Abstract
Objectives
The course of systemic sclerosis (SSc) can differ in female and male patients. According to the literature the incidence rates of diffuse cutaneous SSc, scleroderma renal crisis and digital ulceration are higher in male patients. The aim of the study was to compare selected clinical and serological parameters in male and female patients with SSc.
Material and methods
The study encompassed 101 European Caucasian patients with SSc, including 23 men, hospitalized in the Department of Rheumatology. Patients fulfilled the American Rheumatism Association (ARA) classification criteria for SSc. The study groups of men and women were assessed according to the SSc subtype, incidence of internal organ involvement and presence of antinuclear antibodies considered SSc markers.
Results
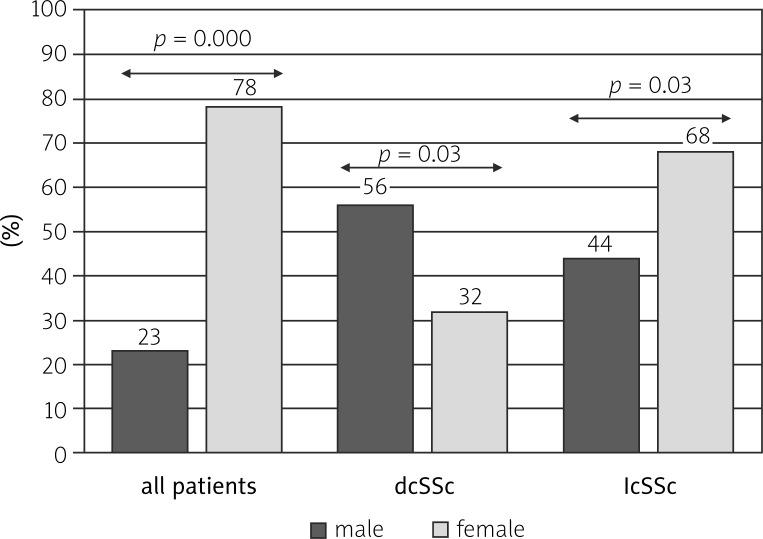
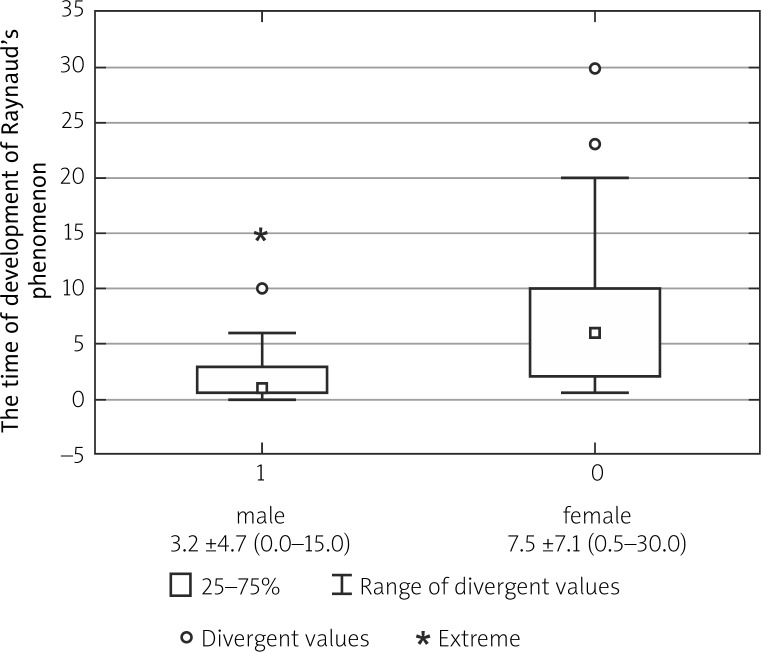
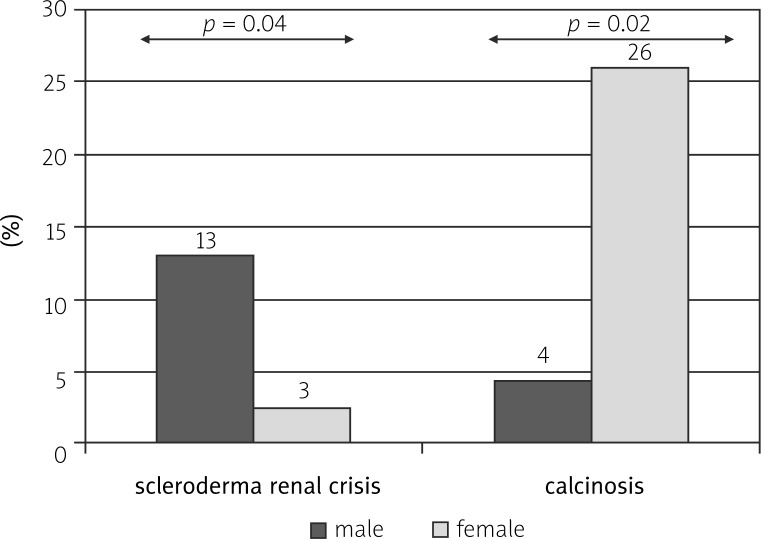
Diffuse cutaneous (dc) SSc was observed more commonly in men than in women (13/23 vs. 25/78; p = 0.03). The time from the development of Raynaud's phenomenon to the diagnosis was significantly shorter in male compared to female patients (3.2 ±4.7 vs. 7.5 ±7.1; p = 0.01). The incidence of scleroderma renal crisis (SRC) was significantly higher (3/23 vs. 2/78; p = 0.04) and of other calcifications significantly lower in the male group compared to the female group (1/23 vs. 20/78; p = 0.02).
Conclusions
We concluded that the incidence of dcSSc is higher in men compared to women. The time from the development of Raynaud's phenomenon to the diagnosis is shorter in the male compare to female group. The incidence of SRC is higher, whereas that of calcifications is lower in SSc men. The serological profiles of female and male patients with SSc are comparable.
Keywords: systemic sclerosis, female, male, clinical parameters
Introduction
Systemic sclerosis (SSc) is a chronic, autoimmune multisystem disorder characterized by vascular damage, inflammation, thickening and fibrosis of the skin and internal organs. It mainly affects women and is associated with significant morbidity, including pain, disability, depression, reduced quality of life and increased mortality [1].
