Abstract
The study investigated direct anthelmintic effects of sesquiterpene lactones (SL)-containing extracts from forage chicory against free-living and parasitic stages of Ostertagia ostertagi. Freeze-dried leaves from chicory cultivars ‘Spadona’ and ‘Puna II’ were extracted using methanol/water. Total SL were further fractionated by solid-phase extraction and resulting extracts were characterised by high-performance liquid chromatography (HPLC). O. ostertagi eggs from faeces of mono-infected calves were hatched and L1 were used in a larval feeding inhibition assay (LFIA), while cultured L3 were used in a larval exsheathment inhibition assay (LEIA). Adult worms were immediately recovered after slaughter and used for motility inhibition assays (AMIA). Electron microscopy (EM) was performed on adult O. ostertagi exposed to 1000 μg extract mL−1 of both chicory cultivars. In all assays, decreasing concentrations of SL-containing extracts in PBS (1% DMSO) were tested in replicates with 1% DMSO in PBS as negative controls. HPLC demonstrated similar concentrations of most SL in both extracts. However, Spadona-extract contained significantly higher concentrations of 11, 13-dihydro-8-deoxylactucin (P = 0.01), while Puna II-extract had increased levels of 11, 13-dihydrolactucin (P < 0.0001). In the LFIA, both extracts reduced larval feeding at increasing concentrations, but Spadona-extract showed higher potency confirmed by significantly lower EC50 (P < 0.0001). In the LEIA, neither of the two extracts interfered with the exsheathment of L3 (P > 0.05). In the AMIA, both SL-containing extracts induced a dose-dependent effect but Spadona-extract showed greater activity and exerted faster worm paralysis than Puna II-extract with significantly lower EC50 (P < 0.0001). No cuticular damage was observed by EM in worms exposed to any of the extracts. We have demonstrated that SL-containing extracts from forage chicory can inhibit feeding of free-living larvae and exert direct effects against parasitic stages of O. ostertagi. Our results may contribute to the identification of natural anti-parasitic compounds and to interpret the in vivo anthelmintic effects of forage chicory.
Keywords: Forage chicory, Sesquiterpene lactones, Anthelmintic, Ostertagia ostertagi, Cattle, Electron microscopy
Graphical abstract
Highlights
-
•
Sesquiterpene lactones (SL)-containing chicory extracts act against Ostertagi ostertagi.
-
•
SL-containing extracts inhibited feeding of L1 and motility of adult nematodes.
-
•
Distinct anti-parasitic potency and SL profile in extracts from 2 chicory cultivars.
-
•
Marked differences between cultivars can help to identify the more active molecules.
1. Introduction
In the face of increasing anthelmintic resistance in parasitic nematodes of ruminants, the development of efficacious novel control strategies that can reduce the reliance on anthelmintics are urgently needed (Waller and Thamsborg, 2004). Feeding of animals with certain plants has shown much promise as a complementary parasite control option, with in vivo results for several forages demonstrating anthelmintic efficacy against a number of gastrointestinal nematode species (Githiori et al., 2006, Hoste et al., 2015). Most of the anti-parasitic activity of these plants is believed to derive from the presence of biologically active compounds, designated as plant secondary metabolites (PSM). Consequently, a comprehensive study of PSM can help to understand the anthelmintic effects of bioactive crops in the field and to potentially detect novel anti-parasitic molecules. One of the plants investigated as a potential anthelmintic forage for ruminants is chicory (Cichorium intybus), which has been linked with significant reductions in adult worm burdens and egg excretion of abomasal nematodes in sheep (Scales et al., 1994, Marley et al., 2003, Tzamaloukas et al., 2005, Heckendorn et al., 2007). Chicory is known to produce a group of biologically active terpenoids, the sesquiterpene lactones (SL), in both leaves and roots (Rees and Harborne, 1985, Price et al., 1990, Foster et al., 2011, Graziani et al., 2015). In chicory leaves, SL are present as glycosides (bound to carbohydrates) and as free molecules (Ferioli et al., 2015). Sesquiterpene lactones are PSM thought to be involved in the protection of plants against herbivore attacks and for signalling between plants (Gershenzon and Dudareva, 2007). In biological systems, SL have demonstrated antioxidant, antibiotic, anticancerigenous and antiprotozoan properties (Bischoff et al., 2004, Cavin et al., 2005, Schmidt, 2006, Barrera et al., 2013).
To date, two studies have provided preliminary evidence of in vitro activity of SL from chicory against free-living stages of parasitic nematodes of ruminants. Molan et al. (2003) reported inhibitory effects of crude SL-extracts from chicory roots on free-living larvae of Dictyocaulus viviparus and mixed-species of gastrointestinal nematodes isolated from red deer, however, detection and characterisation of SL in the tested extracts was not performed. Foster et al. (2011) analysed extracts containing free SL from two chicory cultivars and found differences in the concentration of individual SL and inhibitory effects towards the eclosion of a predominantly Haemonchus contortus egg population. In contrast with free-living stages occurring in the environment, parasitic stages in the host have different biochemical characteristics and detoxification mechanisms, thus the same compound(s)/PSM can exert different effects and affect distinct targets in free-living or adult stages (O'Grady and Kotze, 2004, Hoste et al., 2015). As yet, no studies have explored the direct effects of well-characterised SL-containing extracts from forage chicory on parasitic stages, which are the expected primary targets of dietary SL in the host.
The main objective of our study was to investigate and compare the direct effects of SL-containing extracts isolated from two forage chicory cultivars on key biological processes of free-living and parasitic stages of Ostertagia ostertagi. This abomasal nematode is considered to be the most pathogenic and economically important parasite infecting grazing cattle in temperate areas of the world (Nansen, 1993, Fox, 1993, Charlier et al., 2014). Two chicory cultivars were included in the study in order to account for variations in the activity of extracts derived from different plant cultivars, previously described in chicory (Foster et al., 2011). In relation, a detailed characterisation of the SL profile in the tested chicory extracts was performed.
2. Materials and methods
2.1. Plant materials
Chicory cultivar (cv.) ‘Spadona’ and cv. ‘Puna II’ were sown on 7 May 2013 in two separate fields at the experimental farm of the University of Copenhagen (Tåstrup, Denmark, 55°67′48″N, 12°29′75″E). Both fields were fertilized with 50 kg N/ha in spring (early May). The soil type in both fields was moraine clay loam. Chicory cv. Spadona was sown as a pure sward (7.8 kg seeds/ha) whereas cv. Puna II was mixed with timothy (Phleum pratense; seed rate: 6 kg chicory + 6 kg timothy seeds/ha). Leaves from both chicory cultivars were hand-picked on 23 July 2013 and stored at −20 °C in the dark until extraction of SL. At the moment of collection, all chicory plants were at the vegetative stage.
2.2. Extraction of sesquiterpene lactones from forage chicory
Sesquiterpene lactones were isolated and purified from chicory leaves according to Ferioli and D'Antuono (2012), with some modifications. This extraction method selectively separates total (free and unbound) SL from phenols and other plant compounds. Chicory leaves were freeze-dried overnight, ground into powder, after which pulverized leaf material (2 g) from each cultivar was weighed separately into two 50 mL tubes. Extraction solvent (30 mL) of methanol/Milli-Q-H2O (4/1; v/v) containing 2% formic acid (v/v) was added to each tube. The tubes were vortexed for 1 min, sonicated in a water bath at room temperature for 10 min and centrifuged (10 min, 1540 g). After centrifugation, supernatants from the same cultivar were pooled in a 200 mL round-bottomed glass flask (1 flask per cultivar). Remaining leaf material in the 50 mL tubes was extracted three more times as described above and supernatants were pooled in the corresponding round-bottomed flask. Collected supernatants were concentrated under reduced pressure at 35 °C to evaporate methanol and formic acid and freeze-dried overnight to remove Milli-Q-H2O. The resulting crude extracts were resuspended in methanol (5 mL) and dissolved with 70 mL of a cellulase enzyme solution (10 mg cellulase from Aspergillus niger [Sigma 22178]/mL Milli-Q-H2O) in order to release glycoside (bound) SL. The dissolved extracts with cellulase enzyme solution were then transferred to 15 mL tubes and incubated in a water bath for 2 h at 40 °C. After enzymatic treatment, extracts were distributed into 50 mL tubes (3 tubes per cultivar) and ethyl acetate (25 mL) was added to each tube. The tubes were centrifuged (10 min, 1540 g) and the supernatants (ethyl acetate-extracts) were collected in round-bottomed glass flasks (1 flask per cultivar). Remaining sedimented material in the 50 mL tubes was extracted with ethyl acetate (2 × 25 mL) as described above. The collected ethyl acetate-extracts were evaporated to dryness under reduced pressure (35 °C), redissolved in methanol (8 mL) and transferred to 50 mL tubes (1 tube per cultivar). Final purification of SL from phenols and other plant compounds was performed by solid-phase extraction (SPE). Dichloromethane (28 mL) was added to each tube and centrifuged (10 min, 1540 g). A SPE vacuum manifold was equipped with 12 × 6 mL SPE tubes (Supelclean® LC-Si SPE tubes, Supelco 505374). SPE tubes were conditioned with dichloromethane/i-propanol (6 mL, 1/1; v/v) and equilibrated with dichloromethane (6 mL). Clean 12 × 15 mL tubes (6 tubes per cultivar) were set in the vacuum manifold for collection of SL. Extract mixed with dichloromethane (6 mL) was loaded in each SPE tube and the obtained liquid fractions were transferred into one tared 50 mL glass flask per chicory cultivar. Collected fractions were dried under reduced pressure (35 °C) and resulting purified extracts were weighed. Obtained extracts were highly viscous with low solubility in Milli-Q-H2O or PBS. These purified chicory extracts were therefore resuspended in 100% DMSO at a concentration of 100 mg dry weight extract/mL DMSO (stock solution) and stored at −20 °C until use for in vitro assays and chemical analyses. High-performance liquid chromatography (HPLC) analyses were performed with stock solution of purified chicory extracts after removal of DMSO by freeze-drying and resuspension in methanol (10 mg dry weight extract/mL methanol). Two additional extractions from the same plant material were performed as described above and confirmed the repeatability of the extraction method (data not shown).
