Abstract
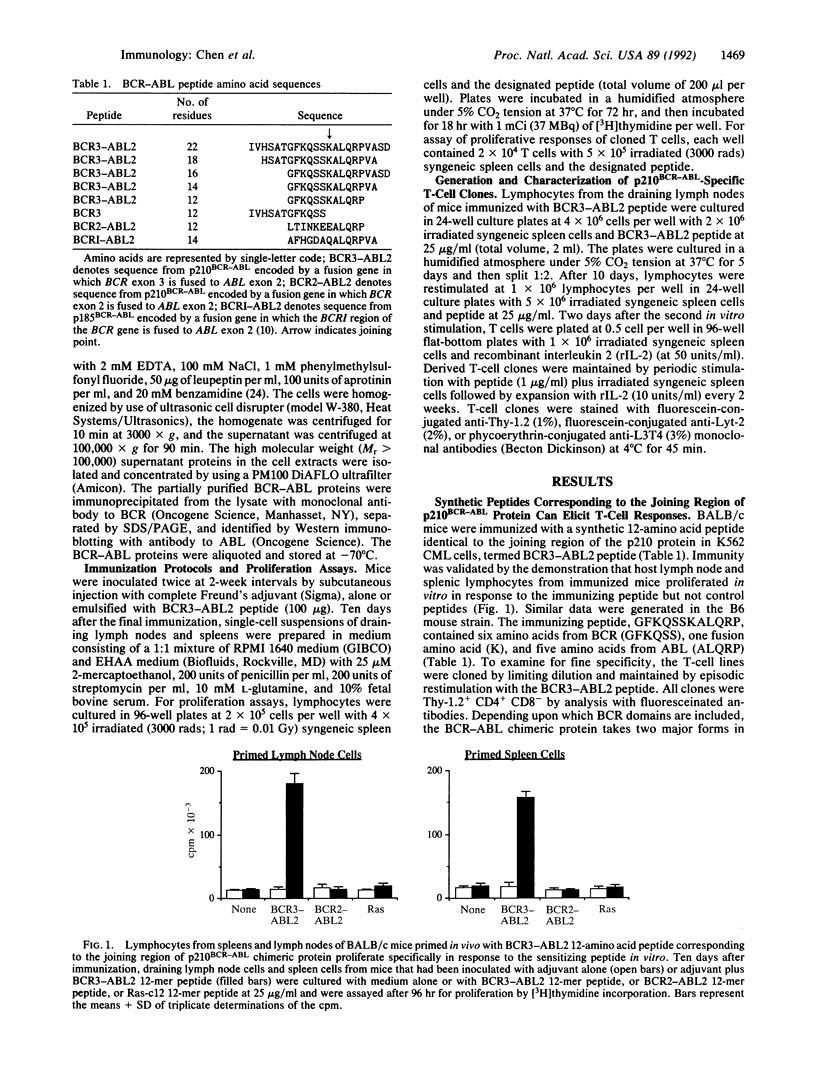
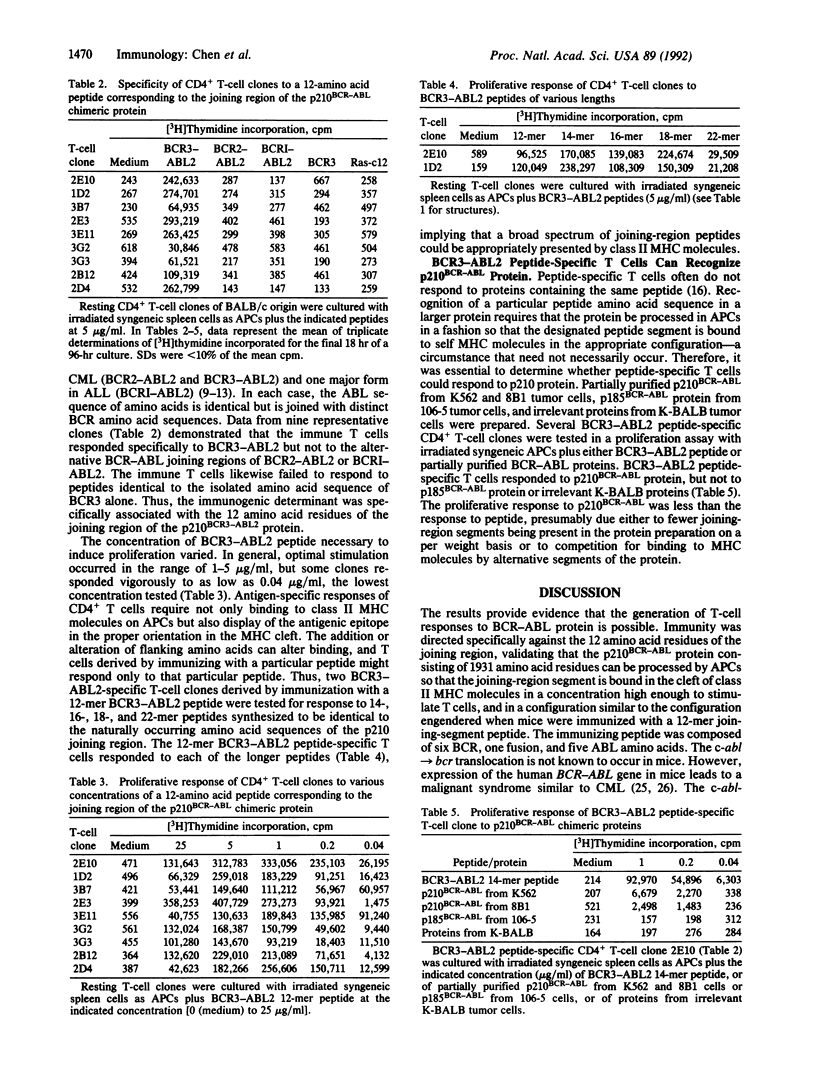
The hallmark of chronic myelogenous leukemia is the translocation of the human c-abl protooncogene (ABL) from chromosome 9 to the specific breakpoint cluster region (bcr) of the BCR gene on chromosome 22. The t(9;22)(q34;q11) translocation results in the formation of a BCR-ABL fusion gene that encodes a 210-kDa chimeric protein with abnormal tyrosine kinase activity. The ABL and BCR genes are expressed by normal cells and thus the encoded proteins are presumably nonimmunogenic. However, the joining-region segment of the p210BCR-ABL chimeric protein is composed of unique sequences of ABL amino acids joined to BCR amino acids that are expressed only by malignant cells. The current study demonstrates that the joining region of BCR-ABL protein is immunogenic to murine T cells. Immunization of mice with synthetic peptides corresponding to the joining region elicited peptide-specific, CD4+, class II major histocompatibility complex-restricted T cells. The BCR-ABL peptide-specific T cells recognized only the combined sequence of BCR-ABL amino acids and not BCR or ABL amino acid sequences alone. Importantly, the BCR-ABL peptide-specific T cells could recognize and proliferate in response to p210BCR-ABL protein. The response of peptide-specific T cells to protein demonstrated that p210BCR-ABL can be processed by antigen-presenting cells so that the joining segment is bound to class II major histocompatibility complex molecules in a configuration similar to that of the immunizing peptide and in a concentration high enough to stimulate the antigen-specific T-cell receptor. Thus, BCR-ABL protein represents a potential tumor-specific antigen related to the transforming event and shared by many individuals with chronic myelogenous leukemia.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartram C. R., Janssen J. W., Becher R., de Klein A., Grosveld G. Persistence of chronic myelocytic leukemia despite deletion of rearranged bcr/c-abl sequences in blast crisis. J Exp Med. 1986 Nov 1;164(5):1389–1396. doi: 10.1084/jem.164.5.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartram C. R., Raghavachar A., Anger B., Stain C., Bettelheim P. T lymphocytes lack rearrangement of the bcr gene in Philadelphia chromosome-positive chronic myelocytic leukemia. Blood. 1987 Jun;69(6):1682–1685. [PubMed] [Google Scholar]
- Ben-Neriah Y., Daley G. Q., Mes-Masson A. M., Witte O. N., Baltimore D. The chronic myelogenous leukemia-specific P210 protein is the product of the bcr/abl hybrid gene. Science. 1986 Jul 11;233(4760):212–214. doi: 10.1126/science.3460176. [DOI] [PubMed] [Google Scholar]