Prognosis is worse in SSc than in other connective tissue disorders, and the risk of mortality is 4–5 times greater than that of an age- and gender-matched population. The known predictors of worse survival in SSc include organ involvement, particularly lung, heart and kidney involvement, older age at diagnosis, ethnicity, elevated serum creatine kinase (CK), diffuse subtype of SSc and male gender [2–9]. It was found that the incidence of diffuse cutaneous dcSSc (subtype of SSc) is higher in African-American and Hispanic populations compared with European Caucasian patients, but it is not clear whether there are differences in subsequent disease course. Although women are more commonly affected by SSc than men, the course of disease may be more progressive in males compared to females. However, the potential impact of gender on the disease course of dcSSc is not well defined. Some studies have shown that males have a greater risk of death due to SSc [7, 10, 11].
Other differences are not clear but include the time to diagnosis after the onset of Raynaud's phenomenon, subtype of disease and visceral organ involvement [3, 12]. It has been suggested that the incidence rates of dcSSc, scleroderma renal crisis and digital ulceration are higher in male patients [13, 14].
The aim of the study was to compare selected clinical and serological parameters in male and female patients with SSc.
Material and methods
The study included 101 SSc European Caucasian patients – 78 female (age 50.9 ±14.9) and 23 male (age 54.5 ±12.7) – hospitalized consecutively in the Department of Rheumatology and Connective Tissue Diseases. Patients fulfilled the American Rheumatism Association (ARA) classification criteria for SSc [15]. According to Le Roy et al. [16] the women and men were classified as having limited cutaneous systemic sclerosis (lcSSc) or dcSSc; moreover, the time from onset of Raynaud's phenomenon to diagnosis was determined (Table I).
