Abstract
Objective
Interferon inducible protein-16 (IFI16) is an intracellular DNA receptor involved in innate immunity. We evaluated the frequency, phenotypic characteristics and clinical associations of anti-IFI16 antibodies in patients with primary Sjögren's syndrome (SS), and quantitated expression levels of IFI16 in SS and control salivary gland lysates.
Methods
Anti-IFI16 antibodies were assayed by ELISA using sera from patients with primary SS (n=133) and from healthy controls (n=47). Sera from SLE patients (n=132) were included as disease controls. Immunoprecipitation of in vitro transcription translated IFI16 was used to determine which portion of IFI16 the antibodies recognized. Expression of IFI16 in salivary gland lysates was quantitated by immunoblotting.
Results
Anti-IFI16 antibodies were present in 38/133 (29%) of SS patient sera compared to 1/47 (2.1%) healthy controls (SS vs controls, p<0.0002) and 31/132 (24%) SLE controls. In SS, anti-IFI16 antibodies were associated with an abnormal Schirmer's test (p=0.003), hyperglobulinemia (p=0.02), ANA≥1:320 (p=0.01), germinal center-like structures in labial salivary gland lymphoid infiltrates (p=0.01), and higher focus scores (3.4 vs 2.4, p=0.005). High titer IFI16 antibodies were directed against an epitope outside the N-terminus in 9/13 (69%) of SS patients. IFI16 was expressed in 4/5 (80%) of SS and 1/6 (17%) of control labial salivary glands.
Conclusion
Anti-IFI16 antibodies are a prominent specificity in primary SS, and are associated with markers of severe disease. IFI16 is expressed at higher levels in SS salivary glands compared to controls. These high levels in disease target tissue may contribute to the ongoing anti-IFI16 immune response.
Human interferon inducible protein-16 (IFI16) is an intracellular DNA receptor that senses DNA from invading pathogens in both the nucleus and cytoplasm and is thus a key component of the innate immune response (1). Its cellular activity is associated with the production of proinflammatory cytokines, such as IFN-β and interleukin-1β. Emerging evidence supports a role for IFI16 in the pathogenesis of several autoimmune disorders, both through the generation of high levels of proinflammatory cytokines and its recognition as an autoantigen (2).
The presence of autoantibodies to IFI16 was first reported in 1994 in 29% of systemic lupus erythematosus (SLE) patients (3). In subsequent studies, antibodies to IFI16 have been reported with a prevalence ranging from 26-63% in SLE (3-7), 21-33% in systemic sclerosis (SSc) (4, 8), 50-70% in SS (4, 5), and 0-13% in rheumatoid arthritis (RA) (3-5). In SLE, these antibodies have been reported to correlate inversely with proteinuria and C3 hypocomplementemia, suggesting that they do not have a pathogenic role in nephritis (6). To date, phenotypic correlations have not been reported in SS.
In this study, we analyzed the prevalence of anti-IFI16 antibodies in SS sera, and show that they are found in 29% of patients. This finding is consistent with previous reports (4, 5) that this is a prominent SS specificity, although the frequency of these antibodies is lower in our cohort. We extend this finding to report for the first time on the detailed clinical characteristics of anti-IFI16 positive SS patients, and show that these antibodies are more prevalent in those SS patients with markers of severe disease. Using immunoprecipitation, we demonstrate that the majority of these high titer autoantibodies are directed against an epitope outside the N-terminus in SS patients. In contrast, most of the high titer anti-IFI16 antibodies in SLE patients are against the N-terminus. IFI16 expression was quantitated by immunoblotting minor salivary gland biopsy lysates, and was found to be elevated in SS compared to controls.
Patients and Methods
Patient cohorts
Sera and clinical data were obtained from SS patients from the Sjögren's International Collaborative Clinical Alliance (SICCA) cohort. As a disease control, patients with SLE from the Hopkins Lupus Cohort were studied. Frozen human salivary gland tissue was obtained from patients (as described below) after informed consent. The Johns Hopkins University School of Medicine Institutional Review Board approved the collection of clinical data, serum and salivary gland tissue from patients and controls for use in this analysis. All patients gave informed consent.
Sjögren's Syndrome
The SICCA registry was conducted during the period of 2003-2013, directed by investigators at the University of California, San Francisco; details of the SICCA registry are provided elsewhere (9). All participants in the SICCA cohort underwent a systematic, extensive assessment of symptoms and signs related to SS. Uniform protocol-driven data collection methods were used at nine global SICCA sites (including Johns Hopkins University) for the completion of questionnaires, recording of clinical examination findings, and the acquisition of biospecimens. Each participant underwent a minor salivary gland biopsy, and the tissue was independently examined by two histopathologists. Germinal-center like structures in the labial salivary gland biopsies were defined on hematoxylin-eosin sections by the presence of at least one defined spherical or ovoid aggregate of mononuclear cells showing an organized zonation of centroblasts and centrocytes surrounded by a dense aggregate of lymphocytes with a small proportion of plasma cells. Complete details of SICCA enrollment forms, protocols and methods may be found at: http://sicca.ucsf.edu/. Informed consent was obtained from all SICCA participants and the study was conducted using protocols approved by the institutional review board at the University of California, San Francisco as well as at each of the eight other global sites.
