Abstract
Chordoid meningioma, World Health Organization grade II, is an uncommon variant of meningioma with a propensity for aggressive behavior and increased likelihood of recurrence. As such, recognition of this entity is important in cases that show similar morphologic overlap with other chondroid/myxoid neoplasms that can arise within or near the central nervous system. A formal comparison of the immunohistochemical features of chordoid meningioma versus tumors with significant histologic overlap has not been previously reported. In this study, immunohistochemical staining was performed with antibodies against D2-40, S100, pankeratin, epithelial membrane antigen (EMA), brachyury, and glial fibrillary acidic protein (GFAP) in 4 cases of chordoid glioma, 6 skeletal myxoid chondrosarcomas, 10 chordoid meningiomas, 16 extraskeletal myxoid chondrosarcoma, 18 chordomas, 22 low-grade chondrosarcomas, and 27 enchondromas. Staining extent and intensity were evaluated semiquantitatively and mean values for each parameter were calculated. Immunostaining with D2-40 showed positivity in 100% of skeletal myxoid chondrosarcomas, 96% of enchondromas, 95% of low-grade chondrosarcomas, 80% of chordoid meningiomas, and 75% of chordoid gliomas. Staining with S100 demonstrated diffuse, strong positivity in all (100%) chordoid gliomas, skeletal myxoid chondrosarcomas, low-grade chondrosarcomas, and enchondromas, 94% of chordomas, and 81% of extraskeletal myxoid chondrosarcomas, with focal, moderate staining in 40% of chordoid meningiomas. Pankeratin highlighted 100% of chordoid gliomas and chordomas, 38% of extraskeletal myxoid chondrosarcomas, and 20% of chordoid meningiomas. EMA staining was positive in 100% of chordoid gliomas, 94% of chordomas, 90% of chordoid meningiomas, and 25% of extraskeletal myxoid chondrosarcomas. Brachyury was positive only in the chordomas (100%), whereas GFAP was positive only in the chordoid gliomas (100%). EMA was the most effective antibody for differentiating chordoid meningioma from skeletal myxoid chondrosarcoma, low-grade chondrosarcoma, and enchondroma, whereas D2-40 was the most effective antibody for differentiating chordoid meningioma from extraskeletal myxoid chondrosarcoma and chordoma. Our findings demonstrate that in conjunction with clinical and radiographic findings, immunohistochemical evaluation with a panel of D2-40, EMA, brachyury, and GFAP is most useful in distinguishing chordoid meningioma from chordoid glioma, skeletal myxoid chondrosarcoma, extraskeletal myxoid chondrosarcoma, chordoma, low-grade chondrosarcoma, and enchondroma. A lack of strong, diffuse S100 reactivity may also be useful in excluding chordoid meningioma. Among the neoplasms evaluated, brachyury and GFAP proved to be both sensitive and specific markers for chordoma and chordoid glioma, respectively. Of note, this study is the first to characterize the D2-40 immunoprofile in extraskeletal myxoid chondrosarcoma, results that could be of utility in differential diagnostic assessment.
Keywords: meningioma, chordoid, myxoid chondrosarcoma, chordoma, chondrosarcoma, enchondroma, immunohistochemistry, D2-40, S100, keratin, EMA, brachyury, GFAP
By World Health Organization criteria,46 histologic features of a meningioma that warrant grade II designation include chordoid, clear cell, or atypical histology, the latter depicted as having ≥4 mitotic figures per 10 high power fields, brain invasion, or 3 of the following features: sheetlike pattern of growth, increased cellularity, small cell morphology, spontaneous necrosis, and prominent nucleoli. Of these 3 categories of grade II meningiomas, the chordoid variant is notable for its rarity, high rate of recurrence, and its historical association with systemic diseases.17,24,38,46
First alluded to in the late 1970 as “myxoid” and “vacuolated” variants of meningioma,20,23 the term “chordoid meningioma” was first coined by Kepes et al in 198838 in a series of young patients with hematologic conditions including Castleman disease. A larger, more recent series failed to identify an association of chordoid meningioma with systemic manifestations; however, the aggressive nature of this neoplasm was confirmed, with 42% of cases (n=14) showing 1 or more recurrences, ranging from 1.8 years to as long as 16 years postoperatively. 17 As such, recognition of this uncommon meningioma variant is paramount.
In the differential diagnosis of a chondroid tumor within or near the central nervous system (CNS), correct identification of the vacuolated trabeculae of neoplastic cells in a myxoid background characteristic of chordoid meningioma is straightforward when accompanied by coexisting areas of typical meningioma. When the chordoid areas predominate or are primarily sampled or when tumors exhibit chondroid metaplasia,49 accurate morphologic diagnosis can be problematic as the differential diagnosis broadens to include chordoma, extraskeletal and skeletal myxoid chondrosarcoma, low-grade chondrosarcoma, enchondroma, chordoid glioma, myxopapillary ependymoma, and metastasis (Figs. 1–3).
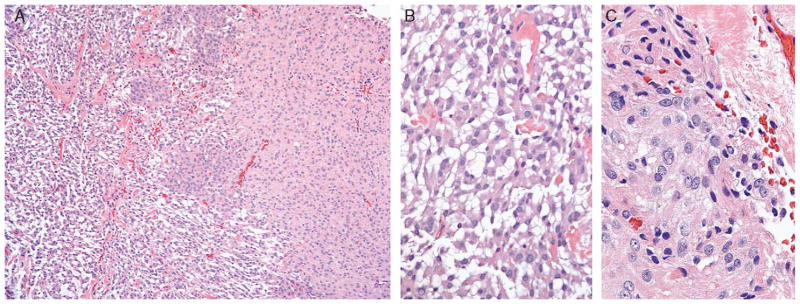
FIGURE 1.
A, Low-power view illustrates chordoid meningioma merging imperceptibly with areas of typical meningioma, making accurate diagnosis straightforward. B, High-power view demonstrates cords of physaliferouslike tumor cells with frequent intracytoplasmic vacuolization characteristic of chordoid meningioma (WHO grade II), contrasting (C) meningothelial meningioma (WHO grade I) morphology. As demonstrated in this case, lymphoplasmacytic inflammation may not always be present. WHO indicates World Health Organization.
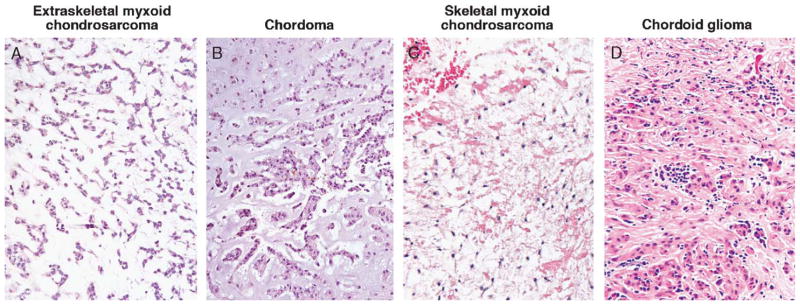
FIGURE 3.
Particularly when present in a CNS/peri-CNS location, corded to nested array of tumor cells with vacuolated cytoplasm embedded in a fibrillar, chondroid, or myxoid matrix makes (A) extraskeletal myxoid chondrosarcoma, (B) chordoma, (C) skeletal myxoid chondrosarcoma, and (D) chordoid glioma plausible entities in the differential diagnosis of chordoid meningioma. CNS indicates central nervous system.
Although several studies have evaluated the use of immunostains in differentiating chondroid tumors within or near the CNS, no previous studies included chordoid meningioma in their immunologic assessment. 1,3,16,27,28,35,50,55–58,67 Moreover, of the case reports and case series that have reported immunostaining results for chordoid meningioma, only 1 report to our knowledge has compared the immunoprofile of chordoid meningioma (2 cases, 1 antibody) with neoplasms of similar morphology.35 The aim of this study was to compare the immunoprofile of a series of chordoid meningiomas versus neoplasms with significant morphologic overlap, using a panel of both novel and traditional antibodies to gauge their potential diagnostic use in the assessment of a chondroid/myxoid tumor arising within or near the CNS.
