We identified five structurally different compounds that trigger the pro-apoptotic protein Bax to permeabilize mitochondrial outer membranes leading to cellular demise. The molecular mechanisms by which these compounds and pro-apoptotic BH3 proteins trigger pore formation by Bax are different.
Keywords: apoptosis, Bax activators, Bax oligomerization, cysteine, mechanism probes, membrane permeabilization
Abstract
The pro-apoptotic protein Bax commits a cell to death by permeabilizing the mitochondrial outer membrane (MOM). To obtain small-molecule probes for elucidating the molecular mechanism(s) of Bax activation, we screened for compounds that induced Bax-mediated liposome permeabilization. We identified five structurally different small molecules that promoted both Bax targeting to and oligomerization at membranes. All five compounds initiated Bax oligomerization in the absence of membranes by a mechanism unlike Bax activation by Bcl-2 homology 3 domain (BH3) proteins. Some of the compounds induced Bax/Bak-dependent apoptosis in cells. Activation of Bax by the most active compound was poorly inhibited by the anti-apoptotic protein Bcl-XL and requires a cysteine residue at position 126 of Bax that is not required for activation by BH3 proteins. Our results reveal a novel pathway for Bax activation independent of pro-apoptotic BH3 proteins that may have important implications for the regulation of Bax activity in cells.
INTRODUCTION
Bcl-2 family proteins function as regulators and executors of apoptotic signals at the mitochondrial outer membrane (MOM) [1]. The fate of a cell is dictated by complex protein–protein and protein–membrane interactions of the pro- and anti-apoptotic Bcl-2 family members [2]. For most cells, MOM permeabilization (MOMP) by the executor Bcl-2 family pro-apoptotic proteins Bax and Bak commits the cell to undergo apoptosis. Since dysregulation of apoptosis is critical not only for tumour initiation, but also for tumour maintenance and survival, small molecules that modulate Bax and Bak activity are of critical importance from a mechanistic and therapeutic point of view [3,4].
It is widely accepted that MOMP occurs when Bax and/or Bak undergo complex conformational changes including the exposure of a buried N-terminal region, insertion of parts of the protein into the bilayer, oligomerization and the formation of a lethal pore [5–7]. However, there is a lack of consensus regarding the activation triggers of Bax and Bak in cells. According to some studies, Bax and Bak are inactive and activation is triggered by pro-apoptotic Bcl-2 homology 3 domain (BH3) proteins such as cBid that target the mitochondria, and recruit and activate Bax and membrane-bound Bak [8–10]. However, results from other studies suggest that both Bax and Bak are constitutively active and held in check by the anti-apoptotic machinery. In this scenario MOMP occurs when BH3 proteins liberate activated Bax or Bak from the anti-apoptotic proteins [11,12]. In these studies, it is unclear how Bax and Bak are activated since, when purified, both proteins are at best poorly active [13,14]. Moreover, most Bax is found in the cytoplasm of asynchronously growing cells [15].
Aside from BH3 proteins, additional reported triggers of Bax activation include: detergents, heat, alterations in pH and in cells, oxidative conditions, proteolytic cleavage and phosphorylation [6,16–21]. Furthermore, three small molecules, BAM7, Compound 106 and SMBA1, have been reported to be activators of Bax [22–24]. All three compounds were identified in virtual docking screens using Bax. Each of these docking studies was designed to identify molecules that can occupy specific sites on Bax. Compound 106 occupies the hydrophobic groove of Bax that binds BH3 proteins, SMBA1 docks in the small pocket around the Ser184 residue in the membrane-binding domain of Bax and BAM7 selectively engages a site that binds a stapled Bim BH3 peptide. Virtual docking at previously identified BH3 peptide-binding sites precludes identification of molecules that modulate Bax activity by binding to a different site and is unlikely to identify molecules that induce Bax activation by a mechanism different from that of the BH3 proteins.
To identify in an unbiased fashion small-molecule probes that can be used to examine the molecular mechanism of Bax activation, we screened for compounds that trigger Bax-mediated liposome permeabilization. We identified five structurally different small molecules that induce both the localization and oligomerization of Bax at membranes. Unexpectedly, all of these molecules activated Bax by a similar mechanism unlike that of Bax activation by BH3 proteins. Further analysis of the molecules in cells revealed, as expected, that some of the compounds induced apoptosis in cells via a Bax/Bak-dependent mechanism. Structure–activity analysis of the most active of these compounds indicated that binding specificity rather than the physicochemical properties of the molecule mediated Bax activation. Surprisingly, activation of Bax by the small molecule requires the cysteine residue at position 126 of Bax, a residue not required for BH3 protein-mediated activation of Bax. These and other data strongly suggesting that BH3 proteins and the small molecules activate Bax by different mechanisms provide insights into alternative mechanisms for regulating Bax activity in cells.
MATERIALS AND METHODS
Materials
8-Aminonaphthalene-1,3,6-trisulfonic acid, disodium salt (ANTS), p-xylene-bis-pyridinium bromide (DPX), Dulbecco's modified Eagle's medium (DMEM) and FBS were obtained from Life Technologies. Terbium III chloride hexahydrate (TBCl3·6H2O) and pyridine-2,6-dicarboxylic acid (also known as dipicolinic acid or DPA) were obtained from Sigma. Lipids were obtained from Avanti Polar Lipids. Disuccinimidyl suberate (DSS) and N-ethylmaleimide (NEM) were obtained from Thermo Fisher. The anti-Bax monoclonal antibodies, 6A7 and 2D2 were gifts from Richard Youle [6]. The sheep polyclonal antibody against cytochrome c was purified in our laboratory [25]. Immunoblotting of Bax and cytochrome c was conducted at a 1:5000 dilution. Immunofluorescence using 6A7 was performed at dilutions of 1:1000. All secondary antibodies were purchased from Jackson ImmunoResearch Laboratories. For immunoblotting, secondary antibodies conjugated to horseradish peroxidase were used at a 1:20 000 dilution. For immunofluorescence, secondary antibodies conjugated to FITC were used at a 1:1000 dilution. The plasmid encoding annexin V was a gift from Seamus Martin (Trinity College Dublin, Dublin, Ireland). Alexa Fluor 488 was obtained from Life Technologies. DRAQ5 was obtained from Biostatus and used a final concentration of 5 μM. Tetramethylrhodamine, ethyl ester (TMRE) was obtained from Life Technologies and used at 10 nM final concentration. Annexin V conjugated to Alexa Fluor 488 was prepared in our laboratory and used as described previously [26]. Baby mouse kidney (BMK) cells and their Bax−/−, Bak−/− and Bax−/− Bak−/− derivatives were gifts from Eileen White (Rutgers University, Piscataway, NJ, U.S.A.). All compounds except for OICR766A and its analogues were obtained from Sigma. OICR766A was obtained from Vitas M Laboratory (catalogue number STL224013). Analogues of OICR766A, SRI-1–SRI-5 were obtained from Enamine (catalogue numbers Z56176185, Z57301731, Z56976836, Z57301713 and Z57301722).
Cell culture and transfection
BMK cells were cultured in DMEM containing 10% FBS. BMK cells stably expressing Smac-(1–56)–mCherry were generated as described previously [27]. To generate BMK cells expressing human Bax, Bax−/− Bak−/− BMK cells were transfected with the mammalian expression vector (pvitro) expressing wild-type (Wt) human Bax. Clones expressing Bax were maintained in 3 μg/ml blasticidin.
