submission of investigator-initiated grants, as well as collaborative efforts between existing programs, registries, and databases. The stakeholders might include many of the specialties and organizations represented at the Working Group, such as federal agencies, advocacy organizations, scientists, patients, and providers. Future collaboration between these organizations, other established research groups, and ACHD investigators will facilitate studies to address the high-priority topics and gaps outlined by the Working Group. Progress in addressing the gaps and priority research topics will be assessed over the coming years to identify successful strategies, develop a pipeline of ACHD clinician-scientists, and promote new areas for innovation.
Conclusions
The NHLBI/ACHA Working Group on emerging research questions in ACHD identified priority research topics and fostered collaboration among researchers in complementary areas of cardiology. The diversity of expertise facilitated identification of opportunities for leveraging and collaboration. The discussions highlighted the challenges facing ACHD research, most prominently the lack of a foundational epidemiological understanding of CHD in the United States and its long-term outcomes, and the paucity of mechanistic and translational research in ACHD. The research topics proposed by the Working Group are important areas of focus that will continue to advance the field, with the goal of increasing the evidence base for care and providing a foundation for lasting and meaningful assessment in the future.
Acknowledgments
The authors acknowledge the following members of the NHLBI staff: Jonathan Kaltman, Ellen Rosenberg, Charlene Schramm, and Monica Shah.
Funding Sources: The National Heart, Lung and Blood Institute, Bethesda, Maryland and the Adult Congenital Heart Association, Philadelphia, Pennsylvania.
Abbreviations and Acronyms
- ACHA
Adult Congenital Heart Association
- ACHD
adult congenital heart disease
- CHD
congenital heart disease
- HF
heart failure
- MCS
mechanical circulatory support
- NHLBI
National Heart, Lung and Blood Institute
- PTSD
post-traumatic stress disorder
- PVR
pulmonary valve replacement
- SCD
sudden cardiac death
- TOF
tetralogy of Fallot
Footnotes
Disclosures: The authors have reported that they have no relationships relevant to the contents of this paper to disclose. The views and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Institutes of Health or the Centers for Disease Control and Prevention.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Moller JH, Taubert KA, Allen HD, et al. Cardiovascular health and disease in children: current status. A Special Writing Group from the Task Force on Children and Youth, American Heart Association. Circulation. 1994;89:923–30. doi: 10.1161/01.cir.89.2.923. [DOI] [PubMed] [Google Scholar]
- 3.Wren C, O'Sullivan JJ. Survival with congenital heart disease and need for follow up in adult life. Heart. 2001;85:438–43. doi: 10.1136/heart.85.4.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marelli AJ, Mackie AS, Ionescu-Ittu R, et al. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007;115:163–72. doi: 10.1161/CIRCULATIONAHA.106.627224. [DOI] [PubMed] [Google Scholar]
- 5.Marelli AJ, Ionescu-Ittu R, Mackie AS, et al. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation. 2014;130:749–56. doi: 10.1161/CIRCULATIONAHA.113.008396. [DOI] [PubMed] [Google Scholar]
- 6.Hoffman JI, Kaplan S, Liberthson RR. Prevalence of congenital heart disease. Am Heart J. 2004;147:425–39. doi: 10.1016/j.ahj.2003.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Nembhard WN, Xu P, Ethen MK, et al. Racial/ethnic disparities in timing of death during childhood among children with congenital heart defects. Birth Defects Res A Clin Mol Teratol. 2013;97:628–40. doi: 10.1002/bdra.23169. [DOI] [PubMed] [Google Scholar]
