Abstract
Objectives
Hospital discharges to post-acute care (PAC) facilities have increased rapidly. This increase may lead to more hospital readmissions from PAC facilities, which are common and poorly understood. We sought to determine the risk factors and timing for hospital readmission from PAC facilities and evaluate the impact of readmission on patient outcomes.
Design
Retrospective analysis of Medicare Current Beneficiary Survey (MCBS) from 2003–2009.
Setting
The MCBS is a nationally-representative survey of beneficiaries matched with claims data.
Participants
Community-dwelling beneficiaries who were hospitalized and discharged to a PAC facility for rehabilitation.
Intervention/Exposure
Potential readmission risk factors included patient demographics, health utilization, active medical conditions at time of PAC admission, and PAC characteristics.
Measurements
Hospital readmission during the PAC stay, return to community residence, and all-cause mortality.
Results
Of 3246 acute hospitalizations followed by PAC facility stays, 739 (22.8%) included at least 1 hospital readmission. The strongest risk factors for readmission included impaired functional status (HR 4.78, 95% CI 3.21–7.10), markers of increased acuity such as need for intravenous medications in PAC (1.63, 1.39–1.92), and for-profit PAC ownership (1.43, 1.21–1.69). Readmitted patients had a higher mortality rate at both 30 days (18.9 vs. 8.6%, p<0.001) and 100 days (39.9 vs. 14.5%, p<0.001) even after adjusting for age, comorbidities, and prior health care utilization (30 days: OR 2.01, 95% CI 1.60–2.54; 100 days: OR 3.79, 95% CI 3.13–4.59).
Conclusions
Hospital readmission from PAC facilities is common and associated with a high mortality rate. Readmission risk factors may signify inadequate transitional care processes or a mismatch between patient needs and PAC resources.
Keywords: post-acute care, readmission, care transition
INTRODUCTION
Medicare’s change to a prospective payment system for hospitals in the 1980s coupled with the rise of managed care in the 1990s resulted in dramatic declines in hospital lengths of stay. This led to increased clinical instability of patients being discharged, and therefore a significant rise in discharges to post-acute care (PAC) facilities (including skilled nursing and rehabilitation facilities).(1–4) The average hospital length of stay has continued to decline and the number of hospitalized patients discharged to PAC facilities has continued to rise since that time, increasing nationally by nearly 50% between 1996 and 2010.(5) PAC is now the most rapidly growing area in Medicare spending;(6,7) spending on care in PAC facilities alone totaled $30.4 billion in 2012.(7)
However, significant quality gaps, including hospital readmission rates that currently exceed those of discharges home, continue to exist in the provision of PAC facility care.(8) The Office of the Inspector General recently reported that 22% of all hospitalized Medicare beneficiaries discharged to PAC facilities experienced an adverse event resulting in harm during their PAC stay (most commonly hospital readmission). Moreover, 60% of the adverse events were considered preventable with better care processes such as enhanced medication reconciliation and improved patient monitoring.(9) PAC facilities with higher readmission rates also have lower rates of patients returning to the community.(10) Reducing the rate of readmissions from PAC may hold significant promise for aligning improvements in the quality of care of older adults with reduced health care costs.(11,12)
However, little is known about risk factors for readmission from PAC facilities, the timing of readmission, or the impact of readmission on patient outcomes. While risk factors for hospital readmission from home have received national attention,(13) comparatively little is known about risk factors for readmission from PAC facilities, and these factors may be quite different due to a dissimilar patient population and care setting. Identifying timing of and risk factors for readmission may provide insight into underlying causes and key areas for future interventions to target.(14–16) For example, early readmissions may reflect inadequate transitional processes of care between the hospital and PAC facility or a mismatch between patient needs and PAC facility resources. Late readmissions may reflect inadequate PAC care processes or resources to identify and treat a worsening condition. We sought to determine the risk factors and timing for hospital readmission from PAC facilities and evaluate the impact of readmission on patient outcomes.
METHODS
Study design and setting
This was a secondary analysis of the Cost and Use and Access to Care modules of the MCBS, a prospective nationally-representative cohort of the Medicare population sponsored by the Centers for Medicare and Medicaid Services. During 2003–2009, a mean of 11,879 beneficiaries per year were surveyed three times annually for a maximum of four years (Access to Care modules); these surveys were matched to Medicare claims data (Cost and Use modules). The MCBS uses a rotating-panel design, adding approximately one-quarter of the cohort annually. The MCBS uniquely allows the ability to follow survey respondents longitudinally across care settings, including movement into and out of the hospital and PAC facilities, combining survey, claims, and nursing home (including Minimum Data Set, MDS) information. The 2009 data was the most recent available and was chosen to maintain continuity with a single MDS version (2.0 was implemented in 2002; version 3.0 in 2010).
We included all hospitalizations in the MCBS that occurred among beneficiaries who were age 65 or older and community-dwelling before hospitalization (N=15,608 hospitalizations), and were discharged to a PAC facility after hospitalization (n=3612). Records missing essential data elements were excluded (n=366 with incomplete PAC facility admission MDS information). PAC facilities were defined as skilled nursing and rehabilitation facilities. Acute inpatient rehabilitation facilities, long-term acute care (LTAC) hospitals, assisted living facilities, swing beds in rural hospitals, and long-term care nursing homes (without skilled care) were excluded. Patients were eligible to be included for more than one hospitalization as long as their hospital-PAC facility episode ended 30 days prior to the next hospitalization; our results are therefore a visit-level rather than patient-level analysis. However, we analyzed only the first readmission during the same PAC facility stay (whether the patient returned to the same PAC facility after hospital readmission or not). Our study was approved from the Colorado Multiple Institutional Review Board.
Analysis of timing of readmission
Our primary outcome was readmission during the PAC facility stay. For those readmitted, we identified the day of readmission, with day 0 reflecting the day of discharge from the hospital to the PAC facility. We report day of readmission as a histogram, calculating summative rates for days 0–7, days 0–14, and days 0–30. We also calculated rates of readmission during these periods of the PAC stay in order to display year-on-year trends. For all analyses, we included PAC stays up to 150 days, the longest stay in our cohort (97% of stays were <100 days).
