Abstract
Sulfate-reducing bacteria (SRB) biofilm formed on metal surfaces can change the physicochemical properties of metals and cause metal corrosion. To enhance understanding of differential gene expression in Desulfovibrio vulgaris under planktonic and biofilm growth modes, a single-cell based RT-qPCR approach was applied to determine gene expression levels of 8 selected target genes in four sets of the 31 individual cells isolated from each growth condition (i.e., biofilm formed on a mild steel (SS) and planktonic cultures, exponential and stationary phases). The results showed obvious gene-expression heterogeneity for the target genes among D. vulgaris single cells of both biofilm and planktonic cultures. In addition, an increased gene-expression heterogeneity in the D. vulgaris biofilm when compared with the planktonic culture was also observed for seven out of eight selected genes at exponential phase, and six out of eight selected genes at stationary phase, respectively, which may be contributing to the increased complexity in terms of structures and morphology in the biofilm. Moreover, the results showed up-regulation of DVU0281 gene encoding exopolysaccharide biosynthesis protein, and down-regulation of genes involved in energy metabolism (i.e., DVU0434 and DVU0588), stress responses (i.e., DVU2410) and response regulator (i.e., DVU3062) in the D. vulgaris biofilm cells. Finally, the gene (DVU2571) involved in iron transportation was found down-regulated, and two genes (DVU1340 and DVU1397) involved in ferric uptake repressor and iron storage were up-regulated in D. vulgaris biofilm, suggesting their possible roles in maintaining normal metabolism of the D. vulgaris biofilm under environments of high concentration of iron. This study showed that the single-cell based analysis could be a useful approach in deciphering metabolism of microbial biofilms.
Keywords: single-cell analysis, gene expression, mild steel, biofilm, planktonic, D. vulgaris
Introduction
Propagation and metabolism of microorganisms on metal surfaces can change the physicochemical properties of these surface and cause the deterioration of metallic materials (Little et al., 1992; Thierry and Sand, 1995; Beech et al., 2000). Some representatives of microorganisms related to metal corrosion in both aerobic and anaerobic environments are the sulfate-reducing bacteria (SRB) (Voordouw, 1995; Hamilton, 2003; Enning and Garrelfs, 2014). The corrosive action of SRB is often associated with bacterial biofilms formed on the metal surfaces (Videla and Characklis, 1992; Dinh et al., 2004; Lewandowski and Beyenal, 2008). Although SRB biofilms have been extensively studied in the past decades and the previous studies have provided insights into SRB metal-reducing physiology and corrosion (Kolter and Losick, 1998; Liu et al., 2000; Fang et al., 2002; Hu, 2004), the differential gene expression between the biofilm and planktonic growth modes was less known until recent years. To accelerate understanding of the molecular mechanisms of SRB biofilm formation and maintenance, a whole-genome oligonucleotide microarray was used to examine differential expression patterns between planktonic populations and mature biofilm of Desulfovibrio vulgaris on a steel surface (Zhang et al., 2007). In addition, an integrated transcriptomic and proteomic analysis was recently conducted on mature D. vulgaris biofilm cells and compared to both batch and reactor planktonic populations (Clark et al., 2012). These results showed that the physiological differences between biofilm and planktonic cultures were caused by altered abundances of genes/proteins associated with carbon flow and extracellular structures; in addition, these studies have revealed the unique metabolic networks related to the formation and maintenance of D. vulgaris biofilm (Zhang et al., 2007; Clark et al., 2012). However, both these studies used population-averaged approach to describe biofilm behavior (Lazazzera, 2005), which did not take into consideration of potential differences (e.g., heterogeneous growth rates) between individual cells or different functional groups of cells in biofilms, and have resulted in possible biased conclusions (Beloin and Ghigo, 2005; An and Parsek, 2007; Stewart and Franklin, 2008; Hellweger and Bucci, 2009). To address this issue, alternative approaches that are able to capture differences between individual cells in micro-scale environments in biofilms need to be developed and evaluated (Lardon et al., 2011).
Recent studies have demonstrated that even homologous populations of microorganisms could have significant cell-to-cell gene expression heterogeneity (Lidstrom and Meldrum, 2003; Brehm-Stecher and Johnson, 2004; Strovas and Lidstrom, 2009; Stepanauskas, 2012; Blainey, 2013; Qi et al., 2014; Shi et al., 2014). In a previous study, the gene-expression levels of some selected genes of D. vulgaris were found to vary as much as 40 folds between cells of the same population, by using quantitative real-time reverse transcription-PCR (RT-qPCR) analysis (Qi et al., 2014). In the case of microbial biofilms, it is expected that such gene-expression heterogeneity between cells may be even more significant due to their obvious morphological, structural and even functional differences. Although works have been conducted in recent years using fluorescent reporter genes to visualize and measure microscale physiological heterogeneity in biofilms (Baty et al., 2000; Chai et al., 2008; Verplaetse et al., 2015), some limitations of the technique (i.e., requiring engineered strains; affecting the cell physiology by the energy required for expression of the reporter genes; and demanding oxygen for the activation of fluorescence) have restricted its application in biofilms (Stewart and Franklin, 2008). However, so far no single-cell based study has been conducted to analyze differential gene expression in D. vulgaris biofilm systems when compared with planktonic cells, and as thus potential gene-expression heterogeneity and its biological relevance in the D. vulgaris biofilm remains unclear.
In this study, with major aims to determine gene-expression heterogeneity between D. vulgaris cells grown in two different environments (i.e., biofilm and planktonic), and to further confirm the relationship between the selected genes and biofilm metabolism at a single-cell level, we applied a real-time reverse-transcription quantitative PCR (RT-qPCR) approach (Zhao et al., 2011; Shi et al., 2013; Qi et al., 2014). To do this, D. vulgaris biofilm was cultivated on mild steel (SS) slides to mimic microenvironments of metal corrosion. As an attempt to quantify heterogeneity levels of gene-expression between single cells in the D. vulgaris biofilm, the study could contribute to the further understanding of biofilm metabolism related to metal corrosion in natural environments.
Materials and Methods
Bacterial Strains and Growth Conditions
Desulfovibrio vulgaris subsp. vulgaris strain Hildenborough DSM 644 used in this study was obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (Braunschweig, Germany) and cultured in mineral medium as described by a previous publication (Zhang et al., 2007). Pure cultivation experiments were conducted in 200 mL serum bottles containing 70 mL of medium with lactate (38 mM) as the electron donor and sulfate (50 mM) as the electron acceptor according to previous publications (Zhang et al., 2006a; Qi et al., 2014). The D. vulgaris biofilm was formed on the surface of immersed SS slides in the same medium. SS slides (BST 503-2 SS slide, 0.7 cm × 5 cm and 1.2 mm thick) were purchased from BioSurface Technologies (Bozeman, USA). The SS slides were cleaned according to a previous study (Zhang et al., 2007) and then put into the medium to allow biofilm formation. The medium (with the immersed SS slides into it) was inoculated (5%) with a bacterial culture at middle-exponential phase (OD595 nm = 0.4), and all cultivation experiments were carried out at 35°C under anaerobic conditions, by using an anaerobic chamber (Fuma, Shanghai, China) for this purpose. These anaerobic conditions were achieved according to our previous publication (Qi et al., 2014). Planktonic cells were the non-attached floating cells under anaerobic cultivation conditions.
Determination of Total Protein and Carbohydrate Levels of Biofilm and Planktonic Cells
Cells collected from the biofilm and planktonic cultures were re-suspended in 1 mL of 50 mM PBS (pH 6.5). For 1 mL cell suspension, 10 μL of phenylmethylsulfonyl fluoride (PMSF) was added. After cell suspensions were repeatedly frozen in liquid nitrogen and thawed at 25°C room temperature for three times, the suspended cells were broken by ultrasonication with an ultrasonic disrupter (Scientz, Ningbo, China) with ultrasonic amplitude transformer probe. The conditions for ultrasonic disruption was 5 s working time, 5 s interval time, 10 min total time under 60% ultrasonic power. Protein and carbohydrate levels were measured for the samples to confirm the growth status (biofilm vs. planktonic). The carbohydrate concentrations were measured using the anthrone-sulfuric acid colorimetry with sucrose as standard (Roe, 1954, 1955). Protein concentrations were determined with the Bradford assay and bovine serum albumin as the standard (Bradford, 1976) (Supplementary Figure S1).
Selection of Target Genes for Single Cell RT-QPCR Analysis
Based on the functions of target genes and the relationships between genes and the D. vulgaris biofilm, 8 target genes (i.e., DVU0281, DVU0434, DVU0588, DVU1340, DVU1397, DVU2571, DVU2410 and DVU3062) were selected for the following single-cell analysis (Supplementary Table S1). The functions of the 8 target genes and 3 candidate internal control genes in the D. vulgaris biofilm have been examined in previous studies, and the results are presented in Table 1. The gene expression levels of DVU0588, DVU0434, and DVU2410 under biofilm-growth conditions were down-regulated when compared to its planktonic culture (Jenney et al., 1999; Zhang et al., 2007); while their expression levels in the biofilm on the glass slides were up-regulated in comparison with planktonic culture in another transcriptomic and proteomic analysis (Clark et al., 2012). In addition, it was proposed that DVU0281 might be related to the formation and metabolism of the D. vulgaris biofilm (Ren et al., 2004). To investigate the role of iron in D. vulgaris biofilm formation process, DVU2571, DVU1340, and DVU1397 were selected in this study. In addition, a previous study showed that expression level of several two-component signal systems was differentially regulated in the D. vulgaris biofilm, suggesting their possible roles related to biofilm formation and maintenance (Zhang et al., 2007). Among the results, DVU3062 was selected, as previous studies suggested that most of hybrid-type histidine kinases found in bacteria could be involved in signal transduction required for cell-cell communication or differentiation (Slater et al., 2000; Takeda et al., 2001; Zhang et al., 2006b).