Protein purification and labelling
Recombinant cBid, Bim, Bax and Bcl-XL were expressed and purified as described previously [8,10,14,25]. During the Bax and Bcl-XL purification, the intein tag was cleaved by incubation of the chitin beads with 100 mM 2-mercaptoethanol for 48 h at 4°C. Only batches of Bax with <15% autoactivity in the liposome permeabilization assay were used. To modify cysteine residues, Bax was labelled with NEM by incubation with a 10-fold molar excess in buffer containing 10 mM Hepes (pH 7.2), 200 mM sodium chloride, 0.1 mM EDTA and 10% glycerol at room temperature for 2 h. Excess NEM was removed by dialysis.
Liposome preparation
Mitochondria like liposomes with a lipid composition of 48% phosphatidylcholine, 28% phosphatidylethanolamine, 10% phosphatidylinositol, 10% dioleoyl phophatidylserine and 4% tetraoleoyl cardiolipin were prepared in assay buffer containing 10 mM Hepes (7.2), 200 mM potassium chloride, 5 mM magnesium chloride and 0.2 mM EDTA as described previously [14,25].
Liposome permeabilization assays
Liposomes encapsulating 12.5 mM ANTS and 45 mM DPX were prepared and assayed as described previously [14,25]. In this assay, background values (F0) were obtained by measuring the fluorescence of liposomes in the presence of compounds at 1 min intervals for at least 10 min at 37°C (Ex: 355 nm and Em: 520 nm). Proteins (cBid and/or Bax) were added at t=0 and fluorescence was measured at 37°C for 3 h. F100 was obtained by adding Triton X-100 at a final concentration of 0.2% (w/v) and measuring fluorescence for 10 min at 37°C. The percentage of ANTS/DPX release was calculated as [(F−F0/F100−F0)]×100. All reactions were carried out in the 96 half-area non-binding plates (Corning # 3686). For the primary screen, compounds from the Canadian Compound Collection and the Ontario Institute for Cancer Research (OICR) diversity subset were screened at 10 μM. Compounds with high background fluorescence were re-assayed with liposomes encapsulating Tb–DPA (0.8 mM TbCl3 and 2.4 mM DPA) prepared in assay buffer without EDTA. The assay was conducted similarly to the ANTS/DPX assay described above except that the liposomes were assayed in assay buffer containing 5 mM EDTA and F100 was obtained by permeabilizing the liposomes with 0.5% CHAPS (Ex: 276 nm and Em: 545 nm).
Mitochondria isolation and permeabilization assay
Heavy membranes containing mitochondria were isolated from BMK cells [27]. Briefly, cells were harvested and washed twice in PBS. Cells were lysed by nitrogen cavitation at 150 psi (1 psi=6.9 kPa) for 10 min on ice in lysis buffer containing 250 mM sucrose, 150 mM potassium chloride, 20 mM Hepes (7.2) and 1 mM EDTA supplemented with Complete protease inhibitor cocktail (Roche). To remove nuclei and cell debris, the lysate was centrifuged at 2000 g for 4 min at 4°C. Mitochondria were then obtained by centrifugation of the supernatant at 13000 g for 10 min at 4°C and washed once in lysis buffer. For Smac–mCherry release assays mitochondria and cytochrome c release assays, mitochondria were diluted to 0.2 and 1 mg/ml protein concentration respectively. Then 50 μl Fifty microlitres of mitochondrial fractions were incubated with the indicated concentrations of compounds and protein at 37°C for 30 min. Another centrifugation step at 13000 g for 10 min was performed after incubation. For Smac–mCherry release assays, fluorometric analysis was conducted on the supernatant (FS) and pellet fractions (FP) (Ex: 580 nm and Em: 610 nm). The percentage release of Smac–mCherry was calculated as (FS/FS + FP) × 100. For cytochrome c release assays, supernatant and pellet fractions were analysed by immunoblotting and densitometry analysis was carried out using ImageJ (NIH).
Membrane-binding assay
Membrane-binding assays were performed as described previously [25]. Briefly, samples containing liposomes (300 μM lipids) were incubated with Bax and the compounds/cBid at 37°C for 2 h. Membrane-bound protein was separated from free protein by gel-filtration chromatography on Sepharose CL-2B resin and fractions were analysed using immunoblotting. Quantitative analysis of the fractions was performed using intensity measurements in ImageJ. Membrane binding was measured by comparing the intensities of membrane-bound proteins (fraction 3–6) with the total protein in all of the fractions.
Cross-linking
Cross-linking studies were carried out as described previously [25]. Briefly, 100 nM Bax and compounds at the indicated concentrations were incubated in the absence or presence of liposomes (300 μM total lipids) for 2 h at 37°C in a low protein- binding plate (Corning 3686). Cross-linking was performed using DSS at a final concentration of 2 mM for 30 min at room temperature. The reaction was subsequently quenched by the addition of Tris/HCl (pH 8) at a final concentration of 20 mM. Bax oligomers were detected on immunoblots using the 2D2 antibody. Quantitative analysis of dimers and higher-order Bax oligomers was performed using intensity measurements in ImageJ.
Liposome partitioning assay
Liposomes encapsulating ANTS/DPX or Tb–DPA were prepared as described above. To assess partitioning of the compounds into liposomes, liposomes were incubated with the compounds at 37°C for 1 h. Membrane-partitioned compounds were separated from free compound by passing the liposomes over a Sepharose CL-2B gel-filtration column. Bax was added to these liposomes and permeabilization was assayed as described above.
Isothermal titration calorimetry (ITC)
ITC runs were performed using the MicroCal ITC200 (Malvern Instruments). For sample preparation, Bax was dialysed against 10 mM Hepes (pH 7.2), 200 mM sodium chloride and 0.1 mM EDTA and diluted to a final concentration of 20 μM. The compounds were diluted in Bax dialysis buffer and titrated from a 150 μM solution. All experiments were performed at 25°C. Data analysis was performed using Origin Software (MicroCal).
Live-cell imaging and analysis
Cells were plated at a cell density of 2000 cells/well in 384-well plates. At 24 h after plating, the cells were treated with the compounds at the indicated concentrations for 24 h. At 30 min prior to imaging, the cells were stained with DRAQ5, TMRE and annexin V–Alexa Fluor 488 in annexin V-binding buffer containing 10 mM Hepes [7], 150 mM sodium chloride, 5 mM potassium chloride, 1 mM magnesium chloride and 1.8 mM calcium chloride [26]. Image acquisition was performed using the Opera High Content Screening System (PerkinElmer) with a ×20 air objective. For every independent experiment, each treatment dose was conducted in a minimum of duplicate wells and a minimum of five different fields of view (∼50 cells/field) were acquired for each well. For each cell, the nucleus and cytoplasm were identified using the DRAQ5 intensity as described previously [28]. Intensity features and morphology features were extracted using a custom Acapella high content imaging and analysis software (PerkinElmer) script available for free download at http://www.andrewslab.ca. Thresholds were determined as the average intensity plus two standard deviations in the annexin V/TMRE channel in DMSO-treated cells.
Immunofluorescence
BMK cells stably expressing human Wt Bax or empty vector were treated for 24 h and, at the end of the treatment, the cells were fixed and immunostained using the 6A7 primary monoclonal antibody and a secondary donkey anti-mouse antibody conjugated to FITC. Cells were stained with DRAQ5 30 min prior to imaging. Image acquisition and analysis was performed as described above.
Cell survival and regrowth
BMK cells were treated with OICR766A for 24 h as described above in 384-well plates. The compound was washed away after treatment and cells were left in regular medium for 24 h. Cells were trypsinized and re-plated in 96-well plates and incubated in growth medium for 3 days. Surviving cells were stained with Crystal Violet and assessed by absorbance measurements at 600 nm.
