Abstract
This overview gives a brief historical summary of key discoveries regarding stem cells of the small intestine. The current concept is that there are two pools of intestinal stem cells (ISCs): an actively cycling pool that is marked by Lgr5, is relatively homogeneous and is responsible for daily turnover of the epithelium; and a slowly cycling or quiescent pool that functions as reserve ISCs. The latter pool appears to be quite heterogeneous and may include partially differentiated epithelial lineages that can reacquire stem cell characteristics following injury to the intestine. Markers and methods of isolation for active and quiescent ISC populations are described as well as the numerous important advances that have been made in approaches to the in vitro culture of ISCs and crypts. Factors regulating ISC biology are briefly summarized and both known and unknown aspects of the ISC niche are discussed. Although most of our current knowledge regarding ISC physiology and pathophysiology has come from studies with mice, recent work with human tissue highlights the potential translational applications arising from this field of research. Many of these topics are further elaborated in the following articles.
Abbreviations
- CBC
crypt‐base columnar
- EE
enteroendocrine
- iHIOs
induced human intestinal organoids
- ISCs
intestinal stem cells
- LRC
label‐retaining cell
- NEC
necrotizing enterocolitis
- PC
Paneth cell
- TA
transit amplifying
Introduction
The following articles summarize a selection of topics on GI stem cells that were presented and discussed at the FASEB Science Research Conference entitled ‘Gastrointestinal Tract XVI: GI homeostasis, the microbiome and the barrier, development and disease’, held 2–7 August 2015 in Steamboat Springs, CO, USA. These reviews illustrate the complexity of our current knowledge regarding physiology and pathophysiology of GI stem cells and point to remaining controversies and to gaps that still need to be explored.
This overview lays the scene for the following articles by presenting a historical context and by touching briefly on the various topics that are covered in detail in the subsequent reviews. While several of these focus solely on the stem cells of the small intestine, as does this overview, others extend to stem cells of diverse regions of the GI tract, including stomach, colon and liver, highlighting the fact that there are both similarities and differences.
Historical perspective of stem cells of the intestinal epithelium
The intestinal epithelium is the most rapidly proliferating tissue in the mammalian body, being entirely replaced every 3–5 days. This turnover is critical to maintaining a healthy epithelium with dual roles of facilitating digestion and absorption as well as maintaining the barrier between the internal and external milieu. The unique crypt–villus architecture with proliferating cells confined to the lower third of the crypt (Fig. 1) has long attracted physiologists, cell biologists and molecular biologists to the challenge of understanding the behaviour of this epithelium. Pioneering studies by Cheng and Leblond in 1974 led to the ‘unitarian hypothesis’ that undifferentiated cells (termed ‘crypt‐base columnar’ (CBC) cells) located in the intestinal crypts just above and between the Paneth cells may serve as multipotent stem cells responsible for the generation of all differentiated lineages of the small intestinal epithelium. These studies relied on the observation that at early times after administration of [3H]thymidine, cells damaged by the local radiation were phagocytosed only by the CBC cells, but subsequently, phagocytic fragments could be detected in cells of all four differentiated lineages (Cheng & Leblond, 1974). While the small numbers of enteroendocrine cells observed in the original study led to some debate as to a separate origin for this lineage, subsequent work confirmed the ‘unitarian hypothesis’ by showing that enteroendocrine cells share a common stem cell with other epithelial lineages (Bjerknes & Cheng, 1981; Thompson et al. 1990; Bjerknes & Cheng, 1999).
Figure 1. Architecture of the small intestinal epithelium .
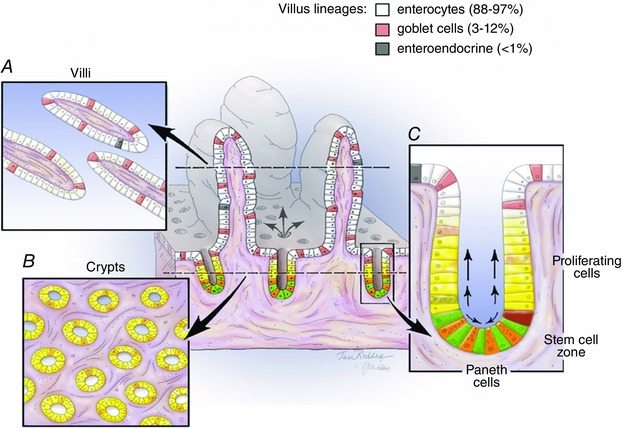
Centre, 3D depiction and typical 2D view as seen in a cross section showing crypt–villus units. Differentiated lineages found on the villi are colour coded as indicated. Left, structures of villi and crypts as seen in sections made along the longitudinal axis of the intestine and illustrates the fact that the villus is surrounded by multiple crypts. Right, an enlargement of the crypt area showing the proliferative transit amplifying (TA) cells (yellow), the stem cell zone and the Paneth cell lineage (orange). Within the stem cell zone, current nomenclature describes the stem cells that are intercalated between the Paneth cells at the crypt base as ‘crypt base columnar’ (CBC) cells (green) and those located immediately above the Paneth cells as ‘+4 cells’ (red).
The explosion of interest in the field of intestinal stem cells (ISCs) is illustrated in Fig. 2, which also highlights several landmark studies. In addition to the work from Hazel Cheng and her colleagues in Toronto, the other major groups in the early years were these of Bruce Ponder and Chris Potten, both in the UK. The Ponder laboratory creatively used chimeric mice to study the clonal behaviour of crypt cells and observed that in adult mice individual crypts were always composed of only one parental type (Ponder et al. 1985). As discussed in a later section, crypt monoclonality has recently been reinvestigated and is now understood at a dynamic level. Potten was a radiobiologist and the exquisite studies from his laboratory on the response of the intestinal epithelium to radiation laid the groundwork of our current understanding of the regenerative capacities of the intestinal epithelium. The microcolony assay originally described by Withers & Elkind (1970) and refined by Potten and his colleagues in the 1980s (Hendry et al. 1984) represented the first functional measure of ISC numbers and is still used today. Likewise, the demonstration that cells in the supra‐Paneth zone, i.e. the so called ‘+4’ position, are capable of long term retention of labelled thymidine analogues (Potten et al. 2002), remains a hallmark of cells in that region. These historical considerations are further discussed in the following review by Smith et al. (2016).
Figure 2. Historical data for publications on intestinal stem cells (ISCs) .
