Abstract
Cryptosporidiosis affects humans of all ages, particularly malnourished children and those with compromised immune systems such as HIV/AIDS. This study investigated the therapeutic effects of acetylspiramycin and garlicin on Cryptosporidium infection in institutionalized male drug users receiving rehabilitative treatment. Examination of stool specimens from 903 drug users via modified acid-fast bacilli staining resulted in 172 positive cases. Among them 151 subjects consented to participate in a randomized trial of acetylspiramycin and garlicin in four groups: acetylspiramycin plus garlicin, acetylspiramycin only, garlicin only, and placebo control. The cryptosporidiosis rate was higher in younger subjects with longer drug use history than subjects who are older with shorter history of drug use. After two segments of treatments, 76.2% of the cases achieved negative test results, with the four groups achieving the rates of 92.1%, 76.7%, 72.2%, and 61.8%, respectively (χ2 = 9.517, P = 0.023). These results indicate clinical potential of garlicin in conjunction with acetylspiramycin in treating cryptosporidiosis.
Keywords: Drug user, Cryptosporidium, Acetylspiramycin, Garlicin, Randomized controlled trial, Therapeutic effects
Graphical abstract
Highlights
-
•
Randomized trial in Cryptosporidium positive drug users in China.
-
•
Positive correlation found between the cryptosporidiosis rate and the drug addicting years.
-
•
Clinical potential of garlicin in conjunction with acetylspiramycin in treating cryptosporidiosis.
1. Introduction
Cryptosporidiosis is an emerging parasitic infection caused by Cryptosporidium spp., and is one of the most common causes of diarrhea in humans of all age groups in China (An et al., 2011, Feng et al., 2012, Liu et al., 2014, Yao et al., 2014) and worldwide (Yoder et al., 2012, Insulander et al., 2013). The 1993 outbreak of cryptosporidiosis in Milwaukee, Wisconsin USA in which over 400,000 people were sickened was the first major waterborne outbreak due to Cryptosporidium infection (Mac Kenzie et al., 1994). Since then cryptosporidiosis has been reported regularly throughout the world for general populations as well as those with HIV/AIDS (Assefa et al., 2009, Bartelt et al., 2013, Uppal et al., 2014). However, little has been reported specifically for drug users (Masarat et al., 2012).
Cryptosporidium parasites are difficult to eradicate because they are resistant to many disinfectants and aren't effectively removed by many filters. They can also survive in the environment for many months at varying temperatures (Kothavade, 2012). Most healthy people recover from infection within 10 days to two weeks without medical attention. Children and people with weakened immune systems, especially those with HIV/AIDS, are prone to having severe and prolonged diarrhea, leading to malnutrition, wasting, or even death. However, there's no reliable treatment for cryptosporidiosis (Vandenberg et al., 2012). The most commonly-used antiparasitic antibiotics such as paromomycin, azithromycin, and spiramycin are only partially effective (Graczyk et al., 2011, Sinkala et al., 2011, Das et al., 2013). Nitazoxanide, a relatively new antiparasitic drug, has been approved for treatment of diarrhea caused by Cryptosporidium in people with healthy immune systems, but is ineffective in immunosuppressed individuals (Mainali et al., 2013, Yacoub et al., 2014). Therefore new drugs against this parasite are urgently needed.
As an extract of garlic, garlicin has been confirmed to have multiple curative effects such as antimicrobial (Tsao and Yin, 2001), antithrombotic (Fukao et al., 2007), hypolipidemic (Asdaq, 2015) and antitumor activities (Thomson and Ali, 2003). Lately, some reports tested the effect of garlicin in the treatment of parasites, such as toxoplasmosis (Liu et al., 2002a, Liu et al., 2002b), trichomoniasis (Ibrahim, 2013) and cryptosporidiosis. In addition, garlicin has been used as a traditional extract of medicinal plant successfully to treat cryptosporidiosis in farm animals (Kadria et al., 2015) and mice (Liu et al., 2002a, Liu et al., 2002b, Gaafar, 2012, Wang and Zhang, 2013). Several Chinese case reports according to garlicin have shown promising anti-Cryptosporidium effect in Infants and young children (Yang and Ge, 1994, Shen and Ge, 1997).
Two Chinese studies (Chen and Shen, 2011, Wang and Zhang, 2014) suggested that spiramycin, a semisynthetic antibiotic recommended for treating toxoplasmosis. Spiramycin is believed to target the apicoplast ribosome (Wang and Zhang, 2014). As one of the macrolide antibiotics, acetylspiramycin is an acetylated derivative of spiramycin, which is better than spiramycin in oral absorption rate and stability, and primarily treats toxoplasmosis (Maeda, 2003, Jiang et al., 2000).