Risk factor analysis
To identify factors associated with readmission, we began by identifying variables in the MCBS that have been linked to complex care transitions in PAC facilities(17) or to hospital readmission in patients discharged home (rather than PAC).(13,18) These included the following patient-level variables contained in Medicare claims data: patient age (dichotomized as <80 or 80 years and older), race (categorized as white vs. non-white), number of hospitalizations in the six months prior to the hospitalization that precipitated the PAC facility stay, degree of medical comorbidity using the Charlson-Deyo method (using diagnoses present within the prior year),(19) payor source (whether dual-eligible with Medicaid as a payor), and primary hospital discharge diagnosis, aggregated from ICD-9 codes into Agency for Healthcare Research and Quality’s Clinical Classification Software categories.(20) We added 2002 MCBS data to allow calculation of prior hospitalizations and Charlson-Deyo comorbidity score for patients hospitalized in 2003. We also utilized two measures that were calculated at the time of PAC facility admission as a part of the MDS: cognitive impairment using the Cognitive Performance Scale(21) and Barthel Index (a functional status measure incorporating Activities of Daily Living and mobility).(22,23)
We also examined facility-level factors using Medicare claims data, including: the number of physician visits a patient received in the PAC facility, the PAC facility length of stay, percent of the facility’s beds that were certified by Medicare, ownership of facility (for-profit vs. non-profit, including government), number of residents in the facility, and cost of the facility stay.
We then evaluated the patient’s active medical conditions and treatment at the time of admission to the PAC facility using MDS data. These included whether the patient had an invasive device (i.e., intravenous catheter, feeding tube, indwelling urinary catheter), an active medical condition or symptom at the time of PAC facility admission (i.e., dyspnea, dehydration, edema, fever, pain, hallucination, internal bleeding, aspiration into the lung, pressure ulcers, or vomiting), was receiving advanced care at the PAC facility (i.e., chemotherapy, dialysis, intravenous medications, monitoring of fluid balance, ostomy care, inhaled oxygen therapy, tracheostomy care, or transfusions), how many different medications the patient received in the last 7 days, and receipt of a high-risk medication captured in the MDS (defined as an antipsychotic or an anti-anxiety/hypnotic medication). None of the variables had more than 3.3% missing data.
We used chi square or Fisher’s exact tests for univariable comparisons of categorical variables and t-tests or Wilcoxon rank-sum tests for parametric and nonparametric continuous variables, respectively, comparing those readmitted with those not readmitted.
We initially included significant factors (p<0.05) from the univariable analysis in a multivariable Cox proportional hazards regression model with readmission as the outcome. This model accounts for potential patient-level clustering. We compared those readmitted and not readmitted using a time-to-event analysis, plotting the cumulative hazard function as survival and Kaplan-Meier curves and censoring for death, the end of PAC stay, or 150 days post-discharge. Survival functions plotted for categorical variables compared the categories of the considered variable for a patient with values at the mean for continuous variables and at the reference for other categorical variables.
Several variables violated the proportional hazards assumption, identified using the supremum test for continuous variables and time interaction terms with the outcome variable for categorical variables. These were transformed or categorized, including index hospital length of stay (log-transformed), Barthel Index (categorized into high function [0–30], moderate function [31–60], and low function [>61]), cognitive performance as measured by the Cognitive Performance Scale (categorized into high cognitive status [0], moderate [1–2], and low [3–6]), and facility size (categorized into small [0–100 residents] versus large [>101 residents]). Despite multiple transformations, cost continued to violate the proportional hazards assumption but was significant in all models and was included. We found that the hazard rate for each $1000 increase in cost increased over time from 0.69 at day 1 to 1.65 at day 100. This means early in the PAC stay patients with higher costs were less likely to be readmitted, and later in the PAC stay patients with higher costs were more likely to be readmitted. Results for cost should be interpreted as an average effect over the time period.
We then used forward selection to identify significant variables for the final model, resulting in 13 independent variables. We then bootstrapped the data 1000 times using the candidate variables in Table 1 for internal validation purposes. This did not identify any additional variables selected ≥60% of the time to force into the final model. It did identify two variables (internal bleeding, ostomy care) that were selected less than 50% of the time and these were dropped from the analysis.
Table 1. Characteristics of hospital discharges to PAC facilities by readmission status.