Table I.
Characteristics of all patients
| Male | Female | |
|---|---|---|
| Number of patients | 23 | 78 |
| Type of SSc: dcSSc lcSSc |
13 10 |
25 53 |
| Age (years) | 50.9 ±14.9 (19.0–72.0) |
54.5 ±12.7 (21.0–81.0) |
| Duration of disease (years) | 5.1 ±5.1 (0.5–23.0) |
7.2 ±5.8 (0.5–23.0) |
Incidence of internal organ involvement was compared between the group of male and female patients. All patients provided written informed consent to participate in the study. Organ involvement was assessed according to the clinical symptoms and the results of diagnostic tests. Interstitial lung disease (ILD) was defined as “a ground glass” pattern or bibasilar pulmonary fibrosis revealed on a high-resolution computer tomography (HRCT) scan [17]. To interpret the pulmonary function the DLCO test (% predicted diffusing capacity for carbon monoxide) was performed [18–20]. Heart involvement was established as arrhythmia, conduction disturbances or heart failure. Pulmonary arterial hypertension (PAH) was defined as systolic pulmonary arterial pressure (sPAP) > 35 in Doppler echocardiography [21].
Myalgia or myositis was assessed as pain or weakness in muscles or increased serum CK levels. Joint involvement was considered as joint tenderness and swelling. Gastrointestinal tract involvement was defined according to clinical symptoms such as dysphagia, heartburn, diarrhea or bloating and was examined by a barium swallow. Renal involvement was defined as the development of scleroderma renal crisis (SRC) or presence of proteinuria and elevated creatinine [22].
Calcinosis and digital erosions were also assessed. Serum samples were obtained from each patient. Moreover, the study groups of men and women were studied according to the presence of antibodies applying two different methods: immunofluorescence to assess patterns of staining – centromeric-nuclear, homogeneous or granular; and a commercial test – EUROLINE Systemic Sclerosis Profile, which is used to determine antibodies against SSc-specific antigens, such as anti-topoisomerase I (anti-topo I) and anti-centromere autoantibodies (ACAs), anti-RNA polymerase III, the more rare anti-PM/Scl, anti-Ku, anti-Th/To and autoantibodies against nucleolus-organizing region-90 (anti-NOR90). Detection and interpretation of results was carried out electronically using the specific program Euroimmun – EUROLINEScan.
All calculations were performed with Statistica 10.0. Data were analyzed using the chi-squared test (variation test) for comparison between groups. A p value < 0.05 was considered statistically significant.
The study was approved by the Ethics Committee of the Medical University of Lublin.
Results
According to our observations, dcSSc was observed more commonly in men than in women (13/23 vs. 25/78; p = 0.03) (Fig. 1). The time from the development of Raynaud's phenomenon to diagnosis was significantly shorter in male patients compared to female patients (3.2 ±4.7 vs. 7.5 ±7.1; p = 0.01) (Fig. 2). The incidence of SRC was significantly higher in the male group compared to females (3/23 vs. 2/78; p = 0.04). On the other hand, the incidence of calcifications was significantly lower in the male group compared to the female group (1/23 vs. 20/78; p = 0.02) (Fig. 3).
Fig. 1.
Comparison of prevalence of subtypes of systemic sclerosis in female and male patients.
Fig. 2.
Comparison of the time from development of Raynaud's phenomenon to diagnosis in female and male patients with systemic sclerosis.
Fig. 3.
Comparison of the incidence of scleroderma renal crisis and calcinosis in female and male patients with systemic sclerosis.
There were no significant intergroup differences in decreased DLCO, ILD, PAH, heart involvement, gastrointestinal tract involvement, prevalence of arthritis or arthralgia, myalgia and digital ulcerations. Furthermore, no statistically significant differences were observed in the prevalence of death between male and female patients. The incidence of overlap syndromes did not differ between the male and female group (Table II).