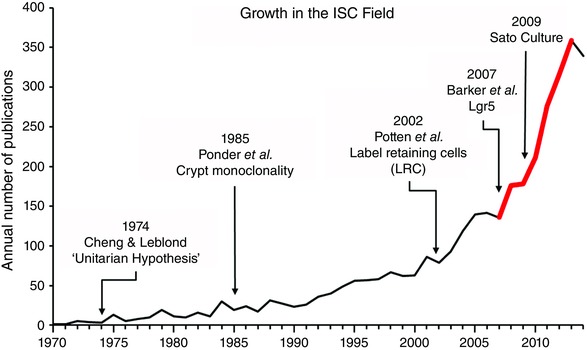
The graph shows the number of papers per year as found in PubMed using ‘stem cell intestine’ as the search term. Arrows show a selection of key papers and the red portion of the line shows the period of identification of various markers for +4 cells.
Markers for ISC populations
Until 2007 the study of ISCs was greatly hampered by a lack of markers. That year a landmark paper by Barker et al. reported the first definitive marker of ISCs, namely the G protein coupled receptor Lgr5, which is expressed specifically in the CBC cells located between Paneth cells at the base of the crypt (Barker et al. 2007). Lineage tracing experiments demonstrated that Lgr5‐labelled actively cycling cells are multipotent for all mature intestinal epithelial lineages, undergo self‐renewal and persist at least 60 days. Additional markers that have subsequently been identified for the Lgr5 positive population include Ascl2, Olfm4 and Sox9 (Gracz & Magness, 2014; Tan & Barker, 2014; Tetteh et al. 2015). There is now a general agreement that these cells are the population normally responsible for daily homeostatic turnover of the epithelium.
As shown in Fig. 2, between 2008 and 2012 there was a flurry of reports of markers for the +4 cells, which represent slowly cycling or quiescent populations of ISCs, specifically: Bmi1, mTert, Dclk1, Sox9hi and Lrig1 (Montgomery & Breault, 2008; Gracz & Magness, 2014; Tan & Barker, 2014). All of these populations appear to function as reserve ISCs in the sense that they can become activated following intestinal damage (such as via radiation or chemotherapy) and play a critical role in the regeneration of the epithelium. HopX also marks slowly cycling cells which function as ISCs; however, the extent to which these cells are activated by damage has not yet been reported (Takeda et al. 2011). The question of whether the various markers of these reserve ISCs identify distinct or overlapping populations remains a matter of debate. Understanding the biology of cells that function as reserve ISCs has become even more complex in view of the current evidence (summarized in Fig. 3 and discussed further in several of the following reviews) indicating that in response to damage to the active ISC population, not only are the various quiescent ISC populations activated, but in addition more differentiated cells such as transit amplifying (TA) cells, secretory progenitors, Paneth cell (PC) precursors and enteroendocrine (EE) precursors appear capable of reverting to an ISC state and regenerating the epithelium (Gracz & Magness, 2014; Philpott & Winton, 2014; Tetteh et al. 2015).
Figure 3. Schematic drawing illustrating the response of the intestine to damage .
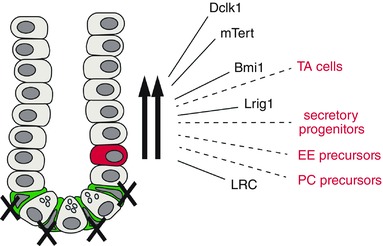
Most data come from studies of the effect of radiation, which ablates the active ISCs (green) and activates reserve ISCs (red). The latter appear to be heterogeneous with subpopulations (shown by black arrows) marked by Dclk1, mTert, Bmi1 and Lrig1, and having the property of label retention (LRC). In addition there is evidence that multiple, more differentiated cells (dashed lines and red labels) are capable of reverting to a stem cell phenotype after damage (TA, transit amplifying cells; EE, enteroendocrine; PC, Paneth cell).
The knowledge summarized above has been generated in mouse models in which lineage tracing with genetically engineered animals has become the ‘gold standard’ for demonstrating stemness. While identification of specific markers for human ISCs has lagged, various investigators have made serendipitous use of naturally occurring mutations, mosaicisms and heterozygous alleles to study the clonal behaviour of cells from the base of the intestinal crypts both at homeostasis and following radiation. A thoughtful and insightful summary of this important translational work can be found in a recent review (Wright, 2012).
Approaches to the isolation of ISCs
The generation of enhanced green fluorescent protein (EGFP) reporter mice for Lgr5 and other markers greatly facilitated the isolation and characterization of putative ISC populations. Likewise the development of the H2B–yellow fluorescent protein (YFP) reporter mouse (Hughes et al. 2012; Buczacki et al. 2013) allowed modern approaches to the study of the label‐retaining cells (LRCs) originally described by the Potten laboratory. In parallel with deployment of various reporter mice, several groups continued to pursue non‐reporter methods to isolate ISCs on the philosophy that such methods are critical to the translation of knowledge generated in mouse models to the human intestinal epithelium. Early studies with side population sorting (Dekaney et al. 2005; Gulati et al. 2008) led to the identification of the membrane protein CD24 as a useful marker for antibody‐based sorting (von Furstenberg et al. 2011). Concurrently, work from the laboratory of Melissa Wong showed that CD166 marks cells at the crypt base and that antibodies to CD166 can be used to isolate these cells (Levin et al. 2010). Some of the pitfalls of antibody‐based sorting were carefully documented in a multicentre study using CD44 to isolate cells from the lower crypt (Magness et al. 2013). Although none of these CD antibodies are specific for ISCs, subsequent studies have shown that careful combinations can be used to yield distinct populations of ISCs from both mouse and human (Gracz et al. 2013; Wang et al. 2013). Interestingly although Lgr5 has an ectodomain, attempts to develop Lgr5 antibodies suitable for sorting have not met with success (Tan & Barker, 2014). At this stage Lrig1 remains the only specific ISC marker for which antibody‐based sorting is possible (Powell et al. 2012). The recent demonstration that different antibodies to Lrig1 mark different subsets of epithelial cells illustrates the challenges of identifying antibodies suitable for genuine ISC isolation (Poulin et al. 2014). In this regard, side population sorting (Dekaney et al. 2005; Gulati et al. 2008) is an attractive option since it does not depend on antibodies. Moreover this approach has recently been further developed and shown to be capable of clearly distinguishing between actively cycling ISCs and quiescent ISCs (von Furstenberg et al. 2013). With the multiple isolation methods now available, the field is poised for cutting edge studies such as single cell transcriptome analyses (Gracz et al. 2015) to assess heterogeneity of ISC populations and further understand their biological properties.