We screened sera from 150 randomly selected SICCA registrants with SS, defined by focal lymphocytic sialadenitis (FLS) with a focus score ≥1 and ocular surface staining score (OSS) ≥3 (10). With these criteria, the SS subjects were selected independently of their a priori autoantibody status. Sixteen subjects were excluded from the SS cohort because of clinical features consistent with RA (8 sera), SLE (4 sera) or with physical examination findings suggestive of systemic sclerosis (4 sera). Another patient did not have an associated serum sample, thus leaving 133 sera for analysis in the SS cohort.
Systemic lupus erythematosus
The Hopkins Lupus Cohort is a prospective cohort in which patients with SLE are followed at least quarterly. Patient inclusion in the cohort is based on the clinical diagnosis of SLE by a member of the Rheumatology Division; 94% of the patients satisfied at least four of the 1982 American College of Rheumatology revised criteria for the classification of SLE (11, 12). At each patient visit, disease activity is assessed by physician's global assessment [0 to 3 visual analog scale and the SELENA (Safety of Estrogens in Lupus Erythematosus: National Assessment) revision of the SLE Disease Activity Index (SLEDAI) (13)] and laboratory tests are performed [complete blood count, erythrocyte sedimentation rate, serum creatinine, cholesterol, urinalysis, urine protein to creatinine ratio, C3, C4, and anti-dsDNA]. For each patient, basic demographic characteristics, presenting and cumulative clinical manifestations, and immunologic markers have been recorded since cohort entry. Secondary SS in this SLE cohort was defined by sicca symptoms in concert with an abnormal Schirmer's or ocular vital dye staining test, absent sublingual salivary pool, or a minor salivary gland biopsy showing focal lymphocytic sialadenitis with a focus score of 1 or higher (14). These data regarding sicca manifestations were collected by chart review as well as from findings elicited at the initial and all subsequent clinic visits. The findings were not collected in a uniform or protocol-driven manner and thus could not be used to classify subjects based on the American-European Consensus group or American College of Rheumatology classification criteria (10, 15)
Control subjects
47 healthy individuals served as control subjects. All gave informed consent.
Human tissue
Excess human minor salivary glands were collected at the time of diagnostic lip biopsy in five patients who had SS and six patients with sicca symptoms who did not prove to have SS. These glands were obtained from patients who were under evaluation in the Johns Hopkins Jerome L. Greene Sjögren's Syndrome Center. All patients gave informed consent for the use of these excess glands for research purposes.
ELISA assays
IFI16 ELISA. Recombinant full-length human IFI16 was expressed and purified as described (1). 96-well ELISA plates were coated overnight at 4°C with 100 ng/well of recombinant full-length human IFI16. Washes were included between this and all subsequent steps, and all further incubations were carried out at room temperature for 1 hour. Wells were blocked with 5% BSA in PBS/0.05% Tween 20 (PBST), followed by incubation with human sera diluted 1:400 in 1% BSA/PBST. Horseradish peroxidase-labeled goat anti-human antibody (1:10,000 dilution, Jackson Immunoresearch) was added to each well, and color development was subsequently performed with SureBlue peroxidase reagent (KPL) before reading absorbances at 450 nm. An arbitrary positive serum (serum SJ149, 1:400 dilution with an OD in the linear range) was included as a reference in every IFI16 ELISA; all absorbances were calibrated relative to this reference absorbance. SSA and SSB ELISAs. SSA (Ro52) and SSB (La) antibodies underwent ELISA using commercially available kits (Inova Diagnostics), per the manufacturer's protocol.
Immunoprecipitation (IP) assays
DNA encoding full length human IFI16 or Ro60 was used in in vitro transcription/translation (IVTT) reactions per the manufacturer's protocol (Promega), generating 35S-methionine labeled protein. Patient sera were used to immunoprecipitate (IP) these labeled proteins to assess antibody status for these antigens as described (16, 17). For IFI16, IPs using these products were also performed using commercial anti-IFI16 antibodies (a goat polyclonal and a mouse monoclonal, both from Santa Cruz Biotechnology). All immunoprecipitates were electrophoresed on 10% sodium dodecyl sulfate polyacrylamide gels, and the labeled proteins were visualized by fluorography. Control sera did not precipitate any of these antigens.