The most common 5 hospital discharge diagnoses were chosen to display. Higher Barthel Index scores are equivalent to higher function. This score was categorized with scores 0–30 = low function, 31–60= moderate function, and >60 high function. In contrast, higher cognitive performance scores connote lower function; these were also categorized as 0 = high cognitive function, 1 = moderate cognitive function, 2–6 = low cognitive function.
| Readmitted N=739 (22.8%) |
Not readmitted N=2507 (77.2%) |
||
|---|---|---|---|
| Characteristics | (95%CI) | (95%CI) | p-value |
| Patient Characteristics | |||
| Age ≥ 80 (%) | 64.4 (61.0–67.9) | 66.2 (64.3–68.0) | 0.37 |
| Male (%) | 38.4 (35.1–42.1) | 34.9 (33.1–36.8) | 0.07 |
| Minority race/ethnicity (%) | 15.0 (12.4–17.6) | 10.5 (9.3–11.7) | <0.001 |
| Dual-eligible (%) | 51.2 (47.6–54.8) | 70.5 (68.7–72.3) | <0.001 |
| Charlson-Deyo score (mean) | 1.2 (1.1–1.3) | 0.8 (0.7–0.8) | <0.001 |
| Hospitalizations in prior 6 mos (mean) | 0.6 (0.5–0.7) | 0.3 (0.3–0.4) | <0.001 |
| Hospital discharge diagnosis (%) | |||
| Congestive heart failure | 6.9 (5.1–8.7) | 4.0 (3.3–4.8) | <0.001 |
| Pneumonia | 6.9 (5.1–8.7) | 5.5 (4.6–6.4) | 0.15 |
| Hip fracture | 2.8 (1.6–3.9) | 5.0 (4.1–5.8) | 0.01 |
| Acute CVA | 2.7 (1.5–3.9) | 2.8 (2.2–3.4) | 0.77 |
| Osteoarthritis | 1.4 (0.6–2.3) | 2.5 (1.9–3.1) | 0.09 |
| Hospital length of stay, days (mean) | 11.9 (10.9–12.9) | 8.8 (8.4–9.2) | <0.001 |
| Functional status on PAC admission | |||
| High function | 6.5 (4.7–8.3) | 14.5 (13.1–15.9) | <0.001 |
| Moderate function | 25.9 (27.7–29.1) | 33.7 (31.9–35.6) | <0.001 |
| Low function | 67.7 (64.3–71.1) | 51.8 (49.8–53.8) | <0.001 |
| Cognitive Performance Score | |||
| High (most disabled) | 55.5 (51.9–59.1) | 50.0 (48.0–52.0) | 0.002 |
| Moderate | 11.6 (9.3–13.9) | 11.3 (10.1–12.5) | 0.80 |
| Low (least disabled) | 32.9 (29.5–36.3) | 39.7 (37.8–41.6) | <0.001 |
| PAC facility characteristics | |||
| For-profit ownership (%) | 72.0 (68.8–75.2) | 62.9 (61.0–64.8) | <0.001 |
| Number of residents (mean) | 142.0 (135.0–149.0) | 130.4 (126.8–134.0) | <0.001 |
| % Medicare-certified beds (mean) | 98.8 (98.3–99.3) | 98.2 (97.8–98.6) | 0.13 |
| Average length of stay, days | 23.1 (21.1–25.1) | 32.2 (31.1–33.4) | <0.001 |
| Average cost of stay, dollars | 7827 (7198–8456) | 11053 (10672–11433) | <0.001 |
| Number of MD visits in PAC (mean) | 3.6 (3.4–3.8) | 2.6 (2.5–2.7) | <0.001 |
| Invasive devices in PAC | |||
| Intravenous catheter (%) | 14.1 (11.6–16.7) | 12.7 (11.4–14.0) | 0.30 |
| Feeding tube (%) | 9.4 (7.3–11.5) | 430 (3.4–4.9) | <0.001 |
| Urinary catheter (%) | 30.4 (27.0–33.7) | 22.9 (21.2–24.5) | <0.001 |
| Advanced care provided in PAC | |||
| Chemotherapy (%) | 0.5 (0.0–1.1) | 0.4 (0.0–0.6) | 0.72 |
| Dialysis (%) | 5.0 (3.4–6.6) | 1.9 (1.4–2.5) | <0.001 |
| Intravenous medications (%) | 64.8 (61.4–68.3) | 58.8 (56.9–60.7) | 0.003 |
| Monitor fluid balance (%) | 55.5 (51.9–59.1) | 51.5 (49.5–53.5) | 0.06 |
| Ostomy care (%) | 7.0 (5.2–8.9) | 3.7 (3.0–4.5) | <0.001 |
| Tracheostomy care (%) | 1.0 (0.3–1.7) | 0.7 (0.4–1.0) | 0.54 |
| Oxygen therapy (%) | 41.3 (37.7–44.8) | 33.4 (31.5–35.2) | <0.001 |
| Transfusions (%) | 8.7 (6.9–8.7) | 7.5 (6.5–8.6) | 0.31 |
| Active medical conditions in PAC | |||
| Dyspnea (%) | 25.2 (22.0–28.3) | 17.6 (16.2–19.1) | <0.001 |
| Dehydration (%) | 3.3 (2.0–4.5) | 4.9 (4.1–5.8) | 0.06 |
| Edema (%) | 36.9 (33.5–40.4) | 34.1 (32.2–35.9) | 0.15 |
| Fever (%) | 7.2 (5.3–9.0) | 5.6 (5.3–9.0) | 0.12 |
| Hallucinations (%) | 3.4 (2.1–4.7) | 2.8 (2.2–3.5) | 0.44 |
| Internal bleeding (%) | 3.9 (2.5–5.3) | 1.6 (1.1–2.1) | <0.001 |
| Lung aspiration (%) | 1.4 (0.5–2.2) | 0.6 (0.3–0.9) | 0.04 |
| Vomiting (%) | 4.3 (2.9–5.8) | 2.3 (1.6–2.8) | 0.002 |
| Presence of surgical wounds (%) | 25.4 (22.3–28.6) | 29.8 (28.0–31.6) | 0.02 |
| Pressure ulcers (%) | 27.5 (24.3–30.7) | 17.4 (15.9–18.8) | <0.001 |
| Active complaints of pain (%) | 56.9 (53.3–60.4) | 59.1 (57.2–61.0) | 0.29 |
| High-risk medications in PAC | |||
| Meds received in last 7 days (mean) | 12.0 (11.7–12.4) | 11.3 (11.1–11.5) | <0.001 |
| Received antipsychotic (%) | 0.9 (0.7–1.0) | 0.8 (0.7–0.9) | 0.33 |
| Received anxiolytic/hypnotic (%) | 1.1 (1.0–1.2) | 1.2 (1.0–1.3) | 0.46 |
Outcomes analysis
Finally, we used multivariable logistic regression to evaluate the effect of hospital readmission on post-PAC outcomes. We evaluated whether the patient returned to the community by 100 days post-PAC discharge, the number of different living situations the patient had in the year following PAC facility discharge, and mortality rate at 30 and 100 days after the index PAC facility discharge. Patients who died during their PAC facility stay were excluded. We included age, gender, Charlson-Deyo score, index hospital length of stay, and number of hospitalizations in the last six months as important potential confounders.(13,17)
RESULTS
Timing of readmissions
Our final cohort included 3246 acute hospitalizations followed by a PAC facility stay among 2921 unique patients. Of these PAC facility stays, 739 (22.8%) had an index readmission during the PAC stay. Readmissions tended to occur earlier in the stay, with 12.6% of all readmissions occurring within 4 days of hospital discharge, 27.5% within 7 days of hospital discharge and 49.5% within 14 days of discharge (Figure 1). One-fifth of readmissions occurred more than 30 days after hospital discharge (22.2%). Year-on-year readmission rates did not demonstrate a clear trend (Supplementary Appendix).
Figure 1.
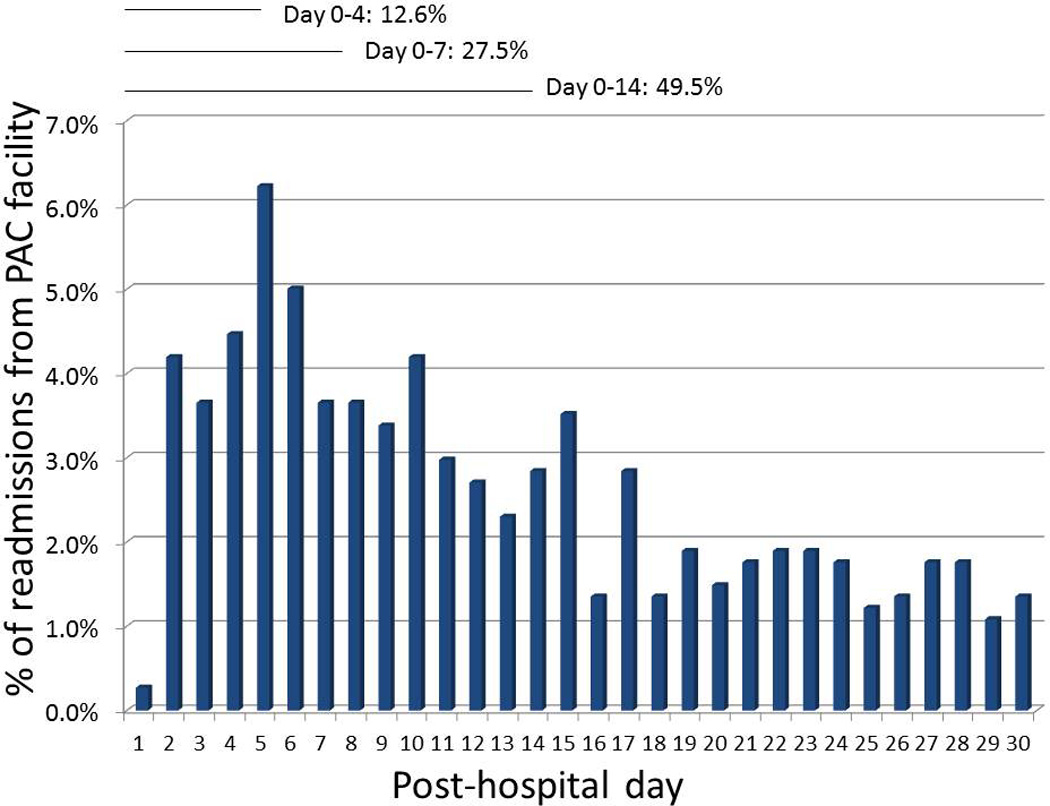
Timing of readmissions from post-acute care are displayed. Day 0 is equivalent to the day of transfer from the hospital to the PAC facility. 22.2% of the 739 readmissions occurred after day 30 and are not displayed here.