Culture of ISCs and crypts
Another critical development in the ISC field (Fig. 2) was the report by Sato et al. in 2009, that isolated Lgr5‐EGFP cells could be clonogenically grown in vitro in 3D cultures using Matrigel with an appropriate cocktail of factors (Sato et al. 2009). Such cultures have now become part of the standard repertoire of ISC investigators. Unfortunately there remains a debate about the appropriate nomenclature for the structures generated in vitro, namely ‘organoids’ (Sato & Clevers, 2013) versus ‘enteroids’ (Stelzner et al. 2012). Nevertheless the growth of these complex self‐renewing structures from isolated ISCs is generally recognized as a useful surrogate for in vivo stemness. While the parent method of Matrigel culture had very low clonogenic efficiencies (less than 1%), modifications which markedly enhance the efficiency have subsequently been developed (Wang et al. 2013; Yin et al. 2014). The method can also be made more affordable by the use of serum and conditioned medium in place of recombinant growth factors (Miyoshi & Stappenbeck, 2013). Initially developed for mouse tissue, these Matrigel‐based approaches have now been applied to culture of isolated human ISCs (Gracz et al. 2013; Wang et al. 2013). Although the original culture method is labour intensive, recent work (Gracz et al. 2015) shows that high throughput adaptations are feasible, which will make ISC culture more amenable to detailed study of ISC biology and to translational applications such as drug screening.
While the ability to grow isolated ISCs in vitro has generated valuable knowledge regarding ISC behaviour, the application of the same technique to the growth of intact crypts may be even more important. The key difference is that in the 3D Matrigel system, the culture efficiency (defined as yield of enteroids versus number of units plated) is 10–100 times greater using crypts as the starting material than when the same conditions are used for isolated ISCs. With crypts from mouse small intestine, efficiencies are typically in the order of 60–80% on initial plating and approach 100% during passaging (Fuller et al. 2012). Efficiencies with human crypts are somewhat lower, but still sufficient to generate abundant cultures from endoscopic biopsies. Enteroid cultures derived from human crypts have been used to study physiological functions as well as host–pathogen interactions (Foulke‐Abel et al. 2014). Such enteroids can be efficiently passaged and frozen, allowing valuable patient samples to be studied indefinitely (Foulke‐Abel et al. 2014; Mahe et al. 2015). At least in mouse, crypts retain a high level of viability even following storage of tissue for 24–30 h at 4°C (Fuller et al. 2013), pointing to the possibility of a useful harvest from postmortem specimens. Exciting recent advances have shown that enteroids grown from crypts can be transfected by both viral and non‐viral methods (Miyoshi & Stappenbeck, 2013; Sato & Clevers, 2013; Foulke‐Abel et al. 2014), thus allowing genetic modification either for studies of basic biology or for therapeutic application (Schwank et al. 2013). As noted by Smith et al. in the following review (Smith et al. 2016), the caveat of all these advances is that the basic culture conditions (Sato et al. 2009) were devised specifically for Lgr5‐ISCs and are probably pharmacological for those cells and possibly totally inappropriate for the reserve ISC populations.
Despite the extent to which 3D Matrigel culture of ISCs has advanced the field, investigators have recently recognized the advantages of 2D culture systems. A promising development in this arena is the report of alternative culture conditions (involving a fibroblast feeder layer) which allow long term growth of ISCs in 2D (Wang et al. 2015). This system, in which differentiation is induced by exposure to an air–liquid interface, may prove more amenable for large scale growth of ISCs and for certain studies of physiological functions. A caveat is that to date the method has only been reported for isolated epithelial cells derived from 20–21 week human fetuses. However, other authors have reported that using crypts from adult intestine as the starting material, conditions can be devised to allow monolayer cultures which are highly polarized and thus suitable for functional studies (Foulke‐Abel et al. 2014; Jabaji et al. 2014).
Generation of intestinal tissue for transplantation
Beyond culture of isolated ISCs and of intestinal crypts, other approaches that can be seen as critical for translational applications are the use of organoid units and of iPS cells to generate intestinal tissue suitable for transplantation. As reviewed recently (Spurrier & Grikscheit, 2013), organoid units have been the choice of investigators in the field of tissue engineering. These units are generated by mild digestion of the intestinal tissue, which allows crypts to be released along with adherent mesenchymal cells. When seeded onto appropriate scaffolds, they can be implanted in the omentum where they grow and vascularize. The resulting structures display fully developed crypts and villi with all four lineages and with functional capacity (Grant et al. 2015). Anastomosis of the neointestine with the native intestine has been demonstrated to yield clinical improvement in a rat model of short bowel syndrome (Spurrier & Grikscheit, 2013). Using a slightly different approach, other investigators have shown that organoid units can be used to ameliorate a rat model of bile acid malabsorption (Avansino et al. 2005). As tissue engineered small intestine has already been generated from human and pig tissue, the roadblocks to clinical application are rapidly being surmounted (Spurrier & Grikscheit, 2013).
An exciting parallel development is the demonstration that intestinal tissue can be generated in vitro by endodermal programming of both hES cells and hIPS cells (McCracken et al. 2011; Spence et al. 2011). Interestingly, the resulting structures spontaneously generate mesenchyme as well as epithelium and thus have appropriately been termed ‘induced human intestinal organoids’ and abbreviated ‘iHIOs’ (Stelzner et al. 2012). When transplanted under the kidney capsule of immune deficient mice, HIOs grew 50–100 times their original size and demonstrated presence of all differentiated lineages as well as digestive and absorptive functions (Watson et al. 2014). In addition these transplanted HIOs were shown to be capable of responding to systemic signals following surgical resection of the host mouse (Watson et al. 2014). Despite these promising results, HIOs have not yet been transplanted into the intestine. Thus, application of the techniques from the tissue engineering field to HIO growth and transplantation is an obvious future avenue.