IFI16 expression in human tissues
Lysates of human minor salivary gland from SS patients were prepared on ice as described previously (18). Equal protein loads were electrophoresed on SDS-polyacrylamide gels, transferred onto nitrocellulose membranes, and probed with a monoclonal anti-IFI16 antibody (Santa Cruz Biotechnology). β-actin was immunoblotted as a loading control. After incubating with appropriate horseradish peroxidase-conjugated secondary antibodies (Jackson Immunoresearch), visualization was performed using an enhanced chemiluminescence detection system (Pierce). For densitometry, X-ray films were scanned using an AGFA Arcus II scanner, and densities were quantified using Bio-Rad Quantity One software. The IFI16 value of each lysate sample was normalized relative to the matched β-actin loading control.
Statistics
We compared the clinical features of patients with and without antibodies against IFI16 using a two-tailed Fisher exact test for categorical variables. Student's t tests were used to compare normally distributed continuous variables between groups while Kruskal-Wallis and Wilcoxon rank-sum tests were used for non-normally distributed variables. P values less than 0.05 were considered statistically significant.
Results
Anti-IFI16 antibodies are a prominent specificity in SS patients
We studied 133 participants in the SICCA registry who had primary SS (Table 1). Of note, the SICCA registry recruited participants at nine global sites, and this is reflected in the ethnic distribution. We set up an ELISA to rapidly screen sera for antibodies against IFI16. Sera were assigned a positive antibody status if the relative absorbance value in the sample was >2 standard deviations higher than the mean value in 47 healthy controls. The levels of serum IFI16 antibody in SS patients and controls are shown in Figure 1. Anti-IFI16 antibodies were present in 38/133 (29%) SS patients, compared to 1/47 (2.1%) of controls, a significant difference (p<0.0002).
Table 1. Demographic characteristics of the 133 SS patients.
| Feature | Number (%)* |
|---|---|
| Mean age at cohort entry, years | 53.0 ± 13.2 |
| Female | 127 (95) |
| Race | |
|
65 (49) |
|
11 (8) |
|
47 (35) |
|
3 (2) |
|
6 (5) |
| Autoantibody status | |
|
109 (82) |
|
98 (74) |
|
68 (51) |
|
38 (29) |
Except where indicated otherwise.
Figure 1. Antibodies against IFI16 are detected in patients with SS, but not in healthy controls.
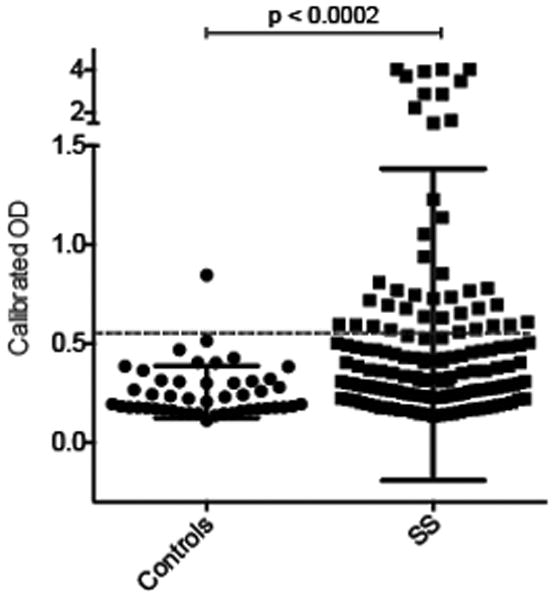
Serum IgG antibodies against IFI16 were assayed by ELISA in patients with SS (n = 133) and healthy controls (n = 47), as described in the Methods and Results sections. Each symbol represents the IFI16 antibody level in a single patient serum. Antibodies were detected in 38/133 (29%) of SS patients, and 1/47 (2.1%) of controls. The dotted line marks the cutoff for assignment of a positive score.
Phenotypic associations of IFI16 antibodies in SS
The phenotypic features of the SS patients in relation to IFI16 antibody status are shown in Table 2. All of the patients had SS as defined by the presence of SICCA ocular surface staining scores of 3 or higher (19) and a “positive lip biopsy” (i.e. focal lymphocytic sialadenitis with a focus score of 1 or higher). The SS patients with anti-IFI16 antibodies, when compared to those without, had a greater prevalence of an abnormal Schirmer's test (63 vs 34%; p=0.003), IgG level >1445 mg/dl (74 vs 49%; p=0.02), ANA ≥1:320 (82 vs 58%; p=0.01), and germinal center-like structures in the labial salivary gland lymphocytic infiltrates (39 vs 18%; p=0.01). Among the patients with anti-IFI16 antibodies compared to those without, focus scores were also significantly higher [median 3.4 (25th-75th percentile 2.6-6.4) vs 2.4 (1.6-3.7), p=0.005]. There was no association of anti-IFI16 antibodies or their levels with anti-SSA or anti-SSB antibodies.
