Abstract
Background:
Periodontitis is an infection of the periodontal complex with severe forms of disease associated with specific bacteria colonizing the subgingival area. Widespread use of drugs has resulted in the emergence of side effects, uncommon infections, and resistance. Plant medicine like Tulsi has been used in many clinical conditions, and it appears to be a suitable alternative to manage conditions affecting the oral cavity. Hence, the objective was to assess the in vitro antimicrobial activity of Tulsi leaves extract (Ocimum sanctum) on periodontal pathogens with doxycycline as standard, as doxycycline has been used as an adjunct to nonsurgical therapy in periodontitis patients.
Materials and Methods:
Ethanolic extract of Tulsi was prepared by cold extraction method. Extract was diluted with an inert solvent, dimethyl formamide, to obtain five different concentrations (0.5%, 1%, 2%, 5%, and 10%). Doxycycline was used as a positive control and dimethyl formamide, as a negative control. The extract and controls were subjected to the microbiological investigation against Aggregatibacter actinomycetemcomitans, Prevotella intermedia, and Porphyromonas gingivalis. Agar well diffusion method was employed to determine the concentration at which Tulsi gave an inhibition zone, similar to doxycycline. Data were analyzed using one-way analysis of variance and Tukey post-hoc test was used for inter- and intra-group comparisons.
Results:
At 5% and 10% concentrations, Tulsi extracts demonstrated antimicrobial activity against A. actinomycetemcomitans, similar to doxycycline with similar inhibition zones (P > 0.05). P. gingivalis and P. intermedia, however, exhibited resistance to Tulsi extract that showed significantly smaller inhibition zones (P < 0.05).
Conclusions:
Tulsi demonstrated effective antimicrobial property against A. actinomycetemcomitans, suggesting its possible use as an effective and affordable “adjunct” along with the standard care in the management of periodontal conditions. However, further research assessing the toxicity, durability, and other assessments followed by clinical trials is necessary to explore the potential of Tulsi in combating oral conditions.
Keywords: Aggregatibacter actinomycetemcomitans, Ocimum sanctum, periodontitis, phytomedicine, Porphyromonas gingivalis, Prevotell intermedia
INTRODUCTION
Oral infections, particularly periodontitis and other periodontal conditions have been long known to be associated with aerobic and anaerobic pathogens. Oral cavity houses more than 500 different types of bacteria that are reportedly associated with different oral diseases.[1] Aggregatibacter actinomycetemcomitans has been recognized as one of the most common micro-organism, responsible for the initiation of periodontitis and is commonly associated with active periodontal lesions.[2] Levels of Porphyromonas gingivalis, Prevotell intermedia, and other anaerobic bacteria have been commonly seen to increase in chronic periodontitis.[3] While P. intermedia is more commonly associated with the advancement of necrotizing ulcerative gingivitis,[4] P. gingivalis is predominantly associated with the development of localized aggressive periodontitis.[5,6,7]
The emergence of drug resistance in human and animal pathogenic bacteria, as well as undesirable side effects of certain antibiotics, has triggered immense interest in the search for new antimicrobial alternatives of plant origin. The most important advantage claimed for therapeutic use of medicinal plants in various ailments is their safety besides being economical, effective, and easy availability.[8]
Medicinal plants are used widely by traditional medical practitioners in their day-to-day practice for curing various diseases. Phytomedicine (use of medicinal herbs for treatment), for oral disorders such as dental caries and periodontal disease, has also been well practiced in traditional medicine of Indian, Egyptian, Greek, and Chinese civilizations.[9,10] Different parts of Ocimum sanctum Linn (known as Tulsi), a small herb seen throughout India, have been used for various medicinal purposes. Tulsi has long been recognized as possessing antioxidant properties,[11] as a COX2 inhibitor,[12] and to provide protection from radiation poisoning[13] and cataracts.[14] Studies have also demonstrated anti-hyperlipidemia and cardioprotective effects of Tulsi in rats fed on a high-fat diet,[15] and it is also known to promote immune system function.[16] A decoction prepared from Tulsi plant is hepatoprotective, immunomodulatory, analgesic, antipyretic, and is used as a diaphoretic in malarial fever.[17] Tulsi has also been used as an important pot herb in folklore practices for a number of ailments and diseases.[18] This scenario is ever applicable to Tulsi for a wide range of conditions ranging from relatively minor illnesses such as cold or a cough to various severe conditions. O. sanctum (Tulsi) has been studied on a major scale and has shown a plethora of biological and pharmacological activities benefiting humans since ages.