This paper reports a randomized trial that investigated the therapeutic effects of acetylspiramycin and garlicin on Cryptosporidium infection in institutionalized drug users enrolled in a drug rehab center in Changsha, Hunan China.
2. Materials and methods
2.1. Population and study subjects
Male drug users were screened for enrollment in 2012 from a drug rehabilitation center in Changsha, Hunan China. Heroin was used among 99% of the subjects. Upon giving written consent, each subject was evaluated for inclusion/exclusion criteria. Procedures at baseline included the medical history, physical examination, collection of stool for parasite examination (including Cryptosporidium), and an assessment of stool characteristics in recent two months (frequency, consistency, presence of mucus or blood). Diarrhea was defined as at least 3 unformed stools per day. Stools were regarded to be unformed if they were soft (taking the shape of the container) or watery (could be poured or soaked into a diaper). Asymptomatic carrier of Cryptosporidium was defined as normal defecation (not more than 2 times a day, without loose or watery stools) but Cryptosporidium spp oocysts positive.
Cases with Cryptosporidium spp oocysts positive in a duplicate detection of stool sample within 7 days before enrollment were eligible for enrollment in the study. Cases were excluded from the study for the following reasons: cryptosporidiosis examination negative the day before study drug administration; hypersensitivity to acetylspiramycin or garlicin; treatment during the 14-day period immediately before study drug administration with any putative anticryptosporidial agent (spiramycin, diclazuril, letrazuril, atovaquone, bovine colostrum, azithromycin, clarithromycin, or octreotide).
2.2. Study design
Informed consent was obtained from all the study participants before this study began. The research protocol was approved by the Institutional Review Board at Xiangya School of Medicine, Central South University. The consenting cryptosporidiosis cases were randomized to four treatment groups under a 2x2 factorial design. Patients in group A (n = 38) received a combination of acetylspiramycin and garlicin; subjects in group B (n = 43) received acetylspiramycin only; cases in group C (n = 36) received garlicin only; and those in group D served as vehicle controls (n = 34).
2.3. Test of cryptosporidiosis
Stool specimens were first smeared into a stool membrane of 2 cm in diameter. After drying, they were fixed with carbine l and stained using the modified acid-fast bacilli staining method. The final diagnosis of cases was made by comparing the shape and colors of sporozoites with reference standards of positive specimens that were developed previously (Huang et al., 1998), and was verified independently by the Parasitology Department of Nanjing Medical University (Ge and Shen, 1991).
2.4. Treatment regimens
Patients in groups A and B took 200 mg acetylspiramycin (200 mg/100000 units, with 99.5% purity, Henan Topfond Pharmaceutical Company, China, catalog number: 100801) 4 times daily. Patients in groups A and C took 40 mg garlicin (with 97.8% purity, Hubei Jin Longfu Medicine co., LTD, in China, catalog number: 110529) 4 times daily. Both drugs and placebo were orally administered in gelatin capsules. Patients in all groups were treated with given drugs or placebo for 7 consecutive days as the first segment, during which no other medications were allowed. A stool sample was collected from participants on the 7th day of treatment. Parasitological response was assessed by examination for the presence of oocysts of Cryptosporidium spp by microscopic examination of the modified acid fast bacilli staining. Three smears per specimen were evaluated. Parasitological response was recorded as either “eradication” (no oocysts observed in either post-treatment stool sample) or “persistence” (oocysts observed in either or both post-treatment stool samples). If all the three parallel stool samples examined on the 7th day were negative, the case was confirmed to be cryptosporidiosis negative and received no further treatment. If the stool samples examined on the 7th day were positive in oocysts of Cryptosporidium spp, cases would to be offered 7 more days of treatment consecutively as the second segment and be evaluated parasitological response again on the 14th day. The endpoint for therapeutic evaluation was the cryptosporidiosis eradication response recorded on day 14 after the start of treatment. If the parasitological response still “persistence” on the 14th day, cases would to be treated for another more 7 days only for consolidation curative effect, but the treatment outcomes in this section would not to be included in the therapeutic evaluation for the last 14 days.
2.5. Statistical analysis
Data were analyzed with the SPSS version 13.0. Chi-squared test and trend tests (Zhu and Fung, 1996) were used to compare rates of infection and resolution of infection. In addition, Bonferroni correction was applied to multiple comparison. Analysis of variance (ANOVA) was used to compare age and mean years of drug use history among four groups. Furthermore, a stepwise logistic regression of recovery rate of cryptosporidiosis was conducted to control for potential confounding effects.
3. Results
3.1. Prevalence of Cryptosporidium infection
Of the 903 participants screened in this study, 172 (19.05%) tested positive for cryptosporidiosis (Fig 1), in which 21 left the rehabilitation center prior to commencement of the trial. The remaining 151 Cryptosporidium positive individuals consented to participate. Prevalence of cryptosporidiosis varied among different demographic groups (Table 1).