Table 2. Phenotypic features of 133 primary SS patients in relation to anti-IFI16 antibody status*.
| Phenotypic feature | IFI16 positive (n=38) | IFI16 negative (n=95) | P value |
|---|---|---|---|
| Age (years), mean±SD | 55.1±13.8 | 52.2±12.9 | 0.27 |
| Female | 37 (97) | 90 (95) | 0.67 |
| Caucasian | 21 (55) | 44 (47) | 0.44 |
| Schirmer <5 mm/5 min | 24 (63) | 32 (34) | 0.003 |
| UWS<0.5 ml/5 min | 32 (84) | 67 (71) | 0.13 |
| WBC<4000 | 9 (24) | 21 (23) | 1.0 |
| C4<16 mg/dl | 11 (29) | 17 (18) | 0.17 |
| IgG>1445 mg/dl | 28 (74) | 47 (49) | 0.02 |
| ANA ≥1:320 | 31 (82) | 55 (58) | 0.01 |
| SSA (Ro52 and/or Ro60) | 28 (74) | 70 (74) | 1.0 |
| SSB | 18 (47) | 50 (53) | 0.70 |
| Extraglandular disorder† | 8 (21) | 18 (19) | 0.81 |
| Germinal center-like structure in biopsy | 15 (39) | 17 (18) | 0.01 |
| Rheumatoid factor | 27 (71) | 56 (59) | 0.24 |
| Focus score, median (25th-75th percentile) | 3.4 (2.6-6.4) | 2.4 (1.6-3.7) | 0.005 |
Except where indicated otherwise, values are the number (%) of patients.
Physician-confirmed diagnoses of Grave's disease, Hashimoto's thyroiditis, interstitial nephritis, primary biliary cirrhosis, autoimmune hepatitis, renal tubular acidosis, glomerulonephritis, and lymphoma
Abbreviations: SS, Sjögren's syndrome; UWS, unstimulated whole saliva flow rate; WBC, white blood count; C4, complement 4; IgG, immunoglobulin G
Most anti-IFI16 antibodies in SS patients are directed against an epitope outside of the N-terminus of the molecule
The 35S-methionine-labeled IFI16 IVTT product reproducibly consisted predominantly of the full-length protein (95 kDa) and a smaller amount of shorter product (85 kDa – Supplementary Figure 1, lane 1 “input”). To determine which region of IFI16 was missing from the shorter product, immunoprecipitations (IP) were performed using an anti-IFI16 monoclonal antibody raised against the N-terminus of IFI16 or a polyclonal anti-IFI16 antibody raised against a peptide at the C-terminus. These IP data demonstrated that the shorter form of IFI16 could only be immunoprecipitated by an antibody directed against the C-terminus of IFI16 (Supplementary Figure 1, lanes 2 and 3), confirming that it lacks the N-terminus and suggesting that it is produced by the use of an alternate translation initiation site.
We used this information to set up the following simple IVTT IP assay to determine whether the anti-IFI16 antibodies in patient sera are directed against the N- or the C-terminus of the protein. Patient sera were used in IVTT IPs, and if they pulled down only full-length IFI16 (pattern similar to the commercial anti-N-terminal antibody in lane 2), the N-terminus is a required component of the epitope, but if they pulled down both the full-length and short forms of the protein (pattern similar to the commercial antibody directed against the C-terminus in lane 3), they recognize an epitope outside the N-terminus. We tested the 13 SS patients with the highest levels of anti-IFI16 antibodies (OD>1) in this assay, and found that anti-IFI16 antibodies were directed at an epitope outside of the N-terminus in 9/13 (69%) of the SS patients.
To give context to these new data on anti-IFI16 antibodies in SS patients, we examined epitopes recognized by anti-IFI16 antibodies in another disease cohort. Since it is well-documented that anti-IFI16 antibodies are found in SLE patients, and the clinical characteristics of these patients have been described (3-6), we selected a well-characterized SLE cohort for this purpose. 132 SLE patients seen at the Hopkins Lupus Clinic (Supplementary Table 1) were tested for anti-IFI16 antibodies. Of these, 31/132 (23.5%) had this specificity (SLE vs controls, p=0.0006). The clinical features of anti-IFI16 positive SLE patients are shown in Supplementary Table 2. As has been shown by some but not all investigators (3, 6), the frequency of anti-IFI16 antibodies was higher among the patients with anti-DNA antibodies (81 vs 48%, p=0.002). An association of anti-IFI16 antibodies with pericarditis (37 vs 15%, p=0.02) was also noted. A renal biopsy was performed at least once in 27 of the patients and the renal histology was lupus nephritis in 24 of these. Proliferative lupus nephritis (WHO class III or IV) was evident in 2/5 (40%) of patients with anti-IFI16 antibodies and in 17/19 (89%) of patients without anti-IFI16 antibodies (p=0.04).