However, literature pertaining to the antimicrobial activity of Tulsi on periodontal bacteria has not been reported earlier. Therefore, the present study was conceptualized as the initial step to comprehensively report the antimicrobial potential of Tulsi by assessing the inhibition zones with agar well diffusion method against three pathogens viz., A. actinomycetemcomitans, P. gingivalis and P. intermedia, which are commonly associated with periodontitis. The findings were then compared with those obtained with doxycycline, a drug used commonly as an adjunct to nonsurgical therapy for treatment of periodontitis, as a standard.[19]
MATERIALS AND METHODS
The study employed an in vitro experimental design. Ethical clearance to conduct the study was obtained from the Institutional Ethics Committee of Manipal College of Dental Sciences, Mangalore (Ref No: MCODS/198/2014).
Tulsi leaves were obtained from courtyards and local market in Mangalore city. Specimens were identified by a botanist and a pharmacognosist for their authenticity. Leaves were separated from the stem, washed in clear water and dried until they were adequately dry to be ground (dried for 7 days). Dried leaves were powdered separately in an electric grinder until a homogenous powder was obtained. Ethanolic extract was prepared from the powder obtained using “cold extraction method.”[20] A total of 250 g of finely powdered Tulsi was macerated with 100% ethanol for 3 days. The alcoholic decoction was subjected to filtration with Whatman #1 filter paper to obtain a clear filtrate. The filtrate thus obtained was reduced at a low temperature of <60°C to obtain a solid residue of Tulsi extract [Figure 1]. From the 250 g of Tulsi powder dissolved in 1 L of ethanol, approximately 18 g of solid residue (extract) was obtained.
Figure 1.

Tulsi (Ocimum sanctum) extract preparation; (a) Ocimum sanctum plant; (b) leaves separated and dried; (c) leaves ground to powder; (d) extract obtained
One gram of this extract was dissolved in 10 ml of dimethyl formamide to obtain a 10% concentration of extract. Similarly, concentrations of 0.5%, 1%, 2%, and 5% of Tulsi extract were obtained by diluting with appropriate amounts of solvent. These extracts were collected in sterile containers and transported for microbiological assays. In this study, doxycycline, one of the drugs commonly employed in the treatment of aggressive periodontitis, was used as a positive control and dimethyl formamide, the solvent used in the extract preparation, was used as a negative control.
Microbiological assay
Agar well diffusion method was used to determine the antimicrobial activity of Tulsi leaves extract in vitro. Blood agar was used to culture different micro-organisms examined in this study. Colonies of microorganisms were transferred to the agar plates using a swab, and their turbidity was visually adjusted with the broth to equal that of a 0.5 McFarland turbidity standard that had been vortexed. Within 15 min of adjusting the inoculum to a McFarland 0.5 turbidity standard, a sterile cotton swab was dipped into the inoculum and rotated against the wall of the tube above the liquid to remove excess inoculum. The entire surface of agar plate was then swabbed 3 times with the cotton swab, transferring the inoculum, while the plates were rotated by approximately 60° between streaks to ensure even distribution. The overall procedure of inoculum preparation and inoculation of culture media remained the same for all three bacteria. Each bacterium was inoculated on five agar plates for five respective concentrations (0.5%, 1%, 2%, 5%, and 10%) of the Tulsi extract. Therefore, a total of 15 plates were inoculated to test all the three bacteria.