Risk factors for readmission
In univariable analysis, patients readmitted from PAC were more likely to be a minority race/ethnicity, had a higher comorbidity score, had more hospitalizations in the six months prior to admission, had a longer index hospital length of stay, were more cognitively and functionally impaired, and were more likely to have a primary diagnosis of heart failure. Those who were not readmitted were more likely to be dual-eligible and to be treated for a hip fracture (Table 1).
In terms of facility factors, readmissions were more common in patients visited more often by PAC physicians and among patients residing in larger, for-profit PAC facilities. Mean PAC facility length of stay was approximately one week less among those readmitted compared to those not readmitted and PAC costs were also correspondingly lower.
Readmitted patients were more likely to be admitted to the PAC facility with an invasive device such as a feeding tube or urinary catheter. They were also more likely to be receiving advanced care such as dialysis, intravenous medications, ostomy care, or oxygen therapy and received more medications overall. Of these, IV medications and oxygen therapy were most commonly provided (>40% of readmitted patients). Readmitted patients were more likely to have dyspnea, aspiration, vomiting, internal bleeding, and pressure ulcers at time of PAC admission. Dyspnea, edema, pain, and active surgical wounds were the only conditions with >25% prevalence in readmitted patients (Table 1).
In multivariable Cox regression analysis, more functionally disabled patients with markers of higher acuity (more hospitalizations in the 6 months prior to the index hospitalization, a longer hospital length of stay before PAC facility transfer, more physician visits in the PAC facility, need for intravenous medications, and heart failure as the primary diagnosis) admitted to larger, for-profit facilities were more likely to be readmitted (Table 2). Patients who were dual-eligible with Medicaid and who were most cognitively impaired were less likely to be readmitted to the hospital (Table 2). Survival curves began to diverge by 25 days (Figure 2, Supplemental Appendix).
Table 2. Significant hazard ratios for readmission in Cox regression analysis.
Cost violates the proportional hazards assumption. Functional status was measured using the Barthel Index, cognitive status using the Cognitive Performance Scale.
| Characteristics | Hazard ratio (95% CI) |
|---|---|
| Functional status | |
| Low vs. high | 4.78 (3.22–7.10) |
| Moderate vs. high | 2.79 (1.87–4.15) |
| Number of physician visits (>1 vs. 0) | 1.82 (1.50–2.21) |
| Intravenous medications | 1.63 (1.39–1.92) |
| Ownership (for-profit vs. non-profit) | 1.43 (1.21–1.69) |
| Index hospital length of stay (log-transformed) | 1.41 (1.28–1.56) |
| Heart failure primary diagnosis | 1.40 (1.06–1.84) |
| Size of facility (>100 beds vs. 100 beds or less) | 1.35 (1.19–1.53) |
| Previous admissions in last 6 months (per admission) | 1.25 (1.16–1.35) |
| Index hospitalization length of stay (log-transformed) | 1.21 (1.10–1.34) |
| Cognitive performance | |
| Low vs. high | 0.80 (0.67–0.96) |
| Moderate vs. high | 0.96 (0.74–1.25) |
| Dual-eligible (Medicare+Medicaid vs Medicare-only) | 0.80 (0.68–0.94) |
| Cost of PAC stay | 0.68 (0.65–0.71) |
Figure 2.
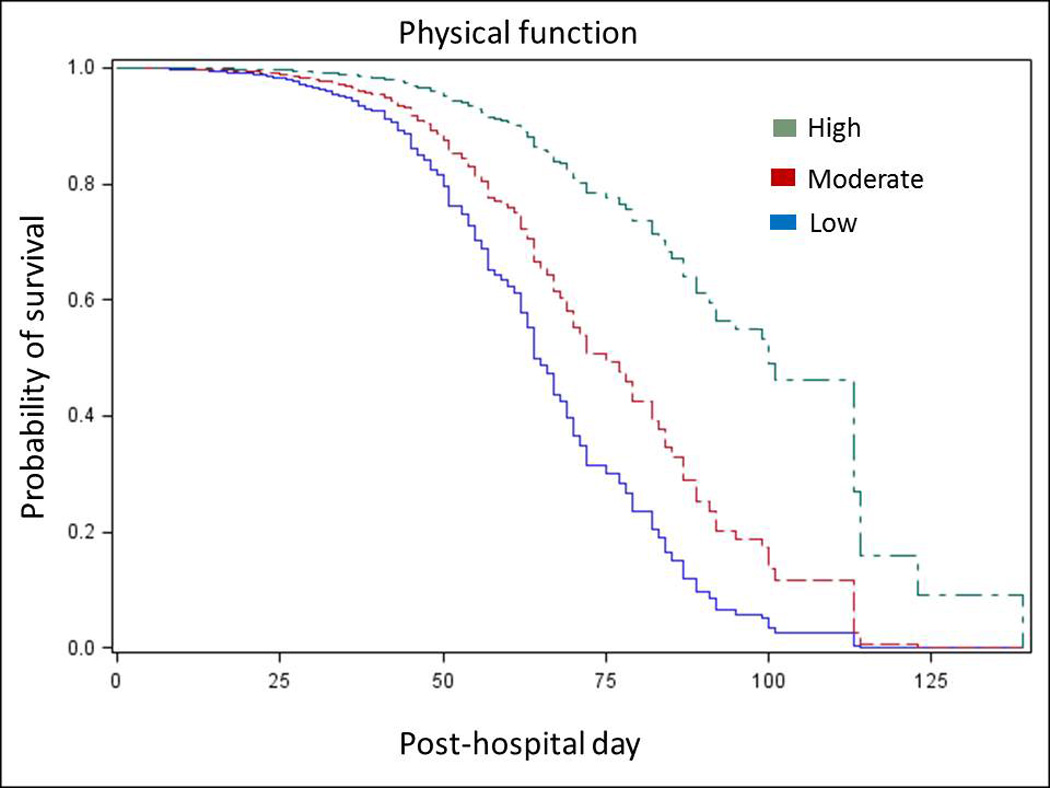
The survival curve for readmission or death for functional status is displayed. The survival curve incorporates values at the mean for continuous variables and at the reference for other categorical variables considered significant in the multivariable regression.