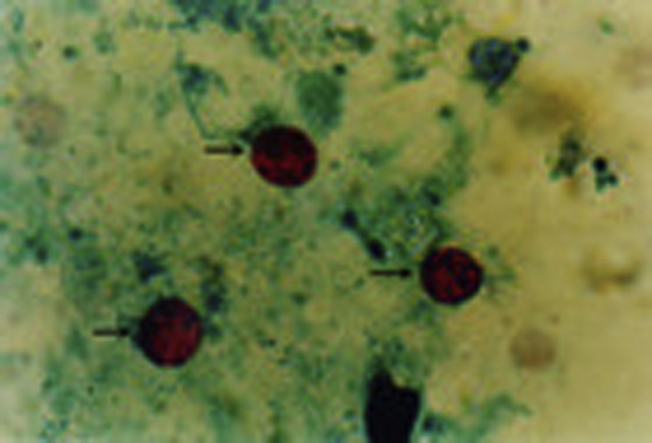
Fig. 1.
Photomicrographs of fecal smears: Oocysts of Cryptosporidium spp. in acid-fast stain (at 100 times magnification). The arrowheads indicate intact oocysts, 4–6 μm in diameter, and round to irregular in shape.
Table 1.
Cryptosporidium infection by demographic groups.
| Total | Infected cases (%) | χ2 (p-value) | ||
|---|---|---|---|---|
| Agea | 20–29 | 350 | 64 (18.29) | 6.701 (0.035) |
| 30–39 | 456 | 98 (21.49) | ||
| 40+ | 97 | 10 (10.31) | ||
| Drug use historyb | 0–5 years | 191 | 31 (16.23) | 3.307 (0.069) |
| 6–10 years | 514 | 95 (18.48) | ||
| 11 and more | 198 | 46 (23.23) | ||
| Sample total | 903 | 172 (19.05) |
Age difference is mostly attributable to that between the groups of 20–39 and 40 + years of age (χ2 = 5.382; p = 0.02), but not between the groups of 20–29 and 30-29 years of age (χ2 = 1.267, p = 0.26).
Trend test based on a quadratic trend with the three levels (0–6 years = 0, 6–11 years = 1, 11+ = 2) of drug use history.
It was highest (21.49%) among 30–39 age group followed by those 20–29 years of age (18.29%), and it was the lowest in the 40+ age group (10.31%). Whereas the difference between the groups of 20–29 and 30–39 years of age was not significant (χ2 = 1.267, p = 0.26), the difference between the groups of 20–39 and 40+ was more pronounced (χ2 = 5.382, p = 0.02). Subjects with junior high school education had the highest infection rate (21.10%), followed by those with high school education or above (16.82%) and those with primary school or below (15.91%). But these differences related to education-attainment were statistically insignificant (χ2 = 0.2.207, p = 0.33). Variation in cryptosporidiosis also varied across occupation. Self-employed and farmers had the highest rates (26.23% and 25.35%, respectively), and government employee and others were among the lowest (16.03% and 15.15%, respectively). After dividing the cases into three drug use history groups, 5 years or less, 6–10 years, and 11 years and more, a trend test yielded χ2 = 3.307 and p-value = 0.069, suggesting a strong trend of increasing rate of cryptosporidiosis with the length of drug use.
Cryptosporidium positive drug users reported none typical symptoms of diarrheal during the 2 months preceding study entry, so all the participants in the therapy study were asymptomatic Cryptosporidium carriers.
3.2. Therapeutic effect of acetylspiramycin and garlicin
Subjects were randomly assigned to the four treatment groups. There were no significant differences in age and length of drug use among the four treatment groups (Table 2). Compliance level was satisfactory among subjects and no adverse events were observed.
Table 2.
Age and drug use history of cryptosporidiosis cases.
| Acetylspiramycin + Garlicin | Acetylspiramycin | Garlicin | Control | F (p-value) | |
|---|---|---|---|---|---|
| Mean age (SD) | 31.13(4.68) | 30.49(5.00) | 33.48(6.18) | 32.25(6.90) | 2.048 (0.110) |
| Mean years of drug use(SD) | 6.60(3.11) | 5.61(2.90) | 7.48(3.28) | 6.50(4.17) | 2.047 (0.110) |
Of those who received acetylspiramycin and garlicin (Group A) 92.1% became negative two weeks after the treatment (Table 3). In contrast, only 61.8% of those controls (group D) were negative at follow-up. Among those receiving acetylspiramycin (group B) alone or garlicin (group C) alone, 76.7% and 72.2% of subjects recovered from cryptosporidiosis, respectively. Pairwise comparisons with Bonferroni correction (significance level α′ = 0.007) suggests that acetylspiramycin and garlicin combined treatment significantly improved recovery rate from cryptosporidiosis compared with the control (p = 0.002). The improvement was also somewhat marked compared with that of taking acetylspiramycin (p = 0.060) alone or garlicin alone (p = 0.025). To control for potential confounding effects, a logistic regression of recovery rate of cryptosporidiosis was conducted with adjustment for age, history of drug use, education and occupation of the subjects, treatment was the only significant factor to the outcome (χ2 = 8.257, p < 0.05).