MATERIALS AND METHODS
Four cases of chordoid glioma, 6 skeletal myxoid chondrosarcomas, 10 chordoid meningiomas, 16 extraskeletal myxoid chondrosarcomas, 18 chordomas, 22 low-grade chondrosarcomas, and 27 enchondromas were retrieved from the pathology archives of Stanford University Medical Center and Emory University Medical Center. Each case was reviewed by 2 of the authors (A.R.S. and M.S.D.), and diagnoses for all cases were confirmed by routine hematoxylin and eosin staining. Cases with ambiguous radiologic findings precluding a definitive histologic diagnosis were excluded. Immunohistochemical staining using antibodies against D2-40, S100, pankeratin, epithelial membrane antigen (EMA), brachyury, and glial fibrillary acidic protein (GFAP) was performed on 1 representative section per case on 4-mm thick formalin-fixed, paraffin-embedded sections mounted on charged slides and baked at 60°C for 1 hour. The primary antibodies used in the study are listed in Table 1. All cases were stained in parallel with appropriate positive and negative controls. Staining extent was semiquantitatively scored as negative (0, <5% cells stained), focally positive (1+, 5% to 10% cells stained), positive (2+, 10% to 50% cells stained), or diffusely positive (3+, >50% cells stained), and a mean extent (range: 0 to 3) calculated. Staining intensity was semiquantitatively scored from 0 to 3+ and a mean intensity (range: 0 to 3) calculated.
TABLE 1.
Antibody Sources and Dilutions
| Antibody | Clone | Dilution | Antigen Retrieval | Source |
|---|---|---|---|---|
| D2-40 | D2-40 | 1:80 | None | Covance (Princeton, NJ) |
| S100 | S100 | 1:1000 | Protease 2 | Dako (Carpinteria, CA) |
| Pankeratin | AE1/AE3; CAM5.2 | 1:50; 1:50 | Protease 2 | Dako (Carpinteria, CA); Becton Dickinson (San Jose, CA) |
| EMA | E29 | 1:20 | None | Dako (Carpinteria, CA) |
| Brachyury | Polyclonal | 1:50 | Citrate | Santa Cruz Biotechnology (Santa Cruz, CA) |
| GFAP | Polyclonal | 1:2000 | None | Dako (Carpinteria, CA) |
EMA indicates epithelial membrane antigen; GFAP, glial fibrillary acidic protein.
Sensitivity, specificity, and overall accuracy of each immunohistochemical stain positive for chordoid meningioma compared with chordoma, extraskeletal and skeletal myxoid chondrosarcoma, low-grade chondrosarcoma, enchondroma, and chordoid glioma were calculated as described elsewhere.4
RESULTS
Clinical Features
The 10 cases of chordoid meningioma demonstrated an equal sex distribution and showed an age range of 7 to 78 years (mean: 43.5 y). The frontal region was the most frequent site of involvement (n=4), followed by the frontotemporal region (n=1), temporal region (n=1), parietal region (n=1), occipital region (n=1), suprasellar region (n=1), and lateral ventricle (n=1). One patient had a history of microcytic anemia, with none having any history of Castleman disease or other hematologic abnormalities. Comparative clinicopathologic features for chordoid glioma, skeletal myxoid chondrosarcoma, chordoid meningioma, extraskeletal myxoid chondrosarcoma, chordoma, low-grade chondrosarcoma, and enchondroma are listed in Table 2.
TABLE 2.
Clinicopathologic Features of Study Cases
| No. Cases | Age (y) | Sex (Male/Female) | Location | |
|---|---|---|---|---|
| Chordoid glioma | 4 | 35 to 50 (mean: 40.5) | 2 M 2 F |
Third ventricle (n=4) |
| Skeletal myxoid chondrosarcoma | 6 | 35to66 (mean: 54.0) | 5 M 1 F |
Femur (n=1) Petroclivus (n=1) Petrous (n=1) Prepontine (n=1) Temporal bone (n=1) Tibia (n=1) |
| Chordoid meningioma | 10 | 7 to 78 (mean: 43.5) | 5 M 5 F |
Frontal region (n=4) Frontotemporal region (n=1) Temporal region (n=1) Parietal region (n=1) Occipital region (n=1) Suprasellar region (n=1) Lateral ventricle (n=1) |
| Extraskeletal myxoid chondrosarcoma | 16 | 39 to 78 (mean: 61.4) | 12 M 4 F |
Thigh (n=7) Abdomen (n=1) Arm (n=1) Chest wall (n=1) Knee (n=1) Lung (n=1) Pelvis (n=1) Perineum (n=1) Scalp (n=1) Suprapubic (n=1) |
| Chordoma | 18 | 32 to 77 (mean: 52.3) | 10 M 8 F |
Clivus (n=4) Coccyx (n=3) Cervical spine (n=2) Soft tissue (n=2) Lumbar spine (n=1) Lung (metastatic) (n=1) Orbit (n=1) Sacrum (n=1) Sella (n=1) Sphenoid (n=1) Thyroid (n=1) |
| Low-grade chondrosarcoma | 22 | 18 to 70 (mean: 50.7) | 10 M 12 F |
Humerus (n=6) Femur (n=3) Skull base (n=2) Tibia (n=2) Fibula (n=1) First ray (n=1) Knee (n=1) Larynx (n=1) Orbit (n=1) Pubic symphysis (n=1) Rib (n=1) Scapula (n=1) Thoracolumbar spine (n=1) |
| Enchondroma | 27 | 3 to 77 (mean: 36) | 8 M 19 F |
Phalynx (n=7) Humerus (n=6) Metacarpal (n=6) Femur (n=3) Acetabulum (n=1) Fibula (n=1) Hand (n=1) Pelvis (n=1) Radius (n=1) |
CNS and/or peri-CNS cases are indicated in italics.
F indicates female; M, male.
Morphologic Features
Review of the 10 chordoid meningioma cases confirmed World Health Organization grade II histology without any anaplastic grade III features (malignant cytology, ≥20 mitotic figures per 10 high power fields, and papillary or rhabdoid morphology).46 Brain invasion was not identified in any cases. A chordoid component was seen in all cases, ranging from 25% to >95%, with 6 cases (60%) demonstrating at least 50% chordoid morphology and 2 (20%) showing near complete (>95%) chordoid morphology (Figs. 1, 2). Lymphoplasmacytic inflammation was present in 7 (70%) of cases, ranging from mild in 2 cases (20%) to minimal in 5 cases (50%). Inflammation was absent in 3 cases (30%).
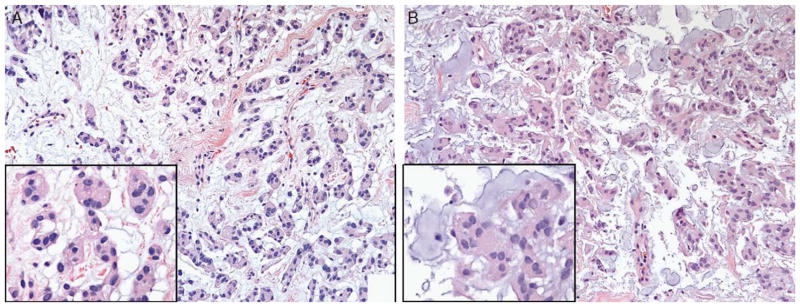
FIGURE 2.
A, Lobulated tumor cells with vacuolated cytoplasm and well-demarcated cell borders characteristic of chordoid meningioma (inset) arising in a delicate fibrous background. B, Chordoid meningioma can also exhibit nested tumor cells (inset) with a more myxoid background. When unaccompanied by areas of typical meningioma or in a diminutive biopsy sample, correct identification of this meningioma variant can be challenging.
Immunohistochemical Results
Immunohistochemical staining results for chordoid glioma, skeletal myxoid chondrosarcoma, chordoid meningioma, extraskeletal myxoid chondrosarcoma, chordoma, low-grade chondrosarcoma, and enchondroma using antibodies D2-40, S100, pankeratin, EMA, brachyury, and GFAP are listed in Table 3.
TABLE 3.
Immunohistochemical Staining Results
| D2-40 (M/C) | S100 (C) | Pankeratin (C) | EMA (C) | Brachyury (N) | GFAP (C) | |
|---|---|---|---|---|---|---|
| Chordoid glioma (n=4) | 3/4 (75%) ME=1.8, MI=1.5 |
4/4 (100%) ME=3, MI=2.5 |
4/4 (100%) ME=2.8, MI=2 |
4/4 (100%) ME=2.3, MI=2 |
0/4 (0%) | 4/4 (100%) ME=3, MI=3 |
| Skeletal myxoid chondrosarcoma (n=6) | 6/6 (100%) ME=2.2, MI=1.8 |
6/6 (100%) ME=3, MI=2.7 |
0/6 (0%) | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) |
| Chordoid meningioma (n=10) | 8/10 (80%) ME=2, MI=1.6 |
4/10 (40%) ME=1, MI=1.5 |
2/10 (20%) ME=2.5, MI=1 |
9/10 (90%) ME=2.3, MI=2.1 |
0/10 (0%) | 0/10 (0%) |
| Extraskeletal myxoid chondrosarcoma (n=16) | 0/16 (0%) | 13/16 (81%) ME=2.4, MI=1.8 |
6/16 (38%) ME=1.8, MI=1.3 |
4/16 (25%) ME=1.8, MI=1.5 |
0/16 (0%) | 0/16 (0%) |
| Chordoma (n=18) | 0/18 (0%) | 17/18 (94%) ME=2.5, MI=2.1 |
18/18 (100%) ME=3, MI=3 |
17/18 (94%) ME=2.6, MI=2.2 |
18/18 (100%) ME=3, MI=2.9 |
0/18 (0%) |
| Low-grade chondrosarcoma (n=22) | 21/22 (95%) ME=2.1, MI=2.4 |
22/22 (100%) ME=2.5, MI=2.2 |
0/22 (0%) | 0/22 (0%) | 0/22 (0%) | 0/22 (0%) |
| Enchondroma (n=27) | 26/27 (96%) ME=2.6, MI=2 |
27/27 (100%) ME=2.7, MI=2.5 |
0/27 (0%) | 0/27 (0%) | 0/27 (0%) | 0/27 (0%) |
C indicates cytoplasmic; EMA, epithelial membrane antigen; GFAP, glial fibrillary acidic protein; M, membranous; ME, mean extent; MI, mean intensity; N, nuclear.