Outcomes of readmission
Patients who experienced hospital readmission during the PAC facility stay were less likely to return to the community and were required to move more often between different living situations compared to patients with PAC stays without a hospital readmission. They also had increased 30-day and 100-day mortality (Table 3a). In multivariable analysis, readmitted patients were twice as likely as non-readmitted patients to die in the 30 days following hospital discharge and nearly four times as likely to die in the 100 days post-hospital discharge (Table 3b).
Table 3.
Living in community refers to residence outside a nursing home at 100 days following index PAC facility discharge. Number of different living situations is calculated at 1 year. Logistic regression used to calculate odds ratios was adjusted for age, gender, Charlson-Deyo score, and index hospitalization length of stay.
| a. Association of readmission with patient outcomes | |||
|---|---|---|---|
| Outcome | Readmitted (95% CI) |
Not readmitted (95% CI) |
P-value |
| Living in community (%) | 45.1 (41.5–48.7) | 64.9 (63.0–66.8) | <0.001 |
| Different living situations (mean) | 5.4 (5.3–5.6) | 3.4 (3.3–3.5) | <0.001 |
| Mortality at 30 days (%) | 18.9 (16.1–21.8) | 8.6 (7.5–9.7) | <0.001 |
| Mortality at 100 days (%) | 39.9 (36.4–43.5) | 14.5 (13.1–15.9) | <0.001 |
| b. Association of readmission with patient outcomes | ||
|---|---|---|
| Outcome | Unadjusted OR (95% CI) |
Adjusted OR (95% CI) |
| Living in community | 0.44 (0.44–0.52) | 0.52 (0.44–0.62) |
| Different living situations, >2 versus 2 or less | 13.96 (5.16–37.73) | 13.15 (4.86–35.58) |
| Mortality (30 days) | 2.18 (1.75–2.72) | 2.01 (1.60–2.54) |
| Mortality (100 days) | 3.92 (3.26–4.72) | 3.79 (3.13–4.59) |
DISCUSSION
In this nationally-representative sample of hospitalized Medicare beneficiaries discharged to PAC facilities, hospital readmissions were frequent, occurred early in the PAC stay, and were independently associated with a mortality rate similar to metastatic cancer in older patients.(24)
Prior literature on readmissions from PAC includes only national descriptions of trends in readmission rates(8) or single-center descriptions of care transition failures.(25–30) Findings from the current analysis complement and advance these prior reports in providing new insights into risk factors, timing, and outcomes of hospital readmission from PAC facilities.
Hospitals and PAC facilities struggle to achieve optimal outcomes for the patients who were most likely to be readmitted: patients with long hospital stays and major disability who often require advanced care (such as intravenous medications) and intensive monitoring (more physician visits for conditions requiring frequent re-evaluation, such as heart failure). Under a prospective payment system, hospitals are incentivized to discharge these patients as early as possible,(1) and in contrast to discharges home, hospitals are not currently penalized for readmissions from PAC facilities. PAC facilities may be substituting for prolonged hospital care in some cases.(31) This may shortly change with passage of legislation authorizing value-based purchasing for skilled nursing facility care, which includes a new 30-day readmission quality measure.(32)
We speculate that financial incentives may also help explain why dual-eligible patients are less likely to be readmitted: they have a payor (Medicaid) for long-term care if they fail to rehabilitate under their Medicare benefit. Otherwise, in our anecdotal experience, they are often readmitted to the hospital so the PAC facility does not bear the financial costs of waiting to establish a payor for long-term (non-rehabilitative) care. Others have found similar rates of readmission among dual-eligibles and non-dual eligibles.(33)
For these high-risk patients, our results suggest two critical interrelated areas to address to improve patient outcomes: patient selection for PAC, and hospital and PAC facility care processes. Discharging physicians (usually hospitalists) have little guidance for selecting patients,(34,35) and often little knowledge of PAC facility care.(36) More evidence regarding which patients benefit most from PAC facility care, and when patients are ready for hospital discharge to PAC is needed. In terms of care processes, it is not clear which PAC facilities deliver the highest-quality care or which hospitals and PAC facilities have the best transitions of care practices; PAC facility quality measures do not correlate with readmission rates.(37) Larger, for-profit PAC facilities were linked to higher readmission rates in our study; prior work has found lower care quality in these facilities compared to not-for-profit facilities.(38,39) Initial efforts by hospitals to identify optimal community nursing facilities partners in Accountable Care Organizations reflect these challenges,(40) and new penalties for readmissions from post-acute care facilities may signal their use as an indicator of the degree to which systems of care are successfully integrated.
Among care processes, the process of rehabilitating patients is most in question. Impaired functional status was associated with the highest risk of readmission in our study; evidence of its impact on patient outcomes in other settings supports this finding.(18,41–45) However, little is known about which patients benefit from skilled rehabilitation therapies,(46) which therapies to utilize,(47–49) or when therapy is most effectively delivered.(50) This has important downstream cost implications as patients who fail to rehabilitate may be confined to nursing homes for long-term care.(51)
While variability in patient selection for PAC and in transitional care processes could not be directly assessed by our data, we focus on these two areas because our results suggest the highest-risk patients for readmission would be those for whom timing of hospital discharge and optimal transitional care processes would be expected to make the largest difference in outcomes. We also find support for this idea in the substantial variability in patient outcomes and regional spending on PAC that exists in the United States. Variability in patient outcomes between different PAC sites is extreme, even after adjusting for patient characteristics 30-day readmission rates range from 0–50%, 100-day community discharge rates range from 0–84% between different PAC facilities).(10) This suggests different care processes are applied at different sites. Similarly, regional spending on PAC is so large it explains 73% of all the regional variability in Medicare spending across the United States.(46,52) This suggests selection of patients for PAC is different in different regions.(34,35)
To this point, it is not known why there are such significant differences in patient outcomes and in spending for PAC across different sites. Discovering the underlying reasons for these would be a major contribution to better outcomes and lower costs, and our results may be helpful in this regard, as they provide preliminary identification of the highest-risk patients during the transition from hospital to PAC facility. Identifying these patients across different sites and following their care trajectory may offer important insights into patient selection and transitional care processes. New financial incentives such as Medicare’s Spending Per Beneficiary (MSPB) may support rigorous examination of patient selection for PAC facility care, transitions of care practices between the hospital and PAC facility, and use of maximally effective therapies in PAC.(53) This measure tracks how many Medicare dollars are spent on individual patients from 3 days before an acute hospitalization to 30 days post-hospitalization, and incorporates this measurement into the hospital’s reimbursement under value-based purchasing. Thus, it incentivizes hospitals to select the right level of care for patients after discharge and to improve care processes across transitions to reduce MSPB during this time period.