Regulation of ISC biology
As detailed in previous reviews (Clevers, 2013; Gracz & Magness, 2014; Tan & Barker, 2014) as well as in the following articles, the combination of in vivo lineage tracing, cell sorting and in vitro culture has led to extensive analysis of the biological properties of both the actively cycling CBC‐ISCs and the +4 quiescent ISCs. Homeostatic balance of self‐renewal and differentiation of CBC‐ISCs is dependent on multiple signalling pathways, including Wnts, EGF, Notch, Bmp and others (Sato & Clevers, 2013; Gracz & Magness, 2014; Tan & Barker, 2014). As noted by Demitrack and Samuelson in a following review (Demitrack & Samuelson, 2016), given that the Lgr5 active ISC population is more numerous and more readily accessed, to date the majority of studies on regulatory pathways have focused on these cells. The populations that serve as quiescent or reserve ISCs may have some regulatory features in common with active ISCs (e.g. Notch signalling), but must also have distinct differences to account for their markedly lower rates of proliferation. Recent studies have begun to document these differences. For example, the elegant work of Yan et al. showed clearly that while the Lgr5 population is stimulated by Wnt signalling, this is not the case for the Bmi1 population (Yan et al. 2012). Intriguingly, a recent report by Roche et al. suggests that differing expression levels of the transcription factor Sox9 may be responsible for quiescence versus active cycling, with the former being maintained by high levels of Sox9 (Roche et al. 2015). The following review by Richmond et al. (Richmond et al. 2016) draws on literature from various tissues to predict the smorgasbord of other factors that are likely to be involved in the maintenance of reserve ISCs in the quiescent state. Since these ISC populations are activated following epithelial damage (such as radiation or chemotherapeutic agents), uncovering the interplay of factors responsible for activation is an area of clear clinical relevance. Likewise, elucidating the mechanisms that return these cells to their quiescent state (which may be distinct from the mechanisms responsible for quiescence during homeostasis) will be critical in the development of ISC based therapies. An intriguing question is whether the various quiescent ISC populations which have been identified to date are regulated via common or distinct mechanisms. This is a particularly challenging question given the evidence summarized above (Fig. 3) and in recent reviews (Clevers, 2013; Gracz & Magness, 2014; Philpott & Winton, 2014) for significant plasticity wherein epithelial cells at various stages of differentiation can reacquire a stem cell state in response to intestinal injury. Interestingly, as reviewed by Aloia et al. in this series of articles, similar plasticity following damage is found in both the stomach and the liver (Aloia et al. 2016).
The ISC niche
Any discussion of the regulation of ISC biology needs to include consideration of the stem cell niche. As reviewed in the following article by Sailaja and Li (Sailaja & Li, 2016) and elsewhere (Shaker & Rubin, 2010; Smith et al. 2012; Tan & Barker, 2014), the ISC niche comprises both epithelial and stromal elements. Cells of the niche can exert their influence on ISCs via cell–cell contact, via paracrine secretions, or via changes in the composition of the extracellular matrix. With respect to the epithelial component of the ISC niche, cells that should be considered as players include Paneth cells, enteroendocrine cells, Tuft cells, goblet cells, intraepithelial lymphocytes and the ISCs themselves. Amongst these, to date by far the most attention has been given to Paneth cells (Clevers, 2013). This is not surprising in view of their close association with both the CBC‐ISCs and the +4‐ISCs (Fig. 1). Paneth cells have been shown to express numerous factors which stimulate ISC proliferation (including Wnt, Notch and EGF signals) and have been shown to enhance ISC growth in vitro (Sato et al. 2011), likely to be via cell–cell contact (Gracz et al. 2015). However, their role in vivo is a matter of debate in view of the reports that mice engineered to have reduced numbers of Paneth cells generally show normal intestinal architecture and normal regenerative responses to epithelial damage (Tan & Barker, 2014). This may reflect redundant sources of key factors such as Wnts (Kabiri et al. 2014; San Roman et al. 2014) as well as activation of reserve ISCs to replace reduced numbers of Lgr5 ISCs (Tan & Barker, 2014). The one aspect of ISC biology that appears to be definitely mediated by Paneth cell signalling (both in vitro and in vivo) is the increase in Lgr5 ISCs that occurs following caloric restriction (Yilmaz et al. 2012). Surprisingly this increase is associated with shorter rather than longer villi, suggesting that normal lineage allocation and differentiation is disrupted by caloric restriction. While this could be viewed as an adaptation to the reduced nutrient load for digestion and absorption, further studies are needed to determine whether these effects reflect luminal signalling or the altered metabolic state elicited by caloric restriction.
Stromal elements of the ISC niche include myofibroblasts, fibroblasts, endothelial cells, smooth muscle cells, neurons and immune cells (Shaker & Rubin, 2010; Smith et al. 2012; Tan & Barker, 2014). Of these, the majority of studies have focused on the intestinal subepithelial myfibroblasts. In vitro these cells have been shown to enhance the growth of human and mouse crypts and of isolated mouse Lgr5 ISCs (Lahar et al. 2011; Lei et al. 2014). Candidate factors responsible for this in vitro effect have been identified as members of the Wnt signalling pathway, including R‐spondin (Lei et al. 2014). In vivo studies have pointed to elevated secretion of the EGF family member amphiregulin as well as the Bmp antagonist chordin‐like 2 in response to epithelial damage (Shao & Sheng, 2010; Seiler et al. 2015). The following review by Mah and Kuo (Mah & Kuo, 2016) highlights the fact that for several factors known to influence ISC behaviour (e.g. individual Wnts and R‐spondins), the precise cellular sources within the ISC niche have not yet been established.
In addition to epithelial and stromal elements, the luminal environment should also be considered as part of the ISC niche. Within the lumen, while pathogens have long been recognized as having dramatic effects on the intestinal epithelium, in the last 20 years commensal microbes have been demonstrated to play numerous roles in the normal physiology of the intestinal epithelium. The presence of microbial sensing receptors (such as the TLR family) within the stem cell zone suggests the possibility that the intestinal microbiota may also influence ISC behaviour (Neal et al. 2012; Brown et al. 2014; Nigro et al. 2014). While in vitro studies with live bacteria (either commensal or pathogenic) remain a challenge, bacterial products have been shown to influence the behaviour of intestinal crypts in culture. To date, the story that has emerged for commensal organisms is somewhat confusing. Two groups have studied the effect of LPS, which acts through TLR4. One of these, using ileal crypts (Neal et al. 2012), reported that LPS reduces crypt proliferation and increases apoptosis, whereas the other study, using jejunal crypts (Davies et al. 2015), found no effect of added LPS. Regional differences are a logical explanation for this apparent discrepancy. The potential clinical significance of the adverse effect of TLR signalling in ileal ISCs was elegantly illustrated by the use of a mouse model of necrotizing enterocolitis (NEC) which showed that when the signalling was blocked, the deleterious effect of the NEC protocol was prevented (Neal et al. 2012). Adverse effects of the viral mimetic polyI:C, which signals via TLR3, have also been reported in crypt cultures (Davies et al. 2015). These authors found evidence of decreased differentiation of jejunal enteroids; however, they did not determine whether these effects reflect direct action on the ISCs. Interestingly, in contrast to the adverse effects of signalling through both TLR3 and TLR4, another microbial product, MDP, acting through Nod2, has the converse effect of eliciting cytoprotection (Nigro et al. 2014), both in crypt cultures and in vivo (using a doxorubicin model of epithelial damage). These studies suggest that overall the effects of the microbiome on ISC biology are complex. This is an area clearly needing further investigation, especially in view of reports that TLR4 receptors are elevated in ileal crypts of patients with Crohn's disease (Brown et al. 2014) and ileal tissue from babies with NEC (Leaphart et al. 2007). Further, Nod2 mutations have been reported to be associated with Crohn's disease (Hugot et al. 2001).