2.3. Chemical analyses of purified chicory extracts
Compounds present in the obtained purified extracts were characterised by HPLC with mass spectrometry (MS) carried out in a Shimadzu Nexera X2 equipment and a Bruker MicrOTOF-Q III mass spectrometer, using an Supelco, Ascentis Express Peptide ES-C18 column (2.7 μm, 160 Å). A 1 mL/min linear gradient from 0 to 100% buffer B over 10 min was employed (buffer A: 0.025% trifluroacetic acid [TFA] in 10% aqueous acetonitrile-MeCN; buffer B: 0.025% TFA in 90% aqueous MeCN). Molecular mass analysis was carried out in conjunction with HPLC on a quadruple mass spectrometer in positive and negative electron spray ionization modes. Individual SL were identified by comparison of the main fragment ions (m/z) detected in all major peaks and retention times from published reports of SL profiles in chicory (Sessa et al., 2000, Ferioli and D'Antuono, 2012, Graziani et al., 2015). To quantify individual SL, HPLC was carried out at a wavelength where the UV absorptions of the different compounds were approximately the same (i.e. isosbetic point). This approximate isosbestic point was determined from the UV absorption spectra of all compounds detected in the purified extracts and occurred at wavelengths of 271 nm for cv. Spadona and 275 nm for cv. Puna II (Supplementary Figs. 1 and 2, respectively). Consequently, each extract was analysed at the mentioned wavelength for determination of individual peak areas. Of the SL known to be present in chicory, only lactucopicrin was commercially available as a pure standard when the experiments were conducted. Therefore, all detected peak areas were quantified by comparison with an external standard calibration curve using pure lactucopicrin. Here, a two-fold dilution of pure lactucopicrin (Extrasynthese 3813) was prepared in methanol (1000–15.6 μg lactucopicrin/mL) and analysed by HPLC-MS at 271 nm and 275 nm under the same conditions as the purified chicory extracts. Two separate SL quantifications were performed on each purified chicory extract.
2.4. Parasite material
Two Jersey calves, aged 5–6 months, were infected with 10,000 or 20,000 third-stage larvae (L3) of an ivermectin-susceptible O. ostertagi strain (Ref Label: OOSG10, Ridgeway Research, UK). The study was approved by the Animal Experiment Inspectorate, Ministry of Food, Agriculture and Fisheries of Denmark (j. No. 2013-15-2934-00763). Nematode eggs were detectable in faeces from both animals at day 16 post-infection. The eggs were recovered and hatched to obtain first-stage larvae (L1) according to Novobilský et al. (2011) and were used immediately after collection for a larval feeding inhibition assay. L3 were obtained from culture of eggs in faeces mixed with vermiculite for 14 days at 25 °C as described by Roepstorff and Nansen (1998). After recovery by baermannisation, L3 were maintained at 12 °C until use in a larval exsheathment inhibition assay. Calves were necropsied at two consecutive days in order to isolate live adult worms for motility inhibition assays. After captive bolt stunning and bleeding, the abomasum was immediately recovered and live O. ostertagi adults were isolated using the agar-migration method (Christensen et al., 1995). Adult worms were allowed to actively migrate from digesta into warm saline for 3 h at 37 °C and were then collected using a 20 μm sieve. Subsequently, worms were transferred into a 50 mL tube in warm saline solution (37 °C) and were washed three times in warm, sterile incubation medium (RPMI 1640 with l-glutamine, Gibco 11875-085), supplemented with 200 U/mL penicillin, 200 μg/mL streptomycin and 2 μg/mL of amphotericin B. After the last wash, worms were transferred to a Petri dish from which they were immediately collected for the assays.
2.5. In vitro assays
2.5.1. Larval feeding inhibition assay (LFIA)
The objective of the LFIA was to investigate the effect of SL-containing extracts on the feeding behaviour of O. ostertagi L1 and performed according with Jackson and Hoste (2010) and Novobilský et al. (2011), with modifications. Fluorescein isothiocyanate (FITC)-labelled Escherichia coli was prepared according to Jackson and Hoste (2010) and preserved at −20 °C until use. After isolation, L1 were diluted in PBS (0.05 M NaCl in Milli-Q-H20, pH 6.9) until achieving a suspension of approximately 100 L1/500 μL of PBS. Stock solutions of purified chicory extracts were serially dissolved in PBS and five concentrations (in triplicates) were prepared for each extract in 1.5 mL Eppendorf tubes (500 μL of diluted extract per tube). Approximately 100 L1 (500 μl of larval suspension) were added to each tube, reaching final concentrations of 500, 250, 100, 50, 10 μg dry extract/mL PBS (final concentration 1% DMSO; total volume in tube = 1000 μL). L1 incubated with ivermectin (IVM, Sigma I8898, 1 mg/mL) and 1% DMSO in PBS were run in triplicates as positive and negative controls, respectively. All tubes were gently shaken and pre-incubated horizontally for 2 h at 25 °C. After pre-incubation, 10 μL of FITC-labelled E. coli were added to each tube. Then, the tubes were gently shaken and incubated horizontally for 18 h at 25 °C. After incubation, the tubes were centrifuged for 1 min at 6000 g and 800 mL of the supernatant was removed. Sediment with L1 was transferred into microscope slides and observed under a fluorescent microscope with a blue filter (Leica DMR A2, band pass filter 450–490 nm, long pass filter 515 nm) at 200× and 400× magnifications. All L1 in each tube were counted and classified as: a) fed larvae, with the presence of green fluorescent FITC-labelled E. coli in the larval oesophagus and/or intestine or b) unfed larvae, lacking FITC-labelled E. coli in the gut or presence of green fluorescence only outside the larval body or around the buccal opening. The number of fed/unfed larvae was used to calculate larval feeding percentages in each replicate, as: % larval feeding = 100 × [(number of fed L1)/(total number of L1)].
2.5.2. Larval exsheathment inhibition assay (LEIA)
This assay measured the potential inhibitory effect of SL-containing extracts on the chemically-induced exsheatment of O. ostertagi L3 and was conducted as described by Jackson and Hoste (2010) with minor modifications. Briefly, ensheathed L3 (2–3 months after isolation) were baermannised for 3 h using a 28 μm nylon mesh in order to isolate only live larvae. Obtained L3 were resuspended in PBS (pH 6.9) until a larval suspension of approximately 1650 L3/mL PBS was achieved. Stock solutions of Spadona and Puna II extracts were serially diluted in 100% DMSO and three decreasing concentrations were prepared for each chicory extract in 1.5 mL Eppendorf tubes (10 μL of each decreasing extract concentration per tube). Extra tubes were prepared with 10 μL of 100% DMSO as negative controls. Approximately 1600 L3 larvae (990 μl of the L3 suspension in PBS) were added to each tube, achieving final concentrations of 1000, 500 and 250 μg dry extract/mL PBS (1% DMSO) and 1% DMSO (negative controls). All tubes were vortex agitated for 10 s and pre-incubated horizontally for 3 h at 22 °C. After pre-incubation, the tubes were centrifuged at 6000 g for 2 min and the supernatants were removed. One mL of PBS was added to each tube and the washing procedure was repeated two more times before L3 were finally resuspended in 1 mL of PBS. A set of 24-well plates were prepared for the artificially induced larval exsheathment by adding 1940 μL of an exsheathment solution per well. Exsheathment solution was prepared by dilution of sodium hypochlorite 10–15% (Sigma 425044) in PBS (1/500; v/v, pH 6.9). Approximately 100 L3 from each pre-incubation tube were then transferred into each well of the 24-well plate containing exsheathment solution (1 well plate per tube). This chemically-induced exsheathment was sequentially stopped by addition of 100 μL of Lugol iodine per well at 15, 30, 45 and 60 min after start of incubation. Exsheathment was replicated four times for each chicory extract concentration and 1% DMSO (negative control) and for each time point. Ensheathed or exsheathed larvae in all wells at each time point were quantified using an inverted microscope at 200 × magnification. Exsheathment rates were calculated for each well using the formula: % exsheathment = 100 × [(number of exsheathed larvae per well)/(total number of larvae per well)].