Table 1.
Selected genes and optimized primers for single-cell analysis.
| Gene ID⋆ | Gene name | Annotation | Primer names | Sequence (5′– > 3′) | Functions of the genes in Desulfovibrio vulgaris biofilm |
|---|---|---|---|---|---|
| DVU0281 | Exopolysaccharide biosynthesis Protein | DVU0281A-fw DVU0281A-rv |
TACCCCTGATTCTACCCGTCA GGTGCGAAATTT GAGGATGTC |
Biofilm formation and metabolism (Ren et al., 2004) | |
| DVU0434 | Ech hydrogenase Subunit EchA |
DVU0434A-fw DVU0434A-rv |
CCTCGGCTACATGAAAGAGCAC AGCGTGGTGATTTCCCAGAAG |
Energy conversion and carbon flow (Jenney et al., 1999; Zhang et al., 2007; Clark et al., 2012) | |
| DVU0588 | Formate dehydrogenase subunit beta | DVU0588C-fw DVU0588C-rv | ATGAACTTCGGCGATGAGCAG CGCATGTTCGTAGAAGTCCTTG | ||
| DVU1340 | Fur family transcriptional regulator | DVU1340B-fw DVU1340B-rv | AAGTCCGCTTCGACGGCAT ACCGGATTGGCTGTCCGAAC | ||
| DVU1397 | bfr | Bacterioferritin | DVU1397D-fw DVU1397D-rv | GCGAAAGTCATCGAAGTGCTGA CGGCAAGTTCTCCGTAGTCCAT | Biofilm formation and maintenance (Zhang et al., 2007; Clark et al., 2012) |
| DVU2571 | feoB | Ferrous iron | DVU2571D-fw | GTCGCGAGAAGCTTGCAACACT | |
| Transport protein | DVU2571D-rv | AAGAAGATGCCGACGATGAGGA | |||
| B | |||||
| DVU2410 | sodB | Superoxide dismutase | DVU2410B-fw DVU2410B-rv | CCATGAGACTCGAAGACGTGGT TCATGCCTGCCCAGTAGAAGGT | Reactive oxygen species or general stress resistance (Clark et al., 2012) |
| DVU3062 | Sensor histidine kinase/response regulator | DVU3062D-fw DVU3062D-rv |
TTGGCGTGCAGGTGAATGA CGTTCAAGCGTTGCAAATCC |
Signal transduction (Slater et al., 2000; Takeda et al., 2001; Zhang et al., 2006b) | |
| DVU0565 | gap-1 | Glyceraldehyde | DVU0565D-fw | TATGACCCGCAGAAGCACCAT | Housekeeping gene |
| 3-phosphate dehydrogenase | DVU0565D-rv | CGATGCCGTACTTCTCTTGGAT | (Marco and Kleerebezem, 2008; Zhao et al., 2011) | ||
| DVU1090 | recA | Recombinase A | DVU1090H-fw DVU1090H-rv | GCCGTCATCTTCATCAACCAG TCCATACGGACGGAACTGTAGA | Housekeeping gene (Qi et al., 2014) |
| Dvl6SA | rrsA | 16S ribosomal | Dvl6SAC-fw Dvl6SAC-rv |
CAACCCCTATTGCCAGTTGCT GCCATGATGACTTGACGTCGT |
Housekeeping gene (Scholten et al., 2007; Plugge et al., 2010) |
⋆IDs of the genes are from Kyoto Encyclopedia of Genes and Genomes.
Selection and Evaluation of Primers
Several previous single-cell studies have found that RT-PCR primers functioning well for bulk cells may not be successfully applied to single-cell analysis (Zhao et al., 2011; Shi et al., 2013; Qi et al., 2014), so that a primer has to be validated at a single-cell level. Typically, for a given target gene, one pair of single-cell primers were screened and validated from 4 to 9 pairs of candidate primers functional well at a bulk-cell level (Supplementary Table S1). The criteria and method for screening and validation of single-cell primers were the same as previously described (Zhao et al., 2011; Shi et al., 2013; Qi et al., 2014). For each single cell, three analytical replicates were analyzed (Shi et al., 2013; Qi et al., 2014).
Selection of Internal Control Genes
To minimize differences between single cells due to sample preparation (i.e., RNA yield, quality and efficiency of the reverse transcription), it was supposed to normalize the resulting threshold cycle (CT) data obtained from RT-qPCR analyses against an internal control gene (Heid et al., 1996; Ståhlberg et al., 2013). So far little was known about the degree of expression stability of these internal control genes in the D. vulgaris biofilm cells. In addition, our previous study showed that cell-to-cell expression levels of several internal control genes widely used for bulk-cell studies, such as DVU1090 encoding recombinase A, DVU0600 encoding L-lactate dehydrogenase, and Dv16SA encoding 16S ribosomal RNA, were different among the single cells from both D. vulgaris monoculture and coculture (Qi et al., 2014). To obtain a suitable internal control gene for the purpose of comparing gene-expression heterogeneity of the D. vulgaris grown in biofilm and planktonic cultures, several potential candidates as internal control genes were evaluated at the single-cell level. According to previous studies about D. vulgaris and other similar species at the bulk-cell level, we selected three housekeeping genes as potential internal control genes for subsequent single-cell analysis, i.e., 16S ribosomal RNA gene (Dv16SA) (Scholten et al., 2007; Plugge et al., 2010), glyceraldehyde 3-phosphate dehydrogenase gene (DVU0565, gap-1), and recombinase A gene (DVU1090, recA) (Marco and Kleerebezem, 2008; Zhao et al., 2011). Toward this goal, we randomly isolated two groups of D. vulgaris single cells (each containing 12 single cells) from D. vulgaris biofilm and planktonic culture, respectively, conducted RT-qPCR analysis of three internal control genes for all single cells, and then calculated the mean and standard deviations (SDs) of CT values from single-cell RT-qPCR using the OriginPro 8.0 software.
Planktonic and Biofilm Cells Collection Procedure
Planktonic cells from the surrounding medium were transferred to centrifuge tubes with O-ring seals under anaerobic conditions in the anaerobic chamber and collected by centrifuging at room temperature (6,000 ×g). To keep their gene expression profiles intact, the planktonic cells were put into a RNALater solution immediately (Ambion, Carlsbad, CA, USA) (Bachoon et al., 2001; Mason et al., 2012; Shi et al., 2013; Qi et al., 2014).
For the D. vulgaris biofilms, superficial cells of the mature biofilm formed on the surface of SS slides were slightly washed off with 50 mM oxygen-free phosphate-buffered saline (PBS, pH 6.5) using a dropper under anaerobic conditions in the anaerobic chamber, then inner mature biofilm cells were scraped using a sterile razor blade from the surface of SS slides. To keep their gene expression profiles intact, the biofilm cells were also put into a RNALater solution immediately (Ambion, Carlsbad, CA, USA) (Zhang et al., 2007). The suspension of biofilm cells was then heavily vortexed to release single D. vulgaris cells to be used for single-cell isolation (Chalmers et al., 2007; Marcy et al., 2007; Zhang et al., 2007).
Single-Cell Isolation, RNA Extraction, cDNA Synthesis and Real-Time Quantitative PCR
Single cells were isolated using an inverted IX71 microscope connected with a single cell manipulator (Olympus Inc, Japan) from the cell suspension according to previous publications (Shi et al., 2013; Qi et al., 2014). Total RNAs were extracted from the single cells by a ZR RNA MicroPrep kit (Zymo Research, Irvine, CA, USA) and then used for synthesize cDNAs by a SuperScript VILO MasterMix (Invitrogen, Carlsbad, CA, USA). Finally, the cDNAs were used as templates for quantitative PCR analysis by using Power SYBR Green PCR master mix (Invitrogen, Carlsbad, CA, USA) on an ABI StepOne real-time PCR system (Applied Biosystems, Foster, CA, USA) as previously described (Qi et al., 2014).
In this study, approximately 31 single D. vulgaris cells were randomly isolated from three culture bottles under the identical growth and subjected to the single-cell RT-qPCR analysis from each biological sample of the biofilm and the planktonic cultures. To ensure no significant change of gene expression profiles, the sample cells were put into a RNALater solution and the single-cell isolation process for 31 cells was completed within 30 min (Bachoon et al., 2001; Mutter et al., 2004; Uhlenhaut and Kracht, 2005; Dekairelle et al., 2007).
Data Analysis
To ensure data reproducibility, all growth experiments and measurements were repeated three times. For single-cell based RT-qPCR analysis, we performed the ΔCT relative quantification method with DVU1090 (recombinase A) as the internal reference to normalize the resulting threshold cycle (CT) data. Non-parametric statistic tests were used to analyze the distribution variation of gene expression levels of single cells (Siegel, 1957). In addition, the Mann–Whitney, Kruskal–Wallis, and Kolmogorov-Smirnov analysis of variance (ANOVA) tests were also utilized to analyze the relationship between four different groups of RT-qPCR measurements (i.e., biofilm and planktonic cultures, exponential and stationary phases) using the OriginPro 8.0 software (OriginLab Corporation, Northampton, MA, USA). Correspondence analysis (CA) was conducted using the IBM SPSS Statistics 20 software (IBM, Chicago, IL, USA) to reveal differences between the various categories in the same variable (genes or growth conditions) and to determine relationships and associations between genes and growth modes (Greenacre, 1984).