- 8.Weidman WH. Second natural history study of congenital heart defects. Circulation. 1993;87:I1–3. [PubMed] [Google Scholar]
- 9.Hörer J, Karl E, Theodoratou G, et al. Incidence and results of reoperations following the Senning operation: 27 years of follow-up in 314 patients at a single center. Eur J Cardiothorac Surg. 2008;33:1061–7. doi: 10.1016/j.ejcts.2007.11.012. discussion 1067-8. [DOI] [PubMed] [Google Scholar]
- 10.Khairy P, Landzberg MJ, Lambert J, et al. Long-term outcomes after the atrial switch for surgical correction of transposition: a meta-analysis comparing the Mustard and Senning procedures. Cardiol Young. 2004;14:284–92. doi: 10.1017/S1047951104003063. [DOI] [PubMed] [Google Scholar]
- 11.Fricke TA, d'Udekem Y, Richardson M, et al. Outcomes of the arterial switch operation for transposition of the great arteries: 25 years of experience. Ann Thorac Surg. 2012;94:139–45. doi: 10.1016/j.athoracsur.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 12.Khairy P, Clair M, Fernandes SM, et al. Cardiovascular outcomes after the arterial switch operation for D-transposition of the great arteries. Circulation. 2013;127:331–9. doi: 10.1161/CIRCULATIONAHA.112.135046. [DOI] [PubMed] [Google Scholar]
- 13.Baumgartner H, Bonhoeffer P, De Groot NM, et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010) Eur Heart J. 2010;31:2915–57. doi: 10.1093/eurheartj/ehq249. [DOI] [PubMed] [Google Scholar]
- 14.Silversides CK, Dore A, Poirier N, et al. Canadian Cardiovascular Society 2009 Consensus Conference on the management of adults with congenital heart disease: shunt lesions. Can J Cardiol. 2010;26:e70–9. doi: 10.1016/s0828-282x(10)70354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silversides CK, Kiess M, Beauchesne L, et al. Canadian Cardiovascular Society 2009 Consensus Conference on the management of adults with congenital heart disease: outflow tract obstruction, coarctation of the aorta, tetralogy of Fallot, Ebstein anomaly and Marfan's syndrome. Can J Cardiol. 2010;26:e80–97. doi: 10.1016/s0828-282x(10)70355-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silversides CK, Salehian O, Oechslin E, et al. Canadian Cardiovascular Society 2009 Consensus Conference on the management of adults with congenital heart disease: complex congenital cardiac lesions. Can J Cardiol. 2010;26:e98–117. doi: 10.1016/s0828-282x(10)70356-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52:e143–263. doi: 10.1016/j.jacc.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Williams RG, Pearson GD, Barst RJ, et al. Report of the National Heart, Lung, and Blood Institute Working Group on research in adult congenital heart disease. J Am Coll Cardiol. 2006;47:701–7. doi: 10.1016/j.jacc.2005.08.074. [DOI] [PubMed] [Google Scholar]
- 19.Khairy P, Hosn JA, Broberg C, et al. Alliance for Adult Research in Congenital Cardiology (AARCC). Multicenter research in adult congenital heart disease. Int J Cardiol. 2008;129:155–9. doi: 10.1016/j.ijcard.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 20.Oster ME, Riehle-Colarusso T, Simeone RM, et al. Public health science agenda for congenital heart defects: report from a Centers for Disease Control and Prevention experts meeting. J Am Heart Assoc. 2013;2:e000256. doi: 10.1161/JAHA.113.000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cotts T, Khairy P, Opotowsky AR, et al. Alliance for Adult Research in Congenital Cardiology (AARCC). Clinical research priorities in adult congenital heart disease. Int J Cardiol. 2014;171:351–60. doi: 10.1016/j.ijcard.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.AARCC. [Accessed February 14, 2016];Alliance for Adult Research in Congenital Cardiology. 2016 Available at: http://www.aarcc.net.