RESULTS AND DISCUSSION
Five small molecules activate Bax in liposomes and enriched mitochondria
In order to identify probes of Bax activation, a screen was conducted for compounds that caused Bax-mediated permeabilization of liposomes encapsulating a fluorophore (ANTS) and a quencher (DPX). Permeabilization of these liposomes results in an increase in fluorescence due to the increase in distance between the released dye and quencher [14]. As a positive control for membrane permeabilization, we used the known Bax activator protein cBid (Supplementary Figure S1A) [8]. To correct for intrinsic fluorescence of the compounds and to identify compounds that lyse liposomes directly, fluorescence intensity was also measured without adding Bax. Compounds with high background fluorescence were re-assayed using liposomes encapsulating the fluorescent complex Tb–DPA in a buffer containing EDTA. In this case, fluorescence is recorded at different wavelengths than for ANTS/DPX and liposome permeabilization causes a decrease in fluorescence because EDTA disrupts the Tb–DPA complex [29].
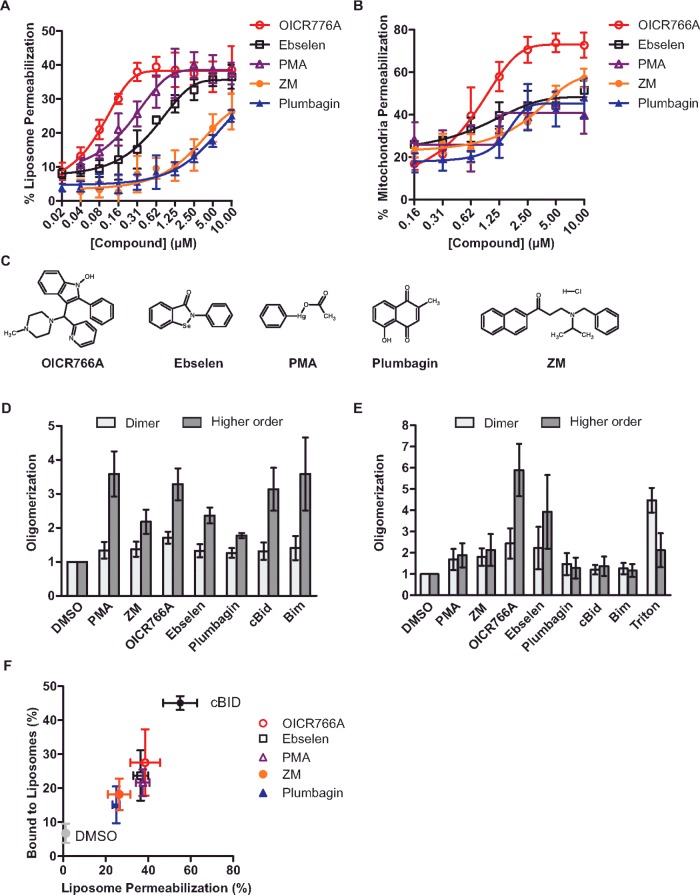
After primary and secondary screening, five compounds met the criteria as bona fide hits including: OICR766A, phenylmercuric acetate (PMA), ebselen, ZM39923 hydrochloride (referred to as ZM hereafter) and plumbagin. The relative potency of the compounds was established by re-assaying them at various concentrations. All five compounds permeabilized liposomes in a Bax- and dose-dependent fashion (Figure 1A). In control experiments, the highest dose of the compound alone had no effect on liposome integrity (Supplementary Figure S1A).
Figure 1. Structurally diverse compounds activate Bax.
(A) Compounds trigger Bax to permeabilize liposomes. Liposomes encapsulating ANTS and DPX were incubated with 100 nM Bax and the indicated concentrations of the compounds. Due to interfering fluorescence from the compound, ZM39923 was assayed using liposomes encapsulating Tb–DPA. Membrane permeabilization was assayed by measuring the increase in ANTS fluorescence or the decrease in Tb–DPA fluorescence. Results are compared with lysis with detergent (100%) and represented as means ± S.D. (n=3). (B) Compounds trigger Bax to permeabilize mitochondria. Mitochondria from Bax−/− Bak−/− BMK cells containing Smac–mCherry were incubated with 20 nM Bax and the indicated concentrations of the compounds. Permeabilization was assayed by pelleting the mitochondria and measuring the fluorescence of the supernatant and pellet fractions. The fraction of Smac–mCherry in the supernatant fraction was determined and results are represented as means ± S.D. (n=3). (C) Structures of the five small-molecule Bax activators. (D) Compound-induced Bax oligomerization in liposomes. Bax at 100 nM was incubated with 20 nM cBid/Bim or 10 μM of the indicated compounds in the presence of liposomes. Cross-linking was performed with DSS followed by immunoblotting (Supplementary Figure S1C). Band intensities of dimer ∼42 kDa and higher-order oligomers (>42 kDa) were quantified using ImageJ. Results are represented as the fold change in Bax intensity relative to the no compound (DMSO) lane ± S.D. (n=3). (E) Compound-induced Bax oligomerization in solution. Quantification of Bax oligomerization assessed by cross-linking in the absence of liposomes as described in (D) (immunoblots shown in Supplementary Figure S1D). As a positive control, Bax was incubated with 1% Triton X-100. (F) Compound-induced Bax binding to liposomes correlates with permeabilization. Bax at 100 nM was incubated with 10 μM of the compounds or 20 nM cBid in the presence of liposomes. Membrane-bound Bax was separated from soluble Bax using gel-filtration chromatography and analysed using immunoblotting. Results are represented as the percentage of membrane-bound Bax compared with the percentage of liposome permeabilization from (A) (error bars show S.D.). Membrane binding was measured by comparing the intensities of membrane-bound proteins (fractions 3–6) with the total protein in all of the fractions (Supplementary Figure S1E; n=3).
To further validate the compounds as Bax activators in biological membranes, we assayed their activity in mitochondria isolated from Bax−/− Bak−/− BMK cells engineered to stably express Smac-(1–56)–mCherry. This fusion protein includes residues 1–56 (the intermembrane mitochondrial targeting signal peptide) of Smac, an intermembrane space protein released during apoptosis, fused to the fluorescence protein mCherry [30]. Bax activated by cBid leads to Smac–mCherry release that can be measured quantitatively using a fluorimeter (Supplementary Figure S1B). Consistent with the results using liposomes, the compounds triggered dose-dependent Bax-mediated permeabilization of mitochondria (Figure 1B). Without Bax, the compounds did not result in significant mitochondrial permeabilization at concentrations up to 10 μM (Supplementary Figure S1B). In both liposomes and mitochondria-based assays, OICR766A had the greatest effect enhancing the pro-apoptotic activity of Bax with EC50 values of ∼0.1 μM and ∼0.9 μM, respectively (Table 1). Interestingly, the five molecules are structurally unrelated demonstrating that Bax can be activated by a variety of molecules (Figure 1C). That Bax responds to such diverse structures suggests that it may also respond to one of more chemical triggers that accumulate in cells in response to stress or intoxication.
Table 1. Bax activation by compounds.
| Compound | EC50 in liposomes (μM) | EC50 in mitochondria (μM) |
|---|---|---|
| OICR766A | 0.1 | 0.9 |
| Ebselen | 0.6 | 1.1 |
| PMA | 0.2 | 1.3 |
| ZM | >3 | 3.5 |
| Plumbagin | >3 | 1.5 |
Compounds induce oligomerization and membrane localization of Bax
To examine the molecular mechanism by which the small molecules induce Bax activation, compound-induced oligomerization and membrane localization of Bax were measured biochemically. To determine whether the compounds were inducing Bax to form oligomers, we performed cross-linking experiments using DSS in reactions containing liposomes [25,31]. As positive controls for Bax oligomerization, we used cBid and Bim which have been shown to induce Bax oligomerization [10]. As expected, the compounds induced the formation of higher- order Bax complexes with OICR766A, and PMA having the most pronounced effects on Bax oligomerization (Figure 1D and Supplementary Figure S1C).