- Berzofsky J. A., Brett S. J., Streicher H. Z., Takahashi H. Antigen processing for presentation to T lymphocytes: function, mechanisms, and implications for the T-cell repertoire. Immunol Rev. 1988 Dec;106:5–31. doi: 10.1111/j.1600-065x.1988.tb00771.x. [DOI] [PubMed] [Google Scholar]
- Chan L. C., Karhi K. K., Rayter S. I., Heisterkamp N., Eridani S., Powles R., Lawler S. D., Groffen J., Foulkes J. G., Greaves M. F. A novel abl protein expressed in Philadelphia chromosome positive acute lymphoblastic leukaemia. Nature. 1987 Feb 12;325(6105):635–637. doi: 10.1038/325635a0. [DOI] [PubMed] [Google Scholar]
- Cheever M. A., Thompson D. B., Klarnet J. P., Greenberg P. D. Antigen-driven long term-cultured T cells proliferate in vivo, distribute widely, mediate specific tumor therapy, and persist long-term as functional memory T cells. J Exp Med. 1986 May 1;163(5):1100–1112. doi: 10.1084/jem.163.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. S., McLaughlin J., Crist W. M., Champlin R., Witte O. N. Unique forms of the abl tyrosine kinase distinguish Ph1-positive CML from Ph1-positive ALL. Science. 1987 Jan 2;235(4784):85–88. doi: 10.1126/science.3541203. [DOI] [PubMed] [Google Scholar]
- Dhut S., Chaplin T., Young B. D. BCR-ABL and BCR proteins: biochemical characterization and localization. Leukemia. 1990 Nov;4(11):745–750. [PubMed] [Google Scholar]
- Fialkow P. J., Jacobson R. J., Papayannopoulou T. Chronic myelocytic leukemia: clonal origin in a stem cell common to the granulocyte, erythrocyte, platelet and monocyte/macrophage. Am J Med. 1977 Jul;63(1):125–130. doi: 10.1016/0002-9343(77)90124-3. [DOI] [PubMed] [Google Scholar]
- Gale R. P., Canaani E. An 8-kilobase abl RNA transcript in chronic myelogenous leukemia. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5648–5652. doi: 10.1073/pnas.81.18.5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehly G. B., Bryant E. M., Lee A. M., Kidd P. G., Thomas E. D. Chimeric BCR-abl messenger RNA as a marker for minimal residual disease in patients transplanted for Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 1991 Jul 15;78(2):458–465. [PubMed] [Google Scholar]
- Germain R. N. Immunology. The ins and outs of antigen processing and presentation. Nature. 1986 Aug 21;322(6081):687–689. doi: 10.1038/322687a0. [DOI] [PubMed] [Google Scholar]
- Greenberg P. D., Kern D. E., Cheever M. A. Therapy of disseminated murine leukemia with cyclophosphamide and immune Lyt-1+,2- T cells. Tumor eradication does not require participation of cytotoxic T cells. J Exp Med. 1985 May 1;161(5):1122–1134. doi: 10.1084/jem.161.5.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey H. M., Sette A., Buus S. How T cells see antigen. Sci Am. 1989 Nov;261(5):56–64. doi: 10.1038/scientificamerican1189-56. [DOI] [PubMed] [Google Scholar]