- 23.Gurvitz M, Valente AM, Broberg C, et al. Prevalence and predictors of gaps in care among adult congenital heart disease patients: HEART-ACHD (The Health, Education, and Access Research Trial) J Am Coll Cardiol. 2013;61:2180–4. doi: 10.1016/j.jacc.2013.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valente AM, Landzberg MJ, Gianola A, et al. Alliance for Adult Research in Congenital Cardiology (AARCC) Investigators and the Adult Congenital Heart Association (ACHA). Improving heart disease knowledge and research participation in adults with congenital heart disease (the Health, Education and Access Research Trial: HEART-ACHD) Int J Cardiol. 2013;168:3236–40. doi: 10.1016/j.ijcard.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Aboulhosn JA, Lluri G, Gurvitz MZ, et al. Left and right ventricular diastolic function in adults with surgically repaired tetralogy of Fallot: a multi-institutional study. Can J Cardiol. 2013;29:866–72. doi: 10.1016/j.cjca.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Broberg CS, Aboulhosn J, Mongeon FP, et al. Alliance for Adult Research in Congenital Cardiology (AARCC). Prevalence of left ventricular systolic dysfunction in adults with repaired tetralogy of Fallot. Am J Cardiol. 2011;107:1215–20. doi: 10.1016/j.amjcard.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 27.Khairy P, Aboulhosn J, Gurvitz MZ, et al. Alliance for Adult Research in Congenital Cardiology (AARCC). Arrhythmia burden in adults with surgically repaired tetralogy of Fallot: a multi-institutional study. Circulation. 2010;122:868–75. doi: 10.1161/CIRCULATIONAHA.109.928481. [DOI] [PubMed] [Google Scholar]
- 28.Valente AM, Gauvreau K, Assenza GE, et al. Contemporary predictors of death and sustained ventricular tachycardia in patients with repaired tetralogy of Fallot enrolled in the INDICATOR cohort. Heart. 2014;100:247–53. doi: 10.1136/heartjnl-2013-304958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gengsakul A, Harris L, Bradley TJ, et al. The impact of pulmonary valve replacement after tetralogy of Fallot repair: a matched comparison. Eur J Cardiothorac Surg. 2007;32:462–8. doi: 10.1016/j.ejcts.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Graham TP, Jr, Bernard Y, Arbogast P, et al. Outcome of pulmonary valve replacements in adults after tetralogy repair: a multi-institutional study. Congenit Heart Dis. 2008;3:162–7. doi: 10.1111/j.1747-0803.2008.00189.x. [DOI] [PubMed] [Google Scholar]
- 31.Stewart GC, Mayer JE., Jr Heart transplantation in adults with congenital heart disease. Heart Fail Clin. 2014;10:207–18. doi: 10.1016/j.hfc.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Maly J, Netuka I, Besik J, et al. Bridge to transplantation with long-term mechanical assist device in adults after the Mustard procedure. J Heart Lung Transplant. 2015;34:1177–81. doi: 10.1016/j.healun.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 33.Ryan TD, Jefferies JL, Zafar F, et al. The evolving role of the total artificial heart in the management of end-stage congenital heart disease and adolescents. ASAIO J. 2015;61:8–14. doi: 10.1097/MAT.0000000000000156. [DOI] [PubMed] [Google Scholar]
- 34.Burchill LJ, Edwards LB, Dipchand AI, et al. Impact of adult congenital heart disease on survival and mortality after heart transplantation. J Heart Lung Transplant. 2014;33:1157–63. doi: 10.1016/j.healun.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Oechslin EN, Harrison DA, Connelly MS, et al. Mode of death in adults with congenital heart disease. Am J Cardiol. 2000;86:1111–6. doi: 10.1016/s0002-9149(00)01169-3. [DOI] [PubMed] [Google Scholar]
- 36.Koyak Z, Harris L, de Groot JR, et al. Sudden cardiac death in adult congenital heart disease. Circulation. 2012;126:1944–54. doi: 10.1161/CIRCULATIONAHA.112.104786. [DOI] [PubMed] [Google Scholar]