Unlike pro-apoptotic BH3 proteins, in the absence of membranes, both OICR766A and ebselen had pronounced effects on the formation of dimers and higher-order Bax oligomers in solution (Figure 1E and Supplementary Figure S1D). In control experiments, no effects on Bax oligomerization were observed upon addition of cBid or Bim to Bax in solution (Figure 1E), a result consistent with our previous observations that Bax inserts into membranes as monomers that then oligomerize [8]. Additionally, in solution, Triton X-100 predominantly induced the formation of dimers with very little higher oligomers, consistent with previous results showing that detergents such as Triton X-100 and octylmaltoside induce the formation of domain-swapped Bax homodimers believed to be inactive [6,32,33]. Taken together, these data suggest that the molecular mechanism by which the compounds activate Bax differs significantly from that by cBid or Triton X-100.
Previously we have shown that, in the presence of liposomes, Bax undergoes a reversible conformational change that exposes a normally hidden epitope recognized by the monoclonal antibody 6A7 [14]. Exposure of this epitope strongly correlates with activation of the oligomerization and pore-forming activities of Bax. It was also possible to detect the formation of reversible Bax oligomers in the presence of liposomes [25]. However, neither exposure of the 6A7 epitope nor the formation of these transient oligomers is sufficient to permeabilize membranes until an activator protein is introduced [8]. Therefore, although the compounds identified here induce changes to Bax in solution, it is possible that a substrate membrane is still required for Bax to undergo all of the functional conformational changes associated with its activation to permeabilize membranes.
In unstressed cells, Bax is typically a cytoplasmic or peripheral membrane protein that binds tightly to membranes only upon activation by a membrane-bound BH3 protein [28]. To determine the effect of the compounds on Bax binding to membranes, Bax was incubated with the compounds in the presence of liposomes and then the reactions were passed over a size-exclusion column to separate membrane-bound Bax from unbound protein. Immunoblots of the fractions revealed that incubation with the compounds or in control reactions with cBid increased the amount of Bax that is eluted in the excluded fractions containing membranes (fraction 3–6) (Supplementary Figure S1E), a result consistent with triggering Bax oligomerization. The fraction of Bax that elutes in the bound fractions varies for each compound and correlates positively with membrane permeabilization (Figure 1F). Taken together, these results indicate that the conformational changes that occur when the compounds trigger Bax oligomerization in solution allow it to bind to membranes productively leading to membrane permeabilization.
Bax activation is not mediated by partitioning of the compounds into liposome membranes
Given that hydrophobic small molecules can partition into membranes, and that membranes trigger transient conformational changes in Bax, we sought to rule out the possibility that membrane permeabilization was due to the compound altering the physical properties of the membranes making them prone to spontaneous fusion such that the altered liposomes mediate Bax activation and oligomerization [34]. To test this hypothesis, we used a modified version of the dye release assay in which liposomes were first incubated with the compounds and then passed over a gel-filtration column to separate the liposomes and any bound compound from unpartitioned compound. Thereafter, membrane permeabilization was compared for Bax and liposomes pre-incubated with the compounds to liposomes in which Bax and the compounds were added simultaneously as a control. Pre-incubation of the liposomes with the compounds did not result in Bax activation, suggesting that that activation of Bax was unlikely to be due to an effect of the compounds on the liposome membranes (Supplementary Figure S1F).
OICR766A induces apoptosis in cells
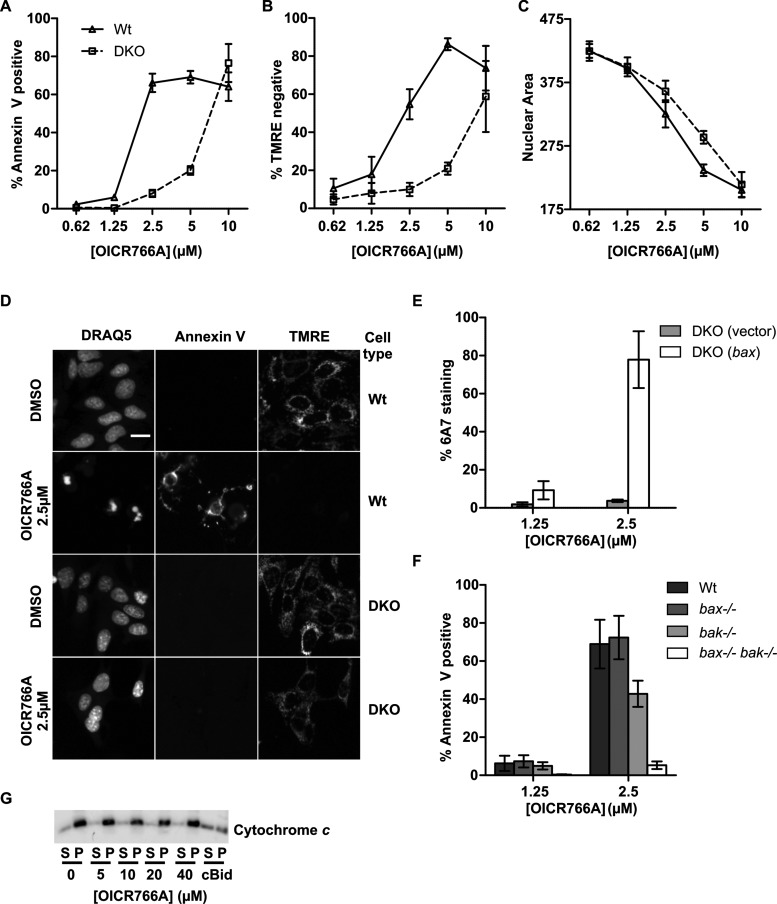
To determine whether the compounds induce Bax/Bak-dependent apoptosis in cells, we examined their effects on Wt and Bax−/− Bak−/− double knockout (DKO) BMK cells. Apoptotic cell death was monitored using triple staining with annexin V, TMRE and DRAQ5 to measure externalization of phosphatidylserine (a hallmark of apoptosis), loss of mitochondrial membrane potential and nuclear/chromatin condensation respectively. Among the five compounds tested, annexin V staining indicated that OICR766A (Figure 2A), PMA and plumbagin (Supplementary Figure S2A) had the most pronounced effects on apoptosis of Wt compared with the DKO cells. Even though ebselen has been reported to kill leukemia cells, we found that it was not toxic to Wt or DKO BMK, cells suggesting that it may be either metabolized or pumped out of the cells [35]. ZM killed both cell types with an EC50 of ∼10 μM, suggesting that in cells the drug has other more toxic activities than Bax activation, a result consistent with previous publications [36]. Since OICR766A was the most effective Bax activator in vitro (Figure 1), and had the largest differential kill between Wt and DKO cells (Figure 2A) we analysed the activity of this compound in more detail.
Figure 2. OICR766A induces cell death by a Bax/Bak-dependent mechanism.