- Heisterkamp N., Jenster G., ten Hoeve J., Zovich D., Pattengale P. K., Groffen J. Acute leukaemia in bcr/abl transgenic mice. Nature. 1990 Mar 15;344(6263):251–253. doi: 10.1038/344251a0. [DOI] [PubMed] [Google Scholar]
- Kelliher M. A., McLaughlin J., Witte O. N., Rosenberg N. Induction of a chronic myelogenous leukemia-like syndrome in mice with v-abl and BCR/ABL. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6649–6653. doi: 10.1073/pnas.87.17.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloetzer W., Kurzrock R., Smith L., Talpaz M., Spiller M., Gutterman J., Arlinghaus R. The human cellular abl gene product in the chronic myelogenous leukemia cell line K562 has an associated tyrosine protein kinase activity. Virology. 1985 Jan 30;140(2):230–238. doi: 10.1016/0042-6822(85)90361-7. [DOI] [PubMed] [Google Scholar]
- Konopka J. B., Watanabe S. M., Witte O. N. An alteration of the human c-abl protein in K562 leukemia cells unmasks associated tyrosine kinase activity. Cell. 1984 Jul;37(3):1035–1042. doi: 10.1016/0092-8674(84)90438-0. [DOI] [PubMed] [Google Scholar]
- Kurzrock R., Kloetzer W. S., Talpaz M., Blick M., Walters R., Arlinghaus R. B., Gutterman J. U. Identification of molecular variants of p210bcr-abl in chronic myelogenous leukemia. Blood. 1987 Jul;70(1):233–236. [PubMed] [Google Scholar]
- Kurzrock R., Shtalrid M., Romero P., Kloetzer W. S., Talpas M., Trujillo J. M., Blick M., Beran M., Gutterman J. U. A novel c-abl protein product in Philadelphia-positive acute lymphoblastic leukaemia. Nature. 1987 Feb 12;325(6105):631–635. doi: 10.1038/325631a0. [DOI] [PubMed] [Google Scholar]
- Lisker R., Casas L., Mutchinick O., Pérez-Chávez F., Labardini J. Late-appearing Philadelphia chromosome in two patients with chronic myelogenous leukemia. Blood. 1980 Nov;56(5):812–814. [PubMed] [Google Scholar]
- McLaughlin J., Chianese E., Witte O. N. Alternative forms of the BCR-ABL oncogene have quantitatively different potencies for stimulation of immature lymphoid cells. Mol Cell Biol. 1989 May;9(5):1866–1874. doi: 10.1128/mcb.9.5.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J., Chianese E., Witte O. N. In vitro transformation of immature hematopoietic cells by the P210 BCR/ABL oncogene product of the Philadelphia chromosome. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6558–6562. doi: 10.1073/pnas.84.18.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niman H. L., Thompson A. M., Yu A., Markman M., Willems J. J., Herwig K. R., Habib N. A., Wood C. B., Houghten R. A., Lerner R. A. Anti-peptide antibodies detect oncogene-related proteins in urine. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7924–7928. doi: 10.1073/pnas.82.23.7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peace D. J., Chen W., Nelson H., Cheever M. A. T cell recognition of transforming proteins encoded by mutated ras proto-oncogenes. J Immunol. 1991 Mar 15;146(6):2059–2065. [PubMed] [Google Scholar]