2.5.3. Adult motility inhibition assay (AMIA)
The AMIAs were designed to investigate the inhibitory effect of SL-containing extracts on the motility of adult O. ostertagi. The assays were carried out following the methods described by Paolini et al. (2004) and Jackson and Hoste (2010), with some modifications. Two independent AMIAs were performed testing decreasing concentrations of each purified chicory extract (four assays in total) using freshly isolated adult worms collected after slaughter (see Section 2.4). For each assay, 48 well-plates were prepared with six decreasing concentrations of Spadona or Puna II extracts dissolved in the incubation medium for adult worms described in Section 2.4. The following concentrations were tested: 1000, 500, 250, 100, 50 and 10 μg dry extract/mL incubation medium (final concentration 1% DMSO; total volume in well = 1000 μL). Every concentration was tested in four replicates per plate. Wells containing IVM (1 mg/mL) and 1% DMSO dissolved in incubation medium were also prepared in four replicates in each plate as positive and negative controls, respectively. Four additional replicate wells were prepared for incubation of worms in 1000 μg dry extract/mL or 1% DMSO for electron microscopy (EM). Immediately after collection from digesta and washing (section 2.4), four to six adult O. ostertagi (males and females) were carefully added to each well. The plates were then placed in an incubator at 37 °C and worm motility was checked after 6, 24 and 48 h of incubation using a stereomicroscope. At each time point, the plates were gently hand-agitated to stimulate worm movement and the number of motile and non-motile (no movement detected during 10 s) individuals in each well were recorded. All observations were made by the same person. After 24 h of incubation, the incubation medium was removed from all wells and replaced by fresh incubation medium (1000 μL; 37 °C) containing identical Spadona or Puna II-extract concentrations. Further, at 24 h worms incubated in the extra replicates at 1000 μg dry extract/mL and 1% DMSO were collected, carefully washed in PBS and fixed in 2% glutaraldehyde in PBS for EM (section 2.6). Motility scores obtained in each AMIA were used to calculate worm motility percentage in each well as: % worm motility = 100 × [(number of motile worms per well)/(total number of worms per well)].
2.6. Electron microscopy
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) investigations were performed to detect ultrastructural damage in adult O. ostertagi exposed to the highest concentration of SL-containing extracts during 24 h. Adult worms incubated with both chicory extracts and 1% DMSO were selected for SEM, while worms exposed to Spadona-extract and 1% DMSO were selected for TEM. Adult worms for SEM were fixed as described by Williams et al. (2014a) and after fixation worms were placed into 99% ethanol and then coated with gold using a Low Vacuum Coater Leica EM ACE200. Scanning electron microscopies were performed using a Phillips XL 30 FEG SEM and image processing was done with Scandium software. Adult worms for TEM were fixed, processed and analysed as described by Williams et al. (2014a).
2.7. Statistical analysis
Concentrations of individual and total compounds, detected in two separate quantifications on each purified extract, were compared between Spadona and Puna II extracts using a pairwise t-test with Bonferroni's correction. In the AMIA and the LFIA the effective concentration of each SL-containing extract able to inhibit the motility or feeding in 50% of adults or L1 (EC50), respectively, was calculated. Tested extract concentrations were log-transformed and motility/feeding percentages obtained in the assays (eight replicates per concentration for AMIA and three replicates per concentration for LFIA) were analysed by non-linear (least squares) regression using the model log (inhibitor) vs. response–variable slope in GraphPad Prism® version 6.05, as described by Demeler et al. (2010). In the AMIA and LFIA the highest extract concentration tested from both extracts achieved a total inhibition (0%) of