Interestingly, when we tested the 15 SLE sera with highest anti-IFI16 antibody levels (OD > 1) by IVTT IP, the findings differed from those in SS. That is, the minority of anti-IFI16 antibodies in SLE patients (4/15, 27%) detected an epitope outside of the N-terminus; representative data are shown in Supplementary Figure 1, lanes 4 and 5. Thus, anti-IFI16 antibodies were directed predominantly against the N-terminus in SLE patients, and against the C-terminus in SS patients. This difference in frequency approached statistical significance (p=0.06, Fisher's exact test).
IFI16 Expression in Human Tissues
Salivary glands are a prominent target tissue in SS. To assess whether the levels of IFI16 expression differ in diseased target tissues compared to their normal counterparts, we used the following quantitative approach. Lysates were generated from frozen biopsies as described (18), and levels of IFI16 were determined by immunoblotting equal protein amounts of the tissue extracts (Figure 2, upper panel) using a monoclonal antibody recognizing the N-terminal portion of IFI16. For each sample, β-actin was immunoblotted as a loading control and IFI16 expression was normalized relative to β-actin in every case. IFI16 bands migrating at both 95 kDa and 85 kDa were detected by immunoblotting. The quantitated data show that expression levels of IFI16 were low/absent in salivary gland lysates made from control subjects, but the levels were significantly elevated in those made from patients with SS (p = 0.004) (Figure 2, lower panel). The inter-individual variation detected in these IFI16 immunoblots is consistent with our experience with other autoantigen expression levels in muscle tissue (18).
Figure 2. Biochemical levels of IFI16 are elevated in salivary gland lysates from SS patients.
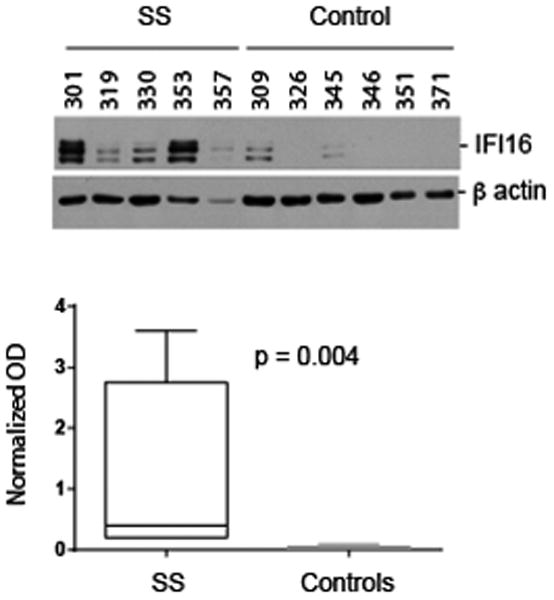
Lysates were made from frozen salivary gland (SS and controls). Equal protein amounts were immunoblotted with an antibody against IFI16 (upper panel). The blotted bands migrated at 95 and 85 kDa. In each lysate, β-actin was immunoblotted as a loading control. The data were quantitated as described in the Methods Section, and IFI16 levels (normalized in each case relative to β-actin in the same lysate) are represented as box plots (lower panel). Levels of IFI16 in SS salivary glands were significantly higher than in control salivary glands (p = 0.004).
Discussion
Phenotypic Characteristics and Clinical Associations of SS patients with anti-IFI 16 Antibodies
We identified anti-IFI16 antibodies in 29% of SS patients in the SICCA registry, each of whom met the histopathologic criterion for disease classification, namely focal lymphocytic sialadenitis with a focus score of one or greater. We are the first to demonstrate that anti-IFI16 antibodies are more prevalent in those SS patients with markers of more severe disease, including a Schirmer's test of tear production showing <5 mm wetting over 5 minutes, ANA titer of 1:320 or greater, hypergammaglobulinemia (IgG>1445 mg/dl), germinal center-like structures in the labial salivary gland lymphocytic infiltrates, and higher minor salivary gland focus scores. Interestingly, anti-IFI16 antibodies did not associate with the presence of anti-SSA or anti-SSB antibodies, a finding which contrasts from that of Uchida et al (5). Among patients with positive SSA/SSB serology, the presence of IFI16 antibodies was associated significantly with hyperglobulinemia and an abnormal Schirmer's test, arguing for an independent association of IFI16 antibodies as a marker for a more severe SS phenotype.