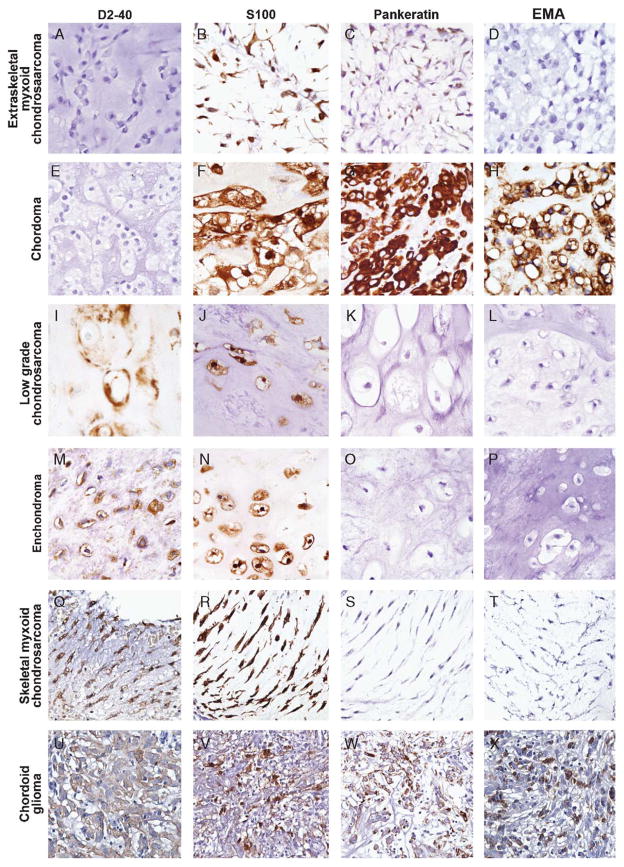
Eight (80%) of 10 chordoid meningiomas stained positive with D2-40 antibody with ample extent and intensity in a membranous to cytoplasmic pattern (Fig. 4A). Although 6 of 6 (100%) skeletal myxoid chondrosarcomas, 26 of 27 (96%) enchondromas, 21 of 22 (95%) low-grade chondrosarcomas, and 3 of 4 (75%) chordoid gliomas demonstrated strong membranous to cytoplasmic D2-40 positivity, all 16 extraskeletal myxoid chondrosarcomas and 18 chordomas were completely negative (Fig. 5). Positive internal control was identified in non-neoplastic bone and cartilage fragments among several of the negatively stained cases.
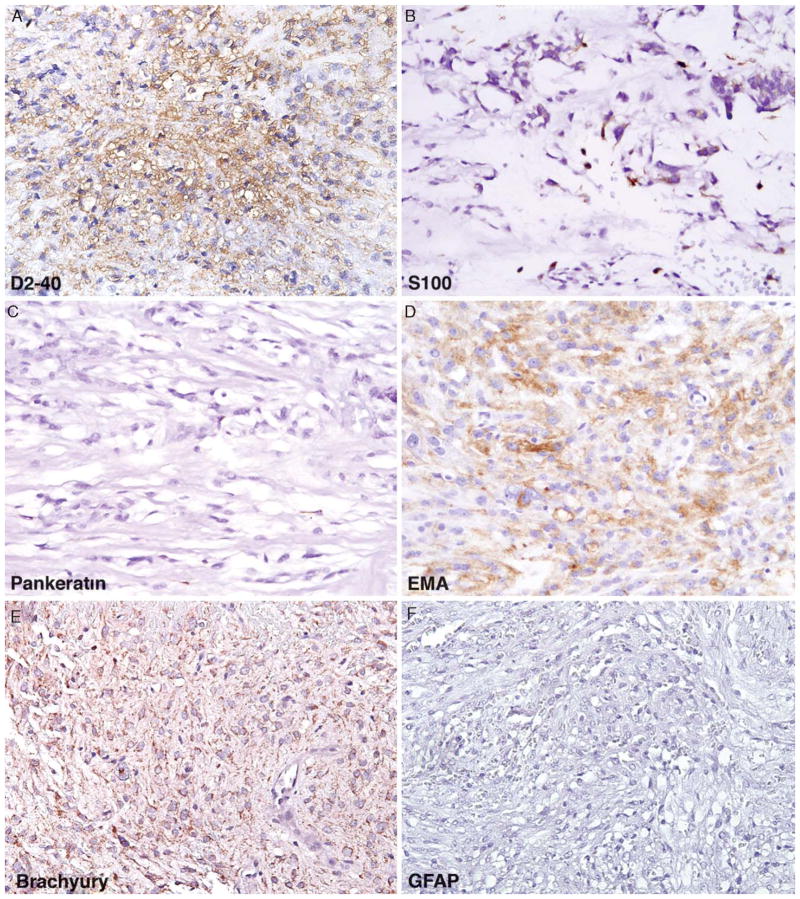
FIGURE 4.
Typical immunoprofile of chordoid meningioma on the basis of the results of this study include (A) consistent membranous to cytoplasmic positivity with D2-40, (B) patchy S100 positivity, (C) predominantly negative staining with pankeratin, (D) strong cytoplasmic positivity for EMA, (E) background cytoplasmic staining (non-nuclear) with brachyury, and (F) negative staining with GFAP. EMA indicates epithelial membrane antigen; GFAP, glial fibrillary acidic protein.
FIGURE 5.
Representative immunoprofile of tumors evaluated in this study included in the morphologic differential diagnosis of chordoid meningioma. Extraskeletal myxoid chondrosarcoma: (A) D2-40 negative, (B) S100 positive, (C) pankeratin positive (patchy, weak), and (D) EMA negative. Chordoma: (E) D2-40 negative, (F) S100 positive, (G) pankeratin positive, and (H) EMA positive. Low-grade chondrosarcoma, enchondroma, and skeletal myxoid chondrosarcoma: (I/M/Q) D2-40 positive, (J/N/R) S100 positive, (K/O/S) pankeratin negative, and (L/P/T) EMA negative. Chordoid glioma: (U) D2-40 positive, (V) S100 positive, (W) pankeratin positive, and (X) EMA positive. EMA indicates epithelial membrane antigen.
Weak extent with average intensity of cytoplasmic S100 staining was identified in 4 of 10 (40%) chordoid meningiomas (Fig. 4B). Strong staining for S100 was seen in 13 of 16 (81%) extraskeletal myxoid chondrosarcomas, 17 of 18 (94%) chordomas, and among all 4 chordoid gliomas, 6 skeletal myxoid chondrosarcomas, 22 low-grade chondrosarcomas, and 27 enchondromas (Fig. 5). S100 staining in chordoma, skeletal myxoid chondrosarcoma, low-grade chondrosarcoma, and enchondroma was both nuclear and cytoplasmic, whereas in chordoid glioma and extraskeletal myxoid chondrosarcoma staining was predominantly cytoplasmic (1 case of each tumor type showed both nuclear and cytoplasmic staining). Similar to D2-40 staining, positive internal control S100 staining was noted in non-neoplastic fragments of osteocytes and chondrocytes.
Weak, patchy pankeratin cytoplasmic staining was identified in 2 of 10 (20%) chordoid meningiomas (Fig. 4C) and 6 of 16 (38%) extraskeletal myxoid chondrosarcomas (Fig. 5C). On the contrary, all 18 chordomas demonstrated maximum (3+) extent and intensity with pankeratin staining; all 4 chordoid gliomas also showed pankeratin reactivity with equal extent but with moderate intensity. All cartilaginous tumors (6 skeletal myxoid chondrosarcomas, 22 low-grade chondrosarcomas, and 27 enchondromas) showed no pankeratin immunoreactivity (Fig. 5).