motility/feeding, with the sole exception of Puna II-extract in the AMIA after 6 h of incubation. Consequently, bottom values were set as 0% and top values were set as the mean motility/feeding percentage observed in negative control replicates. The R squared measure of goodness of fit (R2) was calculated for each dose–response curve. Statistical differences between EC50s obtained with both purified extracts were analysed by extra sum-of-squares F test with a null hypothesis of equal EC50. In the LEIA, the mean exsheathment percentages were calculated for each concentration and time point and were compared with the exsheathment rates obtained in the negative control wells (1% DMSO) by two-way ANOVA. A value of P < 0.05 was considered significant.
3. Results
3.1. Chemical analyses of purified chicory extracts
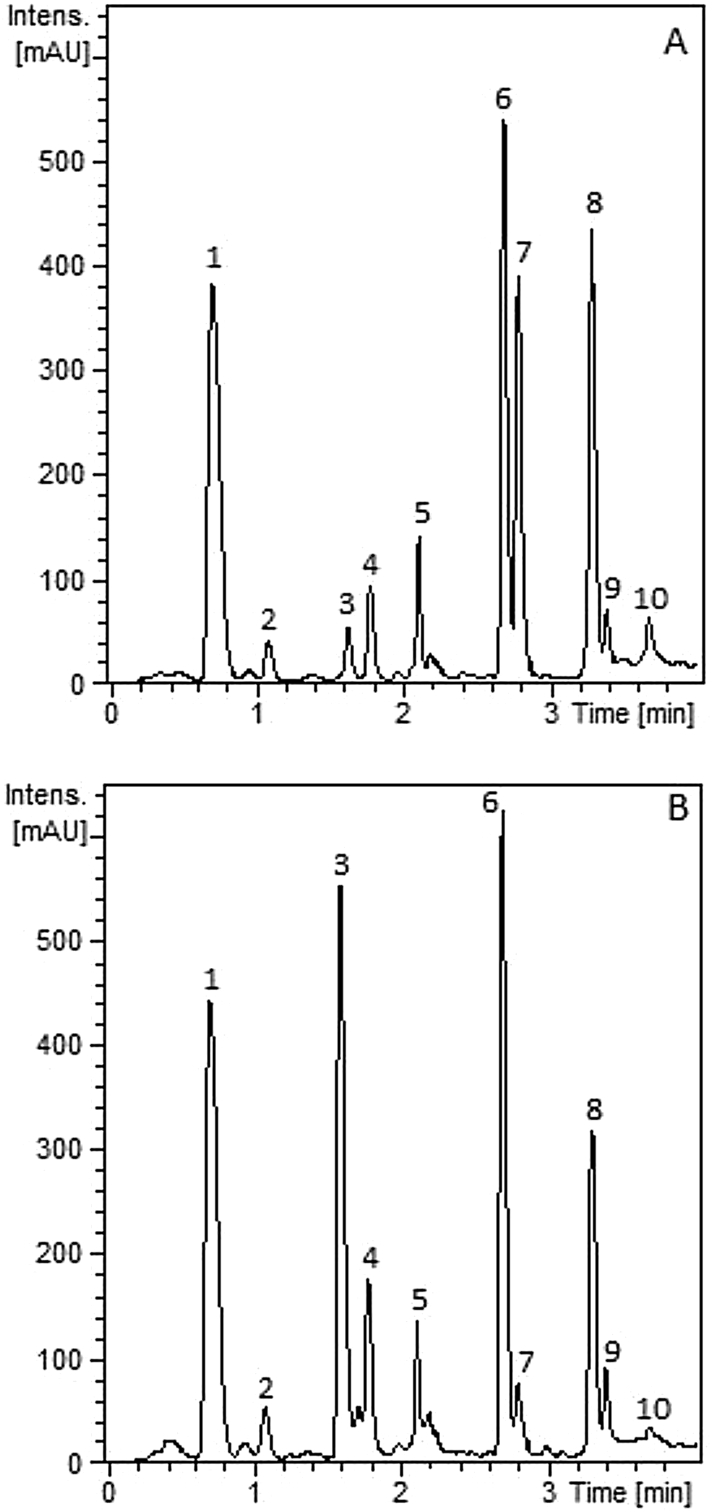
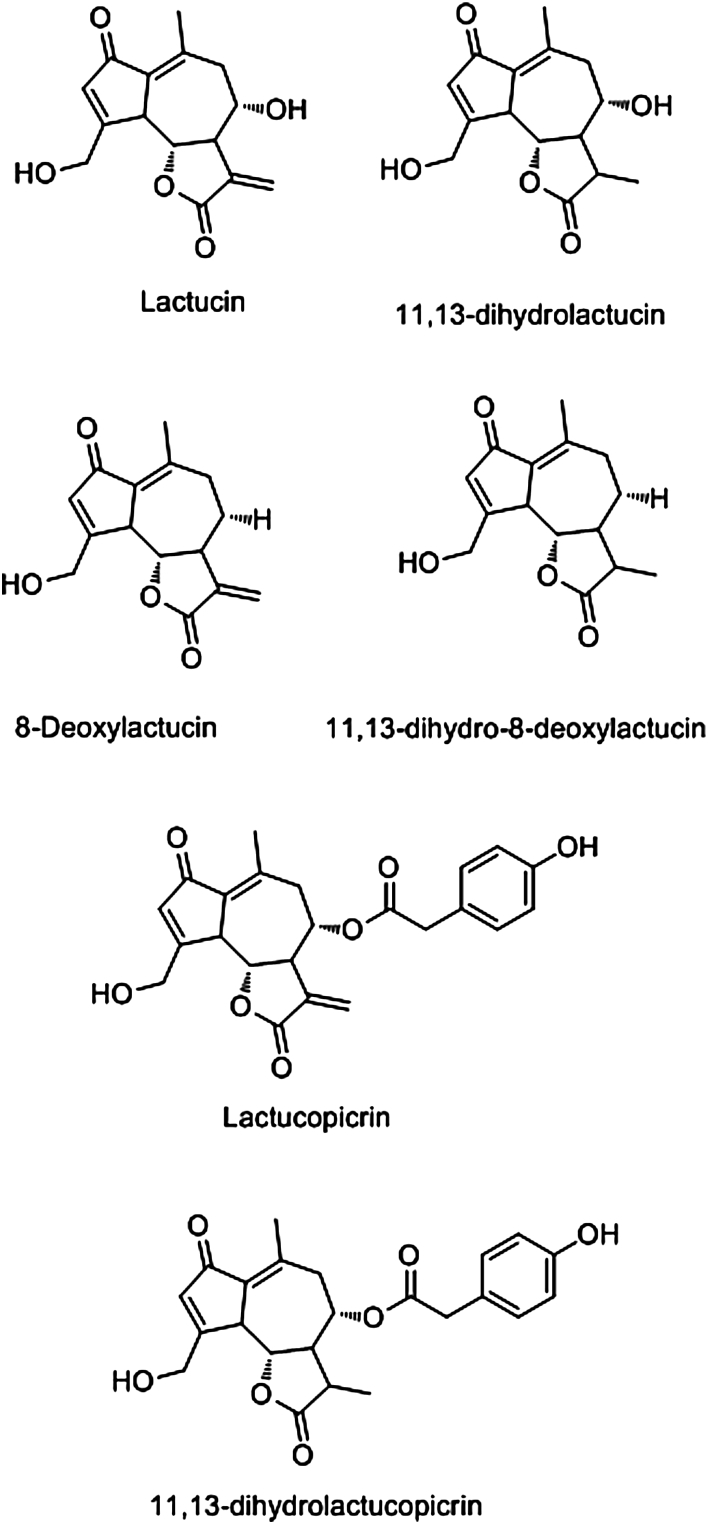
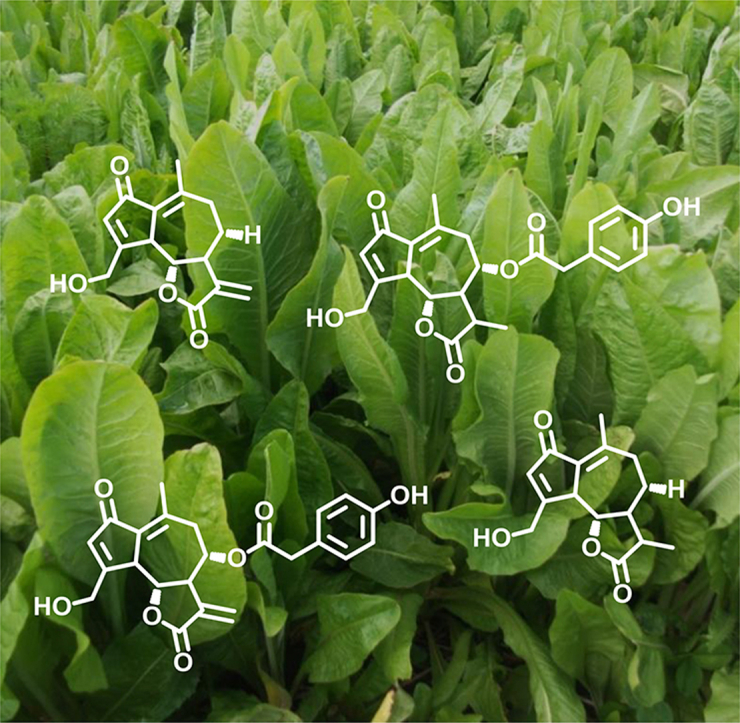
Chromatograms of the purified chicory extracts analysed by HPLC-MS are presented in Fig. 1. Chemical characterisations of detected molecules in two separate quantifications of each chicory extract are presented in Table 1. All peaks in both extracts were detected before 4 min. Identification of m/z ion fragments (positive and negative mode) by MS confirmed that a certain degree of hydrolysis occurred in the SL during the extraction process (Supplementary Table 1). Lactucin (LAC), 8-deoxylactucin (8-DOL) and lactucopicrin (LCP) were detected in extracts from both chicory cultivars, as well as their 11, 13-dihydro (DI)-derivatives: DI-LAC, DI-8-DOL and DI-LCP (Table 1, Fig. 2). In addition, four unknown compounds were detected in both chicory extracts. From the original leaf material, mean (±S.D.) total SL recovered were 9.4 (±1.2) g total SL/kg of dried leaves in Spadona and 11.7 (±1.3) g SL/kg of dried leaves in Puna II. Similar levels of total and individual SL and unknown compounds were detected between extracts, with the exception of DI-LAC and DI-8-DOL which were found in significantly higher concentrations in Puna II and Spadona extracts, respectively (Table 1).
Fig. 1.
Chromatograms obtained of purified extracts from chicory cv. Spadona (A) and cv. Puna II (B) used for in vitro assays with free-living and parasitic stages of Ostertagia ostertagi. Chromatograms are presented at the wavelength where isosbestic points were detected for each extract (271 nm for Spadona and 275 nm for Puna II). 3 = 11, 13-dihydro-lactucin; 4 = lactucin; 6 = 8-deoxy-lactucin; 7 = 11, 13-dihydro-8-deoxylactucin; 8 = 11, 13-dihydro-lactucopicrin; 9 = lactucopicrin. Peaks 1, 2, 5 and 10 = unknown.
Table 1.
Chemical characterisation by high-performance liquid chromatography-mass spectrometry of purified extracts from leaves of chicory cv. Spadona and cv. Puna II used for in vitro assays with free-living and parasitic stages of Ostertagia ostertagi.
| Extract |
Spadona |
Puna II |
Pairwise t-test | |||||
|---|---|---|---|---|---|---|---|---|
| Peak | Compound | % Mean total peak areaa | μg Compound/mg dry extract |
% Mean total peak areaa | μg Compound/mg dry extract |
|||
| Q1b | Q2b | Q1b | Q2b | |||||
| 1 | U1 | 27.4 | 143.0 | 117.0 | 26.4 | 137.7 | 106.7 | N.S. |
| 2 | U2 | 2.1 | 9.6 | 9.4 | 2.4 | 17.7 | 17.3 | N.S. |
| 3 | DI-LAC | 2.3 | 10.7 | 11.4 | 19.9 | 105.3 | 87.2 | **** |
| 4 | LAC | 4.0 | 19.7 | 18.5 | 6.3 | 37.3 | 29.8 | N.S. |
| 5 | U3 | 5.1 | 25.7 | 23.9 | 5.0 | 30.5 | 23.9 | N.S. |
| 6 | 8-DOL | 20.4 | 105.7 | 81.2 | 20.6 | 108.6 | 84.2 | N.S. |
| 7 | DI-8-DOL | 14.8 | 76.5 | 61.6 | 3.0 | 20.4 | 17.4 | *** |
| 8 | DI-LCP | 17.0 | 87.8 | 74.8 | 11.9 | 65.2 | 50.9 | N.S. |
| 9 | LCP | 2.4 | 11.1 | 12.2 | 2.8 | 19.5 | 23.5 | N.S. |
| 10 | U4 | 4.5 | 22.3 | 21.6 | 1.7 | 13.5 | 12.7 | N.S. |
| Total SL | 60.9 | 311.5 | 259.7 | 64.5 | 356.3 | 293.0 | N.S. | |
| Unknown | 39.1 | 200.6 | 171.9 | 35.5 | 199.4 | 160.6 | N.S. | |
Q: quantification; U: Unknown; SL: sesquiterpene lactones; LAC: Lactucin; DI-LAC: 11, 13-dihydrolactucin; 8-DOL: 8-deoxylactucin; DI-8-DOL: 11, 13-dihydro-8-deoxylactucin; LCP: Lactucopicrin; DI-LCP: 11, 13-dihydro-lactucopicrin.