Anti-IFI16 antibodies were present in 23.5% of the SLE patients studied. They were associated with the presence of anti-dsDNA antibodies, an observation consistent with earlier findings made by Seelig et al but not by Caneparo et al (3, 6). Interestingly, this association with anti-dsDNA antibodies did not correlate with an increased risk of proliferative lupus nephritis among our patients with anti-IFI16 antibodies. Similarly, Caneparo et al noted a lower prevalence of proteinuria among their SLE patients with anti-IFI16 antibodies (6).
The prevalence of anti-IFI16 antibodies in our cohorts of SS and SLE patients (29% and 23.5% respectively) is lower than that observed in other cohorts. The prevalence in two other SS cohorts ranged from 50-70% while that in five other cohorts of SLE varied from 26-63%, with a median value of 33% (3-7). These differences may relate to a variety of factors, including the composition of the cohorts and the assay technique. Interestingly, the individuals in our SS cohort all had a “positive lip biopsy”, a selection bias that might have been expected to increase the prevalence of anti-IFI16 antibodies relative to other published SS cohorts (4, 5). The mean age of the patients in our SLE cohort was 10 or more years older than that of other cohorts (where mean age was reported) (4-6). The prevalence of secondary SS in this SLE cohort was also higher relative to other cohorts (20-23). However, we did not note any association of anti-IFI16 antibodies with the age of our patients, the duration of their disease, or the presence of secondary SS.
Antibodies against IFI16 are directed predominantly against different parts of the molecule in SS and SLE patient sera
Of the 15 SLE patients with the highest levels of anti-IFI16 antibodies, we found that in 11 (73%) cases, the antibodies were directed against the N-terminus of IFI16. It is noteworthy that anti-IFI16 antibodies are strongly associated with anti-dsDNA in SLE (p = 0.002 in this study, and also shown by others (3)), and that the DNA binding domain of IFI16 is outside of the N-terminus of the molecule. This raises the interesting possibility that DNA bound to IFI16 may be the immunogen in SLE, hence these anti-IFI16 antibodies recognize the N-terminal exposed part of the molecule, as well as dsDNA. The IFI16 IVTT product reproducibly consisted mostly (>93%) of the full-length product, with a small amount of a shorter form (lacking the N-terminal end) also generated in the reaction. Interestingly, patient sera that immunoprecipitated both forms of IFI16 (i.e., mainly SS sera) did not pull down the products in the same ratio as they existed in the IVTT reaction. Instead, the immunoprecipitations showed a ∼3-fold relative enrichment for the shorter piece (p = 0.002 using data from three different preparations and 11 immunoprecipitations).
Taken together, the enrichment of antibodies directed against the N-terminus (SLE) and an epitope outside the N-terminus (SS), suggests that the autoimmune response in these diseases may be directed against different forms of IFI16 (e.g, resulting from proteolytic cleavage, DNA binding) that could be generated in various physiologic or pathologic states. Future studies are underway to investigate what effects the differential binding of autoantibodies at the N- or C-terminus of IFI16 may have in modulating its activity.
IFI16 expression is elevated in salivary glands from SS patients
We used an immunoblotting approach to quantitate IFI16 expression levels in salivary glands because these are an important target tissue in SS. IFI16 expression (normalized relative to β-actin in every case) was low/absent in salivary gland lysates made from control subjects, but the levels were significantly elevated in those made from patients with SS (p=0.004). This finding is consistent with immunohistochemical observations made by Mondini et al in other autoimmune diseases showing increased levels of IFI16 in the lesional skin of patients with SLE and systemic sclerosis (4). Our immunoblotting data reinforces our earlier observations made in another systemic autoimmune disease (myositis), showing elevated levels of myositis autoantigens in muscle, a prominent target tissue in myositis (18). This suggests that elevated levels of autoantigen expression in a particular disease microenvironment/pathologic state are likely one mechanism for initiating or propagating an autoimmune response. While the mechanisms that make IFI16 a prominent target of the autoimmune response in SLE and SS await elucidation (and may indeed differ in these two diseases), it is tempting to speculate that elevated IFI16 levels, in the setting of DNA binding and apoptosis, create the potential for initiating autoimmunity.
Supplementary Material
Supplementary Figure 1. IFI16 antibodies are directed predominantly against the C-terminus in SS patients, and against the N-terminus in SLE patients 35S-methionine-labeled IFI16, generated by in vitro transcription and translation (IVTT) and visualized by fluorography, consisted predominantly of the full-length protein, with a small amount of a shorter product (lane 1). A monoclonal antibody detecting the N-terminus immunoprecipitated only full-length IFI16 (lane 2), whereas a polyclonal antibody raised against a peptide at the C-terminus immunoprecipitated both IVTT products (lane 3). 9/13 (69%) of the SS patients had an IP pattern consistent with recognition of an epitope outside the N-terminus (serum 63 is a representative example). In contrast, 11/15 (73%) of the SLE patients had an IP pattern consistent with recognition of the N-terminus (serum 1683 is a representative example).