Table II.
Comparison of selected clinical parameters in female and male patients with systemic sclerosis
| Clinical parameters | Male | Female | P |
|---|---|---|---|
| ILD (HRCT) | 13 (56.5%) | 38 (49%) | 0.5 |
| Decreased DLCO | 14 (61%) | 39 (50%) | 0.3 |
| Heart involvement | 9 (39%) | 31 (40%) | 0.9 |
| PAH (ECHO) | 4 (15%) | 21 (27%) | 0.2 |
| Myalgia or myosistis | 5 (22%) | 12 (15%) | 0.4 |
| Arthralgia | 20 (87%) | 60 (77%) | 0.3 |
| Arthritis | 11 (49%) | 32 (41%) | 0.5 |
| Gastrointestinal tract involvement | 16 (69.5%) | 49 (63%) | 0.6 |
| Renal involvement | 8 (35%) | 22 (28%) | 0.5 |
| Digital ulcerations | 3 (13%) | 14 (18%) | 0.6 |
| Death | 6 (26%) | 12 (15%) | 0.2 |
| Overlap syndrome | 4 (17%) | 21 (27%) | 0.3 |
Data were presented as number and percentage. P-value of < 0.05 was considered statistically significant
There were no statistically significant differences in the incidence of anti-Scl-70 and anti-centromere antibodies found in the groups of female and male patients. The presence of anti-RNA polymerase III, anti-Ku, anti-Th/To, anti-PM/Scl and anti-Nor-90 antibodies was comparable in both groups (Table III).
Table III.
Comparison of selected serological parameters in female and male patients with systemic sclerosis
| Serological parameters | Male | Female | P |
|---|---|---|---|
| Antinuclear antibody | 20/22 (87%) | 63/68 (81%) | 0.5 |
| Anti-centromere | 3 (13%) | 24/68 (30%) | 0.1 |
| Anti-nucleoral | 9 (39%) | 24 (30%) | 0.4 |
| Anti-homogenic | 4 (17%) | 8 (10%) | 0.3 |
| Anti–granular | 6 (26%) | 19 (24%) | 0.8 |
| Anti-Scl-70 | 6/20 (26%) | 22/63 (28%) | 0.8 |
| Anti-centromere | 2 (9%) | 16 (20.5%) | 0.2 |
| Anty-RNA polymerase III | 6 (13%) | 4 (5%) | 0.1 |
| Anti-Ku | 1 (4%) | 3 (4%) | 1 |
| Anti-Th/To | 1 (4%) | 4 (5%) | 0.8 |
| Anti-PM-Scl | 4 (17%) | 9 (11.5%) | 0.4 |
| Anti-Nor-90 | 1 (4%) | 4 (5%) | 0.8 |
Data were presented as number and percentage. “P value of < 0.05 was considered statistically significant”
Discussion
Differences in the course of SSc and presence of typical symptoms between the men and women have been reviewed elsewhere. Analysis from three large randomized clinical trials explored the influence of gender and ethnicity on disease course. In this study among the three ethnic groups Caucasians, African-Americans and Hispanics, men in all ethnic groups had lower health assessment questionnaire disability index scores compared with women (p < 0.05), Caucasians were older, African-Americans had lower FVC% predicted and Hispanics had higher tender joint counts (p < 0.05) [23].
The literature data demonstrated that the time to diagnosis was significantly longer in women [3, 24]. Hudson et al. studied 408 patients with SSc and found that the time to diagnosis was significantly longer for women compared to men when the disease onset was measured from the onset of Raynaud's phenomenon; otherwise, when measured from the onset of the first non-Raynaud's disease manifestation, the time to diagnosis was not significantly different [3]. According to their study, the time to diagnosis after the onset of Raynaud's phenomenon was more than twice as long in women (4.6 years) compared with men (2.1 years) with lcSSc; in dcSSc, the median time to diagnosis was 1.0 years in women and 0.7 years in men [3].