Results And Discussion
D. vulgaris Biofilm Formation on Mild Steel Surface and Determination of Total Protein and Carbohydrate Levels
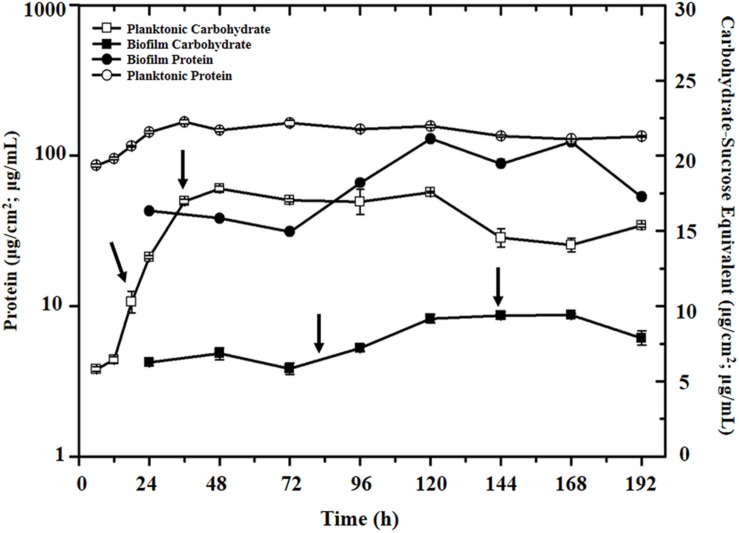
To investigate gene-expression levels of selected target genes potentially associated with SRB biofilm formation and metal corrosion, D. vulgaris biofilm was formed on the immersed SS slides in 200 mL serum bottles with lactate (38 mM) as electron donor and sulfate (50 mM) as electron acceptor. Typically, when D. vulgaris cells was cultured at 35°C for 6 days, the SS slides were completely covered by D. vulgaris biofilm with average 1-2 mm in thickness (Supplementary Figure S2). To monitor growth status of D. vulgaris in both biofilm and planktonic cultures and determine sampling points for further single-cell based analysis, both protein and carbohydrate levels in the biofilm and planktonic cells were measured as previously described (Clark et al., 2012) (Figure 1). The results showed that protein and carbohydrate levels approached steady-state stages at 36 h for planktonic culture and at 144 h for biofilm, respectively (Figure 1). At the steady-state stages, the planktonic cultures had protein concentration of 167 ± 5.2 μg/mL, carbohydrate concentration of 15.9 ± 0.6 μg/mL and a carbohydrate: protein ratio (C:P) of approximately 0.10 (μg/μg), while the biofilm had a protein level of 88 ± 7.0 μg/cm2 and a carbohydrate level of 9.7 ± 0.2 μg/cm2 with an approximately C:P ratio of 0.11 (μg/μg), consistent with a previous conclusion that D. vulgaris did not produce a carbohydrate-rich biofilm matrix (Clark et al., 2007).
FIGURE 1.
Protein and carbohydrate levels in the biofilm and planktonic cells during the growth period. The arrows in the plot indicated the four sampling time points for single-cell RT-qPCR analysis (i.e., 18 and 36 h for planktonic culture, and 84 and 144 h for biofilm, respectively).
To compare gene-expression heterogeneity between biofilm and planktonic cultures, D. vulgaris cells at middle-exponential and stationary phases from both biofilm and planktonic cultures were collected (i.e., 18 and 36 h for planktonic culture, and 84 and 144 h for biofilm, respectively) (Figure 1). To minimize variation during the sampling process, the total sampling time was completed within 2 h from the collection of the samples to the synthesis of the cDNA to keep their gene expression profiles intact (Qi et al., 2014). The cDNAs were stored at –20°C until all the cDNAs at 4 time periods were synthesized, and used for qPCR analysis.
Selection of Target Genes and Optimization of Primers for Single Cell RT-QPCR Analysis
To compare gene-expression heterogeneity between biofilm and planktonic cultures of D. vulgaris, 8 target genes (i.e., DVU0281, DVU0434, DVU0588, DVU1340, DVU1397, DVU2571, DVU2410, and DVU3062) potentially related to formation and metabolism of the D. vulgaris biofilm and three candidate genes of internal reference (i.e., DVU0565, DVU1090, and Dv16SA) were selected (Supplementary Table S1). To improve the success rate of the following single-cell analysis, primers of the eight target genes and three candidate internal reference genes has to be verified at a single-cell level. In this study, we evaluated 49 pairs of primers (a total of 17 pairs for three internal control genes and a total of 32 pairs for eight selected genes) at the single-cell level (Supplementary Table S1). The validation results of single-cell primers for each target gene were listed in Table 1.
Selection and Evaluation of Internal Control Genes
Based on previous studies, three candidate internal control genes (i.e., DVU0565, DVU1090, and Dv16SA) were selected for the following single-cell analysis (Supplementary Table S1). To obtain an optimal reference gene for the single-cell analysis, we evaluated the three candidate internal control genes. The results showed that the SD were 0.74, 1.23, and 0.88 cycles across 12 single cells from the planktonic culture, and 0.83, 2.03, and 1.26 cycles across 12 single cells from the mature biofilm, for internal control gene DVU1090, Dv16SA and DVU0565 genes, respectively (Supplementary Figure S3). Compared with the other two potential internal control genes, the SD of DVU1090 (recA) were lower among individual cells from both biofilm and planktonic cultures (Takle et al., 2007; Marco and Kleerebezem, 2008), so that DVU1090 was selected as the internal control gene for the subsequent single-cell analysis. For Dv16SA gene that was often preferentially selected as an internal control in bulk-cell based studies (Bustin et al., 2009; Plugge et al., 2010), our results showed that it carried a significant gene-expression heterogeneity across single cells, so it may not be optimal as an internal control gene for gene expression analysis at the single-cell level. In addition, the analysis showed that gene-expression heterogeneity of all three internal control genes was greater in the D. vulgaris single cells from biofilm than those from planktonic culture, in agreement with the observation that the biofilm tended to have a higher degree of heterogeneity and complexity as compared with planktonic culture (Stewart and Franklin, 2008).
Reliability Analysis of Single-Cell Gene Expression Data
In this study, one internal control gene (DVU1090) and eight selected target genes (i.e., DVU0281, DVU0434, DVU0588, DVU1340, DVU1397, DVU2410, DVU2571, and DVU3062) were analyzed in each of the 31 individual cells isolated from each growth condition (i.e., biofilm and planktonic cultures, exponential and stationary phases). A quality control was manually conducted for a total 3,348 reactions (i.e., 31 cells × 4 conditions × 9 genes × 3 analytical replicates). As a quality control for reliable data, poor RT-qPCR reactions, i.e., wrong amplification peaks in the melting curves; large variations (i.e., SD > 0.5) among analytical triplicates, were removed, resulting in a total of 29, 30, 28, and 28 cells out of 31 individual cells with all nine genes successfully analyzed at exponential phase in biofilm and planktonic cultures, and at stationary phase in biofilm and planktonic cultures, respectively. The amplification efficiency of all RT-qPCR data was greater than 93%. The results indicated that variations among technical replicates of RT-qPCR analysis were small for all eight genes, suggestive of good reproducibility of the data (Supplementary Table S2).