EMA showed cytoplasmic staining in 9 of 10 (90%) cases of chordoid meningioma (Fig. 5D), 4 of 4 (100%) cases of chordoid gliomas, 17 of 18 (94%) cases of chordoma, 4 of 16 (25%) cases of extraskeletal myxoid chondrosarcoma, and lack of staining in all skeletal myxoid chondrosarcomas (0 of 6), low-grade chondrosarcomas (0 of 22), and enchondromas (0 of 27) (Fig. 5).
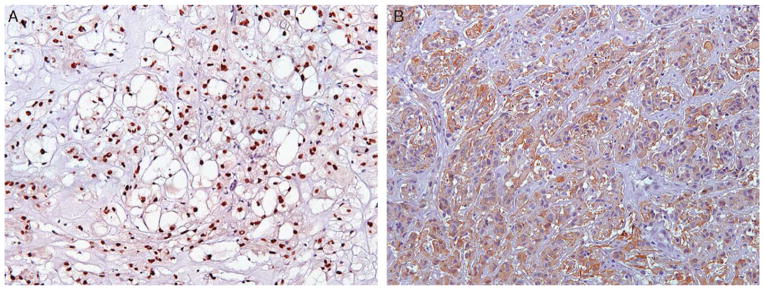
Diffuse, strong nuclear reactivity with brachyury was identified in all 18 chordomas (Fig. 6A), with diffusely negative to patchy cytoplasmic staining seen in all other tumors. Cytoplasmic staining for GFAP was diffusely and strongly positive in 4 of 4 (100%) chordoid gliomas (Fig. 6B), with all other tumors showing no staining.
FIGURE 6.
A, Strong, diffuse nuclear staining for brachyury was specific for chordoma among the neoplasms evaluated in this study and therefore useful in excluding chordoid meningioma. Likewise, (B) strong, diffuse cytoplasmic reactivity with GFAP was specific for chordoid glioma and not identified in chordoma, extraskeletal and skeletal myxoid chondrosarcoma, low-grade chondrosarcoma, or enchondroma. GFAP indicates glial fibrillary acidic protein.
Sensitivity, specificity, and overall accuracy for immunomarkers positive for chordoid meningioma compared with chordoma, extraskeletal and skeletal myxoid chondrosarcoma, low-grade chondrosarcoma, enchondroma, and chordoid glioma are listed in Table 4.
TABLE 4.
Sensitivity and Specificity of Markers Positive for Chordoid Meningioma*
| Sensitivity (%) | Specificity (%) | Overall Accuracy (%) | |
|---|---|---|---|
| D2-40 | 80 | 40 | 44 |
| S100 | 40 | 4 | 8 |
| Pankeratin | 20 | 70 | 65 |
| EMA | 90 | 73 | 75 |
Compared with chordoid glioma, skeletal myxoid chondrosarcoma, chordoma, extraskeletal myxoid chondrosarcoma, low-grade chondrosarcoma, and enchondroma.
EMA indicates epithelial membrane antigen.
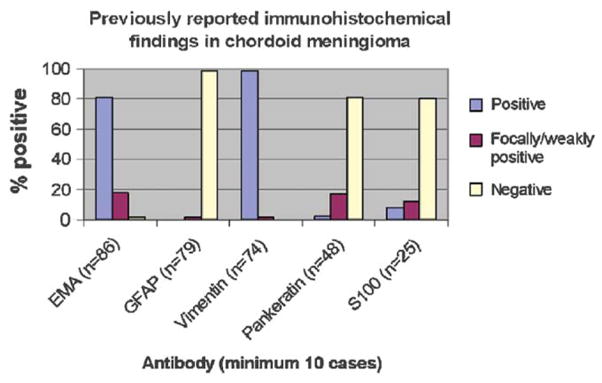
Meta-analysis of Immunohistochemical Results in Chordoid Meningioma From the Published Literature
Meta-analysis results of previously reported immunohistochemical findings in chordoid meningioma6,17,21,22,24,30,35,36,38,40,42,43,45,47– 49,51–54,61,62,70,75,77,80,82,86 (published in English language) are shown in Figure 7. Additional immunohistochemical staining results in chordoid meningioma reported in the literature (which were not included in Fig. 7 owing to small sample size) include 4 of 5 cases positive for CD57, 3 of 3 cases positive for progesterone receptor, and 2 of 2 cases positive for D2-40.35,38,45,52,61 Reported cases of chordoid meningioma have shown no immunohistochemical staining for the following antibodies: carcinoembryonic antigen (n=4), CD34 (n=4), desmin (n=4), smooth muscle actin (n=3), synaptophysin (n=3), chromogranin (n=2), CD56 (n=1), CD99 (n=1), calponin (n=1), 34βE12 (n=1), keratin 14 (n=1), keratin 19 (n=1), factor VIII (n=1), neurofilament (n=1), p63 (n=1), and muscle specific actin (n=1).21,38,40,48,49,52,54,62,70,80
FIGURE 7.
Meta-analysis results of previously reported immunohistochemical findings in chordoid meningioma6,17,21,22,24,30,35,36,38,40,42,43,45,47– 49,51–54,61,62,70,75,77,80,82,86 (published in English language).
DISCUSSION
Although the use of immunohistochemistry in tumor diagnosis is longstanding, few previous studies have been performed to compare the immunoprofile of meningioma with extra-CNS neoplasms.12 Despite several advancements in identification of meningioma staining patterns in the past 30 years, inconsistent case report findings have made it difficult to determine the characteristic immunoprofile of meningioma. Although immunohistochemical staining is frequently a function of tumor differentiation (explaining why a poorly or dedifferentiated neoplasm may exhibit an aberrant or heterogeneous staining pattern), reports of tumor immunoprofiles frequently fail to describe staining degree and/or distribution. Omission of this important information is especially problematic when reporting a “unique” immunoprofile, particularly for pathologists seeking assistance in diagnosis of rare entities. For example, in review of the published immunohistochemical findings of chordoid meningiomas with S100, an antibody whose expression in meningiomas is typically not prominent,46 there is a reported overall positivity rate of 20% (Fig. 7). Of the 5 cases reporting positive S100 staining in chordoid meningioma, only 3 (60%) offer information regarding the distribution of staining (focal), with none describing degree of staining.17,38,48,53 In the absence of a more detailed depiction of S100 staining pattern in chordoid meningioma, a diagnostician seeking ancillary assistance in immunohistochemistry is left with incomplete and potentially misleading data.
In this study, we characterized the immunoprofile of a series of chordoid meningiomas by incorporating semiquantitative analysis of staining intensity and extent while using appropriate statistical tools to determine immunomarker diagnostic utility. We then compared findings with neoplasms exhibiting significant morphologic overlap with chordoid meningioma capable of arising within or near the CNS. Although the differential diagnosis of such chondroid/myxoid neoplasms includes chordoma, extraskeletal myxoid chondrosarcoma, skeletal myxoid chondrosarcoma, low-grade chondrosarcoma, enchondroma, chordoid glioma, myxopapillary ependymoma, and metastasis, we focused our investigation on the first 6 tumors.
Of the neoplasms evaluated in the differential diagnosis of a chordoid tumor, chordoma is potentially the most challenging, not only because of its strikingly similar morphology (Figs. 1–3) but also because of its propensity for midline (CNS/peri-CNS) site of origin.32 The cords and trabeculae of tumor cells with frequent intracytoplasmic vacuoles mimicking the physaliferous cells of chordoma (warranting incorporation of “chordoid” in its name) was identified in all 7 chordoid meningiomas from the original description by Kepes et al in 1988,38 noting that at least one of the cases was originally misdiagnosed as chordoma. Historically exemplified as one of the unique “triple positive” S100/keratin/EMA staining neoplasms in bone and soft tissue pathology,1,16,55 the current study demonstrates identical findings (Table 3). As such, strong EMA positivity in chordoid meningioma is not helpful in differentiating from chordoma. Similarly, because of a moderate number of chordoid meningiomas with weak, inconsistent S100 staining and a few cases with modest extent of pankeratin staining, these 2 antibodies are not helpful in definitive chordoma distinction.
One of the more recently developed markers, D2-40, is a monoclonal antibody recognizing podoplanin, a 38 kd mucin-type transmembrane glycoprotein, and has been used in the clinical setting as a selective marker of lymphatic endothelium25,74 and in the identification of various normal and neoplastic tissues,60 including within the CNS.35,71,76 Only 2 reports of D2-40 immunostaining in meningiomas have been published to our knowledge, 1 demonstrating expression in all cases of unspecified type (n=100)76 and 1 showing 100% immunoreactivity in the chordoid variant of meningioma (n=2).35 Consistent with the findings from the latter report, our series of 10 chordoid meningiomas formally illustrates the utility of positive D2-40 staining in discriminating from chordoma (Table 3).