The inoculated plate was allowed to stand for at least 3 min but no longer than 15 min, before making wells for different compounds to be tested. A hollow tube of 5 mm diameter was heated and pressed above the inoculated agar plates. It was removed immediately by making a well in the plate; likewise, three wells on each plate were made, one each for positive control, negative control and Tulsi extract [Figure 2]. Each well received 5 µl of respective compound assigned for it. Plates were incubated at 37°C in an incubator within 15 min of compound application. Incubation was done for 48 h for both aerobic (A. actinomycetemcomitans) and anaerobic (P. gingivalis and P. intermedia) bacteria. For anaerobic organisms, plates were incubated in the McIntosh and Filde's anaerobic jar, while aerobic micro-organism was cultured in the incubator at 37°C for 48 h.
Figure 2.

Wells made in blood agar for Tulsi, doxycycline, and dimethylformamide
After the incubation period, plates were read only if the lawn of growth was confluent or nearly confluent. The diameter of inhibition zone was measured to the nearest whole millimeter by using a Vernier Calliper. The microbiological procedure was repeated 4 times for each bacterium, and corresponding four values of zones of inhibition for each concentration of Tulsi extract along with doxycycline and dimethyl formamide were obtained for each of the three bacteria. The values so obtained were compared within the group (same concentration of extract) and with different groups (different concentrations of extract) and also with the positive control (doxycycline) for different bacteria [Figure 3].
Figure 3.
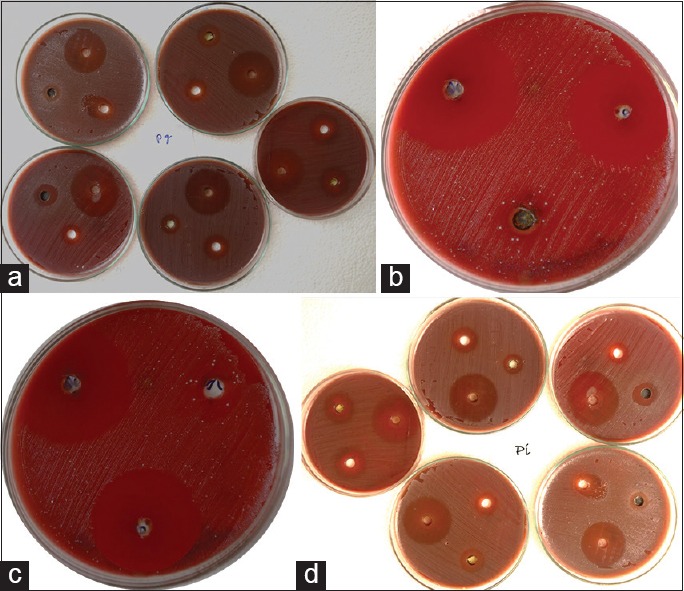
ones of inhibition produced by Tulsi at different concentrations. (a) Against Porphyromonas gingivalis; (b) 5% Tulsi against Aggregatibacter actinomycetemcomitans; (c) 10% Tulsi against Aggregatibacter actinomycetemcomitans; (d) against Prevotella intermedia
Statistical analysis was done using the software Statistical Package for Social Sciences (SPSS, version 16.0; SPSS Inc., Chicago, IL, USA). The disc diffusion values of different concentrations of Tulsi leaves extract, positive and negative controls against all the bacteria were entered in the SPSS software for statistical analysis. Descriptive statistics was retrieved, and data were analyzed using one-way analysis of variance (ANOVA), and Tukey post-hoc test was used for comparison within the group and with different groups. Statistical significance level was established at P < 0.05.