Gene-Expression Heterogeneity in D. Vulgaris Cells from Biofilm and Planktonic Culture
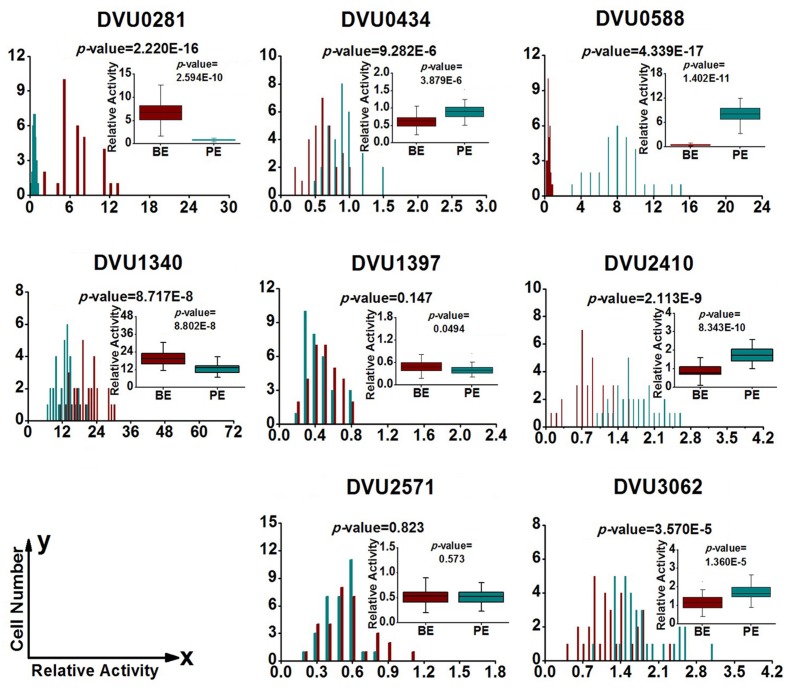
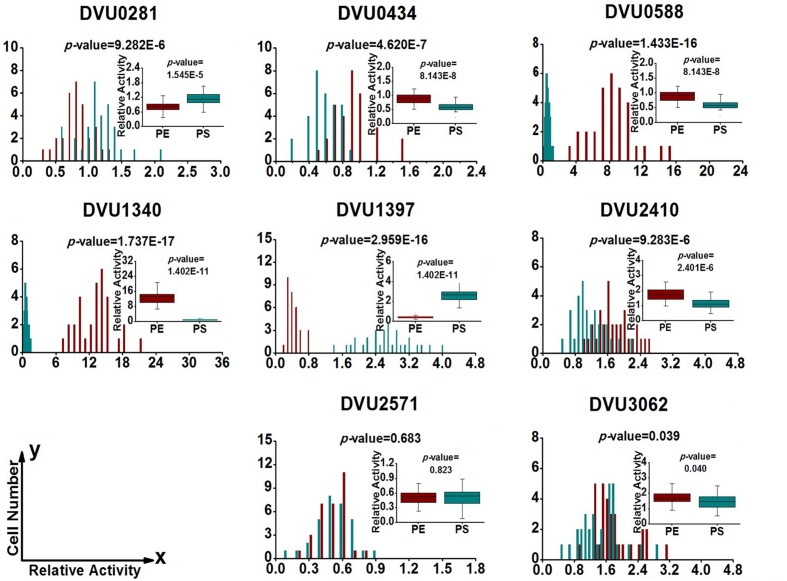
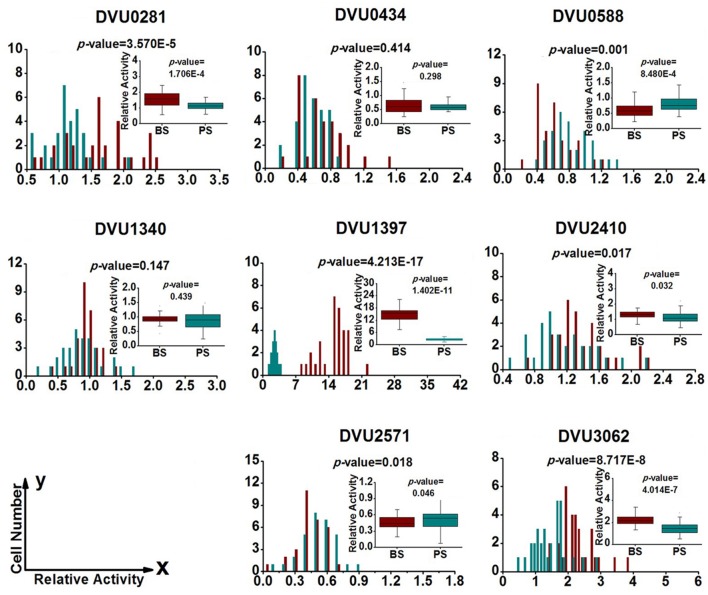
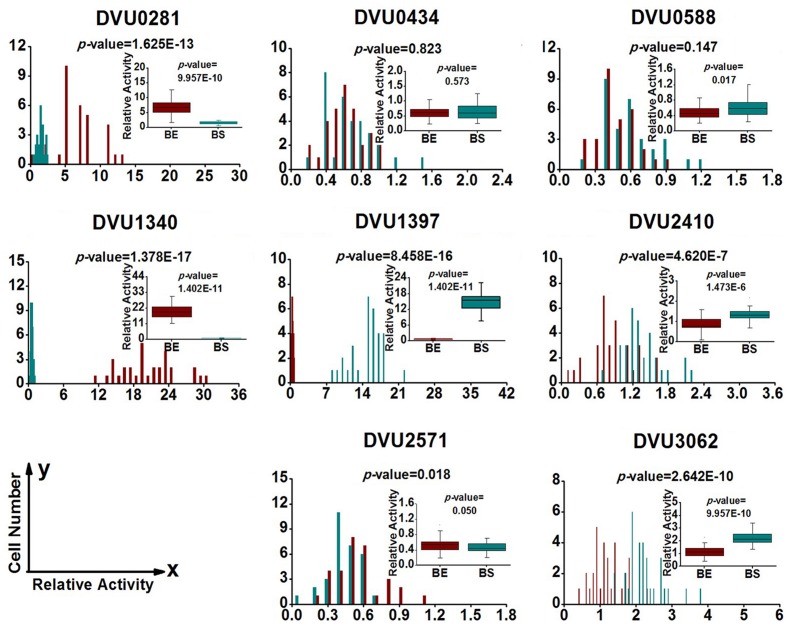
To quantify heterogeneity existed in the D. vulgaris cultures; we drew distribution histograms based on relative activity of all eight target genes under four growth conditions. The relative activity data for all single cells under four conditions was provided in Supplementary Table S3. In addition, to conveniently compare biofilm with planktonic culture, we made four combinations of distribution histograms for four growth conditions (i.e., planktonic vs. biofilm at exponential phase, planktonic vs. biofilm at stationary phase, exponential vs. stationary in planktonic culture, and exponential vs. stationary in biofilm) (Figures 2–5). Similarity between gene-expression distributions was evaluated using p-values determined by non-parametric two-sample Kolmogorov–Smirnov tests. In addition, in the box plots, similarity of gene expression between conditions was evaluated using p-values calculated by two-tailed non-parametric Mann–Whitney statistical significance tests. To more accurately describe the heterogeneity levels, we first defined degree of gene-expression heterogeneity as the fold between the highest and the lowest gene expression levels for each target gene in all single cells under each growth condition, and data was presented in Table 2 accordingly. The results showed that the distribution span of relative gene-expression levels along the horizontal axis reached up to 15, e.g., DVU1397 at stationary phase in the biofilm (Figure 3), suggesting that all target genes carried significant gene-expression heterogeneity in all four conditions. For instance, the differences in terms of relative gene-expression levels could be greater than approximately 80 folds between single cells for DVU0281 in the biofilm at exponential phase, and approximately 50 folds between single cells for DVU3062 at stationary phase in the planktonic culture (Table 2). These results demonstrated that the gene-expression heterogeneity might be a common phenomenon in both biofilm and planktonic cultures. In addition, among all eight genes, the gene-expression heterogeneity for DVU0281 encoding exopolysaccharide biosynthesis protein tended to be the largest among single cells under exponential phase in D. vulgaris biofilm, while DVU2571 involved in ferrous iron transport tended to be the largest under stationary phase in D. vulgaris biofilm (Table 2). Although it is still unknown the specific relationship between the heterogeneity and functionality and physiology of biofilms, high level of heterogeneity for various cellular components is commonly found in biofilm. For example, studies have demonstrated that the distribution of exopolysaccharides in biofilms is highly uneven, which may be related to the formation of the three-dimensional biofilm structures (Laue et al., 2006; Stewart and Franklin, 2008). In addition, the non-uniform multilevel exopolysaccharide matrix is likely one of the main reasons for the stability of biofilms (Jefferson, 2004). Therefore, the highest heterogeneity of DVU0281 (involved in exopolysaccharide biosynthesis) in terms of gene expression might be vital for the D. vulgaris biofilm. Furthermore, according to the previous studies, mature biofilms contain concentration gradients of metabolic substrates and products, including metal ions (Stewart and Franklin, 2008; Chen et al., 2011). Although iron plays significant roles in biofilm formation in many microorganisms, several studies have indicated that excess iron was detrimental to biofilms (Banin et al., 2005; Hindré et al., 2008). Therefore, to maintain appropriate iron concentration in different structure levels of the D. vulgaris biofilm, higher gene-expression heterogeneity of DVU2571 involved in ferrous iron transport may be in agreement with the above observation, especially for D. vulgaris biofilm growth on the SS. By comparing the gene-expression heterogeneity of each target gene under the four growth conditions, the results showed that DVU1340 encoding a Fur regulator and DVU3062 encoding sensor histidine kinase/response regulator were the most heterogeneously expressed at stationary phase in planktonic culture (Table 2). The lower heterogeneity levels of regulatory genes in biofilm might be important in maintaining delicate regulation of the genes involved in biofilm formation and maintenance, as proper regulation and assembly of the matrix components are key determinant to the biofilm formation (Kearns, 2008).
FIGURE 2.
Gene-expression distribution of target genes at exponential phase in the biofilm and planktonic cultures. BE (Dark Red) and PE (Dark Cyan) are abbreviations for exponential phase in the biofilm and the planktonic culture, respectively. P-values in the histograms were determined by using the non-parametric two-sample Kolmogorov–Smirnov test between the biofilm and planktonic cultures (α = 0.05). The x axis shows the relative activity of a specific gene compared with the internal control gene DVU1090 in the same cell, and the y axis shows the number of cells that have the same relative activity. Box plots of single-cell gene expression levels and P-values associated with the differences between biofilm and planktonic culture. P-values in the box plots were calculated by means of the two-tailed non-parametric Mann–Whitney statistical significance test between biofilm and planktonic culture. Box plots show following statistical values: Open square – mean, solid line – median, upper and lower box lines – the 75th and 25th percentiles, respectively. Top and bottom horizontal solid line – maximal and minimal values, respectively.
FIGURE 5.
Gene expression distribution of target genes at exponential and stationary phases in planktonic culture. PE (Dark Red) and PS (Dark Cyan) are abbreviations for exponential phase and stationary phase in planktonic culture, respectively. For statistical analysis, please refer to the figure legend of Figure 2.
Table 2.