Table 3.
Therapeutic effects of acetylspiramycin and garlicin.
| Groups | Total cases | Negative cases (%) | Comparison | χ2 (P) |
|---|---|---|---|---|
| A:acetylspiramycin + garlicin | 38 | 35 (92.11%) | A vs.B | 3.533 (0.060) |
| A vs. C | 5.047 (0.025) | |||
| A vs. D | 9.557 (0.002a) | |||
| B:acetylspiramycin | 43 | 33 (76.74%) | B vs. D | 2.034 (0.154) |
| C:garlicin | 36 | 26 (72.22%) | C vs. D | 0.867 (0.352) |
| D:control | 34 | 21 (61.76%) | ||
| Total | 151 | 115 (76.16%) |
Significant difference after Bonferroni correction for multiple comparison (p < 0.007).
Table 4 gives the adjusted odds ratio of recovery from cryptosporidiosis for each treatment relative to the control group.
Table 4.
Odds ratio of cryptosporidiosis recovery compared with the control group after adjusting for age, length of drug, occupation, and education.
| Independent variables | Coef | S.E. | OR(95%CI) | Wald χ2 | p-value |
|---|---|---|---|---|---|
| Age | −0.018 | 0.039 | 0.982(0.910–1.060) | 0.210 | 0.982 |
| History of drug use | −0.094 | 0.060 | 0.911(0.809–1.025) | 2.414 | 0.12 |
| Education | 0.159 | 0.241 | 1.172(0.730–1.881) | 0.432 | 0.511 |
| Occupation | 0.020 | 0.107 | 1.020(0.827–1.259) | 0.035 | 0.851 |
| Treatment groups | – | – | – | 8.257 | 0.041 |
| Acetylspiramycin + garlicin | 2.014 | 0.704 | 7.490(1.886–29.750) | 8.188 | 0.004 |
| Acetylspiramycin onl | 0.643 | 0.512 | 1.901(0.697–5.185) | 1.576 | 0.209 |
| Garlicin only | 0.581 | 0.526 | 1.788(0.638–5.009) | 1.222 | 0.120 |
| Control | – | – | 1 | – | – |
Overall significance of the model: χ2 = 36.983, p < 0.001.
4. Discussion
The incidence of Cryptosporidium infection is estimated to be 1%–3% in general population in developed countries and 10% in developing countries. More specifically, the incidence among those with diarrhea is about 2.2% (range, 0.26–22%) in immunocompetent persons in developed countries and 6.1% (range, 1.4–41%) in developing countries (Guerrant, 1997). It occurs in up to 7% of children with diarrhea in developed countries and up to 12% in developing countries. The infection rate is estimated at 14% (range, 6–70%) among persons with AIDS and diarrhea in developed countries and 24% (range, 8.7–48%) in developing countries (Guerrant, 1997). Manabe et al. (1998) reported Cyptosporidium infection rate in AIDS patients was between 10% and 15% in the United States.
Ling et al. (2001) reported a 7% infection rate among 186 prisoners with diarrhea. The infection rate of 19% of institutionalized drug users in this study is even higher than 13.6% in a cohort of HIV positive intravenous drug users in Malaysia (Kamel et al., 1994). Repeated and chronic use of drugs may have substantially weakened these users’ immune systems, resulting in a higher rate of infection.
Cryptosporidiosis infection rate appeared to increase with the number of years using drugs among the subjects in this sample. An average of more than six years of drug use may have considerably weakened the immune systems, and led to poorer health in general. Also, cryptosporidiosis rate was significantly higher among those under 40 years of age than those above 40. Given the comparable history of drug use among different age groups, the difference in infection rate suggests that older subjects were perhaps more capable of generating autoimmune activities against cryptosporidiosis because of their more extended exposure to Cryptosporidium.
Cryptosporidium parvum is well well-known to be associated with persistent diarrhea as well as adversely affects nutritional status. Cryptosporidiosis patients often have symptoms similar to those of enteritis, clinicians may thus fail to recognize Cryptosporidium, resulting in clinical misdiagnosis of cryptosporidiosis. When a patient with persistent diarrhea fails to respond to treatment, testing for cryptosporidiosis should be conducted as quickly as possible.