Our study findings were similar to the literature results reported. The time to diagnosis in SSc was found to be longer for women than men after the onset of Raynaud's phenomenon. On the other hand, according to another study, the time to diagnosis was significantly longer for women (median = 1.1 years) than men (median 0.8 = years; p = 0.037) but only with dcSSc following the onset of Raynaud's phenomenon. There were no significant or substantive sex differences in the time to diagnosis after the Raynaud's onset in lcSSc [25]. Still another study showed that men were more likely to have dcSSc than women (67% vs. 32%) and an earlier mean age of diagnosis (41.3 years old vs. 49.7). Moreover, men were found more likely to have died at an earlier age then women (44.6 years vs. 65.6), but there were no significant differences in survival rates between men and women [2]. In our study, dcSSc was also observed more commonly in men than in women. It is known that diffuse disease is a predictor of worse outcomes in SSc and could be one of the causes of more progressive disorders in males compared to females.
Al-Dhaher et al. found that diffuse disease was more frequent among subjects who died, but male sex was not related to survival [2]. Likewise, in our study, we did not find significant differences in the prevalence of death between males and females. However, some studies have shown that males had a greater risk of death due to SSc [10, 11, 23]. In our study we did not collect the data of tobacco smoking by the patients, but such details should be included. There are many literature reports which compare the prevalence of SSc manifestations in men and women. Digital ulcers and SRC are known to occur more frequently in men compared to women [12, 13, 22].
Panopoulos et al. [12] suggested that vasculopathy occurred earlier in men. During the first 3 years digital ulcers developed in 54% of men versus 31% of women (p = 0.036), and SRC developed in 17% of men versus 3% of women (p = 0.006). There were no significant differences in the prevalence of ILD, upper or lower gastrointestinal tract involvement or echocardiographic findings between males and females [12]. According to another study, men were more likely to have digital ulcers (78% vs. 53%); moreover, the association between SRC and death in this men-only subset was stronger than that observed for the women-only subset (50% of those deceased versus 10% of those living) [2]. In our study, the prevalence of SRC was also significantly higher in the male group compared to the female group; otherwise we did not find differences in the presence of digital ulcers and cardiovascular involvement between the groups.
Interestingly, according to the largest worldwide database, the risk of severe cardiovascular involvement in men was higher [14, 26]. This prospective observational study using the latest 2013 data extracted from the EULAR scleroderma trials and research (EUSTAR) cohort investigated 9182 patients with SSc (1321 men). In the multivariate analysis, males were independently associated with a higher risk of dcSSc, higher frequency of digital ulcers and pulmonary hypertension. In the longitudinal analysis, after a follow-up of 2 years, male gender was predictive of a new onset of pulmonary hypertension and heart failure. According to this study, male gender predicted deaths of all origins, but did not significantly account for SSc-related deaths [26].
Another study, which used the Canadian Scleroderma Research Group (CSRG) database, revealed that muscle involvement in SSc had poor prognosis affecting survival, especially in men with early dcSSc with anti-topo 1 and RNP autoantibodies and ILD [27]. In our study, no significant differences in muscle involvement were observed between males and females. The prevalence of calcinosis was found to be higher in female patients. This could be related to the lc subtype of disorder, which is more frequent in women. Calcinosis is known to be common in lcSSc. We did not observe significant differences in the presence of SSc marker antibodies between the two groups. Based on our observations, we conclude that the incidence of dcSSc is higher in men compared to women. The time from the development of Raynaud's phenomenon to diagnosis is shorter in the male than in the female group. The incidence of scleroderma renal crisis is higher, whereas that of calcifications is lower in SSc men compared to SSc women. The serological profiles of female and male patients with SSc are comparable.
Conclusion
The disease is more severely expressed in men than in women. This finding could be associated with the dc subtype of the disorder, which is more frequent in men compared to women, severe organ involvement, particularly higher incidence of SRC, vasculopathy and cardiac involvement [5, 12, 13, 24, 26, 28, 29].