RESULTS
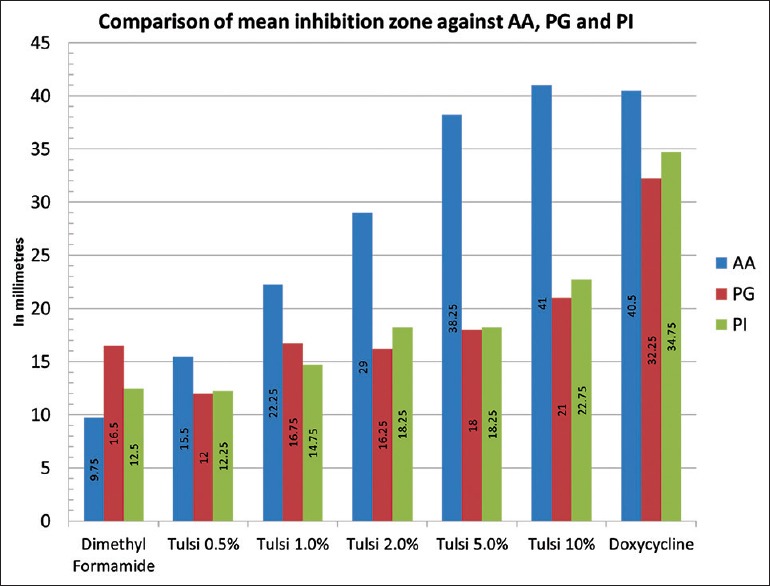
Zones of inhibition displayed by Tulsi extract (at different concentrations), positive and negative controls against A. actinomycetemcomitans, P. gingivalis and P. intermedia are compiled in Table 1. The least zones of inhibition were displayed by the negative control and doxycycline exhibited the widest zones of inhibition against all the three bacteria. Tulsi leaves' extract showed increasing zones of inhibition with increasing concentration against all the three bacteria. Mean zone of inhibition for each concentration and each bacterium was calculated for analysis [Figure 4]. All the concentrations of Tulsi and dimethyl formamide showed minimal inhibitory zones against P. gingivalis and P. intermedia. Against A. actinomycetemcomitans, Tulsi leaves extract at a concentration of 0.5%, 1%, and 2% and dimethyl formamide showed a minimal zone of inhibition. However, Tulsi displayed wide inhibition zones at 5% and 10%. All the bacteria tested showed susceptibility to doxycycline explained by the wide inhibition zones displayed [Table 1].
Table 1.
Zones of inhibition (in mm)
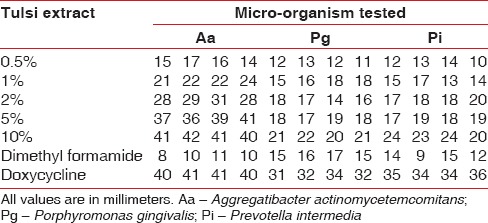
Figure 4.
Mean zones of inhibition by Tulsi, doxycycline and dimethylformamide against Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis and Prevotella intermedi
One-way ANOVA revealed an increase in the mean zones of inhibition against all the three bacteria with increasing concentration of Tulsi, which was statistically significant (P < 0.001). Comparison with doxycycline revealed insignificant inhibition zones by all the concentrations of Tulsi against P. gingivalis and P. intermedia. Similar results were obtained on a comparison of inhibition zones exhibited by Tulsi, at 0.5%, 1%, and 2% concentrations and doxycycline against A. actinomycetemcomitans. However, at 5% and 10%, Tulsi showed wider zones of inhibition against A. actinomycetemcomitans, which were similar to doxycycline in comparison [Table 2].
Table 2.
Antimicrobial efficacy of Tulsi leaf extracts (ANOVA and post-hoc-Tukey)
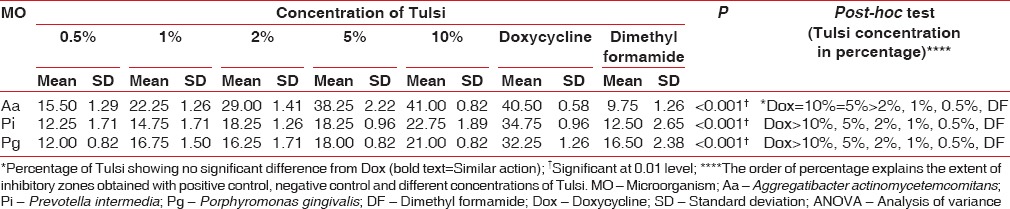
Post-hoc tests revealed a significant difference in the antimicrobial efficacy of doxycycline and Tulsi against P. gingivalis and P. intermedia, with mean zone of inhibition obtained by doxycycline clearly exceeding the mean zones of inhibition obtained by different concentrations of Tulsi. The only exception in this phenomenon was that of Tulsi at a concentration of 5% and 10%, both of which showed antimicrobial efficacy against A. actinomycetemcomitans, similar to that of doxycycline [Table 2].