Maximum gene-expression heterogeneity among 31 single-cells for eight target genes under four growth conditions.
| Growth Conditions | DVU0281 |
DVU0434 |
DVU0588 |
DVU1340 |
DVU1397 |
DVU2410 |
DVU2571 |
DVU3062 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Max-H☆ | Mean-H ± SD⋆ | Max-H | Mean-H ± SD | Mean-H | Max-H ± SD | Max-H | Mean-H ± SD | Max-H | Mean-H ± SD | Mean-H | Max-H ± SD | Max-H | Mean-H ± SD | Mean-H | Max-H ± SD | |
| BE | 80.312 | 4.571 ± 11.503 | 4.607 | 1.626 ± 0.644 | 14.266 | 1.965 ± 0.162 | 2.652 | 1.362 ± 0.301 | 4.452 | 1.613 ± 0.597 | 20.704 | 2.460 ± 2.825 | 5.400 | 1.634 ± 0.635 | 5.746 | 1.642 ± 0.649 |
| PE | 4.312 | 1.461 ± 0.529 | 3.004 | 1.343 ± 0.312 | 4.526 | 1.528 ± 0.531 | 3.102 | 1.38 ± 0.337 | 3.789 | 1.548 ± 0.497 | 2.608 | 1.366 ± 0.303 | 3.522 | 1.445 ± 0.410 | 3.441 | 1.386 ± 0.358 |
| BS | 4.240 | 1.572 ± 0.591 | 5.989 | 1.685 ± 0.665 | 5.149 | 1.607 ± 0.576 | 3.171 | 1.299 ± 0.332 | 2.946 | 1.308 ± 0.313 | 5.198 | 1.431 ± 0.333 | 13.573 | 1.853 ± 1.948 | 2.761 | 1.312 ± 0.283 |
| PS | 3.445 | 1.406 ± 0.390 | 4.394 | 1.499 ± 0.853 | 3.603 | 1.475 ± 0.412 | 7.110 | 1.676 ± 0.784 | 2.919 | 1.338 ± 0.308 | 4.651 | 1.525 ± 0.501 | 11.306 | 1.820 ± 1.469 | 50.071 | 3.095 ± 6.444 |
☆Max-H is abbreviation of maximum gene-expression heterogeneity; The gene-expression heterogeneity values were calculated by dividing the highest gene expression levels by the lowest gene expression levels.
⋆Mean gene-expression heterogeneity ± Standard Deviation. BE and PE are abbreviations of exponential phase for biofilm and planktonic cells, respectively. BS and PS are abbreviations of stationary phase for biofilm and planktonic cells, respectively.
FIGURE 3.
Gene-expression distribution of target genes at stationary phase in the biofilm and planktonic cultures. BS (Dark Red) and PS (Dark Cyan) are abbreviations for stationary phase in the biofilm and the planktonic culture, respectively. For statistical analysis, please refer to the figure legend of Figure 2.
By comparing the gene-expression distributions of the eight target genes, the results showed that gene-expression levels under certain growth conditions were obviously distinct for one certain target gene between different biofilm cells, suggesting significant stochastic gene expression in the D. vulgaris biofilm (Figures 2–5). Although chemical heterogeneity and physiological adaptation to the local environment explain much of the biological heterogeneity in biofilms, it is likely that stochastic gene expression also contributes to the phenotypic heterogeneity (Stewart and Franklin, 2008). Researchers have noted obvious variation in terms of activity of single bacterial cells in biofilms (Davies and Geesey, 1995; Baty et al., 2000). It has been proposed that stochastic gene expression can also commendably explain phenotypic heterogeneity in a biofilm population that is independent of the prevailing environmental conditions (Chai et al., 2008; Stewart and Franklin, 2008). With the increased complexity in biofilms, we hypothesized that increased gene-expression heterogeneity may also occur in the D. vulgaris biofilms when compared with the planktonic culture since the gene-expression heterogeneity could lead to different fates for individual cells (Colman-Lerner et al., 2005; Strovas et al., 2007). To validate this hypothesis, we also calculated the SD for each gene among the single cells as a second indicator. In general, similar trends were observed using both indicators (Table 2). By comparing gene-expression heterogeneity of all eight genes under the four growth conditions, the results indicated that gene-expression heterogeneity of six genes (i.e., DVU0281, DVU0434, DVU0588, DVU1397, DVU2410, and DVU2571) were increased at both exponential and stationary phases for the biofilm when compared with the planktonic culture, and gene-expression heterogeneity of DVU3062 was increased slightly at exponential phase while decreased significantly at stationary phase for the biofilm (Table 2). The only exception was DVU1340 whose gene-expression heterogeneity was decreased at both exponential and stationary phases in the D. vulgaris biofilm (Table 2). In addition, the results showed decreased heterogeneity levels for two regulatory genes (i.e., DVU1347 and DVU30162) at stationary phase in biofilm, which may worth further investigation for possible mechanism. Overall, compared with the planktonic culture, gene-expression heterogeneity of target genes was increased at both exponential and stationary phases for the biofilm. To demonstrate that the gene-expression heterogeneity resulted from the growth states of biofilm instead of the variances among biological replications, we compared the gene-express histograms and heterogeneity boxplots (Supplementary Figure S4) and the heterogeneity frequency distribution histograms (Supplementary Figure S5) for target gene DVU0281 that encodes a exopolysaccharide biosynthesis protein, relevant to biofilm formation) from biological replicates in both planktonic and biofilm cultures. The results showed that the mean gene-expression heterogeneity levels of three biological replicates for the biofilm were about the same (i.e., 7.931, 8.332, and 7.537); similarly, the mean gene-expression heterogeneity levels of three biological replicates for planktonic culture were also about the same (i.e., 1.622, 1.580, and 1.755). Meanwhile, the mean heterogeneity levels between biofilm and planktonic cultures were more than five times different for DVU0281 under the exponential phase, suggesting that different heterogeneity levels were resulted from different growth state (biofilm vs. planktonic) rather than from variation between biological replicates. In addition, based on the studies on aerobic and facultative bacteria, the phenotypic heterogeneity and complexity within biofilm were greater when compared with the planktonic culture (Stewart and Franklin, 2008). Therefore, although further analysis of more genes is still needed, it is speculated that a possible linkage between increased gene-expression heterogeneity and phenotypic heterogeneity and structural complexity in the D. vulgaris biofilm compared to the planktonic culture.
Regulation of Target Genes in D. Vulgaris Cells from Biofilm and Planktonic Culture
To determine the differentially regulated genes between the different growth conditions using single-cell datasets, we established four pairs of comparisons for eight target genes (Figures 2–5), with an emphasis to the first two pairs of comparisons that may reveal genes functionally closely related to the biofilm metabolism. The comparison of planktonic culture vs. biofilm is presented in Figure 2 for the exponential-phase cells, while Figure 3 presents the same comparison for the stationary-phase cells. The results showed that: (i) the distributions of three genes (DVU0281, DVU1340, and DVU1397) were clearly shifted toward right side in the plots at both growth phases in the biofilm, representing possible up-regulation of gene expression in single cells of the D. vulgaris biofilm, suggesting that these genes may be related to formation and metabolism of biofilm (Figures 2 and 3); (ii) both p-values of the distribution and box plots analysis of DVU1340 were greater than 0.05 at stationary phase (Figure 3), suggesting that DVU1340 may be functional primarily at fast-growing exponential phase; (iii) the distributions of three target genes, DVU0434, DVU0588, and DVU2571 were shifted toward left side at both growth phases in the biofilm with compared to the planktonic culture, suggesting that the genes were down-regulated and they may not be directly related to the formation and metabolism of biofilm (Figures 2 and 3); (iv) DVU2410 and DVU3062 were down-regulated at exponential phase in the biofilm but up-regulated at stationary phase in the biofilm (Figures 2 and 3), suggesting the two genes may be related to late-stage function of mature biofilm, such as maintenance, although further proof is still needed.
It has been found that excess iron could be detrimental to biofilms (Johnson et al., 2005; Hindré et al., 2008). In this study, the D. vulgaris biofilm was formed on SS slides, which may lead to a high concentration of iron around the biofilm. Our analysis showed that DVU1340 encoding ferric uptake repressor (Fur) family transcriptional regulators and DVU1397 encoding ion storage protein bacterioferritin were up-regulated, while DVU2571 encoding a ferrous iron transport protein was down-regulated in the D. vulgaris biofilm (Table 3), suggesting that these genes may be important in maintaining the normal metabolism of the D. vulgaris biofilm under a high concentration of iron, although further investigation is still needed to reveal the related mechanism.
Table 3.
The average relative activity among 31 single-cells for eight target genes under four growth conditions.
| Growth conditions | DVU0281 | DVU0434 | DVU0588 | DVU1340 | DVU1397 | DVU2410 | DVU2571 | DVU3062 |
|---|---|---|---|---|---|---|---|---|
| BE | 6.848 ± 3.027⋆ | 0.617 ± 0.210 | 0.461 ± 0.172 | 19.987 ± 4.898 | 0.494 ± 0.167 | 0.850 ± 0.364 | 0.553 ± 0.204 | 1.190 ± 0.432 |
| PE | 0.808 ± 0.218 | 0.924 ± 0.229 | 8.196 ± 2.616 | 12.878 ± 3.231 | 0.432 ± 0.159 | 1.758 ± 0.427 | 0.506 ± 0.137 | 1.779 ± 0.495 |
| BS | 1.605 ± 0.504 | 0.678 ± 0.276 | 0.601 ± 0.224 | 0.934 ± 0.190 | 14.904 ± 3.070 | 1.367 ± 0.340 | 0.446 ± 0.134 | 2.238 ± 0.523 |
| PS | 1.152 ± 0.308 | 0.590 ± 0.167 | 0.812 ± 0.248 | 0.901 ± 0.328 | 2.646 ± 0.596 | 1.179 ± 0.386 | 0.520 ± 0.173 | 1.468 ± 0.547 |
BE and PE are abbreviations of exponential phase for biofilm and planktonic cells, respectively. BS and PS are abbreviations of stationary phase for biofilm and planktonic cells, respectively. ⋆Average Relative Activity±Standard Deviation; The relative activity are calculated by using ΔCT relative quantification method with DVU1090 as the internal reference to normalize the resulting threshold cycle (CT) data.