Brachyury is another novel antibody useful in the distinction of chordoid meningioma from chordoma. This marker, a critical regulator of notochordal development, has recently been shown to be a sensitive and fairly specific antibody for chordoma,33,81,83 with expression also identified in CNS hemangioblastomas and testicular germ cell tumors.81 A lack of nuclear brachyury immunoreactivity among all 10 chordoid meningiomas (Fig. 4E), results that to our knowledge have not been previously reported, in conjunction with diffuse, strong nuclear staining with all 18 chordomas reiterates brachyury’s high specificity among tumors evaluated in this study and supports its efficacy in differentiating chordoid meningioma from chordoma.
Although classically considered a soft tissue neoplasm, extraskeletal myxoid chondrosarcoma can infrequently present in CNS/peri-CNS locations2,13,14,18,29,37,44,72,73,85 that, in conjunction with its morphologic appearance of lobulated to nested tumor cells with occasional vacuolization embedded in a fibrillar to myxoid matrix, can mimic chordoid meningioma (Figs. 1–3). Of the 7 largest published series to date exploring the immunoprofile of extraskeletal myxoid chondrosarcoma (minimum 8 cases) with pankeratin, EMA, or S100 antibodies,3,27,28,50,55,57,58 pankeratin staining ranged from 0% to 12% (mean 3%, n=92), EMA staining ranged from 0% to 25% (mean 10%, n=97), and S100 staining ranged from 13% to 100% (mean 48%, n=146). In review of these meta-analysis results compared with the findings from our study (Table 3), several relevant points emerge. First, the wide range of reported S100 staining results in extraskeletal myxoid chondrosarcoma draws a parallel with its controversial history,3,5,27,28,34,50,56 originally classified as chondroblastic/chondrocytic in nature with more recent studies suggesting otherwise. Likewise, positive D2-40 internal control staining of chondrocytes in extraskeletal myxoid chondrosarcoma without staining in tumor cells may support this contention. Second, although this study demonstrates a somewhat higher rate of positivity with S100, pankeratin, and EMA antibodies compared with historical (mean) results, the applicable deductions are that pankeratin is unlikely to be helpful in distinguishing from chordoid meningioma; a lack of strong, diffuse S100 reactivity may be useful in excluding chordoid meningioma; and that diffuse, strong EMA staining could suggest a diagnosis of chordoid meningioma over extraskeletal myxoid chondrosarcoma (Table 3) in the appropriate clinical and radiologic context. Finally, a lack of immunoreactivity with D2-40 antibody in extraskeletal myxoid chondrosarcoma, results not previously identified, in conjunction with positive staining in chordoid meningioma can be extremely useful in arriving at a correct diagnosis.
The prototypic situation where immunohistochemistry is indispensable occurs in the assessment of a midline (peri-CNS) neoplasm with a nested pattern and vesicular nuclei arising in a myxoid background. The implementation of a panel including S100, pankeratin, and EMA in this scenario is often the only means to separate chordoma (positive for all 3 markers) from chondrosarcoma (positive only for S100 antibody),1,16,55,68 a well-characterized problematic CNS distinction particularly in the skull base.41,68 When the background stroma of neoplasms with this morphology arising in a peri-CNS/CNS anatomic site is more myxoid than chondroid, significant overlap between low-grade chondrosarcoma and skeletal myxoid chondrosarcoma with chordoid meningioma can occur (Figs. 1–3). Moreover, cartilaginous metaplasia in chordoid meningioma legitimizes the potential for morphologic overlap.49 Likewise, as low-grade chondrosarcoma and enchondroma are often histologically indistinguishable requiring skilled radiologic appraisal for accurate diagnosis,84 enchondroma warrants inclusion in a chordoid meningioma differential diagnosis. In review of immunohistochemical staining results from this study, reactivity with EMA is superior to pankeratin in establishing a diagnosis of chordoid meningioma versus low-grade chondrosarcoma/enchondroma or skeletal myxoid chondrosarcoma, with S100 antibody proving the least effective (Tables 3, 4). As expected, low-grade chondrosarcoma and enchondroma demonstrated an identical immunoprofile, consistent with the only published report solely investigating D2-40 antibody in these neoplasms,35 with skeletal myxoid chondrosarcoma also demonstrating equal D2-40 reactivity, results that have not been previously recognized. Importantly, although D2-40 provides no substantial assistance in the differentiation between low-grade chondrosarcoma/enchondroma or skeletal myxoid chondrosarcoma and chordoid meningioma compared with EMA antibody (and potentially pankeratin), it is to our knowledge the only reliable antibody positive in low-grade chondrosarcoma/enchondroma and skeletal myxoid chondrosarcoma and negative in both chordoma and extraskeletal myxoid chondrosarcoma, an immunoprofile not previously reported.
Although chordoid glioma can feature a prominent lymphoplasmacytic infiltrate in combination with a chondroid/myxoid appearance making chordoid meningioma a diagnostic consideration (Fig. 3D) particularly on a diminutive biopsy sample and given reports of chondroid metaplasia in chordoid glioma,11 radiographic findings in combination with characteristic strong GFAP staining8,17,46,63 typically make confusion with chordoid meningioma (normally GFAP negative) unlikely. Diffuse, strong GFAP reactivity in all 4 chordoid gliomas (Fig. 6B) with negative staining in all 10 chordoid meningiomas (Fig. 4F) reiterates its diagnostic utility in this setting. Of note, this study also reaffirms the reported S100+/pankeratin+/EMA+ chordoid glioma immunoprofile from the largest series to date.63 Additionally, the 3 of 4 (75%) cases of chordoid glioma with D2-40 reactivity expands on the only previously reported finding (100%, n=1).76
Additional ancillary methods of differentiating chordoid meningioma from its morphologic mimics include ultrastructural evaluation for characteristic meningioma features, including intermediate filaments, interdigitating cellular processes, and desmosomal intercellular junctions.22,46 Although secretory activity may be identified by electron microscopy in chordoid-predominant areas of chordoid meningioma,22 careful ultrastructural exploration for areas of typical meningioma may be necessary. In addition, a unique unbalanced translocation involving chromosomes 1 and 3 identified in 3 cases of chordoid meningioma not observed in other meningioma subtypes78 suggests that cytogenetic studies and potentially fluorescence in-situ hybridization methodologies could be of diagnostic utility, although further investigation in this area is needed.
Although excluded from our evaluation, myxopapillary ependymoma and metastasis may be considered in the differential diagnosis of a chondroid tumor within or near the CNS. Metastasis can focally mimic chordoid meningioma, particularly when arising from a gastrointestinal (signet ring or mucinous) adenocarcinoma or renal cell carcinoma. In this scenario, clinical and radiographic correlation in conjunction with foci exhibiting typical meningioma morphology is often sufficient for discrimination. Moreover, conventional immunohistochemical panels for primary tumor origin are often useful and adequate. Although a plausible inclusion in the differential diagnosis of chordoid meningioma, myxopapillary ependymoma separates itself from the differential by its typical conus/filum terminale location, encapsulated gross morphology, and papillary arrangement around vascularized stromal cores.46,65 Additionally, strong GFAP staining15 further distinguishes myxopapillary ependymoma from chordoid meningioma (Figs. 6B, 7).
Although vimentin antibody has historically been used as a marker for most meningiomas,46 including the chordoid variant (Fig. 7), we excluded it in our immunohistochemical assessment because of well-known overlap in staining with poor specificity for mesenchymal neoplasms, including those from this study.19 Similarly, although progesterone receptor immunoexpression is well characterized in meningothelial meningioma,46,59 expression in meningioma variants varies from 0% up to 100% in large series,7,10,31,39,59,64,66,69,87 including reports of chordoid meningioma.45,61 Although progesterone receptor expression is not well characterized in enchondromas or chondrosarcomas, expression in chordomas and chordoid gliomas have shown unreliable, mixed results with uncertain diagnostic significance.9,26,79 As such, this marker was also excluded from our study.
In summary, when encountering a chordoid neoplasm within or near the CNS with cytoplasmic vacuolization and a fibrillar to myxoid background that is suggestive of chordoid meningioma, immunohistochemistry in conjunction with clinical and radiologic findings can be useful in distinguishing chordoid meningioma from morphologic mimics (Table 5). Immunoreactivity with D2-40 antibody in chordoid meningioma is useful in differentiation from both extraskeletal myxoid chondrosarcoma and chordoma. Nuclear reactivity with brachyury is also valuable in distinguishing chordoma from chordoid meningioma. Additionally, EMA positivity is superior to pankeratin in discriminating chordoid meningioma from skeletal myxoid chondrosarcoma and low-grade chondrosarcoma/enchondroma. A lack of strong, diffuse S100 reactivity may be useful in excluding chordoid meningioma from neoplasms with morphologic overlap. Although not usually considered in the typical differential diagnosis of chordoid meningioma, chordoid glioma and myxopapillary ependymoma can be dependably evaluated using GFAP antibody. Although chordoid meningioma exhibits a propensity for aggressive behavior and increased recurrence compared with typical meningiomas, it may portend an entirely different prognosis (and subsequent alternative treatment) when compared with other tumors with morphologic overlap, making accurate diagnosis imperative.