Supplementary Table 1: Demographic characteristics of the 132 SLE patients*
Supplementary Table 2. Phenotypic features of SLE patients in relation to anti-IFI16 antibody status*
Significance and Innovation.
Interferon inducible protein-16 (IFI16) is an intracellular DNA receptor involved in innate immunity.
Anti-IFI16 antibodies are a prominent specificity in primary Sjögren's syndrome.
We demonstrate for the first time that anti-IFI16 antibodies associate with markers of more severe Sjögren's syndrome.
IFI16 is expressed at higher levels in the minor salivary glands of patients with Sjögren's syndrome compared to controls.
Acknowledgments
Tao Wung, PhD in the Department of Biophysics and Biophysical Chemistry, Johns Hopkins University School of Medicine, made substantial contributions to the acquisition of data for this study.
Sources of financial support: These studies were supported by NIH grants AR 43727 (MP), R01 DE12354-15A1 (AR), R01-AR-44684 and R56A062615 (LCR). Drs. Baer and Casciola-Rosen's work was supported by the Jerome L. Greene Foundation. Dr Sohn is a Jerome L. Greene Scholar. The Johns Hopkins Rheumatic Disease Research Core Center, where the assays were performed, is supported by NIH grant P30-AR-053503. Some of the data and specimens used in this manuscript are from the Sjögren's International Collaborative Clinical Alliance (SICCA) Biorepository, funded under contract #HHSN26S201300057C by the National Institute of Dental and Craniofacial Research. This manuscript was prepared using a publicly available SICCA data set and does not necessarily reflect the opinions or views of the SICCA investigators, the NIH or NIDCR.
We thank David Fiorentino, MD PhD for critical review of this manuscript.
Footnotes
Conflicts of interest: None of the authors has received any financial support or other benefits from commercial sources for the work reported in this manuscript, nor do any of the authors have any other financial interests which could create a potential conflict of interest, or the appearance thereof.
References
- 1.Morrone SR, Wang T, Constantoulakis LM, Hooy RM, Delannoy MJ, Sohn J. Cooperative assembly of IFI16 filaments on dsDNA provides insights into host defense strategy. Proc Natl Acad Sci U S A. 2014 Jan 7;111(1):E62–71. doi: 10.1073/pnas.1313577111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mondini M, Costa S, Sponza S, Gugliesi F, Gariglio M, Landolfo S. The interferon-inducible HIN-200 gene family in apoptosis and inflammation: implication for autoimmunity. Autoimmunity. 2010 Apr;43(3):226–31. doi: 10.3109/08916930903510922. [DOI] [PubMed] [Google Scholar]
- 3.Seelig HP, Ehrfeld H, Renz M. Interferon-gamma-inducible protein p16. A new target of antinuclear antibodies in patients with systemic lupus erythematosus. Arthritis Rheum. 1994 Nov;37(11):1672–83. doi: 10.1002/art.1780371117. [DOI] [PubMed] [Google Scholar]
- 4.Mondini M, Vidali M, De Andrea M, Azzimonti B, Airo P, D'Ambrosio R, et al. A novel autoantigen to differentiate limited cutaneous systemic sclerosis from diffuse cutaneous systemic sclerosis: the interferon-inducible gene IFI16. Arthritis Rheum. 2006 Dec;54(12):3939–44. doi: 10.1002/art.22266. [DOI] [PubMed] [Google Scholar]
- 5.Uchida K, Akita Y, Matsuo K, Fujiwara S, Nakagawa A, Kazaoka Y, et al. Identification of specific autoantigens in Sjögren's syndrome by SEREX. Immunology. 2005 Sep;116(1):53–63. doi: 10.1111/j.1365-2567.2005.02197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caneparo V, Cena T, De Andrea M, Dell'oste V, Stratta P, Quaglia M, et al. Anti-IFI16 antibodies and their relation to disease characteristics in systemic lupus erythematosus. Lupus. 2013 May;22(6):607–13. doi: 10.1177/0961203313484978. [DOI] [PubMed] [Google Scholar]
- 7.Costa S, Borgogna C, Mondini M, De Andrea M, Meroni PL, Berti E, et al. Redistribution of the nuclear protein IFI16 into the cytoplasm of ultraviolet B-exposed keratinocytes as a mechanism of autoantigen processing. Br J Dermatol. 2011 Feb;164(2):282–90. doi: 10.1111/j.1365-2133.2010.10097.x. [DOI] [PubMed] [Google Scholar]
- 8.Costa S, Mondini M, Caneparo V, Afeltra A, Airo P, Bellisai F, et al. Rheumatology. 4. Vol. 50. Oxford; 2011. Apr, Detection of anti-IFI16 antibodies by ELISA: clinical and serological associations in systemic sclerosis; pp. 674–81. [DOI] [PubMed] [Google Scholar]