ISC clonality, dynamics and numbers
Early studies using mouse chimeras showed that during the first two postnatal weeks of mouse development, crypts are polyclonal, but by the third week they become monoclonal (Schmidt et al. 1988) and remain monoclonal through adulthood (Ponder et al. 1985). Crypt clonality was subsequently confirmed by a somatic mutation approach (Winton et al. 1988; Gordon et al. 1992). Despite these observations, in the following years the generally accepted model of ISC division was that of invariant asymmetry in which one daughter cell is maintained as a stem cell and the other progresses to the TA state and then to one of the differentiated lineages. Not until 2010 was the inconsistency between this model and crypt clonality addressed. In that year, studies in the laboratory of Doug Winton used an inducible lineage tracing approach together with statistical modelling, to study the dynamic behaviour of clonal crypt populations (Lopez‐Garcia et al. 2010). These analyses pointed unambiguously to neutral drift dynamics in which homeostasis is achieved at the population level as a result of stem cells randomly dividing to yield either two stem cells or two TA cells. The same conclusion was simultaneously reached by studies from the laboratory of Hans Clevers using a multicolour lineage tracing approach (Snippert et al. 2010). As noted by Lopez‐Garcia et al., this model ‘provides an inherently flexible assembly in which any stem cell can be deployed to differentiate into one of a number of cell types, to replace stem cells locally, and respond to changing environmental demand’ (Lopez‐Garcia et al. 2010). The following review by Mah and Kuo (Mah & Kuo, 2016) points to the regulation of ISC fate as a key area of future investigation.
Over the years, the precise number of true stem cells in each crypt has been a matter of some debate. Estimates probably depend on the methodology employed and on the likelihood that active and quiescent subpopulations of ISCs may have been differentially detected. That complication notwithstanding, most approaches put the number at five to six (see below) although some studies point to the number being as low as one stem cell per crypt (Ponder et al. 1985; Gordon et al. 1992; Cosentino et al. 1996) and as high as 16 (see below). Interestingly, although the early studies from Hazel Cheng's laboratory have subsequently been viewed as mainly documenting the existence of the active ISC population, whereas those from the laboratory of Chris Potten were focused on the ‘+4’ ISCs, both of these groups concluded there were four to five ISCs per crypt (Potten, 1998; Bjerknes & Cheng, 1999). Subsequently, studies using Lgr5 as a reporter concluded there were 14–16 ISCs per crypt (Lopez‐Garcia et al. 2010; Snippert et al. 2010). More recently an elegant study from the Winton laboratory using a marker‐independent approach, together with mathematical modelling, showed that the number of functional stem cells per crypt in the small intestine is in the order of six (Kozar et al. 2013). These authors concluded that only a subset of the Lgr5 cells behave as functional stem cells. Confirmation of this finding was rapidly forthcoming via a landmark study using multiphoton intravital microscopy, which showed that the behaviour of Lgr5 cells is heterogeneous based on location in the niche, those at the base having a survival advantage and those at the niche boundary being more likely to be lost (Ritsma et al. 2014). Thus, only a fraction of Lgr5 cells retain long term self‐renewal. Higher resolution analysis identified the basis of this by showing that Lgr5 cells at the niche border can become passively displaced from the niche after division of a neighbour. Conversely, when Lgr5 cells were depleted, there was a sporadic transfer of cells from the TA zone into the niche border with acquisition of Lgr5 expression (Ritsma et al. 2014). Hopefully, future studies will use similar imaging approaches to assess the behaviour of the various subpopulations of quiescent ISCs and more differentiated cells (Fig. 3) which are involved in restitution of the epithelium after damage.
Concluding remarks
We hope that this overview together with the following articles will serve as a resource both for those who work in the area of GI stem cells and those with broader interests in the GI epithelium. Taken together the series emphasizes that despite the dramatic explosion of research on GI stem cells in the last 10 years, there are still many gaps in our knowledge and controversial issues to be resolved. Nevertheless, these reviews also point to exciting potential clinical applications and thus to the importance of continued dialogue and collaboration between basic science investigators, physician scientists, and clinicians in order to see these come to fruition.
Additional information
Competing interests
None declared.
Funding
None.
Author contributions
All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Biographies
Susan J Henning is a GI investigator with expertise in intestinal development and intestinal stem cells. She received her PhD from the University of Melbourne, Australia, then did postdoctoral work at Stanford University. She chose to remain in the USA and has held faculty positions at Temple University, University of Houston and Baylor College of Medicine before moving to UNC in 2007. For the last 26 years her primary appointment has been in clinical departments, where in addition to running her own research group, she has spent significant time mentoring physician scientists in the development of their research careers.

Richard von Furstenberg is a research scientist with expertise in flow cytometric isolation and analysis of intestinal stem cells as well as organotypic culture of GI epithelium. He was mentored by Susan Henning at UNC and now studies oesophageal stem cell biology at Duke University.
This review was presented at the “Gastrointestinal Tract XVI: GI homeostasis, the microbiome and the barrier, development and disease” FASEB Summer Research, Steamboat Springs, Colorado, USA between 2–7 August 2015.