(A–C) Cell death measured for Wt and Bax−/− Bak−/− (DKO) BMK cells treated with the indicated concentrations of OICR766A for 24 h prior to imaging. Results are presented as the percentage of cells scored as dead for duplicate wells from three independent experiments ± S.E.M. (A) Externalization of phosphatidylserine. Cell death was assessed by annexin V–Alexa Fluor 488 staining intensity above a calculated threshold. (B) Loss of mitochondrial transmembrane potential. Loss of mitochondrial transmembrane potential was assessed for the same cells from (A) as decreased staining with TMRE below a calculated threshold. (C) Nuclear condensation. Nuclear area was measured for the same cells as in (A) by staining with DRAQ5. (D) Representative images of Wt and DKO BMK cells as indicated on the right, treated with DMSO or OICR766A as indicated on the left for 24 h. Cells were stained with annexin V (to detect externalized phosphatidylserine as indicative of loss of plasma membrane asymmetry), TMRE (mitochondrial membrane potential) and DRAQ5 (nuclear morphology) as indicated above the columns. The scale bar indicates 10 μm. (E) Bax activation. DKO BMK cells stably expressing human Wt Bax or empty vector were treated for 24 h with OICR766A as indicated. Immunofluorescence intensity above a threshold with an antibody (6A7) that binds an epitope exposed during activation of Bax was used to score cells as stained or unstained. Results are represented as the percentage increase in the number of cells with positive 6A7 staining relative to the DMSO control ± S.E.M. of duplicate wells from n=3 independent experiments. (F) Externalization of phosphatidylserine. Wt, Bax−/−, Bak−/− or Bax−/− Bak−/− BMK cells were treated at the indicated concentrations of OICR766A and analysed as in (A) (n=3). (G) Mitochondrial permeabilization. Mitochondria from Bax−/− BMK cells were incubated with OICR766A at the indicated concentrations or 5 nM cBid as a positive control and then the mitochondria were pelleted (P) by centrifugation. Permeabilization results in release of cytochrome c from the mitochondria into the supernatant (S). The blot is representative of three independent experiments.
Annexin V staining revealed that 2.5–5 μM OICR766A killed Wt but had limited activity in DKO cells. However, at 10 μM, it was equally toxic to both Wt and DKO cells, suggesting significant off-target toxicity (Figure 2A). These data were further corroborated by reduced staining of the Wt cells with the membrane potential-sensitive dye TMRE, demonstrating a greater loss in membrane potential in Wt than in the DKO cells at similar doses (Figure 2B). Interestingly, quantitative measurements of nuclear condensation indicated that OICR766A treatment decreased nuclear size of both Wt and DKO cells with only a marginally greater effect on the Wt cells between 2.5 and 5 μM (Figure 2C). Representative fluorescence images displaying staining with annexin V, TMRE and DRAQ5 for cells treated with 2.5 μM OICR766A are shown in Figure 2D. At 2.5 μM OICR766A also killed DKO cells expressing exogenous human Bax (Supplementary Figure S2B). Consistent with the mechanism of cell death at lower concentrations being due to activation of Bax, almost 80% of cells stained with an antibody specific for the active form of Bax (6A7) after treatment with 2.5 μM OICR766A (Figure 2E).
Bax and Bak are functionally equivalent in many cell types, therefore we tested cells expressing Bax or Bak for sensitivity to OICR766A [37,38]. At 2.5 μM the compound killed cells expressing either Bax or Bak (Figure 2F). Off-target activity of the compound in cells could result in indirect activation of Bak, therefore we measured direct activation of Bak by OICR766A in vitro. For these experiments mitochondria isolated from Bax−/− BMK cells were incubated with increasing concentrations of OICR766A. Even at the maximum concentration assayed (40 μM), there was no detectable release of the intermembrane space protein cytochrome c (Figure 2G). This contrasts markedly with the effect of OICR766A on mitochondria with Bax and no Bak (Figure 1B). Thus, these results suggest that in live cells OICR766A indirectly activated Bak to mediate apoptosis of Bax−/− BMK cells. Consistent with this interpretation and the data in Figure 2C, when the Wt and DKO cells were assayed for cell survival and regrowth after OICR766A treatment, no difference was observed in the survival response (Supplementary Figure S2C). Thus, Bax activation is only one mechanism by which OICR766A kills cells, limiting the utility of the compound for cell-based studies.
OICR766A-activated Bax is poorly inhibited by Bcl-XL
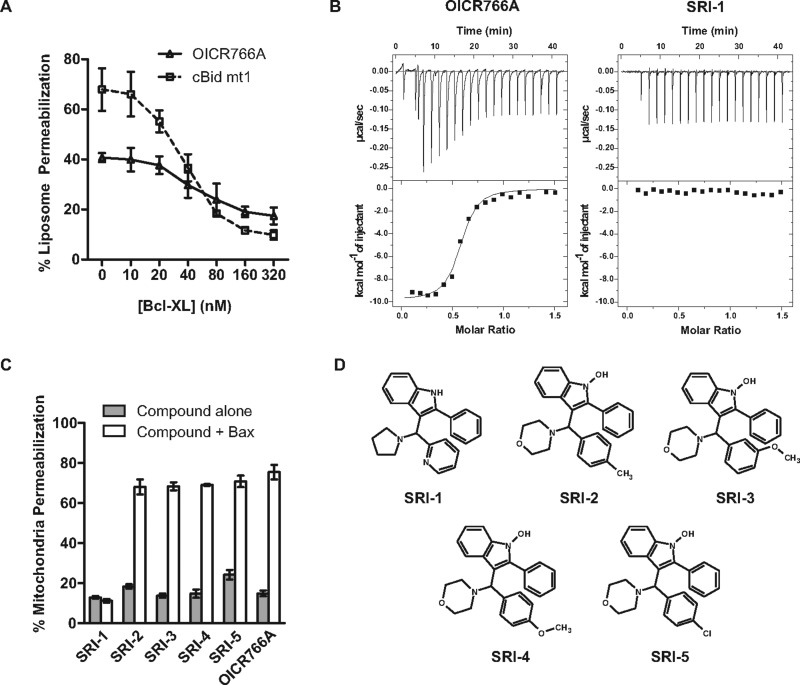
To examine the molecular mechanism of Bax activation by OICR766A in more detail, we measured inhibition of compound-activated Bax by the anti-apoptotic protein Bcl-XL. Bcl-XL functions as a dominant-negative Bax to inhibit membrane permeabilization by binding to Bax directly and inhibiting its oligomerization (Mode 2). However, Bcl-XL also inhibits apoptosis by binding to the BH3 proteins, thereby preventing activation of Bax (Mode 1) [25,39]. The two modes can be differentiated using cBid mt1, a mutant of Bid that activates Bax but does not bind to and therefore cannot be inhibited by Bcl-XL [25,40,41]. Therefore, we compared inhibition of Bax membrane-permeabilizing activity by the anti-apoptotic protein Bcl-XL for Bax activated by OICR766A and cBid mt1. Preliminary experiments demonstrated that Bcl-XL inhibited liposome permeabilization by cBid mt1 and Bax much better than it inhibited OICR766A and Bax. Therefore Bcl-XL-mediated inhibition of liposome permeabilization was compared for reaction conditions in which cBid mt1 and Bax permeabilized ∼70% and OICR766A and Bax permeabilized ∼40% of liposomes, respectively. As the concentration of Bcl-XL was increased, cBid mt1/Bax-mediated liposome permeabilization decreased from ∼70% to ∼10% at 160 nM Bcl-XL (Figure 3A, dotted line). In contrast, 300 nM Bcl-XL was required to decrease liposome permeabilization by OICR766A and Bax from roughly 40% to 18% (Figure 3A, continuous line). Thus, whereas OICR766A-activated Bax mimics cBid mt1-activated Bax, given the difference in sensitivity to Bcl-XL and the data above suggesting the compound activates soluble Bax, we speculated that the mechanism of Bax activation by OICR766A is different from activation by BH3 proteins.
Figure 3. The molecular mechanisms of Bax activation by OICR766A and cBid are different.