While awaiting further insights, hospitals and PAC facilities should focus on transitional care processes. Failures in communication are common, and may cause preventable adverse events and readmissions.(25,27,28) For example, the three most common categories of preventable adverse events in PAC facilities found by the Office of the Inspector General included medication errors, preventable infections (especially catheter-associated), and inadequate patient monitoring. All could arguably be improved with better transitions of care processes from hospital to PAC facility.(9,54) In this context, it is surprising that patients seen more often during their PAC stay by supervising clinicians were also more likely to be readmitted. We think it is most likely that this reflects the underlying illness of these patients; if they were sicker, they were both more likely to be seen more often by clinicians and to be readmitted. However, we plan to evaluate the pattern of timing and frequency of supervising clinician visits between PAC facility admission and hospital readmission to provide insights into the adequacy of patient monitoring among these clinicians. For example, if visits were conducted late in the admission and clustered right before a readmission, perhaps earlier visits would improve outcomes.
We were surprised that patients with lower cognitive function were less likely to be readmitted than those with higher levels of cognitive function. Patients with significant cognitive impairment are frequently discharged for rehabilitation after hospitalization,(55) despite evidence they may be less likely to rehabilitate.(56) Anecdotally, this may be because these patients do not have a payor for needed long-term nursing home care and are discharged for rehabilitation under their Medicare benefit while their application for Medicaid, for example, is processed. They are also more likely to have do-not-hospitalize and do-not-resuscitate orders and are correspondingly less likely to die in the hospital.(57,58)
Our analysis should be interpreted in the context of the data source. There was only a modest number of readmissions from PAC facilities in MCBS data. Hence, derivation and validation of a prediction model for hospital readmission was not possible, and the risk factors identified require validation in a larger sample. Our cost variable violated the proportional hazards assumption despite multiple types of transformation and must be interpreted with caution. We were not able to ascertain other important outcomes of hospital readmission (such as functional status at 100 days post-discharge) as assessments at these time points were not prespecified. PAC facilities during this time period used the MDS 2.0; version 3.0 (implemented in 2010) contains improved assessments of delirium and depression, important potential contributors to readmissions we were unable to assess. A strength of our study was the ability to evaluate potential risk factors for readmission across a variety of domains that may be poorly captured in other data sources in a nationally-representative, longitudinal, claims-based data sample.
CONCLUSION
Our data suggest hospital readmissions from PAC facilities occur early in patients who are more functionally disabled and require more intensive care, such as IV medications; in other words, in patients who most closely resemble elderly hospitalized patients. The consequences of readmission for these patients are dire, including a near-quadrupling of the mortality rate and halving their rate of ever returning to the community, mandating further evaluation of preventable risk factors in this population. While further analyses are needed, our data suggest hospitals and post-acute care facilities could use the risk factors we identified as contributing to hospital readmission to prospectively identify patients at high risk, then use enhanced discharge planning prior to hospital discharge (to allow more time to improve functional status or complete a course of IV medication, for example) and transitions of care processes after discharge (for example, to facilitate more intensive patient monitoring early in the course, when many readmissions occur) with the goal of preventing readmission.
Supplementary Material
REFERENCES
- 1.Halm EA, Magaziner J, Hannan EL, Wang JJ, Silberzweig SB, Boockvar K, et al. Frequency and impact of active clinical issues and new impairments on hospital discharge in patients with hip fracture. Arch Intern Med. 2003 Jan 13;163(1):108–113. doi: 10.1001/archinte.163.1.107. [DOI] [PubMed] [Google Scholar]
- 2.Halm EA, Fine MJ, Kapoor WN, Singer DE, Marrie TJ, Siu AL. Instability on hospital discharge and the risk of adverse outcomes in patients with pneumonia. Arch Intern Med. 2002 Jun 10;162(11):1278–1284. doi: 10.1001/archinte.162.11.1278. [DOI] [PubMed] [Google Scholar]
- 3.Qian X, Russell LB, Valiyeva E, Miller JE. “Quicker and sicker” under Medicare’s prospective payment system for hospitals: new evidence on an old issue from a national longitudinal survey. Bull Econ Res. 2011;63(1):1–27. doi: 10.1111/j.1467-8586.2010.00369.x. [DOI] [PubMed] [Google Scholar]
- 4.Kosecoff J, Kahn KL, Rogers WH, Reinisch EJ, Sherwood MJ, Rubenstein LV, et al. Prospective payment system and impairment at discharge. The “quicker-and-sicker” story revisited. JAMA J Am Med Assoc. 1990 Oct 17;264(15):1980–1983. [PubMed] [Google Scholar]
- 5.Burke RE, Juarez-Colunga E, Levy C, Prochazka AV, Coleman EA, Ginde AA. Rise of post-acute care facilities as a discharge destination of US hospitalizations. JAMA Intern Med. 2015 Feb;175(2):295–296. doi: 10.1001/jamainternmed.2014.6383. [DOI] [PubMed] [Google Scholar]
- 6.Chandra A, Dalton MA, Holmes J. Large increases in spending on postacute care in Medicare point to the potential for cost savings in these settings. Health Aff Proj Hope. 2013 May;32(5):864–872. doi: 10.1377/hlthaff.2012.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Washington (D.C.): Medicare Payment Advisory Commission; 2013. Jun, [cited 2014 Mar 5]. A Data Book: Health care spending and the Medicare Program. [Internet]. Available from: http://www.medpac.gov/documents/Jun13DataBookEntireReport.pdf. [Google Scholar]
- 8.Mor V, Intrator O, Feng Z, Grabowski DC. The revolving door of rehospitalization from skilled nursing facilities. Health Aff Proj Hope. 2010 Feb;29(1):57–64. doi: 10.1377/hlthaff.2009.0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.OIG. Adverse Events in Skilled Nursing Facilities: National Incidence Among Medicare Beneficiaries, OEI-06-11-00370, February 2014. [cited 2014 Mar 5]; [Internet]. Available from: http://oig.hhs.gov/oei/reports/oei-06-11-00370.pdf.