While diarrhea is common among immunocompromised people with cryptosporidiosis, it was worth noting that all the infected drug users in this study had no symptoms of diarrhea, and of the 15 participants who injected heroin within 2 months before enrollment, 10 cases exhibited symptoms of constipation. This is probably not an uncommon phenomenon and is similar to the findings reported by Ravn et a1.(1991), who found the occurrence of asymptomatic carriers of Cryptosporidium to be a frequent occurrence in exposed AIDS of Cryptosporidium infection. Although the exact mechanism is unknown, research suggests that the use of drugs somewhat contribute to the inhibition of bowel's peristalsis. It is also possible that their immunological status is still normal or slightly lower or the infective dose is too small.
Nevertheless these infected drug users acted as asymptomatic carriers should be interpreted cautiously, since them may be a mixture of either HIV-positive or HIV- negative. It is well recognized that intravenous drug use and HIV infection are highly correlated due to the sharing of contaminated needles and syringes (Luo et al., 2015). Injection drug use, particularly heroin, remained a major mode of HIV transmission in China over the past two decades. By the end of 2009, it is estimated that 740,000 (560,000–920,000) people were living with HIV/AIDS (PLHIV) in China, of whom 32.2% were infected through injecting drug use (Ministry of Health, UNAIDS, 2014). However, it is unavoidable that we failed to detect the HIV status within the study population, which is ascribed to the ethical consideration for the protection of patient's rights of privacy. Therefore, it is critical to investigate the prevalence of co-infection with HIV, especially among the IDUs considered to be a high-risk population of co-infection with HIV and Cryptosporidium.
Although there are a large number of antiparasitic antibiotics clinically available, only few are practically used to treat cryptosporidiosis, including specifically paromomycin, azithromycin, spiramycin (Graczyk et al., 2011, Sinkala et al., 2011, Das et al., 2013) and nitazoxanide (Mainali et al., 2013). However, their therapeutic effects are limited (Graczyk et al., 2011, Sinkala et al., 2011; Mainali et al., 2013; Das et al., 2013, Yacoub et al., 2014). There are a few studies on the anti-Cryptosporidium activities of garlicin in China (Han, 1989, Ge et al., 2001, Chen and Shen, 2011). In Han's study, 13 pediatric cases were treated with garlicin alone for 1–6 days. Their stool specimens were examined 2–3 times between one week to two-month after treatment. No Cryptosporidium oocysts were observed in these follow-up examinations. Further, none of the negative cases relapsed after treatment (Han, 1989). In the study of Ge et al. (2001), all of the 202 infected children became cryptosporidiosis negative after three courses of garlicin treatment over 21 days. However, neither study was a placebo controlled. Thus the efficacy of garlicin for treating cryptosporidiosis cannot be ascertained given the likelihood of disinfection among healthy persons without any treatment.
The present study investigated specifically the potential of garlicin in treating cryptosporidiosis in conjunction with acetylspiramycin. Results of this study show that 61.8% of cases in the control group became negative without medical treatment, whereas in the group of garlicin alone and acetylspiramycin alone the rates are 72.2% and 76.7%, respectively. These differences suggest a marked improvement in using garlicin or acetylspiramycin to reduce and eliminate Cryptosporidium activities. Combined use of garlicin and acetylspiramycin elevated the cure rate to an even higher level (92.1%). These results suggest the potential of garlicin in treating cryptosporidiosis either alone or in combination with other antiparasitic antibioticis. However, the mechanisms are unknown in the literature of garlicin's activity against Cryptosporidium spp in conjunction with the use of acetyspiramycin. The production of diallyl disulfide (DADS) and diallyl trisulfide (DATS), major components of garlicin, may have the effect of disinfectant and fungicide, thus endowing garlicin a killing effect to the Cryptosporidium (Anthony et al., 2005). Another possible mode of action is that garlicin could markedly enhance the phagocytosis of peritoneal macrophage, promote the transformation of T lymphocyte, and reinforce the activity of NK cell. Because poor immune function of the human host is the most important opportunity for cryptosporidiosis, improvement of the immune function of the patients may be the most important contributor to the efficacy of garlicin in treating Cryptosporidium infection. It remains unknown how the synergy between garlicin and acetylspiramycin in therapy, hence further clinical investigation is under design along this line.
Drug use appears to be a risk factor to cryptosporidiosis. Special measures of prevention and control of cryptosporidiosis are needed to protect drug users. It is of public health importance to control and reduce exposure to Cryptosporidium, and it is equally important to effectively treat infected cases to prevent the spreading of Cryptosporidium. Because diarrhea remains a common illness among the Chinese population and can be caused by many human pathogens such as helminth, cryptosporidiosis is easily misdiagnosed among patients with diarrhea. Public awareness and knowledge about of cryptosporidiosis and its treatment are low, resulting in another impediment to effective prevention, control, and treatment of cryptosporidiosis. Thus timely diagnosis and effective treatment are critically important. This study is a part of the effort to developing effective and accessible treatment of cryptosporidiosis in the Chinese population.