N.S: p > 0.05; ***P = 0.01; ****P < 0.0001.
Area percentage of the respective peak of the total peak area in the chromatograms presented in Fig. 1.
Quantification of individual compounds was performed using an external standard curve with a pure lactucopicrin standard.
Fig. 2.
Structures of sesquiterpene lactones detected in purified extracts from chicory cv. Spadona and cv. Puna II and tested in vitro against free-living and parasitic stages of Ostertagia ostertagi.
3.2. LFIA
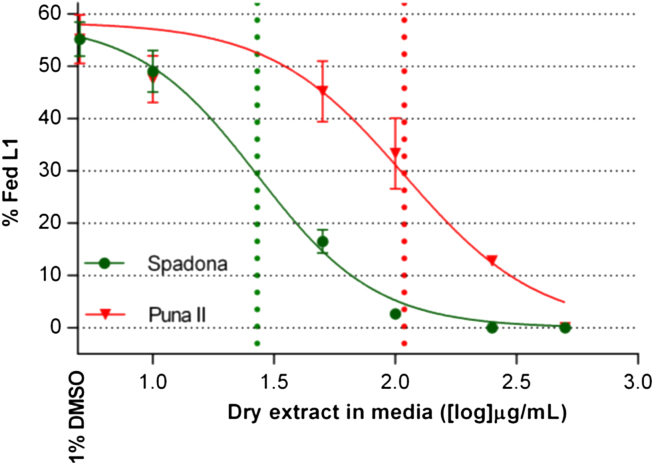
In negative controls, 55.2% (±3.3%) of L1 incubated with 1% DMSO had fed at the end of the incubation. All fed larvae of the negative controls were motile while all unfed larvae were dead. In contrast, all L1 incubated with IVM (positive control) were dead and unfed. Larvae exposed to SL-containing extracts, however, showed a distinct pattern as all fed L1 were motile while unfed larvae were either motile or dead. No morphological damage was observed in L1 exposed to the SL-containing extracts at any tested concentration. Dose-response curves obtained in the LFIA with the tested extracts are depicted in Fig. 3. Larvae incubated with the lowest concentration of SL-containing extracts had similar feeding percentage as observed in the negative controls, while the highest concentration tested (500 μg dry extract/mL) of both extracts induced a total suppression of larval feeding. Nevertheless, at intermediate concentrations, Spadona-extract exerted a remarkably higher potency than Puna II-extract, tested at equal concentrations, illustrated by significantly lower EC50 values (EC50 [95% confidence interval] in μg dry extract/mL: Spadona = 26.9 [22.3–32.4] and Puna II = 108.7 [80.9–146.2], P < 0.0001).
Fig. 3.
Dose–response curves obtained in the larval feeding inhibition assay with Ostertagia ostertagi L1 incubated at different concentrations of SL-containing extracts ([log] μg dry extract/ml) from two chicory cultivars. Error bars represent S. D. between replicates (n = 3 X concentration per extract). Data points with no error bars indicate that the variation among values was 0 or close to 0. Doted vertical lines represent EC50 values for each extract.
3.3. LEIA
No significant differences were observed in the exsheathment rates between SL-containing extracts at all tested concentrations and negative controls (P > 0.05). All negative control L3 (pre-incubated with 1% DMSO) were exsheathed after 45 min of incubation with the exsheathment solution. Similarly, >99% of L3 exposed to any of the SL-containing extracts were exsheathed after 45 min of incubation.
3.4. AMIA
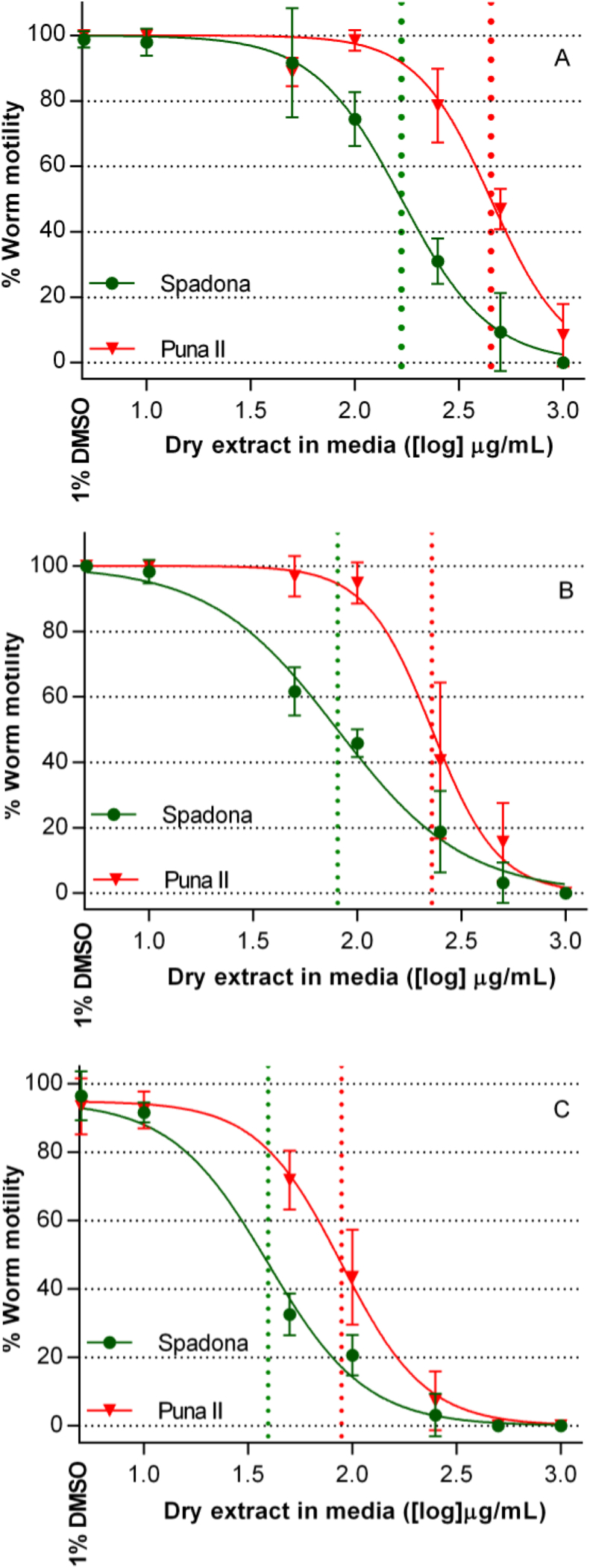
In negative controls, a mean worm motility of 100% was observed at 6 and 24 h of incubation with 1% DMSO. After 48 h of incubation, mean (±S.D.) worm motility percentage in the negative controls was 94.8% (±2.6%). Dose-response curves obtained in the AMIAs at 6, 24 and 48 h of incubation are presented in Fig. 4. Mean motility of worms incubated with the lowest concentration of both SL-containing extracts was not different from negative controls at any time point. In contrast, and after 6 h of incubation with the highest concentration, Spadona and Puna II extracts induced a paralysis in 100 and 92% of the incubated worms, respectively. However, at intermediate concentrations Spadona-extract induced a greater paralysis in exposed worms, reflected in significantly lower EC50 values at all time points, as compared with worms incubated with the same concentrations of Puna II-extract (P < 0.0001, Table 2). In addition, a time-dependent effect of the inhibitory activity of the SL-containing extracts was observed, represented as decreasing EC50 values for both extracts as the incubation period progressed.
Fig. 4.
Dose–response curves obtained in adult motility inhibition assays with Ostertagia ostertagi at 6 h (A), 24 h (B) and 48 h (C) after incubation with different concentrations of SL-containing extracts ([log] μg dry extract/ml) from two chicory cultivars. Each data point in the graphs represents the mean motility percentage of 8 replicates (n = 4–6 adult worms per replicate) for each concentration obtained in two independent assays. Error bars represent S.D. between replicates. Data points with no error bars indicate that the variation among values was 0 or close to 0. Doted vertical lines represent EC50 values for each chicory extract.
Table 2.
Effective concentration able to induce 50% inhibition in worm motility (EC50) by purified extracts from leaves of chicory cv. Spadona and cv. Puna II at different incubation times in adult motility inhibition assays with Ostertagia ostertagi.
| Incubation time | 6 h | 24 h | 48 h | |||
|---|---|---|---|---|---|---|
| Extract | Spadona | Puna II | Spadona | Puna II | Spadona | Puna II |
| EC50 (μg dry extract/mL) | 167.6∗∗∗∗ | 449.4 | 80.3∗∗∗∗ | 228.2 | 35.7∗∗∗∗ | 82.4 |
| 95% CI | 146.7–191.3 | 406.1–497.4 | 70.5–91.6 | 198.6–262.1 | 31.5–40.3 | 72.3–93.4 |
| R2 | 0.96 | 0.95 | 0.97 | 0.95 | 0.98 | 0.96 |
CI = confidence interval; R2 = goodness-of-fit; ∗∗∗∗P < 0.0001.