References
- Aloia L, Mckie MA & Huch M (2016). Cellular plasticity in the adult liver and stomach. J Physiol 594, 4815–4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avansino JR, Chen DC, Hoagland VD, Woolman JD, Haigh WG & Stelzner M (2005). Treatment of bile acid malabsorption using ileal stem cell transplantation. J Am Coll Surg 201, 710–720. [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den BM, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ & Clevers H (2007). Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007. [DOI] [PubMed] [Google Scholar]
- Bjerknes M & Cheng H (1981). The stem‐cell zone of the small intestinal epithelium. III. Evidence from columnar, enteroendocrine, and mucous cells in the adult mouse. Am J Anat 160, 77–91. [DOI] [PubMed] [Google Scholar]
- Bjerknes M & Cheng H (1999). Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology 116, 7–14. [DOI] [PubMed] [Google Scholar]
- Brown M, Hughes KR, Moossavi S, Robins A & Mahida YR (2014). Toll‐like receptor expression in crypt epithelial cells, putative stem cells and intestinal myofibroblasts isolated from controls and patients with inflammatory bowel disease. Clin Exp Immunol 178, 28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczacki SJ, Zecchini HI, Nicholson AM, Russell R, Vermeulen L, Kemp R & Winton DJ (2013). Intestinal label‐retaining cells are secretory precursors expressing Lgr5. Nature 495, 65–69. [DOI] [PubMed] [Google Scholar]
- Cheng H & Leblond CP (1974). Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian theory of the origin of the four epithelial cell types. Am J Anat 141, 537–562. [DOI] [PubMed] [Google Scholar]
- Clevers H (2013). The intestinal crypt, a prototype stem cell compartment. Cell 154, 274–284. [DOI] [PubMed] [Google Scholar]
- Cosentino L, Shaver‐Walker P & Heddle JA (1996). The relationships among stem cells, crypts, and villi in the small intestine of mice as determined by mutation tagging. Dev Dyn 207, 420–428. [DOI] [PubMed] [Google Scholar]
- Davies JM, Santaolalla R, von Furstenberg RJ, Henning SJ & Abreu MT (2015). The viral mimetic polyinosinic: polycytidylic acid alters the growth characteristics of small intestinal and colonic crypt cultures. PLoS One 10, e0138531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekaney CM, Rodriguez JM, Graul MC & Henning SJ (2005). Isolation and characterization of a putative intestinal stem cell fraction from mouse jejunum. Gastroenterology 129, 1567–1580. [DOI] [PubMed] [Google Scholar]
- Demitrack ES & Samuelson LC (2016). Notch regulation of gastrointestinal stem cells. J Physiol 594, 4791–4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulke‐Abel J, In J, Kovbasnjuk O, Zachos NC, Ettayebi K, Blutt SE, Hyser JM, Zeng XL, Crawford SE, Broughman JR, Estes MK & Donowitz M (2014). Human enteroids as an ex‐vivo model of host‐pathogen interactions in the gastrointestinal tract. Exp Biol Med 239, 1124–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller MK, Faulk DM, Sundaram N, Mahe MM, Stout KM, von Furstenberg RJ, Smith BJ, McNaughton KK, Shroyer NF, Helmrath MA & Henning SJ (2013). Intestinal stem cells remain viable after prolonged tissue storage. Cell Tissue Res 354, 441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller MK, Faulk DM, Sundaram N, Shroyer NF, Henning SJ & Helmrath MA (2012). Intestinal crypts reproducibly expand in culture. J Surg Res 178, 48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JI, Schmidt GH & Roth KA (1992). Studies of intestinal stem cells using normal, chimeric, and transgenic mice. FASEB J 6, 3039–3050. [DOI] [PubMed] [Google Scholar]
- Gracz AD, Fuller MK, Wang F, Li L, Stelzner M, Dunn JC, Martin MG & Magness ST (2013). Brief report: CD24 and CD44 mark human intestinal epithelial cell populations with characteristics of active and facultative stem cells. Stem Cells 31, 2024–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracz AD & Magness ST (2014). Defining hierarchies of stemness in the intestine: evidence from biomarkers and regulatory pathways. Am J Physiol Gastrointest Liver Physiol 307, G260–G273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracz AD, Williamson IA, Roche KC, Johnston MJ, Wang F, Wang Y, Attayek PJ, Balowski J, Liu XF, Laurenza RJ, Gaynor LT, Sims CE, Galanko JA, Li L, Allbritton NL & Magness ST (2015). A high‐throughput platform for stem cell niche co‐cultures and downstream gene expression analysis. Nat Cell Biol 17, 340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant CN, Mojica SG, Sala FG, Hill JR, Levin DE, Speer AL, Barthel ER, Shimada H, Zachos NC & Grikscheit TC (2015). Human and mouse tissue‐engineered small intestine both demonstrate digestive and absorptive function. Am J Physiol Gastrointest Liver Physiol 308, G664–G677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati AS, Ochsner SA & Henning SJ (2008). Molecular properties of side population‐sorted cells from mouse small intestine. Am J Physiol Gastrointest Liver Physiol 294, G286–G294. [DOI] [PubMed] [Google Scholar]
- Hendry JH, Moore JV & Potten CS (1984). The proliferative status of microcolony‐forming cells in mouse small intestine. Cell Tissue Kinet 17, 41–47. [DOI] [PubMed] [Google Scholar]
- Hughes KR, Gandara RM, Javkar T, Sablitzky F, Hock H, Potten CS & Mahida YR (2012). Heterogeneity in histone 2B‐green fluorescent protein‐retaining putative small intestinal stem cells at cell position 4 and their absence in the colon. Am J Physiol Gastrointest Liver Physiol 303, G1188–G1201. [DOI] [PubMed] [Google Scholar]
- Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent‐Puig P, Gower‐Rousseau C, Macry J, Colombel JF, Sahbatou M & Thomas G (2001). Association of NOD2 leucine‐rich repeat variants with susceptibility to Crohn's disease. Nature 411, 599–603. [DOI] [PubMed] [Google Scholar]
- Jabaji Z, Brinkley GJ, Khalil HA, Sears CM, Lei NY, Lewis M, Stelzner M, Martin MG & Dunn JC (2014). Type I collagen as an extracellular matrix for the in vitro growth of human small intestinal epithelium. PLoS One 9, e107814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabiri Z, Greicius G, Madan B, Biechele S, Zhong Z, Zaribafzadeh H, Edison, Aliyev J , Wu Y, Bunte R, Williams BO, Rossant J & Virshup DM (2014). Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development 141, 2206–2215. [DOI] [PubMed] [Google Scholar]