- 37.Khairy P, Van Hare GF, Balaji S, et al. PACES/HRS expert consensus statement on the recognition and management of arrhythmias in adult congenital heart disease: developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology (ACC), the American Heart Association (AHA), the European Heart Rhythm Association (EHRA), the Canadian Heart Rhythm Society (CHRS), and the International Society for Adult Congenital Heart Disease (ISACHD) Can J Cardiol. 2014;30:e1–e63. doi: 10.1016/j.cjca.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Khairy P, Harris L, Landzberg MJ, et al. Implantable cardioverter-defibrillators in tetralogy of Fallot. Circulation. 2008;117:363–70. doi: 10.1161/CIRCULATIONAHA.107.726372. [DOI] [PubMed] [Google Scholar]
- 39.Aboulhosn J, Child JS. Left ventricular outflow obstruction: subaortic stenosis, bicuspid aortic valve, supravalvar aortic stenosis, and coarctation of the aorta. Circulation. 2006;114:2412–22. doi: 10.1161/CIRCULATIONAHA.105.592089. [DOI] [PubMed] [Google Scholar]
- 40.Report of the New England Regional Infant Cardiac Program. Pediatrics. 1980;65:375–461. [PubMed] [Google Scholar]
- 41.Cohen M, Fuster V, Steele PM, et al. Coarctation of the aorta. Long-term follow-up and prediction of outcome after surgical correction. Circulation. 1989;80:840–5. doi: 10.1161/01.cir.80.4.840. [DOI] [PubMed] [Google Scholar]
- 42.Choudhary P, Canniffe C, Jackson DJ, et al. Late outcomes in adults with coarctation of the aorta. Heart. 2015;101:1190–5. doi: 10.1136/heartjnl-2014-307035. [DOI] [PubMed] [Google Scholar]
- 43.Bocelli A, Favilli S, Pollini I, et al. Prevalence and long-term predictors of left ventricular hypertrophy, late hypertension, and hypertensive response to exercise after successful aortic coarctation repair. Pediatr Cardiol. 2013;34:620–9. doi: 10.1007/s00246-012-0508-0. [DOI] [PubMed] [Google Scholar]
- 44.Correia AS, Gonçalves A, Paiva M, et al. Long-term follow-up after aortic coarctation repair: the unsolved issue of exercise-induced hypertension. Rev Port Cardiol. 2013;32:879–83. doi: 10.1016/j.repc.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 45.Botto LD, Correa A, Erickson JD. Racial and temporal variations in the prevalence of heart defects. Pediatrics. 2001;107:E32. doi: 10.1542/peds.107.3.e32. [DOI] [PubMed] [Google Scholar]
- 46.Kim YY, Gauvreau K, Bacha EA, et al. Resource use among adult congenital heart surgery admissions in pediatric hospitals: risk factors for high resource utilization and association with inpatient death. Circ Cardiovasc Qual Outcomes. 2011;4:634–9. doi: 10.1161/CIRCOUTCOMES.111.963223. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez FH, 3rd, Moodie DS, Parekh DR, et al. Outcomes of heart failure-related hospitalization in adults with congenital heart disease in the United States. Congenit Heart Dis. 2013;8:513–9. doi: 10.1111/chd.12019. [DOI] [PubMed] [Google Scholar]
- 48.Diller GP, Giardini A, Dimopoulos K, et al. Predictors of morbidity and mortality in contemporary Fontan patients: results from a multicenter study including cardiopulmonary exercise testing in 321 patients. Eur Heart J. 2010;31:3073–83. doi: 10.1093/eurheartj/ehq356. [DOI] [PubMed] [Google Scholar]
- 49.Opotowsky AR, Landzberg MJ, Earing MG, et al. Abnormal spirometry after the Fontan procedure is common and associated with impaired aerobic capacity. Am J Physiol Heart Circ Physiol. 2014;307:H110–7. doi: 10.1152/ajpheart.00184.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valente AM, Bhatt AB, Cook S, et al. AARCC (Alliance for Adult Research in Congenital Cardiology) Investigators. The CALF (Congenital Heart Disease in Adults Lower Extremity Systemic Venous Health in Fontan Patients) study. J Am Coll Cardiol. 2010;56:144–50. doi: 10.1016/j.jacc.2010.02.048. [DOI] [PubMed] [Google Scholar]