TABLE 5.
Useful Immunoprofile in the Differential Diagnosis of Chordoid/Myxoid Neoplasms in a CNS/peri-CNS Anatomic Location
| Neoplasm* | Antibody
|
|||||
|---|---|---|---|---|---|---|
| D2-40 | EMA | Pankeratin | GFAP | Brachyury | S100† | |
| Chordoid glioma | + | + | + | + | − | + |
| Chordoid meningioma | + | + | ± | − | − | − |
| Chordoma‡ | − | + | + | − | + | + |
| Enchondroma§ | + | − | − | − | − | + |
| Extraskeletal myxoid chondrosarcoma‡ | − | ± | ± | − | − | ± |
| Low-grade chondrosarcoma§ | + | − | − | − | − | + |
| Skeletal myxoid chondrosarcoma | + | − | − | − | − | + |
In conjunction with clinical and radiographic findings, metastasis should always be excluded.
Diffuse, strong S100 staining.
Can also be immunohistochemically differentiated using keratin 5/6 (CK5/6) and keratin 19 (CK19).46
Because histology can be identical, radiologic assessment is often the only means of differentiation.67
CNS indicates central nervous system; EMA, epithelial membrane antigen; GFAP, glial fibrillary acidic protein.
Acknowledgments
The authors thank Anet James for her excellent technical assistance in photomicrograph preparation. They also thank Dr Jesse McKenney for his helpful suggestions to the manuscript.
Footnotes
Presented in part at the 97th meeting of the United States and Canadian Academy of Pathology, Denver, CO, March, 2008.
References
- 1.Abenoza P, Sibley RK. Chordoma: an immunohistologic study. Hum Pathol. 1986;17:744–747. doi: 10.1016/s0046-8177(86)80185-x. [DOI] [PubMed] [Google Scholar]
- 2.Acero J, Escrig M, Gimeno M, et al. Extraskeletal myxoid chondrosarcoma of the infratemporal fossa: a case report. Int J Oral Maxillofac Surg. 2003;32:342–345. doi: 10.1054/ijom.2002.0335. [DOI] [PubMed] [Google Scholar]
- 3.Aigner T, Oliveira AM, Nascimento AG. Extraskeletal myxoid chondrosarcomas do not show a chondrocytic phenotype. Mod Pathol. 2004;17:214–221. doi: 10.1038/modpathol.3800036. [DOI] [PubMed] [Google Scholar]
- 4.Alberg AJ, Park JW, Hager BW, et al. The use of “overall accuracy” to evaluate the validity of screening or diagnostic tests. J Gen Intern Med. 2004;19:460–465. doi: 10.1111/j.1525-1497.2004.30091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonescu CR, Argani P, Erlandson RA, et al. Skeletal and extraskeletal myxoid chondrosarcoma: a comparative clinicopathologic, ultrastructural, and molecular study. Cancer. 1998;83:1504–1521. doi: 10.1002/(sici)1097-0142(19981015)83:8<1504::aid-cncr5>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 6.Arima T, Natsume A, Hatano H, et al. Intraventricular chordoid meningioma presenting with Castleman disease due to overproduction of interleukin-6. Case report. J Neurosurg. 2005;102:733–737. doi: 10.3171/jns.2005.102.4.0733. [DOI] [PubMed] [Google Scholar]
- 7.Bozzetti C, Camisa R, Nizzoli R, et al. Estrogen and progesterone receptors in human meningiomas: biochemical and immunocytochemical evaluation. Surg Neurol. 1995;43:230–233. doi: 10.1016/0090-3019(95)80003-y. discussion 234. [DOI] [PubMed] [Google Scholar]
- 8.Brat DJ, Scheithauer BW, Staugaitis SM, et al. Third ventricular chordoid glioma: a distinct clinicopathologic entity. J Neuropathol Exp Neurol. 1998;57:283–290. doi: 10.1097/00005072-199803000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Camacho-Arroyo I, Gonzalez-Aguero G, Gamboa-Dominguez A, et al. Progesterone receptor isoforms expression pattern in human chordomas. J Neurooncol. 2000;49:1–7. doi: 10.1023/a:1006412000726. [DOI] [PubMed] [Google Scholar]
- 10.Carroll RS, Glowacka D, Dashner K, et al. Progesterone receptor expression in meningiomas. Cancer Res. 1993;53:1312–1316. [PubMed] [Google Scholar]
- 11.Castellano-Sanchez AA, Schemankewitz E, Mazewski C, et al. Pediatric chordoid glioma with chondroid metaplasia. Pediatr Dev Pathol. 2001;4:564–567. doi: 10.1007/s10024001-0087-1. [DOI] [PubMed] [Google Scholar]
- 12.Catalano LW, Jr, Harter DH, Hsu KC. Common antigen in meningioma-derived cell cultures. Science. 1972;175:180–182. doi: 10.1126/science.175.4018.180. [DOI] [PubMed] [Google Scholar]
- 13.Ceylan K, Kizilkaya Z, Yavanoglu A. Extraskeletal myxoid chondrosarcoma of the nasal cavity. Eur Arch Otorhinolaryngol. 2006;263:1044–1047. doi: 10.1007/s00405-006-0102-2. [DOI] [PubMed] [Google Scholar]
- 14.Charabi S, Engel P, Bonding P. Myxoid tumours in the temporal bone. J Laryngol Otol. 1989;103:1206–1209. doi: 10.1017/s0022215100111351. [DOI] [PubMed] [Google Scholar]
- 15.Coffin CM, Swanson PE, Wick MR, et al. An immunohistochemical comparison of chordoma with renal cell carcinoma, colorectal adenocarcinoma, and myxopapillary ependymoma: a potential diagnostic dilemma in the diminutive biopsy. Mod Pathol. 1993;6:531–538. [PubMed] [Google Scholar]
- 16.Coindre JM, Rivel J, Trojani M, et al. Immunohistological study in chordomas. J Pathol. 1986;150:61–63. doi: 10.1002/path.1711500110. [DOI] [PubMed] [Google Scholar]
- 17.Couce ME, Aker FV, Scheithauer BW. Chordoid meningioma: a clinicopathologic study of 42 cases. Am J Surg Pathol. 2000;24:899–905. doi: 10.1097/00000478-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Cummings TJ, Bridge JA, Fukushima T. Extraskeletal myxoid chondrosarcoma of the jugular foramen. Clin Neuropathol. 2004;23:232–237. [PubMed] [Google Scholar]
- 19.Dabbs D. Diagnostic Immunohistochemistry. Philadelphia: Churchill Livingstone Elsevier; 2006. pp. 787–792. [Google Scholar]
- 20.Dahmen HG. Studies on mucous substances in myxomatous meningiomas. Acta Neuropathol. 1979;48:235–237. doi: 10.1007/BF00690527. [DOI] [PubMed] [Google Scholar]
- 21.Denaro L, Di Rocco F, Gessi M, et al. Pyrogenic cytokine interleukin-6 expression by a chordoid meningioma in an adult with a systemic inflammatory syndrome. Case report and review of the literature. J Neurosurg. 2005;103:555–558. doi: 10.3171/jns.2005.103.3.0555. [DOI] [PubMed] [Google Scholar]
- 22.Donato G, Ferraro G, Signorelli F, et al. Chordoid meningioma: case report and literature review. Ultrastruct Pathol. 2006;30:309–314. doi: 10.1080/01913120600820591. [DOI] [PubMed] [Google Scholar]
- 23.Eimoto T, Hashimoto K. Vacuolated meningioma. A light and electron microscopic study. Acta Pathol Jpn. 1977;27:557–566. [PubMed] [Google Scholar]
- 24.Epari S, Sharma MC, Sarkar C, et al. Chordoid meningioma, an uncommon variant of meningioma: a clinicopathologic study of 12 cases. J Neurooncol. 2006;78:263–269. doi: 10.1007/s11060-005-9092-y. [DOI] [PubMed] [Google Scholar]
- 25.Evangelou E, Kyzas PA, Trikalinos TA. Comparison of the diagnostic accuracy of lymphatic endothelium markers: Bayesian approach. Mod Pathol. 2005;18:1490–1497. doi: 10.1038/modpathol.3800457. [DOI] [PubMed] [Google Scholar]
- 26.Fasig JH, Dupont WD, Olson SJ, et al. Steroid hormone receptor and COX-2 expression in chordoma. Am J Clin Pathol. 2007;128:375–381. doi: 10.1309/8T2NPHLK5X5WQ3E7. [DOI] [PubMed] [Google Scholar]