Our study has several limitations. Firstly, the participants in our study were asymptomatic Cryptosporidium carriers, which may impose restrictions on treatment efficacy among symptomatic patients. Additionally, we failed to detect the HIV status and the genotype of Cryptosporidium within the study population, which may confound study results in assessment of efficacy. So a design of trial of treatment for HIV-related cryptosporidiosis and genotypes should be aided by the experience gained from this trial. Important aspects include the necessity of therapeutic evaluation stratified based on the HIV status, genotypes, severity of diarrheal and clinical end points.
Authors' contributions
Min-Zhu Huang designed the study protocol and drafted the manuscript; Jin Li carried out the treatment effect assessment and statistical analysis; Deng-qing Li and Xin-Min Nie participated in the field investigation, data collection, and data annlysis; Lan Guan and Rong Gui carried out the test of the stool specimens for cryptosporidiosis and the interpretation of these data; Xia Chen contributed to data analysis, interpretation, and drafted the manuscript. All authors read and approved the final manuscript.
Conflicts of interest
None
Ethical approval
All participants had been required to formally, through signature on the “Permission and Information Sheet”, indicate their consent to participating in the research process.
And they had been given the opportunity to withdraw from the research at any time prior to the publication of the research findings.
The matter of how data collected and stored, with reference to the Data Protection legislation had been clarified for participants, with information being stored in locked cabinets or on IT hardware protected with the highest security software.
There is no indication that the research processes will result in any harm or discomfort. The research protocol was approved by the Institutional Review Board at Xiangya School of Medicine, Central South University.
Acknowledgments
The authors thank all patients and guardians involved in the study.
Contributor Information
Min-Zhu Huang, Email: huangminzhu_2002@163.com.
Jin Li, Email: lincoln0221@126.com.
Lan Guan, Email: guanlan7@163.com.
Deng-Qing Li, Email: lidengqing@aliyun.com.
Xin-Min Nie, Email: niexinmin19840702@163.com.
Rong Gui, Email: aguirong@163.com.
Xia Chen, Email: chen_1987_com@163.com.
References
- An W., Zhang D., Xiao S., Yu J., Yang M. Quantitative health risk assessment of Cryptosporidium in rivers of southern China based on continuous monitoring. Environ. Sci. Technol. 2011;45:4951–4958. doi: 10.1021/es103981w. [DOI] [PubMed] [Google Scholar]
- Anthony J.P., Fyfe L., Smith H. Plant active components–a resource for antiparasitic agents? Trends Parasitol. 2005;21:462–468. doi: 10.1016/j.pt.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Asdaq S.M. Antioxidant and hypolipidemic potential of aged garlic extract and its constituent, s-allyl cysteine, in rats. Evid. Based Complement. Altern. Med. – eCAM. 2015 doi: 10.1155/2015/328545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assefa S., Erko B., Medhin G., Assefa Z., Shimelis T. Intestinal parasitic infections in relation to HIV/AIDS status, diarrhea and CD4 T-cell count. BMC Infect. Dis. 2009;9:155. doi: 10.1186/1471-2334-9-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartelt L.A., Sevilleja J.E., Barrett L.J., Warren C.A., Guerrant R.L., Bessong P.O., Samie A. High anti-Cryptosporidium parvum IgG seroprevalence in HIV-infected adults in Limpopo, South Africa. Am. J. Trop. Med. Hyg. 2013;89:531–534. doi: 10.4269/ajtmh.12-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Shen K.F. Progress in prevention and treatment of cryptosporidiosis with traditional Chinese medicine. Chin. J. Vet. Drug. 2011;10:017. [Google Scholar]
- Das J.K., Ali A., Salam R.A., Bhutta Z.A. Antibiotics for the treatment of cholera, Shigella and Cryptosporidium in children. BMC Public Health. 2013;13:S10. doi: 10.1186/1471-2458-13-S3-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Wang L., Duan L., Gomez-Puerta L.A., Zhang L., Zhao X., Xiao L. Extended outbreak of cryptosporidiosis in a pediatric hospital, China. Emerg. Infect. Dis. 2012;18:312. doi: 10.3201/eid1802.110666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao H., Yoshida H., Tazawa Y.I. Antithrombotic effects of odorless garlic powder both in vitro and in vivo. Biosci. Biotechnol. Biochem. 2007;71(1):84–90. doi: 10.1271/bbb.60380. [DOI] [PubMed] [Google Scholar]