To determine the functioning periods of the genes in biofilm and planktonic cultures, the gene-expression distribution was also compared between exponential and stationary phases (Figures 4 and 5). The results showed that the distribution patterns of 4 out of the 8 genes (i.e., DVU0281, DVU0588, DVU1340, and DVU2571) were shifted toward right at the exponential phase in the biofilm (Figure 4), with statistical significances of both non-parametric two-sample Mann–Whitney and Kolmogorov–Smirnov tests less than 0.05, suggesting that they might be functional primarily in exponential phase in the D. vulgaris biofilm. Meanwhile, the distribution patterns of three genes (DVU1397, DVU2410, and DVU3062) shifted toward left at exponential phase in the biofilm with the p-values less than 0.05 (Figure 4).
FIGURE 4.
Gene expression distribution of target genes at exponential and stationary phases in the biofilm. BE (Dark Red) and BS (Dark Cyan) are abbreviations for exponential phase and stationary phase in biofilm, respectively. For statistical analysis, please refer to the figure legend of Figure 2.
Correspondence Analysis of Single-Cell Data
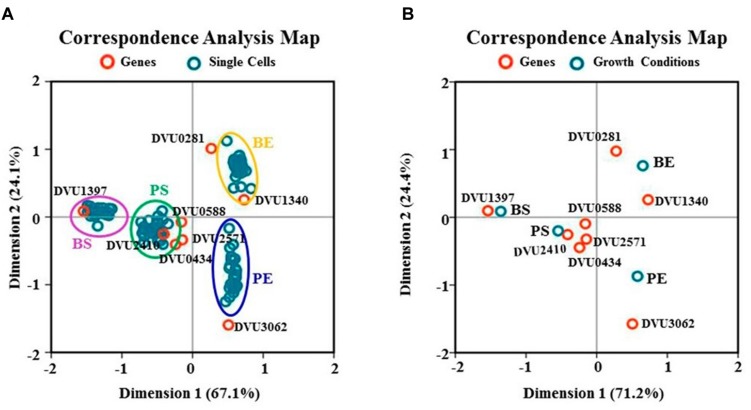
A statistical analysis technique of multivariate dependent-variables, namely CA, was also applied to the single-cell datasets (Kishino and Waddell, 2000). Two CA plots were generated independently; one was based on the relationship between target genes and all single cells of four growth conditions (Figure 6A), and another was based on the relationship between target genes and four growth conditions where the conditions were represented by the CT means of all 31 single cells (Figure 6B). The CA analysis showed that a cumulative inertia (that represents the explanation levels of each dimension to the differences between the various categories of the variables) of 91.2 and 95.6% could be explained by the first two dimensions in Figures 6A,B, respectively, suggesting that the plots explained most of the variance quite well. Several key features can be observed from the plots: (i) all target genes were well separated in the plots (Figure 6A), confirmed by the Kruskal–Wallis ANOVA tests with p-values significantly less than 0.05 (Table 4), suggesting that the RT-qPCR and CA analysis was able to differentiate the independent gene-expression distribution patterns exhibited by each of the target genes (Theodorsson-Norheim, 1986; Shi et al., 2013; Qi et al., 2014); (ii) in the CA plot (Figure 6A), single cells at exponential phase all distributed in the left, while single cells at stationary phase all distributed in the right of the CA plots. In addition, single cells in the biofilm located in the upper of the CA plot, while single cells in the planktonic culture located in the bottom of the CA plot, indicating significant differences between single cells from four growth conditions and the distributions of four growth conditions in the CA plot were independent as also confirmed by the statistical analyses; (iii) in the CA plot based on the relationship between target genes and four growth conditions (Figure 6B), DVU0281 and DVU1340 genes were more close to the exponential phase in the biofilm, while DVU1397 was more close to the stationary phase in the biofilm, suggesting that they could be functionally related to the corresponding condition according to the CA theory (Greenacre, 1984; Yelland, 2010). Similarly, DVU3062 was far away from both BE and BS conditions in the plot, suggesting it might play little role in the formation and metabolism of the D. vulgaris biofilm.
FIGURE 6.
Correspondence analysis. (A) CA plot of eight target genes and 124 individual cells under four growth conditions; (B) CA plots of eight target genes and four growth conditions. BE and PE are abbreviations for exponential phase in the biofilm and the planktonic culture, respectively. BS and PS are abbreviations for stationary phase in the biofilm and the planktonic culture, respectively.
Table 4.
P values of Kruskal–Wallis tests at the 95% Confidence level.
| Gene ID | P-value |
|---|---|
| DVU0281 | 5.156E-18 |
| DVU0434 | 2.790E-7 |
| DVU0588 | 1.729E-18 |
| DVU1340 | 1.856E-21 |
| DVU1397 | 1.673E-22 |
| DVU2410 | 9.803E-12 |
| DVU2571 | 0.016 |
| DVU3062 | 2.862E-11 |
Conclusion
In this study, we applied a single-cell RT-qPCR based approach to compare gene-expression levels of selected target genes in D. vulgaris grown in biofilm and planktonic cultures. While significant gene-expression heterogeneity was found for the selected genes in both the D. vulgaris biofilm and planktonic culture, the analysis showed that gene-expression heterogeneities of 7 out of 8 selected genes were clearly increased in single cells isolated from the D. vulgaris biofilm when compared with those from the planktonic culture, implying a possible linkage between gene-expression heterogeneity and structural and phenotypic complexities of the D. vulgaris biofilms. Interestingly, the analysis showed that compared to planktonic culture, expression levels of DVU1340 encoding ferric uptake repressor family transcriptional regulators and DVU1397 encoding the iron storage protein bacterioferritin were up-regulated for the D. vulgaris biofilms, while the expression level of DVU2571 encoding ferrous iron transport protein were down-regulated, suggesting their roles in maintaining the normal metabolism of the D. vulgaris biofilm under high concentration of iron. Finally, the results demonstrated that single-cell RT-qPCR analysis could be a valuable tool to reveal cell-level and micro-scale differences in the D. vulgaris biofilm, which could be applied in the future to understand molecular mechanism related to growth, maintenance and functions of various microbial biofilms.
Author Contributions
ZQ performed experiments and data analysis, and wrote the manuscript. LC conceived, designed experiments and wrote the manuscript. WZ conceived and designed experiments, analyzed the data and wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The research was supported by grants from the National Science Foundation of China (NSFC) (No. 31170043, 31270086, and 31370115), the National Basic Research Program of China (National “973” program) (No. 2014CB745101) and the Tianjin Municipal Science and Technology Commission. We would also like to thank Prof. Jiangxin Wang of Shenzhen University and Mr. Zixi Chen and Mr. Guangsheng Pei in our laboratory for the useful discussion throughout the project, and Mr. Yang Zhang in our laboratory for help with the SEM analysis.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2016.00597
Standard curve to determine protein and carbohydrate levels for biofilm and planktonic cells of D. vulgaris. (A) Standard curve of carbohydrate by using the anthrone-sulfuric acid colorimetry with sucrose as the standard. (B) Standard curve of protein by using the Bradford assay with bovine serum albumin as the standard. Correlation coefficients (square values – R2) and correlation equation are shown.
Scanning electron microscope imaging of D. vulgaris biofilm grown on mild steel slides. Image was taken on a Hitachi S-4800 SEM scanning electron microscope. Magnification is 10,000 × and th magnification bar (in micrometers) is 5 μm. Experimental procedures: slides were rinsed once with 50 mM PBS (PH 6.5) and then placed in a fixative overnight. The biofilms were washed with ddH2O, and then dried at the critical point with a CO2 critical point drier.
Evaluation of three candidates of internal control genes for the biofilm and planktonic cultures. CT is the number of qPCR quantification cycle, the fractional cycle number where fluorescence increases above the threshold. Each set of 12 cells from biofilm and planktonic culture were used to evaluate the consistency of the internal control genes. The standard deviations (SD) and means of the CT values were calculated using the OriginPro 8.0 software.
Gene-expression histograms and heterogeneity boxplots of biological replication of DVU0281 under the exponential phase in both biofilm and planktonic culture. BE and PE are abbreviations for exponential phase in the biofilm and the planktonic culture, respectively. (A) Gene-express histograms among biological replications of DVU0281 under BE and PE, respectively. (B) Gene-express histograms of DVU0281 in all 31 single cells under BE and PE, respectively. (C) Gene-expression heterogeneity boxplots of biological replication of DVU0281 under BE and PE, respectively.
Frequency distribution histogram of gene-expression heterogeneities for biological replications of DVU0281 under the exponential phase in both biofilm and planktonic cultures.