(A) Bcl-XL poorly inhibits OICR766A compared with cBid mt1-mediated activation of Bax. Bax at 100 nM and 1 μM OICR766A or 20 nM cBid mt1 were added to liposomes encapsulating ANTS/DPX and the indicated concentrations of Bcl-XL. Membrane permeabilization was assayed by the increase in fluorescence compared with liposome lysis with detergent (100%) as in Figure 1A (n=3). (B) OICR766A binds to Bax. ITC analysis of 20 μM Bax with successive additions of 2 μl from a 150 μM stock of OICR766A (left panel) and SRI-1 (right panel). Raw injection heats are shown in the top panel and corresponding isotherms fitted to a one-site model in the bottom panel. Shown here is one representative titration of least three independent replicates. (C) Mitochondrial permeabilization. Mitochondria from Bax−/− Bak−/− BMK cells expressing Smac–mCherry were incubated with 10 μM OICR766A analogue and 20 nM Bax and assayed as described in Figure 1B (n=3). (D) Structures of OICR766A analogues tested in (C). Names are indicated below the structures.
OICR766A binds to Bax
To determine whether OICR766A binds directly to Bax and if so with what stoichiometry, we monitored the binding thermodynamics by ITC. Titration of OICR766A into 20 μM Bax yielded a dissociation constant (KD) of 255 nM ± 58 nM and a compound/Bax stoichiometry of no more than 1:1 (Figure 3B, left panel). A structural analogue of OICR766A, SRI-1, with similar physicochemical properties, failed to induce Bax mediated permeabilization in mitochondria (Figures 3C and 3D). This compound also displayed no detectable binding to Bax in the ITC measurements (Figure 3B, right panel). In contrast four other analogues of OICR677A (SRI-2–SRI-5, Figure 3D) all activated Bax to permeabilize mitochondria (Figure 3C). These data demonstrate that OICR766A-mediated Bax activation is induced by direct binding of OICR766A and that this interaction is structure-specific.
Cysteine 126 of Bax is required for activation by OICR766A
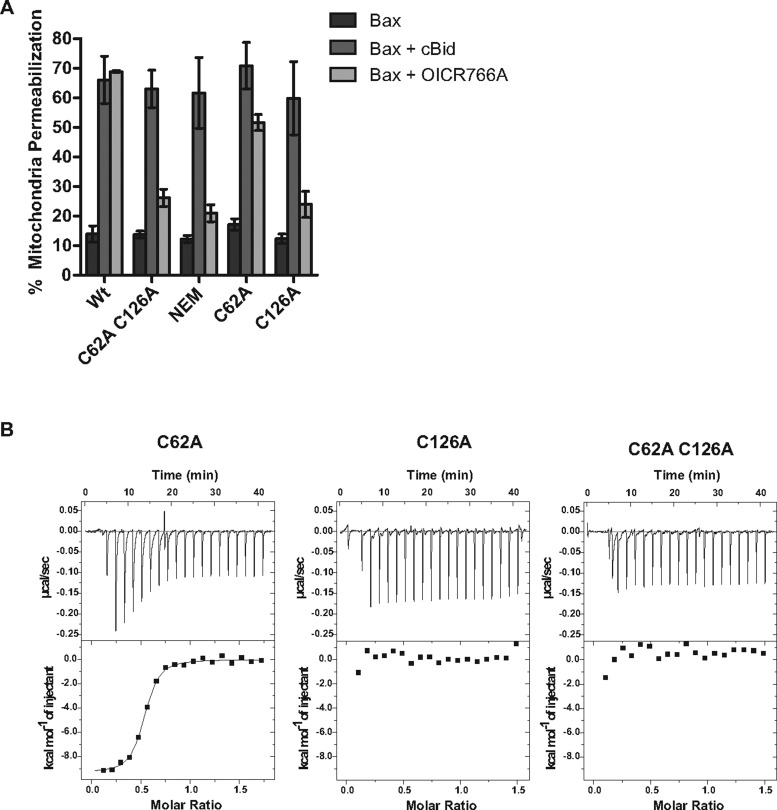
Bax has two cysteines located at positions 62 and 126 [42]. Conflicting reports exist on the role of the cysteines in Bax activation and oligomerization. It has been reported that for Bax activation in cells, Cys62 is required under oxidative conditions whereas Cys126 appears to be required for activation by DHHC protein acyltransferase family members or prostaglandins [18,43,44]. In contrast, data from other studies suggest that in cells neither cysteine is required during Bax-mediated cell death in response to etoposide or staurosporine [45]. Because all of these measurements were made in cells, it is difficult to evaluate the relative importance of direct and indirect effects. To investigate the involvement of Bax cysteines for compound-mediated Bax activation directly, we used genetic elimination or chemical modification of the cysteine residues with NEM. Both treatments dramatically reduced activation of Bax by OICR766A, but not by cBid (Figure 4A) or Bim (Supplementary Figure S3A). To examine the individual cysteine residues, each was mutated to alanine. Bax C62A was activated, whereas C126A Bax was almost completely resistant to chemical activation by OICR766A and the other two compounds that activated Bax at mitochondria, ZM and ebselen (Figure 4A and Supplementary Figure S3B). Although it is possible that all three compounds react with Cys126, the differences in the structures of the compounds suggest that they are interacting with the Cys126 through distinct binding modes. Of note, covalent modification of Cys126 with other compounds (e.g. fluorophores [8]) is not sufficient to activate Bax. These results suggest that Cys126 is most likely to be part of the compound-binding site and modification or substitution alters the binding site sufficiently to prevent the compounds from activating the protein.
Figure 4. OICR766A mediated Bax activation requires Cys126.
(A) Mitochondrial permeabilization. Mitochondria from Bax−/− Bak−/− BMK cells containing Smac–mCherry were assayed with 20 nM Bax and 10 μM OICR766A or 2 nM cBid as a positive control and assayed as described in Figure 1B (n=3). (B) OICR766A-Bax binding. ITC analysis of 20 μM Bax mutants C62A, C126A and C62A C126A titrated with OICR766A as described in Figure 3C.
To determine whether Cys126 is required for compound binding to Bax or for some other aspect of Bax activation (e.g. insertion into membranes) we measured OICR766A binding to the cysteine mutants using ITC. Consistent with Cys126 forming part of the compound-binding site, a direct interaction between Bax and OICR766A was observed for Bax with a cysteine residue at this position (C62A) with a KD of 266±136 nM but not for Bax C126A or for the double cysteine mutant (Figure 4B). Taken together, these results indicate that Cys126 is required for OICR766A to bind to Bax. We could not address the cysteine requirement for activation of Bax by OICR766A in cells due to off-target effects. However, it is likely that the mechanism of Bax activation by the compound is similar in cells.
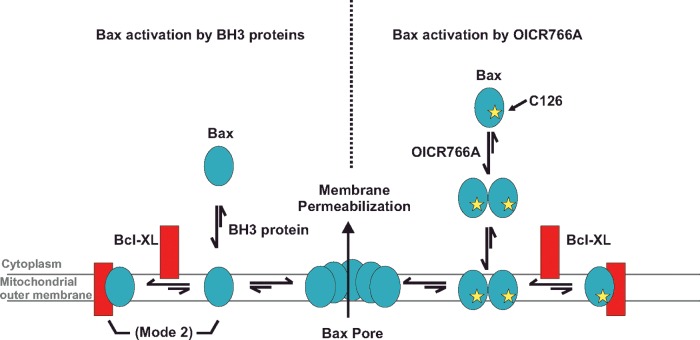
In summary, our data show that, although cBid, Bim and OICR766A function to activate Bax, they differ in the mechanism by which this happens. Unlike cBid and Bim, OICR766A triggers oligomerization of Bax in the absence of membranes, is poorly inhibited by Bcl-XL and requires Bax Cys126 (Figure 5). Thus multiple mechanisms exist for Bax activation and we speculate that the mechanism of Bax activation by small molecules allows Bax to respond to cellular signals, drug intoxication and/or metabolites independently of BH3 proteins. Our results may also explain why, in some cells, Bax and Bak appear to be constitutively activated without up-regulation of BH3 proteins [11,46]. In cells, dual-activation pathways may be particularly important for the integration of multiple stimuli. Insights gained into alternative mechanisms for targeting Bax using the small molecules here revealed a novel pathway for the activation of Bax that is druggable and may prove to be an advantageous therapeutic target in some disease states including cancers where cells are dependent on sequestration of BH3 proteins (Mode 1) inhibition of apoptosis.