- 27.Fletcher CD, Powell G, McKee PH. Extraskeletal myxoid chondrosarcoma: a histochemical and immunohistochemical study. Histopathology. 1986;10:489–499. doi: 10.1111/j.1365-2559.1986.tb02500.x. [DOI] [PubMed] [Google Scholar]
- 28.Goh YW, Spagnolo DV, Platten M, et al. Extraskeletal myxoid chondrosarcoma: a light microscopic, immunohistochemical, ultrastructural and immuno-ultrastructural study indicating neuroendocrine differentiation. Histopathology. 2001;39:514–524. doi: 10.1046/j.1365-2559.2001.01277.x. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez-Lois C, Cuevas C, Abdullah O, et al. Intracranial extraskeletal myxoid chondrosarcoma: case report and review of the literature. Acta Neurochir (Wien) 2002;144:735–740. doi: 10.1007/s00701-002-0949-y. [DOI] [PubMed] [Google Scholar]
- 30.Hasegawa S, Yoshioka S, Urabe S, et al. Rapidly enlarging chordoid meningioma with abundant mucin production. Neuropathology. 2006;26:438–441. doi: 10.1111/j.1440-1789.2006.00708.x. [DOI] [PubMed] [Google Scholar]
- 31.Hasselblatt M, Nolte KW, Paulus W. Angiomatous meningioma: a clinicopathologic study of 38 cases. Am J Surg Pathol. 2004;28:390–393. doi: 10.1097/00000478-200403000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Healey JH, Lane JM. Chordoma: a critical review of diagnosis and treatment. Orthop Clin North Am. 1989;20:417–426. [PubMed] [Google Scholar]
- 33.Henderson SR, Guiliano D, Presneau N, et al. A molecular map of mesenchymal tumors. Genome Biol. 2005;6:R76. doi: 10.1186/gb-2005-6-9-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hinrichs SH, Jaramillo MA, Gumerlock PH, et al. Myxoid chondrosarcoma with a translocation involving chromosomes 9 and 22. Cancer Genet Cytogenet. 1985;14:219–226. doi: 10.1016/0165-4608(85)90187-6. [DOI] [PubMed] [Google Scholar]
- 35.Huse JT, Pasha TL, Zhang PJ. D2-40 functions as an effective chondroid marker distinguishing true chondroid tumors from chordoma. Acta Neuropathol. 2007;113:87–94. doi: 10.1007/s00401-006-0140-2. [DOI] [PubMed] [Google Scholar]
- 36.Ibrahim A, Galloway M, Leung C, et al. Cervical spine chordoid meningioma. Case report. J Neurosurg Spine. 2005;2:195–198. doi: 10.3171/spi.2005.2.2.0195. [DOI] [PubMed] [Google Scholar]
- 37.Jawad J, Lang J, Leader M, et al. Extraskeletal myxoid chondrosarcoma of the maxillary sinus. J Laryngol Otol. 1991;105:676–677. doi: 10.1017/s0022215100117001. [DOI] [PubMed] [Google Scholar]
- 38.Kepes JJ, Chen WY, Connors MH, et al. “Chordoid” meningeal tumors in young individuals with peritumoral lymphoplasmacellular infiltrates causing systemic manifestations of the Castleman syndrome. A report of seven cases. Cancer. 1988;62:391–406. doi: 10.1002/1097-0142(19880715)62:2<391::aid-cncr2820620226>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 39.Khalid H. Immunohistochemical study of estrogen receptor-related antigen, progesterone and estrogen receptors in human intracranial meningiomas. Cancer. 1994;74:679–685. doi: 10.1002/1097-0142(19940715)74:2<679::aid-cncr2820740221>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 40.Kobata H, Kondo A, Iwasaki K, et al. Chordoid meningioma in a child. Case report. J Neurosurg. 1998;88:319–323. doi: 10.3171/jns.1998.88.2.0319. [DOI] [PubMed] [Google Scholar]
- 41.Korten AG, ter Berg HJ, Spincemaille GH, et al. Intracranial chondrosarcoma: review of the literature and report of 15 cases. J Neurol Neurosurg Psychiatry. 1998;65:88–92. doi: 10.1136/jnnp.65.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kozler P, Benes V, Netuka D, et al. Chordoid meningioma: presentation of two case reports, review of the literature, and plea for data standardisation. J Neurooncol. 2008;88:115–120. doi: 10.1007/s11060-008-9541-5. [DOI] [PubMed] [Google Scholar]
- 43.Lee DK, Kim DG, Choe G, et al. Chordoid meningioma with polyclonal gammopathy. Case report. J Neurosurg. 2001;94:122–126. doi: 10.3171/jns.2001.94.1.0122. [DOI] [PubMed] [Google Scholar]
- 44.Liu-Shindo M, Rice DH, Sherrod AE. Extraskeletal myxoid chondrosarcoma of the head and neck: a case report. Otolaryngol Head Neck Surg. 1989;101:485–488. doi: 10.1177/019459988910100414. [DOI] [PubMed] [Google Scholar]
- 45.Liu AJ, Wang FL, Li XH. Chordoid meningioma: a report of two cases. Chin Med J (Engl) 2007;120:726–728. [PubMed] [Google Scholar]
- 46.Louis DN, Wiestler O, Cavenee W. World Health Organization Classification of Tumours of the Central Nervous System. Geneva: IARC Press; 2007. pp. 72–73.pp. 90–91.pp. 164–172. [Google Scholar]
- 47.Lui PC, Chau TK, Wong SS, et al. Cytology of chordoid meningioma: a series of five cases with emphasis on differential diagnoses. J Clin Pathol. 2007;60:1024–1028. doi: 10.1136/jcp.2006.040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marhx-Bracho A, Rueda-Franco F, Ibarra-de la Torre A, et al. Chordoid meningioma of the foramen magnum in a child: a case report and review of the literature. Childs Nerv Syst. 2008;24:623–627. doi: 10.1007/s00381-007-0568-2. [DOI] [PubMed] [Google Scholar]
- 49.Matyja E, Grajkowska W, Lazarczyk M, et al. Chordoid meningiomas of a different histopathological pattern: a report of two cases. Folia Neuropathol. 2006;44:34–41. [PubMed] [Google Scholar]
- 50.Meis-Kindblom JM, Bergh P, Gunterberg B, et al. Extraskeletal myxoid chondrosarcoma: a reappraisal of its morphologic spectrum and prognostic factors based on 117 cases. Am J Surg Pathol. 1999;23:636–650. doi: 10.1097/00000478-199906000-00002. [DOI] [PubMed] [Google Scholar]
- 51.Mitsuhashi T, Ono S, Inohara T, et al. Chordoid meningioma—case report. Neurol Med Chir (Tokyo) 2006;46:37–40. doi: 10.2176/nmc.46.37. [DOI] [PubMed] [Google Scholar]
- 52.Mori S, Oka K, Hakozaki H, et al. Chordoid meningioma. A case report. Pathol Res Pract. 2001;197:515–518. doi: 10.1078/0344-0338-00120. discussion 519. [DOI] [PubMed] [Google Scholar]
- 53.Mullassery D, O’Brien DF, Williams D, et al. Malignant disseminated chordoid meningioma in a 12-year-old child: a role for early cranial and spinal radiation treatment after subtotal resection. Childs Nerv Syst. 2006;22:1344–1350. doi: 10.1007/s00381-006-0096-5. [DOI] [PubMed] [Google Scholar]
- 54.Murali R, Ng T. Chordoid meningioma masquerading as chordoma. Pathology. 2004;36:198–201. doi: 10.1080/00313020410001672074. [DOI] [PubMed] [Google Scholar]
- 55.O’Hara BJ, Paetau A, Miettinen M. Keratin subsets and monoclonal antibody HBME-1 in chordoma: immunohistochemical differential diagnosis between tumors simulating chordoma. Hum Pathol. 1998;29:119–126. doi: 10.1016/s0046-8177(98)90220-9. [DOI] [PubMed] [Google Scholar]
- 56.Okajima K, Honda I, Kitagawa T. Immunohistochemical distribution of S-100 protein in tumors and tumor-like lesions of bone and cartilage. Cancer. 1988;61:792–799. doi: 10.1002/1097-0142(19880215)61:4<792::aid-cncr2820610425>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 57.Okamoto S, Hisaoka M, Ishida T, et al. Extraskeletal myxoid chondrosarcoma: a clinicopathologic, immunohistochemical, and molecular analysis of 18 cases. Hum Pathol. 2001;32:1116–1124. doi: 10.1053/hupa.2001.28226. [DOI] [PubMed] [Google Scholar]