- 9.Daniels TE, Criswell LA, Shiboski C, Shiboski S, Lanfranchi H, Dong Y, et al. An early view of the international Sjögren's syndrome registry. Arthritis Rheum. 2009 May 15;61(5):711–4. doi: 10.1002/art.24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiboski SC, Shiboski CH, Criswell L, Baer A, Challacombe S, Lanfranchi H, et al. Arthritis Care Res. 4. Vol. 64. Hoboken; 2012. Apr, American College of Rheumatology classification criteria for Sjögren's syndrome: a data-driven, expert consensus approach in the Sjögren's International Collaborative Clinical Alliance cohort; pp. 475–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 12.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997 Sep;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 13.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992 Jun;35(6):630–40. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 14.Bloch KJ, Buchanan WW, Wohl MJ, Bunim JJ. Medicine. Vol. 44. Baltimore; 1965. May, Sjögren's Syndrome A Clinical, Pathological, and Serological Study of Sixty-Two Cases; pp. 187–231. [PubMed] [Google Scholar]
- 15.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002 Jun;61(6):554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiorentino D, Chung L, Zwerner J, Rosen A, Casciola-Rosen L. The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM-140): a retrospective study. J Am Acad Dermatol. 2011 Jul;65(1):25–34. doi: 10.1016/j.jaad.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiorentino DF, Chung LS, Christopher-Stine L, Zaba L, Li S, Mammen AL, et al. Most patients with cancer-associated dermatomyositis have antibodies to nuclear matrix protein NXP-2 or transcription intermediary factor 1gamma. Arthritis Rheum. 2013 Nov;65(11):2954–62. doi: 10.1002/art.38093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casciola-Rosen L, Nagaraju K, Plotz P, Wang K, Levine S, Gabrielson E, et al. Enhanced autoantigen expression in regenerating muscle cells in idiopathic inflammatory myopathy. J Exp Med. 2005 Feb 21;201(4):591–601. doi: 10.1084/jem.20041367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitcher JP, Shiboski CH, Shiboski SC, Heidenreich AM, Kitagawa K, Zhang S, et al. A simplified quantitative method for assessing keratoconjunctivitis sicca from the Sjögren's Syndrome International Registry. Am J Ophthalmol. 2010 Mar;149(3):405–15. doi: 10.1016/j.ajo.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manoussakis MN, Georgopoulou C, Zintzaras E, Spyropoulou M, Stavropoulou A, Skopouli FN, et al. Sjögren's syndrome associated with systemic lupus erythematosus: clinical and laboratory profiles and comparison with primary Sjögren's syndrome. Arthritis Rheum. 2004 Mar;50(3):882–91. doi: 10.1002/art.20093. [DOI] [PubMed] [Google Scholar]
- 21.Pan HF, Ye DQ, Wang Q, Li WX, Zhang N, Li XP, et al. Clinical and laboratory profiles of systemic lupus erythematosus associated with Sjögren syndrome in China: a study of 542 patients. Clin Rheumatol. 2008 Mar;27(3):339–43. doi: 10.1007/s10067-007-0720-0. [DOI] [PubMed] [Google Scholar]
- 22.Nossent JC, Swaak AJ. Systemic lupus erythematosus VII: frequency and impact of secondary Sjogren's syndrome. Lupus. 1998;7(4):231–4. doi: 10.1191/096120398678920046. [DOI] [PubMed] [Google Scholar]
- 23.Jonsson H, Nived O, Sturfelt G, Norberg R. Symptomatic secondary Sjögren's syndrome in patients with systemic lupus erythematosus (SLE). Relation to anti-SS-A and anti-SS-B autoantibodies. Scand J Rheumatol Suppl. 1986;61:166–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. IFI16 antibodies are directed predominantly against the C-terminus in SS patients, and against the N-terminus in SLE patients 35S-methionine-labeled IFI16, generated by in vitro transcription and translation (IVTT) and visualized by fluorography, consisted predominantly of the full-length protein, with a small amount of a shorter product (lane 1). A monoclonal antibody detecting the N-terminus immunoprecipitated only full-length IFI16 (lane 2), whereas a polyclonal antibody raised against a peptide at the C-terminus immunoprecipitated both IVTT products (lane 3). 9/13 (69%) of the SS patients had an IP pattern consistent with recognition of an epitope outside the N-terminus (serum 63 is a representative example). In contrast, 11/15 (73%) of the SLE patients had an IP pattern consistent with recognition of the N-terminus (serum 1683 is a representative example).
Supplementary Table 1: Demographic characteristics of the 132 SLE patients*
Supplementary Table 2. Phenotypic features of SLE patients in relation to anti-IFI16 antibody status*