Figure 5. Comparison of Bax activation by BH3 proteins and OICR766A.
Left: BH3 proteins recruit and activate Bax at the membrane. Upon activation, Bax either integrates into the membrane and oligomerizes or binds to and is inhibited by Bcl-XL. Oligomerization of Bax permeabilizes the membrane by an unknown mechanism. Right: OICR766A activates Bax in the cytoplasm. OICR766A-mediated Bax oligomerization and activation requires Cys126 of Bax. Activated Bax dimers and oligomers (for simplicity only dimers are shown) then bind to and integrate into membranes by a process inefficiently inhibited by Bcl-XL. Bax oligomers permeabilize the membrane by an unknown mechanism.
Acknowledgments
We thank Dr Eileen White for providing the BMK cells used in the study, Dr Richard Youle for providing the 2D2 and 6A7 antibodies, Dr Seamus Martin for providing the plasmid encoding annexin V, and Jarkko Ylanko, Mina Falcone, Erin Wang and Annie Dahlgran for conducting the screen to identify Bax activators.
Abbreviations
- ANTS
8-aminonaphthalene-1,3,6-trisulfonic acid, disodium salt
- BH3
Bcl-2 homology 3 domain
- BMK
baby mouse kidney
- DKO
double knockout
- DMEM
Dulbecco's modified Eagle's medium
- DPA
pyridine-2,6-dicarboxylic acid (dipicolinic acid)
- DPX
p-xylene-bis-pyridinium bromide
- DSS
disuccinimidyl suberate
- ITC
isothermal titration calorimetry
- MOM
mitochondrial outer membrane
- MOMP
mitochondrial outer membrane permeabilization
- NEM
N-ethylmaleimide
- OICR
Ontario Institute for Cancer Research
- PMA
phenylmercuric acetate
- Tb–DPA
terbium–dipicolinic acid
- TMRE
tetramethylrhodamine, ethyl ester
- Wt
wild-type
AUTHOR CONTRIBUTION
Hetal Brahmbhatt, Brian Leber and David Andrews designed the experiments, analysed the data and wrote the paper. Rima Al-awar and David Uehling assisted with the OICR766A analogues. David Andrews and Brian Leber directed the project, Hetal Brahmbhatt performed the experiments.
FUNDING
This work was supported by the Canadian Institute of Health Research [grant number FRN12517 (to D.W.A. and B.L.)]; the Ontario Institute for Cancer Research with funding from the Government of Ontario through the Ontario Ministry of Research and Innovation/Terry Fox Research Institute Grant for Selective Therapies [grant number 1010121].
References
- 1.Antonsson B. Mitochondria and the Bcl-2 family proteins in apoptosis signaling pathways. Mol. Cell. Biochem. 2004;256–257:141–155. doi: 10.1023/B:MCBI.0000009865.70898.36. [DOI] [PubMed] [Google Scholar]
- 2.Leber B., Lin J., Andrews D.W. Embedded together: the life and death consequences of interaction of the Bcl-2 family with membranes. Apoptosis. 2007;12:897–911. doi: 10.1007/s10495-007-0746-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Certo M., Del Gaizo Moore V., Nishino M., Wei G., Korsmeyer S., Armstrong S.A., Letai A. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 5.Annis M.G., Soucie E.L., Dlugosz P.J., Cruz-Aguado J.A., Penn L.Z., Leber B., Andrews D.W. Bax forms multispanning monomers that oligomerize to permeabilize membranes during apoptosis. EMBO J. 2005;24:2096–2103. doi: 10.1038/sj.emboj.7600675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu Y.T., Youle R.J. Nonionic detergents induce dimerization among members of the Bcl-2 family. J. Biol. Chem. 1997;272:13829–13834. doi: 10.1074/jbc.272.21.13829. [DOI] [PubMed] [Google Scholar]
- 7.Alsop A.E., Fennell S.C., Bartolo R.C., Tan I.K., Dewson G., Kluck R.M. Dissociation of Bak alpha1 helix from the core and latch domains is required for apoptosis. Nat. Commun. 2015;6:6841. doi: 10.1038/ncomms7841. [DOI] [PubMed] [Google Scholar]
- 8.Lovell J.F., Billen L.P., Bindner S., Shamas-Din A., Fradin C., Leber B., Andrews D.W. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell. 2008;135:1074–1084. doi: 10.1016/j.cell.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Kuwana T., Mackey M.R., Perkins G., Ellisman M.H., Latterich M., Schneiter R., Green D.R., Newmeyer D.D. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–342. doi: 10.1016/S0092-8674(02)01036-X. [DOI] [PubMed] [Google Scholar]
- 10.Sarosiek K.A., Chi X., Bachman J.A., Sims J.J., Montero J., Patel L., Flanagan A., Andrews D.W., Sorger P., Letai A. BID preferentially activates BAK while BIM preferentially activates BAX, affecting chemotherapy response. Mol. Cell. 2013;51:751–765. doi: 10.1016/j.molcel.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willis S.N., Chen L., Dewson G., Wei A., Naik E., Fletcher J.I., Adams J.M., Huang D.C. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L., Willis S.N., Wei A., Smith B.J., Fletcher J.I., Hinds M.G., Colman P.M., Day C.L., Adams J.M., Huang D.C. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol. Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 13.Leshchiner E.S., Braun C.R., Bird G.H., Walensky L.D. Direct activation of full-length proapoptotic BAK. Proc. Natl. Acad. Sci. U.S.A. 2013;110:E986–E995. doi: 10.1073/pnas.1214313110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yethon J.A., Epand R.F., Leber B., Epand R.M., Andrews D.W. Interaction with a membrane surface triggers a reversible conformational change in Bax normally associated with induction of apoptosis. J. Biol. Chem. 2003;278:48935–48941. doi: 10.1074/jbc.M306289200. [DOI] [PubMed] [Google Scholar]
- 15.Gilmore A.P., Metcalfe A.D., Romer L.H., Streuli C.H. Integrin-mediated survival signals regulate the apoptotic function of Bax through its conformation and subcellular localization. J. Cell Biol. 2000;149:431–446. doi: 10.1083/jcb.149.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pagliari L.J., Kuwana T., Bonzon C., Newmeyer D.D., Tu S., Beere H.M., Green D.R. The multidomain proapoptotic molecules Bax and Bak are directly activated by heat. Proc. Natl. Acad. Sci. U.S.A. 2005;102:17975–17980. doi: 10.1073/pnas.0506712102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cartron P.F., Oliver L., Mayat E., Meflah K., Vallette F.M. Impact of pH on Bax alpha conformation, oligomerisation and mitochondrial integration. FEBS Lett. 2004;578:41–46. doi: 10.1016/j.febslet.2004.10.080. [DOI] [PubMed] [Google Scholar]
- 18.Nie C., Tian C., Zhao L., Petit P.X., Mehrpour M., Chen Q. Cysteine 62 of Bax is critical for its conformational activation and its proapoptotic activity in response to H2O2-induced apoptosis. J. Biol. Chem. 2008;283:15359–15369. doi: 10.1074/jbc.M800847200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D'Alessio M., De Nicola M., Coppola S., Gualandi G., Pugliese L., Cerella C., Cristofanon S., Civitareale P., Ciriolo M.R., Bergamaschi A., et al. Oxidative Bax dimerization promotes its translocation to mitochondria independently of apoptosis. FASEB J. 2005;19:1504–1506. doi: 10.1096/fj.04-3329fje. [DOI] [PubMed] [Google Scholar]