- 58.Oliveira AM, Sebo TJ, McGrory JE, et al. Extraskeletal myxoid chondrosarcoma: a clinicopathologic, immunohistochemical, and ploidy analysis of 23 cases. Mod Pathol. 2000;13:900–908. doi: 10.1038/modpathol.3880161. [DOI] [PubMed] [Google Scholar]
- 59.Omulecka A, Papierz W, Nawrocka-Kunecka A, et al. Immunohistochemical expression of progesterone and estrogen receptors in meningiomas. Folia Neuropathol. 2006;44:111–115. [PubMed] [Google Scholar]
- 60.Ordonez NG. Podoplanin: a novel diagnostic immunohistochemical marker. Adv Anat Pathol. 2006;13:83–88. doi: 10.1097/01.pap.0000213007.48479.94. [DOI] [PubMed] [Google Scholar]
- 61.Ozen O, Sar A, Atalay B, et al. Chordoid meningioma: rare variant of meningioma. Neuropathology. 2004;24:243–247. doi: 10.1111/j.1440-1789.2004.00551.x. [DOI] [PubMed] [Google Scholar]
- 62.Park SC, Oh DE, Suh YL, et al. Orbital chordoid meningioma: a rare subtype of meningioma. Ophthal Plast Reconstr Surg. 2007;23:246–248. doi: 10.1097/IOP.0b013e31803eb1b9. [DOI] [PubMed] [Google Scholar]
- 63.Pasquier B, Peoc’h M, Morrison AL, et al. Chordoid glioma of the third ventricle: a report of two new cases, with further evidence supporting an ependymal differentiation, and review of the literature. Am J Surg Pathol. 2002;26:1330–1342. doi: 10.1097/00000478-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 64.Perrot-Applanat M, Groyer-Picard MT, Kujas M. Immunocytochemical study of progesterone receptor in human meningioma. Acta Neurochir (Wien) 1992;115:20–30. doi: 10.1007/BF01400586. [DOI] [PubMed] [Google Scholar]
- 65.Prayson RA. Myxopapillary ependymomas: a clinicopathologic study of 14 cases including MIB-1 and p53 immunoreactivity. Mod Pathol. 1997;10:304–310. [PubMed] [Google Scholar]
- 66.Probst-Cousin S, Villagran-Lillo R, Lahl R, et al. Secretory meningioma: clinical, histologic, and immunohistochemical findings in 31 cases. Cancer. 1997;79:2003–2015. doi: 10.1002/(sici)1097-0142(19970515)79:10<2003::aid-cncr23>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 67.Reifenberger G, Weber T, Weber RG, et al. Chordoid glioma of the third ventricle: immunohistochemical and molecular genetic characterization of a novel tumor entity. Brain Pathol. 1999;9:617–626. doi: 10.1111/j.1750-3639.1999.tb00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rosenberg AE, Nielsen GP, Keel SB, et al. Chondrosarcoma of the base of the skull: a clinicopathologic study of 200 cases with emphasis on its distinction from chordoma. Am J Surg Pathol. 1999;23:1370–1378. doi: 10.1097/00000478-199911000-00007. [DOI] [PubMed] [Google Scholar]
- 69.Roser F, Samii M, Ostertag H, et al. The Ki-67 proliferation antigen in meningiomas. Experience in 600 cases. Acta Neurochir (Wien) 2004;146:37–44. doi: 10.1007/s00701-003-0173-4. discussion 44. [DOI] [PubMed] [Google Scholar]
- 70.Rowsell C, Sirbovan J, Rosenblum MK, et al. Primary chordoid meningioma of lung. Virchows Arch. 2005;446:333–337. doi: 10.1007/s00428-004-1192-0. [DOI] [PubMed] [Google Scholar]
- 71.Roy S, Chu A, Trojanowski JQ, et al. D2-40, a novel monoclonal antibody against the M2A antigen as a marker to distinguish hemangioblastomas from renal cell carcinomas. Acta Neuropathol. 2005;109:497–502. doi: 10.1007/s00401-005-0999-3. [DOI] [PubMed] [Google Scholar]
- 72.Salcman M, Scholtz H, Kristt D, et al. Extraskeletal myxoid chondrosarcoma of the falx. Neurosurgery. 1992;31:344–348. doi: 10.1227/00006123-199208000-00021. [DOI] [PubMed] [Google Scholar]
- 73.Sato K, Kubota T, Yoshida K, et al. Intracranial extraskeletal myxoid chondrosarcoma with special reference to lamellar inclusions in the rough endoplasmic reticulum. Acta Neuropathol. 1993;86:525–528. doi: 10.1007/BF00228591. [DOI] [PubMed] [Google Scholar]
- 74.Schacht V, Dadras SS, Johnson LA, et al. Up-regulation of the lymphatic marker podoplanin, a mucin-type transmembrane glycoprotein, in human squamous cell carcinomas and germ cell tumors. Am J Pathol. 2005;166:913–921. doi: 10.1016/S0002-9440(10)62311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scott A, Sharkawi E, Micallef C, et al. Chordoid meningioma presenting as painful orbital apex syndrome in pregnancy. Int Ophthalmol. 2008;28:355–357. doi: 10.1007/s10792-007-9135-9. [DOI] [PubMed] [Google Scholar]
- 76.Shibahara J, Kashima T, Kikuchi Y, et al. Podoplanin is expressed in subsets of tumors of the central nervous system. Virchows Arch. 2006;448:493–499. doi: 10.1007/s00428-005-0133-x. [DOI] [PubMed] [Google Scholar]
- 77.Soo MY, Ng T, Gomes L, et al. Skull base chordoid meningioma: imaging features and pathology. Australas Radiol. 2004;48:233–236. doi: 10.1111/j.1440-1673.2004.01278.x. [DOI] [PubMed] [Google Scholar]
- 78.Steilen-Gimbel H, Niedermayer I, Feiden W, et al. Unbalanced translocation t(1;3)(p12–13;q11) in meningiomas as the unique feature of chordoid differentiation. Genes Chromosomes Cancer. 1999;26:270–272. [PubMed] [Google Scholar]
- 79.Takei H, Bhattacharjee MB, Adesina AM. Chordoid glioma of the third ventricle: report of a case with cytologic features and utility during intraoperative consultation. Acta Cytol. 2006;50:691–696. doi: 10.1159/000326044. [DOI] [PubMed] [Google Scholar]
- 80.Takei H, Rivera A, Suzuki H, et al. Jugular foramen chordoid meningioma. Pathol Int. 2006;56:397–401. doi: 10.1111/j.1440-1827.2006.01976.x. [DOI] [PubMed] [Google Scholar]
- 81.Tirabosco R, Mangham DC, Rosenberg AE, et al. Brachyury expression in extra-axial skeletal and soft tissue chordomas: a marker that distinguishes chordoma from mixed tumor/myoepithelioma/parachordoma in soft tissue. Am J Surg Pathol. 2008;32:572–580. doi: 10.1097/PAS.0b013e31815b693a. [DOI] [PubMed] [Google Scholar]
- 82.Varma DR, Rao BR, Parameswaran S, et al. Chordoid meningioma: a report of two cases. Neurol India. 2003;51:522–524. [PubMed] [Google Scholar]
- 83.Vujovic S, Henderson S, Presneau N, et al. Brachyury, a crucial regulator of notochordal development, is a novel biomarker for chordomas. J Pathol. 2006;209:157–165. doi: 10.1002/path.1969. [DOI] [PubMed] [Google Scholar]
- 84.Wang XL, De Beuckeleer LH, De Schepper AM, et al. Low-grade chondrosarcoma vs enchondroma: challenges in diagnosis and management. Eur Radiol. 2001;11:1054–1057. doi: 10.1007/s003300000651. [DOI] [PubMed] [Google Scholar]
- 85.Worley GA, Wareing MJ, Sergeant RJ. Myxoid chondrosarcoma of the external auditory meatus. J Laryngol Otol. 1999;113:742–743. doi: 10.1017/s0022215100145074. [DOI] [PubMed] [Google Scholar]
- 86.Yeon JY, Lee JI, Kim JH, et al. Chordoid meningioma: a case report. J Korean Med Sci. 2003;18:768–771. doi: 10.3346/jkms.2003.18.5.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zorludemir S, Scheithauer BW, Hirose T, et al. Clear cell meningioma. A clinicopathologic study of a potentially aggressive variant of meningioma. Am J Surg Pathol. 1995;19:493–505. [PubMed] [Google Scholar]