- 10.Kramer A, Fish R, Min S. Washington (D.C.): Medicare Payment Advisory Commission; 2013. Apr, [cited 2014 Mar 24]. Community Discharge and Rehospitalization Outcome Measures. [Internet]. Available from: http://medpac.gov/documents/Apr13_CommunityDischarge_CONTRACTOR.pdf. [Google Scholar]
- 11.Mechanic R. Post-acute care--the next frontier for controlling Medicare spending. N Engl J Med. 2014 Feb 20;370(8):692–694. doi: 10.1056/NEJMp1315607. [DOI] [PubMed] [Google Scholar]
- 12.Ackerly DC, Grabowski DC. Post-acute care reform--beyond the ACA. N Engl J Med. 2014 Feb 20;370(8):689–691. doi: 10.1056/NEJMp1315350. [DOI] [PubMed] [Google Scholar]
- 13.Kansagara D, Englander H, Salanitro A, Kagen D, Theobald C, Freeman M, et al. Risk prediction models for hospital readmission: a systematic review. JAMA J Am Med Assoc. 2011 Oct 19;306(15):1688–1698. doi: 10.1001/jama.2011.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dharmarajan K, Hsieh AF, Lin Z, Bueno H, Ross JS, Horwitz LI, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA J Am Med Assoc. 2013 Jan 23;309(4):355–363. doi: 10.1001/jama.2012.216476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ottenbacher KJ, Karmarkar A, Graham JE, Kuo Y-F, Deutsch A, Reistetter TA, et al. Thirty-day hospital readmission following discharge from postacute rehabilitation in fee-for-service Medicare patients. JAMA J Am Med Assoc. 2014 Feb 12;311(6):604–614. doi: 10.1001/jama.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham KL, Wilker EH, Howell MD, Davis RB, Marcantonio ER. Differences between early and late readmissions among patients: a cohort study. Ann Intern Med. 2015 Jun 2;162(11):741–749. doi: 10.7326/AITC201506020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coleman EA, Min S, Chomiak A, Kramer AM. Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res. 2004 Oct;39(5):1449–1465. doi: 10.1111/j.1475-6773.2004.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greysen SR, Stijacic Cenzer I, Auerbach AD, Covinsky KE. Functional impairment and hospital readmission in medicare seniors. JAMA Intern Med. 2015 Apr 1;175(4):559–565. doi: 10.1001/jamainternmed.2014.7756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li B, Evans D, Faris P, Dean S, Quan H. Risk adjustment performance of Charlson and Elixhauser comorbidities in ICD-9 and ICD-10 administrative databases. BMC Health Serv Res. 2008;8:12. doi: 10.1186/1472-6963-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cowen ME, Dusseau DJ, Toth BG, Guisinger C, Zodet MW, Shyr Y. Casemix adjustment of managed care claims data using the clinical classification for health policy research method. Med Care. 1998 Jul;36(7):1108–1113. doi: 10.1097/00005650-199807000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Chodosh J, Edelen MO, Buchanan JL, Yosef JA, Ouslander JG, Berlowitz DR, et al. Nursing home assessment of cognitive impairment: development and testing of a brief instrument of mental status. J Am Geriatr Soc. 2008 Nov;56(11):2069–2075. doi: 10.1111/j.1532-5415.2008.01944.x. [DOI] [PubMed] [Google Scholar]
- 22.Mahoney FI, Barthel DW. FUNCTIONAL EVALUATION: THE BARTHEL INDEX. Md State Med J. 1965 Feb;14:61–65. [PubMed] [Google Scholar]
- 23.Levy C, Kheirbek R, Alemi F, Wojtusiak J, Sutton B, Williams AR, et al. Predictors of six-month mortality among nursing home residents: diagnoses may be more predictive than functional disability. J Palliat Med. 2015;18(2):100–106. doi: 10.1089/jpm.2014.0130. [DOI] [PubMed] [Google Scholar]
- 24.Salpeter SR, Malter DS, Luo EJ, Lin AY, Stuart B. Systematic review of cancer presentations with a median survival of six months or less. J Palliat Med. 2012 Feb;15(2):175–185. doi: 10.1089/jpm.2011.0192. [DOI] [PubMed] [Google Scholar]
- 25.King BJ, Gilmore-Bykovskyi AL, Roiland RA, Polnaszek BE, Bowers BJ, Kind AJH. The consequences of poor communication during transitions from hospital to skilled nursing facility: a qualitative study. J Am Geriatr Soc. 2013 Jul;61(7):1095–1102. doi: 10.1111/jgs.12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ouslander JG, Lamb G, Perloe M, Givens JH, Kluge L, Rutland T, et al. Potentially avoidable hospitalizations of nursing home residents: frequency, causes, and costs: [see editorial comments by Drs. Jean F. Wyman and William R. Hazzard, pp 760–761] J Am Geriatr Soc. 2010 Apr;58(4):627–635. doi: 10.1111/j.1532-5415.2010.02768.x. [DOI] [PubMed] [Google Scholar]
- 27.Boockvar K, Fishman E, Kyriacou CK, Monias A, Gavi S, Cortes T. Adverse events due to discontinuations in drug use and dose changes in patients transferred between acute and long-term care facilities. Arch Intern Med. 2004 Mar 8;164(5):545–550. doi: 10.1001/archinte.164.5.545. [DOI] [PubMed] [Google Scholar]
- 28.Boockvar KS, Fridman B, Marturano C. Ineffective communication of mental status information during care transfer of older adults. J Gen Intern Med. 2005 Dec;20(12):1146–1150. doi: 10.1111/j.1525-1497.2005.00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Popejoy L, Galambos C, Vogelsmeier A. Hospital to nursing home transition challenges: perceptions of nursing home staff. J Nurs Care Qual. 2014 Jun;29(2):103–109. doi: 10.1097/NCQ.0000000000000051. [DOI] [PubMed] [Google Scholar]
- 30.Tjia J, Bonner A, Briesacher BA, McGee S, Terrill E, Miller K. Medication discrepancies upon hospital to skilled nursing facility transitions. J Gen Intern Med. 2009 May;24(5):630–635. doi: 10.1007/s11606-009-0948-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burke RE, Juarez-Colunga E, Levy C, Prochazka AV, Coleman EA, Ginde AA. Patient and Hospitalization Characteristics Associated With Increased Postacute Care Facility Discharges From US Hospitals. Med Care. 2015 Jun;53(6):492–500. doi: 10.1097/MLR.0000000000000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skilled Nursing Facility Readmission Measure (SNFRM) NQF #2510: All-Cause Risk-Standardized Readmission Measure - SNFRM-Technical-Report-3252015.pdf. [cited 2015 Sep 11]; [Internet]. Available from: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/NursingHomeQualityInits/Downloads/SNFRM-Technical-Report-3252015.pdf.