3.5. Electron microscopy
At the time of collection for electron microscopy (after 24 h of incubation) all worms incubated at 1% DMSO were motile and all worms incubated with the SL-containing extracts were dead. Examinations by SEM showed no noticeable differences between negative control nematodes and worms exposed to SL-containing extracts, with no obvious structural damage in the buccal opening or cuticle of worms exposed to Spadona-extract (Supplementary Fig. 3a, b) or Puna II-extract. Ultrastructural examination of thin sections of O. ostertagi adults by TEM did not demonstrate substantial differences between nematodes. Negative control nematodes and nematodes incubated with Spadona-extract had integral exterior borders of the cuticle and an intact basal layer and hypodermis. The only apparent difference was a slight decolouration and subtle erosion of the epicuticle in worms incubated with Spadona-extract (Supplementary Fig. 3c).
4. Discussion
Here, we have demonstrated direct and dose-dependent anti-parasitic effects of SL-containing extracts of forage chicory on the motility and feeding of O. ostertagi adults and L1, respectively. In contrast, no effects were observed on the exsheathment of L3 at any of the tested concentrations. Marked differences in anthelmintic activity were detected in SL-containing extracts from two forage chicory cultivars, with Spadona-extract exerting higher anti-parasitic effects compared with Puna II-extract. Despite the similar content of total SL and unknown molecules in the two tested extracts, the increased potency of Spadona-extract may be associated with its distinctive composition of individual compounds, which is further discussed below.
In the AMIA, nonetheless the rapid lethal effect of high concentrations of SL-containing extracts on adult worms, EM investigations did not reveal any morphological damage of SL-exposed worms, with the exception of a decoloured and slightly eroded epicuticle, suggesting a possible early stage of cuticle degeneration. These results are in contrast with studies describing marked disruptions in the cuticle and ultrastructure of nematodes exposed to other bioactive plant compounds like cysteine proteinases and condensed tannins (Stepek et al., 2004, Brunet et al., 2011, Martínez-Ortíz-de-Montellano et al., 2013, Williams et al., 2014a, Williams et al., 2014b). Our findings therefore suggest that the mode of action of SL is different from that of the above mentioned PSM and this needs to be further explored.
In the LFIA, mean percentage of fed larvae in the negative controls (1% DMSO in PBS) were similar to published reports of LFIA with O. ostertagi L1 using 100% PBS as negative control medium (Novobilský et al., 2011). All unfed larvae in the negative controls were dead and similar low control values were observed by the authors in preliminary studies with L1 from the same O. ostertagi strain, but the reasons for this are unknown. In larvae exposed to SL, both extracts demonstrated a dose-dependent inhibitory effect on the feeding of O. ostertagi L1. Nonetheless, Spadona-extract inhibited larval feeding at much lower concentrations than Puna II-extract, which correlates with results from the AMIA. EC50 values obtained in the LFIA with both chicory extracts were much lower than those observed in the AMIA, confirming that inhibition of larval feeding required a much lower concentration than the inhibition of adult worm motility, as observed previously in studies with IVM (Geary et al., 1993, Gill et al., 1995). This indicates that the inhibition of the pharyngeal muscles appears to occur at a much lower extract concentration than the inhibition of motility, suggesting that pharyngeal muscles could be a more sensitive target for SL.
In the LEIA, SL-containing extracts did not affect the exsheathment kinetics of exposed O. ostertagi L3. Exsheathment is a precondition for the later embedding of L3 in the abomasal mucosa and our results agree with in vivo findings that showed no effect of forage chicory (cv. Grasslands Puna) on the establishment of Teladorsagia circumcincta L3 in infected lambs (Tzamaloukas et al., 2005). In the LEIA, all L3 incubated with the highest concentration of both chicory extracts were alive and motile at the end of the assay. These results are further supported by preliminary data from our group indicating that there is no effect of the same SL-containing extracts on the motility of O. ostertagi L3, even after incubation for 24–48 h with very high concentrations (2000 μg dry extract/mL, data not published).
At sampling, chicory plants were at the vegetative stage and leaves from both cultivars were hand-picked on the same occasion in order to standardise the collection procedure. Early findings by Rees and Harborne (1985) described that free SL are present in the latex of chicory roots and leaves and that levels of individual SL vary in different parts of the chicory plant according to the growth stage. Nevertheless, these findings need to be confirmed in different chicory cultivars as well as by screening of other sections of the aerial part of the plant (i.e. stems and flowers), which can also be grazed by cattle (Peña-Espinoza et al., unpublished observations). With respect to the quantification of compounds in the extracts, this was performed using an external calibration curve with pure lactucopicrin, the only SL of chicory commercially available as a pure standard at the time of the experiments. Our approach was a quantification of the compounds in the extracts (unknown concentrations) by comparison with the calibration curve (known concentrations), using peak areas and assuming similar chromatographic properties in increasing concentrations of compounds in the extract and in the standard. A quantification using pure standards of all SL and their dihydro-derivatives would have increased the sensitivity of the method. However, this was an initial attempt to understand the SL profile in these particular extracts using, as a standard, a compound actually present in the extracts (lactucopicrin). For comparison, previous studies have added santonin (another SL not present in chicory) as internal standard for quantification of SL in chicory (Ferioli and D'Antuono, 2012, Graziani et al., 2015).
Our results are the first evidence of anthelmintic activity of SL-containing extracts against larval feeding and adult motility, contributing to expand our knowledge on the role of SL in the anti-parasitic properties of chicory. Previously, Molan et al. (2003) were the first to suggest the potential anthelmintic effect of SL in chicory and reported the inhibitory effects of a crude extract from chicory roots against free-living larvae. However, these authors did not report the SL characterisation of the tested extract or the chicory cultivar used. More recently, Foster et al. (2011) described the dose-dependent inhibition of nematode egg hatching by free SL-containing extracts from forage chicory cv. Grasslands Puna and cv. Forage Feast. These authors reported the presence of only the three major SL of chicory in the tested extracts and detected an increased anthelmintic effect of Grasslands Puna-extract. In contrast to the present investigations, Foster et al. (2011) used much larger test concentrations (0–10 mg dry extract/mL). In our study the range of tested concentrations was based on preliminary in vitro studies with O. ostertagi by the authors (data not published) and was selected with the aim to detect a dose-dependent response. Scarce literature exists on the metabolism of SL from chicory in the digestive tract of ruminants and therefore, at the moment, it is difficult to accurately estimate the concentration of dietary SL that could reach the abomasum, the predilection site of O. ostertagi in cattle. However, in a preliminary study we have observed that 4 months-old calves are able to consume daily up to 3 kg dry matter of chicory silage (with 5.3 g SL/kg dry matter), and assuming an abomasum volume of 5 L (Nickel et al., 1979) and that SL are not inactivated in the forestomach, this would roughly correspond to a maximum of 3.18 mg SL/mL in the abomasum (Peña-Espinoza et al., unpublished results).
In our study, the three well-known guaianolide SL of chicory were also detected in leaf extracts of cv. Spadona and cv. Puna II: lactucin, 8-deoxylactucin and lactucopicrin, as reported for other chicory cultivars (Rees and Harborne, 1985, Price et al., 1990, Foster et al., 2011, Ferioli and D'Antuono, 2012, Graziani et al., 2015). Furthermore, the 11, 13-dihydro-derivatives of the mentioned SL were also detected: 11, 13-dihydro-lactucin, 11, 13-dihydro-8-deoxylactucin and 11, 13-dihydro-lactucopicrin, which have been also described in salad chicory cultivars (Ferioli and D'Antuono, 2012, Wulfkuehler et al., 2014, Ferioli et al., 2015). Spadona and Puna II extracts induced dose-dependent anthelmintic effects in the LFIA and the AMIA, suggesting that anti-parasitic compounds were present in both extracts. In addition, unknown compounds were also detected in both chicory extracts, which may have contributed to the observed anthelmintic effects. The HPLC-MS analyses revealed similar quantities of unknown compounds and of total and individual SL between the tested extracts, except for 11, 13-dihydro-lactucin and 11, 13-dihydro-8-deoxylactucin, which were found in significantly higher concentrations in Puna II and Spadona extracts, respectively. Based on results from the in vitro assays, our data suggest that the increased concentration of 11, 13-dihydro-8-deoxylactucin in Spadona-extract (3.6-fold increase) correlates, to some extent, with the increased potency and lower EC50 values exerted by Spadona-extract in the LFIA (3.3-fold lower EC50) and in the AMIA (2.6-fold lower EC50), in comparison with Puna II-extract. These findings suggest that 11, 13-dihydro-8-deoxylactucin is a likely candidate to explain the higher anthelmintic effects of the Spadona-extract. In contrast, 11, 13-dihydro-lactucin, with increased levels in the less-potent Puna II-extract, seems not to be particularly related with an anthelmintic effect. However, unknown compounds were also present in the extract and no pure SL were tested in our study. Therefore we cannot confirm a direct relation between concentration of a particular SL and anthelmintic effect at the moment. To confirm these findings, further fractionation, isolation and testing of individual molecules in the extracts would identify the most active compound(s). Nevertheless, and to the best of our knowledge, this is the first report of the SL profiles of forage chicory cv. Spadona and cv. Puna II and the first study describing the in vitro anti-parasitic activity of forage chicory extracts containing a distinctive profile of dihydro-derivatives of SL. Previously, Foster et al. (2011) linked the higher in vitro activity of Grassland Puna-extract to the higher content of 8-deoxylactucin, which in our study was present in similar quantities in Spadona and Puna II extracts. This suggests that, if SL are responsible for the anti-parasitic effects of chicory in vivo, only some SL may be considered as candidates for novel anthelmintic compounds and for selection of forage chicory cultivars with increased anthelmintic activity.