Our study was limited due to the small number of men in the SSc group. We will continue the study on larger cohorts of patients, and we will consider the analysis of clinical and serological parameters in subgroups of patients with lcSSc and dcSSc.
The authors declare no conflict of interest.
References
- 1.BellandoRandone S, Guiducci S, MatucciCerinic M. Very early diagnosis of systemic sclerosis. Pol Arch Med Wewn. 2012;122:18–23. [PubMed] [Google Scholar]
- 2.Al-Dhaher FF, Pope JE, Ouimet JM. Determinants of morbidity and mortality of systemic sclerosis in Canada. Semin Arthritis Rheum. 2010;39:269–277. doi: 10.1016/j.semarthrit.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Hudson M, Thombs B, Baron M. Canadian Scleroderma Research Group: Time to diagnosis in systemic sclerosis: is sex a factor? Arthritis Rheum. 2009;61:274–278. doi: 10.1002/art.24284. [DOI] [PubMed] [Google Scholar]
- 4.Mendoza F, Derk CT. Systemic sclerosis mortality in the United States: 1999-2002 implications for patient care. J Clin Rheumatol. 2007;13:187–192. doi: 10.1097/RHU.0b013e318124a89e. [DOI] [PubMed] [Google Scholar]
- 5.Lambova S. Cardiac manifestations in systemic sclerosis. World J Cardiol. 2014;26:993–1005. doi: 10.4330/wjc.v6.i9.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sierakowska M, Sierakowski S, Doroszkiewicz H, et al. Symptoms from the internal organs of patients with systemic sclerosis in the light of selected diagnostic tests. Pol Merkur Lekarski. 2011;30:116–120. [PubMed] [Google Scholar]
- 7.Widuchowska M, Głowacka M, Kopeć-Mędrek M, et al. Postępująca twardzina układowa o niepomyślnym przebiegu u mężczyzn. Reumatologia. 2010;48:45–48. [Google Scholar]
- 8.Hasegawa M, Hatta Y, Matsushita T, et al. Clinical and laboratory features dependent on age at onset in Japanese systemic sclerosis. Mod Rheumatol. 2013;23:913–919. doi: 10.1007/s10165-012-0764-0. [DOI] [PubMed] [Google Scholar]
- 9.Chan PT, Mok CC, Chan KL, et al. Functioning and health-related quality of life in Chinese patients with systemic sclerosis: a case-control study. Clin Rheumatol. 2014;33:659–666. doi: 10.1007/s10067-014-2525-2. [DOI] [PubMed] [Google Scholar]
- 10.Reveille JD, Fischbach M, McNearney T, et al. Systemic sclerosis in 3USethnic groups: a comparison of clinical, sociodemographic, serologic, and immunogenetic determinants. Semin Arthritis Rheum. 2001;30:332–346. doi: 10.1053/sarh.2001.20268. [DOI] [PubMed] [Google Scholar]
- 11.Ohta A, Nagai M, Nishina M, et al. Age at onset and gender distribution of systemic lupus erythematosus, polymyositis/dermatomyositis, and systemic sclerosis in Japan. Mod Rheumatol. 2013;23:759–764. doi: 10.1007/s10165-012-0733-7. [DOI] [PubMed] [Google Scholar]
- 12.Panopoulos ST, Bournia VK, Sfikakis PP. Is vasculopathy associated with systemic sclerosis more severe in men? J Rheumatol. 2013;40:46–51. doi: 10.3899/jrheum.120667. [DOI] [PubMed] [Google Scholar]
- 13.Mihai C, Landewé R, van der Heijde D, et al. Digital ulcers predict a worse disease course in patients with systemic sclerosis. Ann Rheum Dis. 2015 doi: 10.1136/annrheumdis-2014-205897. [DOI] [PubMed] [Google Scholar]
- 14.Chung L, Farber HW, Benza R, et al. Unique predictors of mortality in patients with pulmonary arterial hypertension associated with systemic sclerosis in the REVEAL registry. Chest. 2014;146:1494–1504. doi: 10.1378/chest.13-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Preliminary criteria for the classification of systemic sclerosis (scleroderma) Arthritis Rheum. 1980;23:581–590. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 16.LeRoy EC, Black C, Fleishmajer R. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–204. [PubMed] [Google Scholar]