- 51.Ciliberti P, Schulze-Neick I, Giardini A. Modulation of pulmonary vascular resistance as a target for therapeutic interventions in Fontan patients: focus on phosphodiesterase inhibitors. Future Cardiol. 2012;8:271–84. doi: 10.2217/fca.12.16. [DOI] [PubMed] [Google Scholar]
- 52.Hebert A, Mikkelsen UR, Thilen U, et al. Bosentan improves exercise capacity in adolescents and adults after Fontan operation: the TEMPO (Treatment With Endothelin Receptor Antagonist in Fontan Patients, a Randomized, Placebo-Controlled, Double-Blind Study Measuring Peak Oxygen Consumption) study. Circulation. 2014;130:2021–30. doi: 10.1161/CIRCULATIONAHA.113.008441. [DOI] [PubMed] [Google Scholar]
- 53.Marino BS, Lipkin PH, Newburger JW, et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation. 2012;126:1143–72. doi: 10.1161/CIR.0b013e318265ee8a. [DOI] [PubMed] [Google Scholar]
- 54.Cassidy AR, White MT, DeMaso DR, et al. Executive function in children and adolescents with critical cyanotic congenital heart disease. J Int Neuropsychol Soc. 2015;21:34–49. doi: 10.1017/S1355617714001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Toren P, Horesh N. Psychiatric morbidity in adolescents operated in childhood for congenital cyanotic heart disease. J Paediatr Child Health. 2007;43:662–6. doi: 10.1111/j.1440-1754.2007.01183.x. [DOI] [PubMed] [Google Scholar]
- 56.Connolly D, McClowry S, Hayman L, et al. Posttraumatic stress disorder in children after cardiac surgery. J Pediatr. 2004;144:480–4. doi: 10.1016/j.jpeds.2003.12.048. [DOI] [PubMed] [Google Scholar]
- 57.Kovacs AH, Saidi AS, Kuhl EA, et al. Depression and anxiety in adult congenital heart disease: predictors and prevalence. Int J Cardiol. 2009;137:158–64. doi: 10.1016/j.ijcard.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 58.Norozi K, Zoege M, Buchhorn R, et al. The influence of congenital heart disease on psychological conditions in adolescents and adults after corrective surgery. Congenit Heart Dis. 2006;1:282–8. doi: 10.1111/j.1747-0803.2006.00048.x. [DOI] [PubMed] [Google Scholar]
- 59.Ong L, Nolan RP, Irvine J, et al. Parental overprotection and heart-focused anxiety in adults with congenital heart disease. Int J Behav Med. 2011;18:260–7. doi: 10.1007/s12529-010-9112-y. [DOI] [PubMed] [Google Scholar]
- 60.Kuhl EA, Fauerbach JA, Bush DE, et al. Relation of anxiety and adherence to risk-reducing recommendations following myocardial infarction. Am J Cardiol. 2009;103:1629–34. doi: 10.1016/j.amjcard.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 61.Wu JR, Frazier SK, Rayens MK, et al. Medication adherence, social support, and event-free survival in patients with heart failure. Health Psychol. 2013;32:637–46. doi: 10.1037/a0028527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Drenthen W, Boersma E, Balci A, et al. ZAHARA Investigators. Predictors of pregnancy complications in women with congenital heart disease. Eur Heart J. 2010;31:2124–32. doi: 10.1093/eurheartj/ehq200. [DOI] [PubMed] [Google Scholar]
- 63.Siu SC, Sermer M, Colman JM, et al. Cardiac Disease in Pregnancy (CARPREG) Investigators. Prospective multicenter study of pregnancy outcomes in women with heart disease. Circulation. 2001;104:515–21. doi: 10.1161/hc3001.093437. [DOI] [PubMed] [Google Scholar]
- 64.Balint OH, Siu SC, Mason J, et al. Cardiac outcomes after pregnancy in women with congenital heart disease. Heart. 2010;96:1656–61. doi: 10.1136/hrt.2010.202838. [DOI] [PubMed] [Google Scholar]
- 65.Siu SC, Sermer M, Harrison DA, et al. Risk and predictors for pregnancy-related complications in women with heart disease. Circulation. 1997;96:2789–94. doi: 10.1161/01.cir.96.9.2789. [DOI] [PubMed] [Google Scholar]
- 66.Patel MS, Kogon BE. Care of the adult congenital heart disease patient in the United States: a summary of the current system. Pediatr Cardiol. 2010;31:511–4. doi: 10.1007/s00246-009-9629-5. [DOI] [PubMed] [Google Scholar]