- Gaafar M.R. Efficacy of Allium sativum (garlic) against experimental cryptosporidiosis. Alexandria J. Med. 2012;48(1):59–66. [Google Scholar]
- Ge J.J., Shen J.P. A detection of Cryptosporidium oocysts in stool with modified two-step acid-fast bacilli staining method. Chin. Med. J. 1991;71:567. [Google Scholar]
- Ge J.J., Shen J.P., Jiang X.R., Yu F., Gong G.Q. Experimentations and clinic study of Cryptosporidium enteritis among children. Chin. J. Zoonoses. 2001;17:121–122. [Google Scholar]
- Graczyk Z., Chomicz L., Kozłowska M., Kazimierczuk Z., Graczyk T.K. Novel and promising compounds to treat Cryptosporidium parvum infections. Parasitol. Res. 2011;109:591–594. doi: 10.1007/s00436-011-2290-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant R.L. Cryptosporidiosis: an emerging, highly infectious threat. Emerg. Infect. Dis. 1997;3:51. doi: 10.3201/eid0301.970106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han F. Cryptosporidiosis was found among infants in China. Chin. J. Pediatr. 1989;27:75–76. [Google Scholar]
- Huang M.Z., Zhou C.X., Guan L., Li D.Q. Study on condition of Cryptosporidium infection among adult of diarrhea patients. Curr. Physician. 1998;3:42–43. [Google Scholar]
- Ibrahim A.N. Comparison of in vitro activity of metronidazole and garlic-based product (tomex®) on trichomonas vaginalis. Parasitol. Res. 2013;112(5):2063–2067. doi: 10.1007/s00436-013-3367-6. [DOI] [PubMed] [Google Scholar]
- Insulander M., Silverlas C., Lebbad M., Karlsson L., Mattsson J.G., Svenungsson B. Molecular epidemiology and clinical manifestations of human cryptosporidiosis in Sweden. Epidemiol. Infect. 2013;141:1009–1020. doi: 10.1017/S0950268812001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Wen L., Ling X. The clinical evaluation of curative effect of acetylspiramycin on toxoplasmosis infection during pregnancy. Chin. J. Pract. Gynecol. Obstet. 2000;2:107–108. [Google Scholar]
- Kadria N., Hammam A.M., Morsy G.H., Khalil A.M., Seliem M.M.E., Aboelsoued Dina. Control of cryptosporidiosis in Buffalo calves using garlic (Allium sativum)and nitazoxanide with special reference to some biochemical parameters. Glob. Vet. 2015;14(5):646–655. [Google Scholar]
- Kamell A.M., Nurahan Maning S., Murad S., Nasuruddin A., Lail K.P.F. Cryptosporidiosis among HIV positive intravenous drug users in Malaysia. Malay. 1994;72:27–28. [PubMed] [Google Scholar]
- Mac Kenzie W.R., Hoxie N.J., Proctor M.E., Gradus M.S., Blair K.A., Peterson D.E., Davis J.P. A massive outbreak in Milwaukee of Cryptosporidium infection transmitted through the public water supply. N. Engl. J. Med. 1994;331:161–167. doi: 10.1056/NEJM199407213310304. [DOI] [PubMed] [Google Scholar]
- Kothavade R.J. Potential molecular tools for assessing the public health risk associated with waterborne Cryptosporidium oocysts. J. Med. Microbiol. 2012;61:1039–1051. doi: 10.1099/jmm.0.043158-0. [DOI] [PubMed] [Google Scholar]
- Ling X.M., Chen H., Yue W., Mao X.R., Song J.J. An investigation of Cryptosporidium infection in special population groups. Chin. J. Parasit. Dis. Control. 2001;14:240. [Google Scholar]
- Liu J.H., Chen X.C., Zhou S.C. Effect of allitridin on macrophage function in mice. J. Taishan Med. Coll. 2002;23(2):141–142. [Google Scholar]
- Liu P.M., Shen L., Zheng K., Yang X.Z. Protective effect of garlicin combined with compound sulfamethoxazole against toxoplasma infection of mice. Chin. J. New Drugs & Clin. Remedies. 2002;21(4):226–228. [Google Scholar]
- Liu H., Shen Y., Yin J., Yuan Z., Jiang Y., Xu Y., Cao J. Prevalence and genetic characterization of Cryptosporidium, Enterocytozoon, Giardia and Cyclospora in diarrheal outpatients in China. BMC Infect. Dis. 2014;14:25. doi: 10.1186/1471-2334-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W., Wu Z., Poundstone K., Mcgoogan J.M., Dong W., Pang L. Needle and syringe exchange programmes and prevalence of HIV infection among intravenous drug users in China. Addiction. 2015;110(Suppl. 1(Supplement S1)):61–67. doi: 10.1111/add.12783. [DOI] [PubMed] [Google Scholar]