- 17.Ariani A, Silva M, Bravi E, et al. Operator-independent quantitative chest computed tomography versus standard assessment of interstitial lung disease related to systemic sclerosis: A multi-centric study. Mod Rheumatol. 2015;30:1–7. doi: 10.3109/14397595.2015.1016200. [DOI] [PubMed] [Google Scholar]
- 18.Patiwetwitoon S, Wangkaew S, Euathrongchit J, et al. High-resolution computed tomographic findings in systemic sclerosis-associated interstitial lung disease: comparison between diffuse and limited systemic sclerosis. J Clin Rheumatol. 2012;18:229–233. doi: 10.1097/RHU.0b013e318261176f. [DOI] [PubMed] [Google Scholar]
- 19.Guarnieri G, Zanatta E, Mason P, et al. Determinants of impairment in lung diffusing capacity in patients with systemic sclerosis. Clin Exp Rheumatol. 2015;33(4 Suppl 91):S80–S86. [PubMed] [Google Scholar]
- 20.Schoenfeld SR, Castelino FV. Interstitial lung disease in scleroderma. Rheum Dis Clin North Am. 2015;41:237–248. doi: 10.1016/j.rdc.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foocharoen C, Mahakkanukrauh A, Pussadhamma B, et al. Incidence of pulmonary hypertension in patients with systemic sclerosis and no pulmonary symptoms: is annual echocardiographic screening necessary? J Clin Rheumatol. 2014;20:268–274. doi: 10.1097/RHU.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 22.Wielosz E, Dryglewska M, Majdan M. Antiphospholipid antibodies and kidney involvement in patients with systemic sclerosis. Clin Rheumatol. 2009;28:955–259. doi: 10.1007/s10067-009-1188-x. [DOI] [PubMed] [Google Scholar]
- 23.Nashid M, Khanna PP, Furst DE, et al. Gender and ethnicity differences in patients with diffuse systemic sclerosis – analysis from three large randomized clinical trials. Rheumatology (Oxford) 2011;50:335–342. doi: 10.1093/rheumatology/keq294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaultier JB, Hot A, Cathébras P, et al. Systemic sclerosis in men. Rev Med Interne. 2008;29:181–186. doi: 10.1016/j.revmed.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Delisle VC, Hudson M, Baron M, et al. Sex and time to diagnosis in systemic sclerosis: an updated analysis of 1,129 patients from the Canadian scleroderma research group registry. Clin Exp Rheumatol. 2014;32:10–14. [PubMed] [Google Scholar]
- 26.Elhai M, Avouac J, Walker UA, et al. A gender gap in primary and secondary heart dysfunctions in systemic sclerosis: a EUSTAR prospective study. Ann Rheum Dis. 2016;75:163–169. doi: 10.1136/annrheumdis-2014-206386. [DOI] [PubMed] [Google Scholar]
- 27.Jung M, Bonner A, Hudson M, et al. Myopathy is a poor prognostic feature in systemic sclerosis: results from the Canadian Scleroderma Research Group (CSRG) cohort. Scand Rheum. 2014;43:217–220. doi: 10.3109/03009742.2013.868512. [DOI] [PubMed] [Google Scholar]
- 28.Frech T, Walker AE, Barrett-O'Keefe Z, et al. Systemic sclerosis induces pronounced peripheral vascular dysfunction characterized by blunted peripheral vasoreactivity and endothelial dysfunction. Clin Rheumatol. 2015;34:905–913. doi: 10.1007/s10067-014-2834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strickland G, Pauling J, Cavill C, et al. Mortality in systemic sclerosis – a single centre study from the UK. Clin Rheumatol. 2013;32:1533–1539. doi: 10.1007/s10067-013-2289-0. [DOI] [PubMed] [Google Scholar]