It is known that guaianolide SL can exert potent cytotoxic activities, which have been mainly attributed to the presence of an α-methylene (CH2) functional group linked to the γ-lactone in the SL molecule (Simonsen et al., 2013). This α-methylene group is known to react with sulfhydryl (thiol) groups in cysteine and cysteine-containing peptides by a Michael-type addition, affecting cell signalling, cell replication, apoptosis and mitochondrial respiration (Schmidt, 2006, Simonsen et al., 2013). As an example, it has been reported that natural SL can reduce the intracellular concentration of free glutathione in Leishmania mexicana mexicana, which led to a toxic intra-cellular accumulation of reactive oxygen species and blocked cell proliferation (Barrera et al., 2013). However, dihydro-derivatives of SL lack this α-methylene group and still can exert biological effects (Lee et al., 1977, Schmidt, 2006, Ghantous et al., 2010), which clearly indicates that there is not just one single biological mechanism of effect for all SL. Ren et al. (2005) reported that 11, 13-β-dihydro-lactucopicrin and 11, 13-β-dihydro-lactucin from Mulgedium tatarica, missing the α-methylene group but having an ester group at C-8 instead, exerted a higher toxicity towards human nasopharyngeal and liver cancer cells. Currently, the molecular mechanisms of action of SL and their dihydro-derivatives towards nematodes are unknown and need to be further investigated. Moreover, it is uncertain whether the anti-parasitic effects observed in our study correspond to the action of single (known and/or unknown) molecules or a synergistic effect between different plant compounds, as indicated by recent findings with condensed tannins and flavonoids (Klongsiriwet et al., 2015).
Our study indicates that the tested compounds isolated from forage chicory could affect O. ostertagi populations in cattle by reducing the feeding and motility/survival of worms. In addition, the higher anti-parasitic effects exerted by Spadona-extract, and the dose-dependent effect of both extracts, suggest that potential in vivo effects could be dependent on the chicory cultivar under investigation and the concentration of active molecules that reach the abomasum. However, compounds present in the tested extracts may not be the same molecules available at the site of infection of O. ostertagi. Although little is known about the pharmacokinetics of SL in ruminants, Ferreira and Gonzalez (2008) demonstrated that artemisinin, a purified SL widely used to treat malaria in humans, given to goats as an oral capsule (23 mg artemisinin/kg body weight), was partly metabolised in the rumen and was detected as dihydroartemisinin in plasma after 4 h of treatment. Moreover, in vivo trials in sheep indicate that the active compounds do not seem to be degraded or inactivated in the rumen and can reach the abomasum to exert their anti-parasitic activity (Scales et al., 1994, Tzamaloukas et al., 2005). Yet, extensive research is needed to elucidate the fate of dietary SL and other bioactive plant compounds in the digestive tract of ruminants.
In conclusion, we have demonstrated that SL-containing extracts from forage chicory can inhibit larval feeding and exert direct anti-parasitic effects against adult stages of O. ostertagi, whereas no effects were observed on larval exsheathment. We observed substantial differences in the anti-parasitic activity between extracts of two forage chicory cultivars and this may be related to variations in the concentration of individual SL. The distinctive anti-parasitic activities of extracts from different cultivars with particular molecular profiles can help to target the identification of the responsible compound(s). However, further studies are warranted not only to confirm the PSM/compound(s) responsible for the anti-parasitic activity in the tested chicory extracts, but also to assess the in vivo anthelmintic effects of a chicory-based diet in cattle infected with gastrointestinal nematodes.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
The EMIDA ERA-NET project “Coping with anthelmintic resistance in ruminants” (CARES; 3405-11-0430/32) and CONICYT Chile (Becas Chile Scholarship) are acknowledged for financial support. We gratefully acknowledge to Dr. Federico Ferioli from University of Bologna, Italy, for guidance in the isolation of sesquiterpene lactones from chicory leaves. We also thank the anonymous reviewers that helped to improve the quality and clarity of the original draft. Likewise, Sahar Mirsharghi, Olivier Desrues, Hans Skåning, Leif Eiersted and Esben Møller Xu are thanked for technical guidance and valuable help.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ijpddr.2015.10.002.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Barrera P., Sülsen V.P., Lozano E., Rivera M., Beer M.F., Tonn C., Martino V.S., Sosa M.A. Natural sesquiterpene lactones induce oxidative stress in Leishmania mexicana. Evid. Based. Complement. Altern. Med. 2013;2013:163404. doi: 10.1155/2013/163404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff T.A., Kelley C.J., Karchesy Y., Laurantos M., Nguyen-Dinh P., Arefi A.G. Antimalarial activity of lactucin and lactucopicrin: sesquiterpene lactones isolated from Cichorium intybus L. J. Ethnopharmacol. 2004;95:455–457. doi: 10.1016/j.jep.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Brunet S., Fourquaux I., Hoste H. Ultrastructural changes in the third-stage, infective larvae of ruminant nematodes treated with sainfoin (Onobrychis viciifolia) extract. Parasitol. Int. 2011;60:419–424. doi: 10.1016/j.parint.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Cavin C., Delannoy M., Malnoe A., Debefve E., Touché A., Courtois D., Schilter B. Inhibition of the expression and activity of cyclooxygenase-2 by chicory extract. Biochem. Biophys. Res. Commun. 2005;327:742–749. doi: 10.1016/j.bbrc.2004.12.061. [DOI] [PubMed] [Google Scholar]
- Charlier J., van der Voort M., Kenyon F., Skuce P., Vercruysse J. Chasing helminths and their economic impact on farmed ruminants. Trends Parasitol. 2014;30:361–367. doi: 10.1016/j.pt.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Christensen C., Barnes E., Nansen P., Roepstorff A., Slotved H. Experimental Oesophagostomum dentatum infection in the pig: worm populations resulting from single infections with three doses of larvae. Int. J. Parasitol. 1995;25:1491–1498. doi: 10.1016/0020-7519(95)00085-2. [DOI] [PubMed] [Google Scholar]
- Demeler J., Küttler U., von Samson-Himmelstjerna G. Adaptation and evaluation of three different in vitro tests for the detection of resistance to anthelmintics in gastro intestinal nematodes of cattle. Vet. Parasitol. 2010;170:61–70. doi: 10.1016/j.vetpar.2010.01.032. [DOI] [PubMed] [Google Scholar]
- Ferioli F., D'Antuono L.F. An update procedure for an effective and simultaneous extraction of sesquiterpene lactones and phenolics from chicory. Food Chem. 2012;135:243–250. [Google Scholar]
- Ferioli F., Manco M., D'Antuono L. Variation of sesquiterpene lactone and phenolic content in chicory and endive germplasm. J. Food Compos. Anal. 2015;39:77–86. [Google Scholar]
- Ferreira J., Gonzalez J. Chemical and biological stability of artemisinin in bovine rumen fluid and its kinetics in goats (Capra hircus) Braz. J. Vet. Parasitol. 2008;17:103–109. [PubMed] [Google Scholar]
- Foster J., Cassida K., Turner K. In vitro analysis of the anthelmintic activity of forage chicory (Cichorium intybus L.) sesquiterpene lactones against a predominantly Haemonchus contortus egg population. Vet. Parasitol. 2011;180:298–306. doi: 10.1016/j.vetpar.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Fox M.T. Pathophysiology of infection with Ostertagia ostertagi in cattle. Vet. Parasitol. 1993;46:143–158. doi: 10.1016/0304-4017(93)90055-r. [DOI] [PubMed] [Google Scholar]
- Geary T., Sims S., Thomas E., Vanover L., Davis J., Winterrowd C., Klein R., Ho N., Thompson D. Haemonchus contortus: ivermectin-induced paralysis of the pharynx. Exp. Parasitol. 1993;77:88–96. doi: 10.1006/expr.1993.1064. [DOI] [PubMed] [Google Scholar]