References
- An D., Parsek M. R. (2007). The promise and peril of transcriptional profiling in biofilm communities. Curr. Opin. Microbiol. 10 292–296. 10.1016/j.mib.2007.05.011 [DOI] [PubMed] [Google Scholar]
- Bachoon D. S., Chen F., Hodson R. E. (2001). RNA recovery and detection of mRNA by RT-PCR from preserved prokaryotic samples. FEMS Microbiol. Lett. 201 127–132. 10.1111/j.1574-6968.2001.tb10745.x [DOI] [PubMed] [Google Scholar]
- Banin E., Vasil M. L., Greenberg E. P. (2005). Iron and Pseudomonas aeruginosa biofilm formation. Proc. Natl. Acad. Sci. U.S.A. 102 11076–11081. 10.1073/pnas.0504266102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baty A. M., III, Eastburn C. C., Techkarnjanaruk S., Goodman A. E., Geesey G. G. (2000). Spatial and temporal variations in chitinolytic gene expression and bacterial biomass production during chitin degradation. Appl. Environ. Microbiol. 66 3574–3585. 10.1128/AEM.66.8.3574-3585.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech I., Mollica A., Flemming H.-C., Scotto V., Sand W. (2000). Simple Methods for the Investigation of the Role of Biofilms in Corrosion. Brite Euram Thematic Network on MIC of Industrial Materials, Biofilm Publication, (London: IWA Publishing; ), 1–27. [Google Scholar]
- Beloin C., Ghigo J. M. (2005). Finding gene-expression patterns in bacterial biofilms. Trends Microbiol. 13 16–19. 10.1016/j.tim.2004.11.008 [DOI] [PubMed] [Google Scholar]
- Blainey P. C. (2013). The future is now: single-cell genomics of bacteria and archaea. FEMS Microbiol. Rev. 37 407–427. 10.1111/1574-6976.12015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72 248–254. 10.1006/abio.1976.9999 [DOI] [PubMed] [Google Scholar]
- Brehm-Stecher B. F., Johnson E. A. (2004). Single-cell microbiology: tools, technologies, and applications. Microbiol. Mol. Biol. Rev. 68 538–559. 10.1128/MMBR.68.3.538-559.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin S. A., Benes V., Garson J. A., Hellemans J., Huggett J., Kubista M., et al. (2009). The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55 611–622. 10.1373/clinchem.2008.112797 [DOI] [PubMed] [Google Scholar]
- Chai Y., Chu F., Kolter R., Losick R. (2008). Bistability and biofilm formation in Bacillus subtilis. Mol. Microbiol. 67 254–263. 10.1111/j.1365-2958.2007.06040.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers N. I., Palmer R. J., Du-Thumm L., Sullivan R., Shi W., Kolenbrander P. E. (2007). Use of quantum dot luminescent probes to achieve single-cell resolution of human oral bacteria in biofilms. Appl. Environ. Microbiol. 73 630–636. 10.1128/AEM.02164-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G. C., Chen X. C., Yang Y. Q., Hay A. G., Yu X. H., Chen Y. X. (2011). Sorption and distribution of copper in unsaturated Pseudomonas putida CZ1 biofilms as determined by X-ray fluorescence microscopy. Appl. Environ. Microbiol. 77 4719–4727. 10.1128/Aem.00125-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M. E., Edelmann R. E., Duley M. L., Wall J. D., Fields M. W. (2007). Biofilm formation in Desulfovibrio vulgaris hildenborough is dependent upon protein filaments. Environ. Microbiol. 9 2844–2854. 10.1111/j.1462-2920.2007.01398.x [DOI] [PubMed] [Google Scholar]
- Clark M. E., He Z., Redding A. M., Joachimiak M. P., Keasling J. D., Zhou J. Z., et al. (2012). Transcriptomic and proteomic analyses of Desulfovibrio vulgaris biofilms: carbon and energy flow contribute to the distinct biofilm growth state. BMC Genomics 13:138 10.1186/1471-2164-13-138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman-Lerner A., Gordon A., Serra E., Chin T., Resnekov O., Endy D., et al. (2005). Regulated cell-to-cell variation in a cell-fate decision system. Nature 437 699–706. 10.1038/nature03998 [DOI] [PubMed] [Google Scholar]
- Davies D. G., Geesey G. G. (1995). Regulation of the alginate biosynthesis gene algC in Pseudomonas aeruginosa during biofilm development in continuous culture. Appl. Environ. Microbiol. 61 860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekairelle A. F., Van Der Vorst S., Tombal B., Gala J. L. (2007). Preservation of RNA for functional analysis of separated alleles in yeast: comparison of snap-frozen and RNALater (R) solid tissue storage methods. Clin. Chem. Lab. Med. 45 1283–1287. 10.1515/Cclm.2007.281 [DOI] [PubMed] [Google Scholar]
- Dinh H. T., Kuever J., Mussmann M., Hassel A. W., Stratmann M., Widdel F. (2004). Iron corrosion by novel anaerobic microorganisms. Nature 427 829–832. 10.1038/nature02321 [DOI] [PubMed] [Google Scholar]
- Enning D., Garrelfs J. (2014). Corrosion of iron by sulfate-reducing bacteria: new views of an old problem. Appl. Environ. Microbiol. 80 1226–1236. 10.1128/AEM.02848-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang H. H., Xu L. C., Chan K. Y. (2002). Effects of toxic metals and chemicals on biofilm and biocorrosion. Water Res. 36 4709–4716. 10.1016/S0043-1354(02)00207-5 [DOI] [PubMed] [Google Scholar]
- Greenacre M. J. (1984). Theory and Applications of Correspondence Analysis. London: Academic Press. [Google Scholar]
- Hamilton W. A. (2003). Microbially influenced corrosion as a model system for the study of metal microbe interactions: a unifying electron transfer hypothesis. Biofouling 19 65–76. 10.1080/0892701021000041078 [DOI] [PubMed] [Google Scholar]
- Heid C. A., Stevens J., Livak K. J., Williams P. M. (1996). Real time quantitative PCR. Genome Res. 6 986–994. 10.1101/gr.6.10.986 [DOI] [PubMed] [Google Scholar]
- Hellweger F. L., Bucci V. (2009). A bunch of tiny individuals—Individual-based modeling for microbes. Ecol. Modell. 220 8–22. 10.1016/j.ecolmodel.2008.09.004 [DOI] [Google Scholar]
- Hindré T., Brüggemann H., Buchrieser C., Héchard Y. (2008). Transcriptional profiling of Legionella pneumophila biofilm cells and the influence of iron on biofilm formation. Microbiology 154 30–41. 10.1099/mic.0.2007/008698-0 [DOI] [PubMed] [Google Scholar]
- Hu A. (2004). Investigation of Sulfate-Reducing Bacteria Growth Behavior for the Mitigation of Microbiologically Influenced Corrosion (MIC). Ohio: Ohio University. [Google Scholar]
- Jefferson K. K. (2004). What drives bacteria to produce a biofilm? FEMS Microbiol. Lett. 236 163–173. 10.1111/j.1574-6968.2004.tb09643.x [DOI] [PubMed] [Google Scholar]
- Jenney F. E., Verhagen M. F. J. M., Cui X. Y., Adams M. W. W. (1999). Anaerobic microbes: oxygen detoxification without superoxide dismutase. Science 286 306–309. 10.1126/science.286.5438.306 [DOI] [PubMed] [Google Scholar]
- Johnson M., Cockayne A., Williams P. H., Morrissey J. A. (2005). Iron-responsive regulation of biofilm formation in staphylococcus aureus involves fur-dependent and fur-independent mechanisms. J. Bacteriol. 187 8211–8215. 10.1128/JB.187.23.8211-8215.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns D. B. (2008). Division of labour during Bacillus subtilis biofilm formation. Mol. Microbiol. 67 229–231. 10.1111/j.1365-2958.2007.06053.x [DOI] [PubMed] [Google Scholar]
- Kishino H., Waddell P. J. (2000). Correspondence analysis of genes and tissue types and finding genetic links from microarray data. Genome Inform. Ser. Workshop Genome Inform. 11 83–95. [PubMed] [Google Scholar]
- Kolter R., Losick R. (1998). Microbiology - one for all and all for one. Science 280 226–227. 10.1126/science.280.5361.226 [DOI] [PubMed] [Google Scholar]
- Lardon L. A., Merkey B. V., Martins S., Dötsch A., Picioreanu C., Kreft J. U., et al. (2011). iDynoMiCS: next-generation individual-based modelling of biofilms. Environ. Microbiol. 13 2416–2434. 10.1111/j.1462-2920.2011.02414.x [DOI] [PubMed] [Google Scholar]
- Laue H., Schenk A., Li H., Lambertsen L., Neu T. R., Molin S., et al. (2006). Contribution of alginate and levan production to biofilm formation by Pseudomonas syringae. Microbiology 152 2909–2918. 10.1099/mic.0.28875-0 [DOI] [PubMed] [Google Scholar]
- Lazazzera B. A. (2005). Lessons from DNA microarray analysis: the gene expression profile of biofilms. Curr. Opin. Microbiol. 8 222–227. 10.1016/j.mib.2005.02.015 [DOI] [PubMed] [Google Scholar]
- Lewandowski Z., Beyenal H. (2008). “Mechanisms of microbially influenced corrosion,” in Marine and Industrial Biofouling, eds H.-C. Flemming, P. S. Murthy, R. Venkatesan, and K. E. Cooksey (Berlin: Springer), 35–65. 10.1007/978-3-540-69796-1_3 [DOI] [Google Scholar]
- Lidstrom M. E., Meldrum D. R. (2003). Life-on-a-chip. Nat. Rev. Microbiol. 1 158–164. 10.1038/nrmicro755 [DOI] [PubMed] [Google Scholar]
- Little B., Wagner P., Mansfeld F. (1992). An overview of microbiologically influenced corrosion. Electrochim. Acta 37 2185–2194. 10.1016/0013-4686(92)85110-7 [DOI] [Google Scholar]
- Liu H., Xu L., Zeng J. (2000). Role of corrosion products in biofilms in microbiologically induced corrosion of carbon steel. Br. Corrosion J. 35 131–135. 10.1179/000705900101501155 [DOI] [Google Scholar]
- Marco M., Kleerebezem M. (2008). Assessment of real-time RT-PCR for quantification of Lactobacillus plantarum gene expression during stationary phase and nutrient starvation. J. Appl. Microbiol. 104 587–594. 10.1111/j.1365-2672.2007.03578.x [DOI] [PubMed] [Google Scholar]