- 20.Wood D.E., Newcomb E.W. Cleavage of Bax enhances its cell death function. Exp. Cell. Res. 2000;256:375–382. doi: 10.1006/excr.2000.4859. [DOI] [PubMed] [Google Scholar]
- 21.Kim B.J., Ryu S.W., Song B.J. JNK- and p38 kinase-mediated phosphorylation of Bax leads to its activation and mitochondrial translocation and to apoptosis of human hepatoma HepG2 cells. J. Biol. Chem. 2006;281:21256–21265. doi: 10.1074/jbc.M510644200. [DOI] [PubMed] [Google Scholar]
- 22.Gavathiotis E., Reyna D.E., Bellairs J.A., Leshchiner E.S., Walensky L.D. Direct and selective small-molecule activation of proapoptotic BAX. Nat. Chem. Biol. 2012;8:639–645. doi: 10.1038/nchembio.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao G., Zhu Y., Eno C.O., Liu Y., Deleeuw L., Burlison J.A., Chaires J.B., Trent J.O., Li C. Activation of the proapoptotic Bcl-2 protein Bax by a small molecule induces tumor cell apoptosis. Mol. Cell. Biol. 2014;34:1198–1207. doi: 10.1128/MCB.00996-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xin M., Li R., Xie M., Park D., Owonikoko T.K., Sica G.L., Corsino P.E., Zhou J., Ding C., White M.A., et al. Small-molecule Bax agonists for cancer therapy. Nat. Commun. 2014;5:4935. doi: 10.1038/ncomms5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Billen L.P., Kokoski C.L., Lovell J.F., Leber B., Andrews D.W. Bcl-XL inhibits membrane permeabilization by competing with Bax. PLoS Biol. 2008;6:e147. doi: 10.1371/journal.pbio.0060147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Logue S.E., Elgendy M., Martin S.J. Expression, purification and use of recombinant annexin V for the detection of apoptotic cells. Nat. Protoc. 2009;4:1383–1395. doi: 10.1038/nprot.2009.143. [DOI] [PubMed] [Google Scholar]
- 27.Shamas-Din A., Satsoura D., Khan O., Zhu W., Leber B., Fradin C., Andrews D.W. Multiple partners can kiss-and-run: Bax transfers between multiple membranes and permeabilizes those primed by tBid. Cell Death Dis. 2014;5:e1277. doi: 10.1038/cddis.2014.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shamas-Din A., Bindner S., Zhu W., Zaltsman Y., Campbell C., Gross A., Leber B., Andrews D.W., Fradin C. tBid undergoes multiple conformational changes at the membrane required for Bax activation. J. Biol. Chem. 2013;288:22111–22127. doi: 10.1074/jbc.M113.482109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilschut J., Duzgunes N., Fraley R., Papahadjopoulos D. Studies on the mechanism of membrane fusion: kinetics of calcium ion induced fusion of phosphatidylserine vesicles followed by a new assay for mixing of aqueous vesicle contents. Biochemistry. 1980;19:6011–6021. doi: 10.1021/bi00567a011. [DOI] [PubMed] [Google Scholar]
- 30.Du C., Fang M., Li Y., Li L., Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/S0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 31.Eskes R., Desagher S., Antonsson B., Martinou J.C. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol. Cell. Biol. 2000;20:929–935. doi: 10.1128/MCB.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu Y.T., Youle R.J. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J. Biol. Chem. 1998;273:10777–10783. doi: 10.1074/jbc.273.17.10777. [DOI] [PubMed] [Google Scholar]
- 33.Czabotar P.E., Westphal D., Dewson G., Ma S., Hockings C., Fairlie W.D., Lee E.F., Yao S., Robin A.Y., Smith B.J., et al. Bax crystal structures reveal how BH3 domains activate Bax and nucleate its oligomerization to induce apoptosis. Cell. 2013;152:519–531. doi: 10.1016/j.cell.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 34.Mondal Roy S., Sarkar M. Membrane fusion induced by small molecules and ions. J. Lipids. 2011;2011:528784. doi: 10.1155/2011/528784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown R.D., Burke G.A., Brown G.C. Dependence of leukemic cell proliferation and survival on H2O2 and L-arginine. Free Radic. Biol. Med. 2009;46:1211–20. doi: 10.1016/j.freeradbiomed.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Lai T.S., Liu Y., Tucker T., Daniel K.R., Sane D.C., Toone E., Burke J.R., Strittmatter W.J., Greenberg C.S. Identification of chemical inhibitors to human tissue transglutaminase by screening existing drug libraries. Chem. Biol. 2008;15:969–978. doi: 10.1016/j.chembiol.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei M.C., Zong W.X., Cheng E.H., Lindsten T., Panoutsakopoulou V., Ross A.J., Roth K.A., MacGregor G.R. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Degenhardt K., Sundararajan R., Lindsten T., Thompson C., White E. Bax and Bak independently promote cytochrome C release from mitochondria. J. Biol. Chem. 2002;277:14127–14134. doi: 10.1074/jbc.M109939200. [DOI] [PubMed] [Google Scholar]
- 39.Llambi F., Moldoveanu T., Tait S.W., Bouchier-Hayes L., Temirov J., McCormick L.L., Dillon C.P., Green D.R. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol. Cell. 2011;44:517–531. doi: 10.1016/j.molcel.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang K., Yin X.M., Chao D.T., Milliman C.L., Korsmeyer S.J. BID: a novel BH3 domain-only death agonist. Genes Dev. 1996;10:2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- 41.Desagher S., Osen-Sand A., Nichols A., Eskes R., Montessuit S., Lauper S., Maundrell K., Antonsson B., Martinou J.C. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J. Cell Biol. 1999;144:891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suzuki M., Youle R.J., Tjandra N. Structure of Bax: coregulation of dimer formation and intracellular localization. Cell. 2000;103:645–654. doi: 10.1016/S0092-8674(00)00167-7. [DOI] [PubMed] [Google Scholar]
- 43.Frohlich M., Dejanovic B., Kashkar H., Schwarz G., Nussberger S. S-palmitoylation represents a novel mechanism regulating the mitochondrial targeting of BAX and initiation of apoptosis. Cell Death Dis. 2014;5:e1057. doi: 10.1038/cddis.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lalier L., Cartron P.F., Olivier C., Loge C., Bougras G., Robert J.M., Oliver L., Vallette F.M. Prostaglandins antagonistically control Bax activation during apoptosis. Cell Death Differ. 2011;18:528–537. doi: 10.1038/cdd.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dewson G., Ma S., Frederick P., Hockings C., Tan I., Kratina T., Kluck R.M. Bax dimerizes via a symmetric BH3:groove interface during apoptosis. Cell Death Differ. 2012;19:661–670. doi: 10.1038/cdd.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willis S.N., Fletcher J.I., Kaufmann T., van Delft M.F., Chen L., Czabotar P.E., Ierino H., Lee E.F., Fairlie W.D., Bouillet P., et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]