- 33.Rahman M, Tyler D, Thomas KS, Grabowski DC, Mor V. Higher Medicare SNF care utilization by dual-eligible beneficiaries: can Medicaid long-term care policies be the answer? Health Serv Res. 2015 Feb;50(1):161–179. doi: 10.1111/1475-6773.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kane RL. Finding the right level of posthospital care: “We didn’t realize there was any other option for him.”. JAMA J Am Med Assoc. 2011 Jan 19;305(3):284–293. doi: 10.1001/jama.2010.2015. [DOI] [PubMed] [Google Scholar]
- 35.Kane RL, Bershadsky B, Bershadsky J. Who recommends long-term care matters. The Gerontologist. 2006 Aug;46(4):474–482. doi: 10.1093/geront/46.4.474. [DOI] [PubMed] [Google Scholar]
- 36.Ward KT, Eslami MS, Garcia MB, McCreath HE. Do internal medicine residents know enough about skilled nursing facilities to orchestrate a good care transition? J Am Med Dir Assoc. 2014;15(11):841–843. doi: 10.1016/j.jamda.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neuman MD, Wirtalla C, Werner RM. Association between skilled nursing facility quality indicators and hospital readmissions. JAMA. 2014 Oct 15;312(15):1542–1551. doi: 10.1001/jama.2014.13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Neill C, Harrington C, Kitchener M, Saliba D. Quality of care in nursing homes: an analysis of relationships among profit, quality, and ownership. Med Care. 2003 Dec;41(12):1318–1330. doi: 10.1097/01.MLR.0000100586.33970.58. [DOI] [PubMed] [Google Scholar]
- 39.Hillmer MP, Wodchis WP, Gill SS, Anderson GM, Rochon PA. Nursing home profit status and quality of care: is there any evidence of an association? Med Care Res Rev MCRR. 2005 Apr;62(2):139–166. doi: 10.1177/1077558704273769. [DOI] [PubMed] [Google Scholar]
- 40.Lage DE, Rusinak D, Carr D, Grabowski DC, Ackerly DC. Creating a network of high-quality skilled nursing facilities: preliminary data on the postacute care quality improvement experiences of an accountable care organization. J Am Geriatr Soc. 2015 Apr;63(4):804–808. doi: 10.1111/jgs.13351. [DOI] [PubMed] [Google Scholar]
- 41.Barnes DE, Mehta KM, Boscardin WJ, Fortinsky RH, Palmer RM, Kirby KA, et al. Prediction of recovery, dependence or death in elders who become disabled during hospitalization. J Gen Intern Med. 2013 Feb;28(2):261–268. doi: 10.1007/s11606-012-2226-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoyer EH, Needham DM, Atanelov L, Knox B, Friedman M, Brotman DJ. Association of impaired functional status at hospital discharge and subsequent rehospitalization. J Hosp Med Off Publ Soc Hosp Med. 2014 May;9(5):277–282. doi: 10.1002/jhm.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peel NM, Navanathan S, Hubbard RE. Gait speed as a predictor of outcomes in post-acute transitional care for older people. Geriatr Gerontol Int. 2014 Oct;14(4):906–910. doi: 10.1111/ggi.12191. [DOI] [PubMed] [Google Scholar]
- 44.Sager MA, Franke T, Inouye SK, Landefeld CS, Morgan TM, Rudberg MA, et al. Functional outcomes of acute medical illness and hospitalization in older persons. Arch Intern Med. 1996 Mar 25;156(6):645–652. [PubMed] [Google Scholar]
- 45.Shih SL, Gerrard P, Goldstein R, Mix J, Ryan CM, Niewczyk P, et al. Functional Status Outperforms Comorbidities in Predicting Acute Care Readmissions in Medically Complex Patients. J Gen Intern Med. 2015 May 9; doi: 10.1007/s11606-015-3350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kane RL, Lin W-C, Blewett LA. Geographic variation in the use of post-acute care. Health Serv Res. 2002 Jun;37(3):667–682. doi: 10.1111/1475-6773.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lenze EJ, Host HH, Hildebrand MW, Morrow-Howell N, Carpenter B, Freedland KE, et al. Enhanced medical rehabilitation increases therapy intensity and engagement and improves functional outcomes in postacute rehabilitation of older adults: a randomized-controlled trial. J Am Med Dir Assoc. 2012 Oct;13(8):708–712. doi: 10.1016/j.jamda.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Falvey JR, Mangione KK, Stevens-Lapsley JE. Rethinking Hospital-Associated Deconditioning: Proposed Paradigm Shift. Phys Ther. 2015 Apr 23; doi: 10.2522/ptj.20140511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jette DU, Warren RL, Wirtalla C. The relation between therapy intensity and outcomes of rehabilitation in skilled nursing facilities. Arch Phys Med Rehabil. 2005 Mar;86(3):373–379. doi: 10.1016/j.apmr.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 50.Walsh TS, Salisbury LG, Merriweather JL, Boyd JA, Griffith DM, Huby G, et al. Increased Hospital-Based Physical Rehabilitation and Information Provision After Intensive Care Unit Discharge: The RECOVER Randomized Clinical Trial. JAMA Intern Med. 2015 Jun 1;175(6):901–910. doi: 10.1001/jamainternmed.2015.0822. [DOI] [PubMed] [Google Scholar]
- 51.Goodwin JS, Howrey B, Zhang DD, Kuo Y-F. Risk of continued institutionalization after hospitalization in older adults. J Gerontol A Biol Sci Med Sci. 2011 Dec;66(12):1321–1327. doi: 10.1093/gerona/glr171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Newhouse JP, Garber AM. Geographic variation in health care spending in the United States: insights from an Institute of Medicine report. JAMA J Am Med Assoc. 2013 Sep 25;310(12):1227–1228. doi: 10.1001/jama.2013.278139. [DOI] [PubMed] [Google Scholar]
- 53.Schumacher DN, Dobkin ED. Medicare spending per beneficiary. Health Aff Proj Hope. 2014 Oct;33(10):1878. doi: 10.1377/hlthaff.2014.0919. [DOI] [PubMed] [Google Scholar]
- 54.Ouslander JG, Lamb G, Tappen R, Herndon L, Diaz S, Roos BA, et al. Interventions to reduce hospitalizations from nursing homes: evaluation of the INTERACT II collaborative quality improvement project. J Am Geriatr Soc. 2011 Apr;59(4):745–753. doi: 10.1111/j.1532-5415.2011.03333.x. [DOI] [PubMed] [Google Scholar]
- 55.Givens JL, Mitchell SL, Kuo S, Gozalo P, Mor V, Teno J. Skilled nursing facility admissions of nursing home residents with advanced dementia. J Am Geriatr Soc. 2013 Oct;61(10):1645–1650. doi: 10.1111/jgs.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barnes C, Conner D, Legault L, Reznickova N, Harrison-Felix C. Rehabilitation outcomes in cognitively impaired patients admitted to skilled nursing facilities from the community. Arch Phys Med Rehabil. 2004 Oct;85(10):1602–1607. doi: 10.1016/j.apmr.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 57.Levy CR, Fish R, Kramer A. Do-not-resuscitate and do-not-hospitalize directives of persons admitted to skilled nursing facilities under the Medicare benefit. J Am Geriatr Soc. 2005 Dec;53(12):2060–2068. doi: 10.1111/j.1532-5415.2005.00523.x. [DOI] [PubMed] [Google Scholar]
- 58.Levy CR, Fish R, Kramer AM. Site of death in the hospital versus nursing home of Medicare skilled nursing facility residents admitted under Medicare’s Part A Benefit. J Am Geriatr Soc. 2004 Aug;52(8):1247–1254. doi: 10.1111/j.1532-5415.2004.52352.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