- Maeda T. Toxoplasmosis. Jpn. J. Clin. Med. 2003;61:603–607. [PubMed] [Google Scholar]
- Mainali N.R., Quinlan P., Ukaigwe A., Amirishetty S. Cryptosporidial diarrhea in an immunocompetent adult: role of nitazoxanide. J. community Hosp. Intern. Med. Perspect. 2013;3:3–4. doi: 10.3402/jchimp.v3i3-4.21075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe Y.C., Clark D.P., Moore R.D., Dahlman H.R., Belitsos P.C., Chaisson R.E., Sears C.L. Cryptosporidiosis in patients with AIDS: correlates of disease and survival. Clin. Infect. Dis. 1998;27:536–542. doi: 10.1086/514701. [DOI] [PubMed] [Google Scholar]
- Masarat S., Ahmad F., Chisti M., Hamid S., Sofi B.A. Prevalence of Cryptosporidium species among HIV positive asymptomatic and symptomatic immigrant population in Kashmir, India. Iran. J. Microbiol. 2012;4:35. [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health, UNAIDS, WHO . 2010. 2009 estimates for the HIV/AIDS epidemic in China.http://www.unaids.org.cn/download/2009%20China%20Estimation%20Report-En.pdf Beijing, China. Available: (accessed 09.02.14) [Google Scholar]
- Ravn P., Lundgren J.D., Poul K. Nosocomial outbreak of cryptosporidiosis in AIDS patients. Br. Med. J. 1991;302:277–280. doi: 10.1136/bmj.302.6771.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L.J., Ge J.J. Allicin treatment of cryptosporidiosis in infants. Jiangsu Med. J. 1997;8(23):571. [Google Scholar]
- Sinkala E., Katubulushi M., Sianongo S., Obwaller A., Kelly P. In a trial of the use of miltefosine to treat HIV-related cryptosporidiosis in Zambian adults, extreme metabolic disturbances contribute to high mortality. Ann. Trop. Med. Parasitol. 2011;105:129–134. doi: 10.1179/136485911X12899838683160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson M., Ali M. Garlic Allium sativum: a review of its potential use as an anti-cancer agent. Curr. Cancer Drug Targ. 2003;3:67–81. doi: 10.2174/1568009033333736. [DOI] [PubMed] [Google Scholar]
- Tsao S.M., Yin M.C. In-vitro antimicrobial activity of four diallyl sulphides occurring naturally in garlic and chinese leek oils. J. Med. Microbiol. 2001;50(7):646–649. doi: 10.1099/0022-1317-50-7-646. [DOI] [PubMed] [Google Scholar]
- Uppal B., Singh O., Chadha S., Jha A.K. A comparison of nested PCR assay with conventional techniques for diagnosis of intestinal cryptosporidiosis in AIDS cases from Northern India. J. Parasitol. Res. 2014:706105. doi: 10.1155/2014/706105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg O., Robberecht F., Dauby N., Moens C., Talabani H., Dupont E., Levy J. Management of a Cryptosporidium hominis outbreak in a day-care center. Pediatr. Infect. Dis. J. 2012;31:10–15. doi: 10.1097/INF.0b013e318235ab64. [DOI] [PubMed] [Google Scholar]
- Wang D., Zhang Y.Y. Therapeutic efficacy of allitridin and azithromycin on Cryptosporidium infection in Mice. Chin. J. Parasitol. Parasit. Dis. 2013;6(31):447–459. [PubMed] [Google Scholar]
- Wang D., Zhang Y.Y. Intestinal pathological changes of Kunming mice infected by Cryptosporidium and the therapeutic efficacy of spiramycin on infected mice. Chin. J. Parasitol. Parasit. Dis. 2014;32:225–228. [PubMed] [Google Scholar]
- Yacoub A.T., Jones L., Coppola D., Smith K., Sandin R.L., Vincent A.L., Greene J.N. Nitazoxanide for cryptosporidiosis after hematopoietic stem cell transplantation: a case series and review of literature. Infect. Dis. Clin. Pract. 2014;22:257–259. [Google Scholar]
- Yang S.P., Ge J.J. Effect of garlicin on Cryptosporidium infectious diarrhea in infants and young children: 172 cases of clinical report. Chin. Med. J. 1994;3(9):49. [Google Scholar]
- Yao Y.X., Chen H.F., Liu X., Xiao N., Xiao Y., Huang Y.H., Yu S.Y. Molecular epidemiological studies of Cryptosporidiosis diarrhea in children of Guangzhou sentinel hospital. J. Trop. Med. 2014;1:017. [Google Scholar]
- Yoder J.S., Wallace R.M., Collier S.A., Beach M.J., Hlavsa M.C., Centers for Disease Control and Prevention (CDC). Cryptosporidiosis surveillance—United states, 2009–2010. MMWR Surveill. Summ. 2012;61:1–12. [PubMed] [Google Scholar]
- Zhu Y.L., Fung K. Statistical methods in developmental toxicity risk assessment. In: Fan A.M., Chang L.W., editors. Toxicology Risk Assessment. 1996. pp. 413–446. [Google Scholar]