- Gershenzon J., Dudareva N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007;3:408–414. doi: 10.1038/nchembio.2007.5. [DOI] [PubMed] [Google Scholar]
- Ghantous A., Gali-Muhtasib H., Vuorela H., Saliba N.A., Darwiche N. What made sesquiterpene lactones reach cancer clinical trials? Drug Discov. Today. 2010;15:668–678. doi: 10.1016/j.drudis.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Gill J., Redwin J., Van Wyk J., Lacey E. Avermectin inhibition of larval development in Haemonchus contortus – effects of ivermectin resistance. Int. J. Parasitol. 1995;25:463–470. doi: 10.1016/0020-7519(94)00087-5. [DOI] [PubMed] [Google Scholar]
- Githiori J.B., Athanasiadou S., Thamsborg S.M. Use of plants in novel approaches for control of gastrointestinal helminths in livestock with emphasis on small ruminants. Vet. Parasitol. 2006;139:308–320. doi: 10.1016/j.vetpar.2006.04.021. [DOI] [PubMed] [Google Scholar]
- Graziani G., Ferracane R., Sambo P., Santagata S., Nicoletto C., Fogliano V. Profiling chicory sesquiterpene lactones by high resolution mass spectrometry. Food Res. Int. 2015;67:193–198. [Google Scholar]
- Heckendorn F., Häring D.A., Maurer V., Senn M., Hertzberg H. Individual administration of three tanniferous forage plants to lambs artificially infected with Haemonchus contortus and Cooperia curticei. Vet. Parasitol. 2007;146:123–134. doi: 10.1016/j.vetpar.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Hoste H., Torres-Acosta J.F.J., Sandoval-Castro C.A., Mueller-Harvey I., Sotiraki S., Louvandini H., Thamsborg S.M., Terrill T.H. Tannin containing legumes as a model for nutraceuticals against digestive parasites in livestock. Vet. Parasitol. 2015;212:5–17. doi: 10.1016/j.vetpar.2015.06.026. [DOI] [PubMed] [Google Scholar]
- Jackson F., Hoste H. In vitro methods for the primary screening of plant products for direct activity against ruminant gastrointestinal nematodes. In: Vercoe P.E., Makkar H.P.S., Schlink A.C., editors. In Vitro Screening of Plant Resources for Extra-nutritional Attributes in Ruminants: Nuclear and Related Methodologies. FAO/International Atomic Energy Agency. Springer; Dordrecht: 2010. pp. 25–45. [Google Scholar]
- Klongsiriwet C., Quijada J., Williams A.R., Mueller-Harvey I., Williamson E.M., Hoste H. Synergistic inhibition of Haemonchus contortus exsheathment by flavonoid monomers and condensed tannins. Int. J. Parasitol. Drugs Drug Resist. 2015;5:127–134. doi: 10.1016/j.ijpddr.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K., Ibuka T., Rong-Yang W., Geissman T. Structure-antimicrobial activity relationships among the sesquiterpene lactones and related compounds. Phytochemistry. 1977;16:1177–1181. [Google Scholar]
- Marley C., Cook R., Keatinge R., Barrett J., Lampkin N. The effect of birdsfoot trefoil (Lotus corniculatus) and chicory (Cichorium intybus) on parasite intensities and performance of lambs naturally infected with helminth parasites. Vet. Parasitol. 2003;112:147–155. doi: 10.1016/s0304-4017(02)00412-0. [DOI] [PubMed] [Google Scholar]
- Martínez-Ortíz-de-Montellano C., Arroyo-López C., Fourquaux I., Torres-Acosta J.F.J., Sandoval-Castro C.A., Hoste H. Scanning electron microscopy of Haemonchus contortus exposed to tannin-rich plants under in vivo and in vitro conditions. Exp. Parasitol. 2013;133:281–286. doi: 10.1016/j.exppara.2012.11.024. [DOI] [PubMed] [Google Scholar]
- Molan A.L., Duncan A.J., Barry T.N., McNabb W.C. Effects of condensed tannins and crude sesquiterpene lactones extracted from chicory on the motility of larvae of deer lungworm and gastrointestinal nematodes. Parasitol. Int. 2003;52:209–218. doi: 10.1016/s1383-5769(03)00011-4. [DOI] [PubMed] [Google Scholar]
- Nansen P. Current and future prospects for control of ostertagiasis in northern Europe — examples from Denmark. Vet. Parasitol. 1993;46:3–21. doi: 10.1016/0304-4017(93)90044-n. [DOI] [PubMed] [Google Scholar]
- Nickel R., Schummer A., Seiferle S. second ed. Springer-Verlag; Berlin Heidelberg: 1979. The Viscera of the Domestic Mammals. [Google Scholar]
- Novobilský A., Mueller-Harvey I., Thamsborg S.M. Condensed tannins act against cattle nematodes. Vet. Parasitol. 2011;182:213–220. doi: 10.1016/j.vetpar.2011.06.003. [DOI] [PubMed] [Google Scholar]
- O'Grady J., Kotze A.C. Haemonchus contortus: in vitro drug screening assays with the adult life stage. Exp. Parasitol. 2004;106:164–172. doi: 10.1016/j.exppara.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Paolini V., Fouraste I., Hoste H. In vitro effects of three woody plant and sainfoin extracts on 3rd-stage larvae and adult worms of three gastrointestinal nematodes. Parasitology. 2004;129:69–77. doi: 10.1017/s0031182004005268. [DOI] [PubMed] [Google Scholar]
- Price K., DuPont M., Shepherd R., Chan H., Fenwick G. Relationship between the chemical and sensory properties of exotic salad crops−coloured lettuce (Lactuca sativa) and chicory (Cichorium intybus) J. Sci. Food Agric. 1990;53:185–192. [Google Scholar]
- Rees S., Harborne J. The role of sesquiterpene lactones and phenolics in the chemical defence of the chicory plant. Phytochemistry. 1985;24:2225–2231. [Google Scholar]
- Ren Y., Zhou Y., Chen X., Ye Y. Discovery, structural determination and anticancer activities of lactucin-like guaianolides. Lett. Drug Des. Discov. 2005;2:444–450. [Google Scholar]
- Roepstorff A., Nansen P. FAO Animal Heath Manual; Rome: 1998. Epidemiology, Diagnosis and Control of Helminth Parasites of Swine; pp. 56–60. [Google Scholar]
- Scales G.H., Knight T.L., Saville D.J. Effect of herbage species and feeding level on internal parasites and production performance of grazing lambs. N. Z. J. Agric. Res. 1994;38:237–247. [Google Scholar]
- Schmidt T.J. Structure-activity relationships of sesquiterpene lactones. Stud. Nat. Prod. Chem. 2006;33:309–392. [Google Scholar]
- Sessa R.A., Bennett M.H., Lewis M.J., Mansfield J.W., Beale M.H. Metabolite profiling of sesquiterpene lactones from Lactuca species. J. Biol. Chem. 2000;275:26877–26884. doi: 10.1074/jbc.M000244200. [DOI] [PubMed] [Google Scholar]
- Simonsen H., Weitzel C., Christensen S. Guaianolide sesquiterpenoids: pharmacology and biosynthesis. In: Ramawat K., Mérillon J., editors. Natural Products. Springer; Berlin Heidelberg: 2013. pp. 3069–3098. [Google Scholar]
- Stepek G., Behnke J.M., Buttle D.J., Duce I.R. Natural plant cysteine proteinases as anthelmintics? Trends Parasitol. 2004;20:322–327. doi: 10.1016/j.pt.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Tzamaloukas O., Athanasiadou S., Kyriazakis I., Jackson F., Coop R.L. The consequences of short-term grazing of bioactive forages on established adult and incoming larvae populations of Teladorsagia circumcincta in lambs. Int. J. Parasitol. 2005;35:329–335. doi: 10.1016/j.ijpara.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Waller P.J., Thamsborg S.M. Nematode control in “green” ruminant production systems. Trends Parasitol. 2004;20:493–497. doi: 10.1016/j.pt.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Williams A.R., Fryganas C., Ramsay A., Mueller-Harvey I., Thamsborg S.M. Direct anthelmintic effects of condensed tannins from diverse plant sources against Ascaris suum. PLoS One. 2014;9:e97053. doi: 10.1371/journal.pone.0097053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A.R., Ropiak H.M., Fryganas C., Desrues O., Mueller-Harvey I. Assessment of the anthelmintic activity of medicinal plant extracts and purified condensed tannins against free-living and parasitic stages of Oesophagostomum dentatum. Parasit. Vectors. 2014;7:518. doi: 10.1186/s13071-014-0518-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulfkuehler S., Gras C., Carle R. Influence of light exposure during storage on the content of sesquiterpene lactones and photosynthetic pigments in witloof chicory (Cichorium intybus L. var. foliosum Hegi) LWT−Food Sci. Technol. 2014;58:417–426. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.