- Pendergast A. M., Clark R., Kawasaki E. S., McCormick F. P., Witte O. N. Baculovirus expression of functional P210 BCR-ABL oncogene product. Oncogene. 1989 Jun;4(6):759–766. [PubMed] [Google Scholar]
- Perkins D. L., Lai M. Z., Smith J. A., Gefter M. L. Identical peptides recognized by MHC class I- and II-restricted T cells. J Exp Med. 1989 Jul 1;170(1):279–289. doi: 10.1084/jem.170.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbard J. B., Taylor W. R. A sequence pattern common to T cell epitopes. EMBO J. 1988 Jan;7(1):93–100. doi: 10.1002/j.1460-2075.1988.tb02787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtivelman E., Lifshitz B., Gale R. P., Canaani E. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature. 1985 Jun 13;315(6020):550–554. doi: 10.1038/315550a0. [DOI] [PubMed] [Google Scholar]
- Stam K., Heisterkamp N., Reynolds F. H., Jr, Groffen J. Evidence that the phl gene encodes a 160,000-dalton phosphoprotein with associated kinase activity. Mol Cell Biol. 1987 May;7(5):1955–1960. doi: 10.1128/mcb.7.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczylik C., Skorski T., Nicolaides N. C., Manzella L., Malaguarnera L., Venturelli D., Gewirtz A. M., Calabretta B. Selective inhibition of leukemia cell proliferation by BCR-ABL antisense oligodeoxynucleotides. Science. 1991 Aug 2;253(5019):562–565. doi: 10.1126/science.1857987. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Allen P. M. The basis for the immunoregulatory role of macrophages and other accessory cells. Science. 1987 May 1;236(4801):551–557. doi: 10.1126/science.2437650. [DOI] [PubMed] [Google Scholar]
- Young J. C., Witte O. N. Selective transformation of primitive lymphoid cells by the BCR/ABL oncogene expressed in long-term lymphoid or myeloid cultures. Mol Cell Biol. 1988 Oct;8(10):4079–4087. doi: 10.1128/mcb.8.10.4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Denderen J., Hermans A., Meeuwsen T., Troelstra C., Zegers N., Boersma W., Grosveld G., van Ewijk W. Antibody recognition of the tumor-specific bcr-abl joining region in chronic myeloid leukemia. J Exp Med. 1989 Jan 1;169(1):87–98. doi: 10.1084/jem.169.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Denderen J., van der Plas D., Meeuwsen T., Zegers N., Boersma W., Grosveld G., van Ewijk W. Immunologic characterization of the tumor-specific bcr-abl junction in Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 1990 Jul 1;76(1):136–141. [PubMed] [Google Scholar]