- Marcy Y., Ouverney C., Bik E. M., Lösekann T., Ivanova N., Martin H. G., et al. (2007). Dissecting biological “dark matter” with single-cell genetic analysis of rare and uncultivated TM7 microbes from the human mouth. Proc. Natl. Acad. Sci. U.S.A. 104 11889–11894. 10.1073/pnas.0704662104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason O. U., Hazen T. C., Borglin S., Chain P. S., Dubinsky E. A., Fortney J. L., et al. (2012). Metagenome, metatranscriptome and single-cell sequencing reveal microbial response to Deepwater Horizon oil spill. ISME J. 6 1715–1727. 10.1038/ismej.2012.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutter G. L., Zahrieh D., Liu C. M., Neuberg D., Finkelstein D., Baker H. E., et al. (2004). Comparison of frozen and RNALater solid tissue storage methods for use in RNA expression microarrays. BMC Genomics 5:88 10.1186/1471-2164-5-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plugge C. M., Scholten J. C., Culley D. E., Nie L., Brockman F. J., Zhang W. (2010). Global transcriptomics analysis of the Desulfovibrio vulgaris change from syntrophic growth with Methanosarcina barkeri to sulfidogenic metabolism. Microbiology 156 2746–2756. 10.1099/mic.0.038539-0 [DOI] [PubMed] [Google Scholar]
- Qi Z., Pei G., Chen L., Zhang W. (2014). Single-cell analysis reveals gene-expression heterogeneity in syntrophic dual-culture of Desulfovibrio vulgaris with Methanosarcina barkeri. Sci. Rep. 4:7478 10.1038/srep07478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D., Bedzyk L. A., Thomas S. M., Ye R. W., Wood T. K. (2004). Gene expression in Escherichia coli biofilms. Appl. Microbiol. Biotechnol. 64 515–524. 10.1007/s00253-003-1517-y [DOI] [PubMed] [Google Scholar]
- Roe J. H. (1954). The determination of dextran in blood and urine with anthrone reagent. J. Biol. Chem. 208 889–896. [PubMed] [Google Scholar]
- Roe J. H. (1955). The determination of sugar in blood and spinal fluid with anthrone reagent. J. Biol. Chem. 212 335–343. [PubMed] [Google Scholar]
- Scholten J. C., Culley D. E., Brockman F. J., Wu G., Zhang W. (2007). Evolution of the syntrophic interaction between Desulfovibrio vulgaris and Methanosarcina barkeri: involvement of an ancient horizontal gene transfer. Biochem. Biophys. Res. Commun. 352 48–54. 10.1016/j.bbrc.2006.10.164 [DOI] [PubMed] [Google Scholar]
- Shi X., Gao W., Chao S. H., Zhang W., Meldrum D. R. (2013). Monitoring the single-cell stress response of the diatom Thalassiosira pseudonana by quantitative real-time reverse transcription-PCR. Appl. Environ. Microbiol. 79 1850–1858. 10.1128/AEM.03399-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X., Gao W., Wang J., Chao S. H., Zhang W., Meldrum D. R. (2014). Measuring gene expression in single bacterial cells: recent advances in methods and micro-devices. Crit. Rev. Biotechnol. 35 448–460. 10.3109/07388551.2014.899556 [DOI] [PubMed] [Google Scholar]
- Siegel S. (1957). Nonparametric statistics. Am. Statist. 11 13–19. 10.2307/2685679 [DOI] [Google Scholar]
- Slater H., Alvarez-Morales A., Barber C. E., Daniels M. J., Dow J. M. (2000). A two-component system involving an HD-GYP domain protein links cell–cell signalling to pathogenicity gene expression in Xanthomonas campestris. Mol. Microbiol. 38 986–1003. 10.1046/j.1365-2958.2000.02196.x [DOI] [PubMed] [Google Scholar]
- Ståhlberg A., Rusnakova V., Forootan A., Anderova M., Kubista M. (2013). RT-qPCR work-flow for single-cell data analysis. Methods 59 80–88. 10.1016/j.ymeth.2012.09.007 [DOI] [PubMed] [Google Scholar]
- Stepanauskas R. (2012). Single cell genomics: an individual look at microbes. Curr. Opin. Microbiol. 15 613–620. 10.1016/j.mib.2012.09.001 [DOI] [PubMed] [Google Scholar]
- Stewart P. S., Franklin M. J. (2008). Physiological heterogeneity in biofilms. Nat. Rev. Microbiol. 6 199–210. 10.1038/nrmicro1838 [DOI] [PubMed] [Google Scholar]
- Strovas T. J., Lidstrom M. E. (2009). Population heterogeneity in Methylobacterium extorquens AM1. Microbiology 155 2040–2048. 10.1099/mic.0.025890-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strovas T. J., Sauter L. M., Guo X., Lidstrom M. E. (2007). Cell-to-cell heterogeneity in growth rate and gene expression in Methylobacterium extorquens AM1. J. Bacteriol. 189 7127–7133. 10.1128/JB.00746-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S. I., Fujisawa Y., Matsubara M., Aiba H., Mizuno T. (2001). A novel feature of the multistep phosphorelay in Escherichia coli: a revised model of the RcsC→ YojN→ RcsB signalling pathway implicated in capsular synthesis and swarming behaviour. Mol. Microbiol. 40 440–450. 10.1046/j.1365-2958.2001.02393.x [DOI] [PubMed] [Google Scholar]
- Takle G. W., Toth I. K., Brurberg M. B. (2007). Evaluation of reference genes for real-time RT-PCR expression studies in the plant pathogen Pectobacterium atrosepticum. BMC Plant Biol. 7:50 10.1186/1471-2229-7-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodorsson-Norheim E. (1986). Kruskal-Wallis test: BASIC computer program to perform nonparametric one-way analysis of variance and multiple comparisons on ranks of several independent samples. Comput. Methods Prog. 23 57–62. 10.1016/0169-2607(86)90081-7 [DOI] [PubMed] [Google Scholar]
- Thierry D., Sand W. (1995). Microbially Influenced Corrosion. New York, NY: Marcel Dekker. [Google Scholar]
- Uhlenhaut C., Kracht M. (2005). Viral infectivity is maintained by an RNA protection buffer. J. Virol. Methods 128 189–191. 10.1016/j.jviromet.2005.05.002 [DOI] [PubMed] [Google Scholar]
- Verplaetse E., Slamti L., Gohar M., Lereclus D. (2015). Cell differentiation in a Bacillus thuringiensis Population during Planktonic Growth. Biofilm Format. Host Infection. MBio 6:e138 10.1128/mBio.00138-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videla H. A., Characklis W. G. (1992). Biofouling and microbially influenced corrosion. Int. Biodeterior. Biodegrad. 29 195–212. 10.1016/0964-8305(92)90044-O [DOI] [Google Scholar]
- Voordouw G. (1995). The genus desulfovibrio: the centennial. Appl. Environ. Microbiol. 61 2813–2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelland P. M. (2010). An introduction to correspondence analysis. Math. J. 12 1–23. 10.3888/tmj.12-4 [DOI] [Google Scholar]
- Zhang W., Culley D. E., Nie L., Scholten J. C. (2007). Comparative transcriptome analysis of Desulfovibrio vulgaris grown in planktonic culture and mature biofilm on a steel surface. Appl. Microbiol. Biotechnol. 76 447–457. 10.1007/s00253-007-1014-9 [DOI] [PubMed] [Google Scholar]
- Zhang W., Culley D. E., Scholten J. C., Hogan M., Vitiritti L., Brockman F. J. (2006a). Global transcriptomic analysis of Desulfovibrio vulgaris on different electron donors. Antonie Van Leeuwenhoek 89 221–237. 10.1007/s10482-005-9024-z [DOI] [PubMed] [Google Scholar]
- Zhang W., Culley D. E., Wu G., Brockman F. J. (2006b). Two-component signal transduction systems of Desulfovibrio vulgaris: structural and phylogenetic analysis and deduction of putative cognate pairs. J. Mol. Evol. 62 473–487. 10.1007/s00239-005-0116-1 [DOI] [PubMed] [Google Scholar]
- Zhao W., Li Y., Gao P., Sun Z., Sun T., Zhang H. (2011). Validation of reference genes for real-time quantitative PCR studies in gene expression levels of Lactobacillus casei Zhang. J. Ind. Microbiol. Biotechnol. 38 1279–1286. 10.1007/s10295-010-0906-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Standard curve to determine protein and carbohydrate levels for biofilm and planktonic cells of D. vulgaris. (A) Standard curve of carbohydrate by using the anthrone-sulfuric acid colorimetry with sucrose as the standard. (B) Standard curve of protein by using the Bradford assay with bovine serum albumin as the standard. Correlation coefficients (square values – R2) and correlation equation are shown.
Scanning electron microscope imaging of D. vulgaris biofilm grown on mild steel slides. Image was taken on a Hitachi S-4800 SEM scanning electron microscope. Magnification is 10,000 × and th magnification bar (in micrometers) is 5 μm. Experimental procedures: slides were rinsed once with 50 mM PBS (PH 6.5) and then placed in a fixative overnight. The biofilms were washed with ddH2O, and then dried at the critical point with a CO2 critical point drier.
Evaluation of three candidates of internal control genes for the biofilm and planktonic cultures. CT is the number of qPCR quantification cycle, the fractional cycle number where fluorescence increases above the threshold. Each set of 12 cells from biofilm and planktonic culture were used to evaluate the consistency of the internal control genes. The standard deviations (SD) and means of the CT values were calculated using the OriginPro 8.0 software.
Gene-expression histograms and heterogeneity boxplots of biological replication of DVU0281 under the exponential phase in both biofilm and planktonic culture. BE and PE are abbreviations for exponential phase in the biofilm and the planktonic culture, respectively. (A) Gene-express histograms among biological replications of DVU0281 under BE and PE, respectively. (B) Gene-express histograms of DVU0281 in all 31 single cells under BE and PE, respectively. (C) Gene-expression heterogeneity boxplots of biological replication of DVU0281 under BE and PE, respectively.
Frequency distribution histogram of gene-expression heterogeneities for biological replications of DVU0281 under the exponential phase in both biofilm and planktonic cultures.