- Kozar S, Morrissey E, Nicholson AM, van der Heijden M, Zecchini HI, Kemp R, Tavare S, Vermeulen L & Winton DJ (2013). Continuous clonal labeling reveals small numbers of functional stem cells in intestinal crypts and adenomas. Cell Stem Cell 13, 626–633. [DOI] [PubMed] [Google Scholar]
- Lahar N, Lei NY, Wang J, Jabaji Z, Tung SC, Joshi V, Lewis M, Stelzner M, Martin MG & Dunn JC (2011). Intestinal subepithelial myofibroblasts support in vitro and in vivo growth of human small intestinal epithelium. PLoS One 6, e26898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaphart CL, Cavallo J, Gribar SC, Cetin S, Li J, Branca MF, Dubowski TD, Sodhi CP & Hackam DJ (2007). A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol 179, 4808–4820. [DOI] [PubMed] [Google Scholar]
- Lei NY, Jabaji Z, Wang J, Joshi VS, Brinkley GJ, Khalil H, Wang F, Jaroszewicz A, Pellegrini M, Li L, Lewis M, Stelzner M, Dunn JC & Martin MG (2014). Intestinal subepithelial myofibroblasts support the growth of intestinal epithelial stem cells. PLoS One 9, e84651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin TG, Powell AE, Davies PS, Silk AD, Dismuke AD, Anderson EC, Swain JR & Wong MH (2010). Characterization of the intestinal cancer stem cell marker, CD166/activated leukocyte cell adhesion molecule, in the human and mouse gastrointestinal tract. Gastroenterology 139, 2072–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez‐Garcia C, Klein AM, Simons BD & Winton DJ (2010). Intestinal stem cell replacement follows a pattern of neutral drift. Science 330, 822–825. [DOI] [PubMed] [Google Scholar]
- Mah AT, Yan KS & Kuo CJ (2016). Wnt pathway regulation of intestinal stem cells. J Physiol 594, 4837–4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken KW, Howell JC, Wells JM & Spence JR (2011). Generating human intestinal tissue from pluripotent stem cells in vitro. Nat Protoc 6, 1920–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magness ST, Puthoff BJ, Crissey MA, Dunn J, Henning SJ, Houchen C, Kaddis JS, Kuo CJ, Li L, Lynch J, Martin MG, May R, Niland JC, Olack B, Qian D, Stelzner M, Swain JR, Wang F, Wang J, Wang X, Yan K, Yu J & Wong MH (2013). A multicenter study to standardize reporting and analyses of fluorescence‐activated cell‐sorted murine intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 305, G542–G551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahe MM, Sundaram N, Watson CL, Shroyer NF & Helmrath MA (2015). Establishment of human epithelial enteroids and colonoids from whole tissue and biopsy. J Vis Exp 97, doi: 10.3791/52483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi H & Stappenbeck TS (2013). In vitro expansion and genetic modification of gastrointestinal stem cells in spheroid culture. Nat Protoc 8, 2471–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery RK & Breault DT (2008). Small intestinal stem cell markers. J Anat 213, 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal MD, Sodhi CP, Jia H, Dyer M, Egan CE, Yazji I, Good M, Afrazi A, Marino R, Slagle D, Ma C, Branca MF, Prindle T Jr, Grant Z, Ozolek J & Hackam DJ (2012). Toll‐like receptor 4 is expressed on intestinal stem cells and regulates their proliferation and apoptosis via the p53 up‐regulated modulator of apoptosis. J Biol Chem 287, 37296–37308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigro G, Rossi R, Commere PH, Jay P & Sansonetti PJ (2014). The cytosolic bacterial peptidoglycan sensor Nod2 affords stem cell protection and links microbes to gut epithelial regeneration. Cell Host Microbe 15, 792–798. [DOI] [PubMed] [Google Scholar]
- Philpott A & Winton DJ (2014). Lineage selection and plasticity in the intestinal crypt. Curr Opin Cell Biol 31, 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponder BAJ, Schmidt GH, Wilkinson MM, Wood MJ, Monk M & Reid A (1985). Derivation of mouse intestinal crypts from single progenitor cells. Nature 313, 689–691. [DOI] [PubMed] [Google Scholar]
- Potten CS (1998). Stem cells in gastrointestinal epithelium: numbers, characteristics and death. Philos Trans R Soc Lond B Biol Sci 353, 821–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS, Owen G & Booth D (2002). Intestinal stem cells protect their genome by selective segregation of template DNA strands. J Cell Sci 115, 2381–2388. [DOI] [PubMed] [Google Scholar]
- Poulin EJ, Powell AE, Wang Y, Li Y, Franklin JL & Coffey RJ (2014). Using a new Lrig1 reporter mouse to assess differences between two Lrig1 antibodies in the intestine. Stem Cell Res 13, 422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell AE, Wang Y, Li Y, Poulin EJ, Means AL, Washington MK, Higginbotham JN, Juchheim A, Prasad N, Levy SE, Guo Y, Shyr Y, Aronow BJ, Haigis KM, Franklin JL & Coffey RJ (2012). The pan‐ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell 149, 146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond CA, Shah MS, Carlone DL & Breault DT (2016). Factors regulating quiescent stem cells: insights from the intestine and other self‐renewing tissues. J Physiol 594, 4805–4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritsma L, Ellenbroek SI, Zomer A, Snippert HJ, de Sauvage FJ, Simons BD, Clevers H & van Rheenen J (2014). Intestinal crypt homeostasis revealed at single‐stem‐cell level by in vivo live imaging. Nature 507, 362–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KC, Gracz AD, Liu XF, Newton V, Akiyama H & Magness ST (2015). SOX9 maintains reserve stem cells and preserves radio‐resistance in mouse small intestine. Gastroenterology 149, 1553–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailaja BS, He XC & Li L (2016). The regulatory niche in intestinal stem cells. J Physiol 594, 4827–4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Roman A, Jayewickreme C, Murtaugh C & Shivdasani R (2014). Wnt secretion from epithelial cells and subepithelial myofibroblasts is not required in the mouse intestinal stem cell niche in vivo. Stem Cell Reports 2, 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T & Clevers H (2013). Growing self‐organizing mini‐guts from a single intestinal stem cell: mechanism and applications. Science 340, 1190–1194. [DOI] [PubMed] [Google Scholar]
- Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M & Clevers H (2011). Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469, 415–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ & Clevers H (2009). Single Lgr5 stem cells build crypt‐villus structures in vitro without a mesenchymal niche. Nature 459, 262–265. [DOI] [PubMed] [Google Scholar]
- Schmidt GH, Winton DJ & Ponder BAJ (1988). Development of the pattern of cell renewal in the crypt‐villus unit of chimaeric mouse small intestine. Development 103, 785–790. [DOI] [PubMed] [Google Scholar]
- Schwank G, Koo BK, Sasselli V, Dekkers JF, Heo I, Demircan T, Sasaki N, Boymans S, Cuppen E, van der Ent CK, Nieuwenhuis EE, Beekman JM & Clevers H (2013). Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 13, 653–658. [DOI] [PubMed] [Google Scholar]
- Seiler KM, Schenhals EL, von Furstenberg RJ, Allena BK, Smith BJ, Scaria D, Bresler MN, Dekaney CM & Henning SJ (2015). Tissue underlying the intestinal epithelium elicits proliferation of intestinal stem cells following cytotoxic damage. Cell Tissue Res 361, 427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaker A & Rubin DC (2010). Intestinal stem cells and epithelial‐mesenchymal interactions in the crypt and stem cell niche. Transl Res 156, 180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao J & Sheng H (2010). Amphiregulin promotes intestinal epithelial regeneration: roles of intestinal subepithelial myofibroblasts. Endocrinology 151, 3728–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith N, Davies P, Silk A & Wong M (2012). Epithelial and mesenchymal contribution to the niche: a safeguard for intestinal stem cell homeostasis. Gastroenterology 143, 1426–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NR, Gallagher AC & Wong MH (2016). Defining a stem cell hierarchy in the intestine: markers, caveats and controversies. J Physiol 594, 4781–4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon‐Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD & Clevers H (2010). Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143, 134–144. [DOI] [PubMed] [Google Scholar]
- Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, Shroyer NF & Wells JM (2011). Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470, 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurrier RG & Grikscheit TC (2013). Tissue engineering the small intestine. Clin Gastroenterol Hepatol 11, 354–358. [DOI] [PubMed] [Google Scholar]
- Stelzner M, Helmrath M, Dunn JCY, Henning SJ, Houchen CW, Kuo C, Lynch J, Li L, Magness ST, Martin MG, Wong MH & Yu J (2012). A nomenclature for intestinal in vitro cultures. Am J Physiol Gastrointest Liver Physiol 302, 1359–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda N, Jain R, LeBoeuf MR, Wang QH, Lu MM & Epstein JA (2011). Interconversion between intestinal stem cell populations in distinct niches. Science 334, 1420–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan D & Barker N (2014). Intestinal stem cells and their defining niche. Curr Topics Dev Biol 107, 77–107. [DOI] [PubMed] [Google Scholar]
- Tetteh PW, Farin HF & Clevers H (2015). Plasticity within stem cell hierarchies in mammalian epithelia. Trends Cell Biol 25, 100–108. [DOI] [PubMed] [Google Scholar]
- Thompson M, Fleming KA, Evans DJ, Fundele R, Surani MA & Wright NA (1990). Gastric endocrine cells share a clonal origin with other gut cell lineages. Development 110, 477–481. [DOI] [PubMed] [Google Scholar]
- von Furstenberg RJ, Buczacki SJ, Smith BJ, Seiler KM, Winton DJ & Henning SJ (2013). Side population sorting separates subfractions of cycling and non‐cycling intestinal stem cells. Stem Cell Res 12, 364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Furstenberg RJ, Gulati AS, Baxi A, Doherty JM, Stappenbeck TS, Gracz AD, Magness ST & Henning SJ (2011). Sorting mouse jejunal epithelial cells with CD24 yields a population with characteristics of intestinal stem cells. Am J Physiol Gastrointest Liver Physiol 300, G409–G417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Scoville D, He XC, Mahe MM, Box A, Perry JM, Smith NR, Lei NY, Davies PS, Fuller MK, Haug JS, McClain M, Gracz AD, Ding S, Stelzner M, Dunn JC, Magness ST, Wong MH, Martin MG, Helmrath M & Li L (2013). Isolation and characterization of intestinal stem cells based on surface marker combinations and colony‐formation assay. Gastroenterology 145, 383–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Yamamoto Y, Wilson LH, Zhang T, Howitt BE, Farrow MA, Kern F, Ning G, Hong Y, Khor CC, Chevalier B, Bertrand D, Wu L, Nagarajan N, Sylvester FA, Hyams JS, Devers T, Bronson R, Lacy DB, Ho KY, Crum CP, McKeon F & Xian W (2015). Cloning and variation of ground state intestinal stem cells. Nature 522, 173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CL, Mahe MM, Munera J, Howell JC, Sundaram N, Poling HM, Schweitzer JI, Vallance JE, Mayhew CN, Sun Y, Grabowski G, Finkbeiner SR, Spence JR, Shroyer NF, Wells JM & Helmrath MA (2014). An in vivo model of human small intestine using pluripotent stem cells. Nat Med 20, 1310–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winton DJ, Blount MA & Ponder BAJ (1988). A clonal marker induced by mutation in mouse intestinal epithelium. Nature 333, 463–466. [DOI] [PubMed] [Google Scholar]
- Withers HR & Elkind MM (1970). Microcolony survival assay for cells of mouse intestinal mucosa exposed to radiation. Int J Radiat Biol Relat Stud Phys Chem Med 17, 261–267. [DOI] [PubMed] [Google Scholar]
- Wright N (2012). Stem cell identification – in vivo lineage analysis versus in vitro isolation and clonal expansion. J Pathol 227, 255–266. [DOI] [PubMed] [Google Scholar]
- Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, Su N, Luo Y, Heilshorn SC, Amieva MR, Sangiorgi E, Capecchi MR & Kuo CJ (2012). The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci USA 109, 466–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz OH, Katajisto P, Lamming DW, Gultekin Y, Bauer‐Rowe KE, Sengupta S, Birsoy K, Dursun A, Yilmaz VO, Selig M, Nielsen GP, Mino‐Kenudson M, Zukerberg LR, Bhan AK, Deshpande V & Sabatini DM (2012). mTORC1 in the Paneth cell niche couples intestinal stem‐cell function to calorie intake. Nature 486, 490–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Farin HF, van Es JH, Clevers H, Langer R & Karp JM (2014). Niche‐independent high‐purity cultures of Lgr5+ intestinal stem cells and their progeny. Nat Methods 11, 106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]