DISCUSSION
With due consideration to available evidence pertaining to side effects and emergence of uncommon infections with the usage of synthetic antimicrobial agents,[21,22] including tetracyclines,[23] particularly doxycycline[24,25] which is used most commonly for management of aggressive periodontitis; this study was conducted in the quest of identifying Tulsi as a possible alternative or an “adjunct” in the treatment of aggressive periodontitis. Several plant products such as Tulsi, neem, lemon, and others have been tested for their antimicrobial properties in the past[26,27,28,29,30] with considerable success. Resistance to currently used chemotherapeutics is the major factor that necessitates the search for alternative safe, efficacious, and cost-effective treatment options, particularly in developing countries.[31]
In this study, we attempted to obtain information on the antimicrobial efficacy of Tulsi, particularly against three periodontal pathogens namely A. actinomycetemcomitans, P. intermedia and P. gingivalis; as these microbes are more commonly associated with initiation and progression of various periodontal diseases, especially aggressive periodontitis.[2,32,33,34,35,36,37,38] Results in this in vitro experiment showed that Tulsi at a concentration of 5% and 10% can effectively inhibit the growth of A. actinomycetemcomitans, comparable to that of doxycycline. Different mechanisms of action of Tulsi have been proposed by many authors earlier. Vishwabhan et al.[39] proposed the antimicrobial activity of Tulsi by virtue of its essential oil content. Various other studies also project the idea that Tulsi yields various essential oils which are responsible for the medicinal uses including antimicrobial, antioxidant, antifungal, and anti-inflammatory activities that can probably explain its activity against the microbes discussed so far.[40,41,42] It has also been postulated that Tulsi has an immunomodulatory effect and acts by increasing the levels of interferon, interleukin-4 and T helper cells that can strengthen host response to infections.[16] Singhal et al.[41] proposed that the antibacterial activity of Tulsi leaf extract could be attributed to its ability to reduce silver ions to silver nanoparticles that possess antibacterial properties against both Gram-negative and Gram-positive bacteria.
Although many previous studies like those conducted by Agarwal et al.,[20,26] Rathod[43] Shah et al.[44] and Prasannabalaji[45] have all shown the antimicrobial properties of Tulsi against different organisms, and there also is evidence claiming plant products being used as effective therapy against periodontitis.[46] The present study is one of the first to assess the antimicrobial efficacy of Tulsi leaves extract against periodontal pathogens, in particular associated with aggressive periodontitis. Comparisons with previous studies are not justified here due to variation in the organisms tested against Tulsi for its antimicrobial effect. Since there were scarce literature available that could depict the efficacy of Tulsi against periodontal microbes specifically, the present study encourages researchers to carry out further studies assessing toxicity, durability and other assessments followed by clinical trials to provide an insight into the activity of Tulsi against periodontal pathogens on a transient as well as longitudinal basis to establish clear implications of Tulsi in periodontal disease management. With the basic limitations of the study design, generalizability is a possibility with further accumulation of evidence in this regard. However, within the limitations of the present study it could be concluded that Tulsi, as an adjunct, “if” found effective and safe on further research would be considered as a potential “adjunct” along with the standard care in the management of periodontitis to overcome the side effects of synthetic drugs, especially in this era of ever advancing clinical dentistry.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
Acknowledgement
Dr. Ashok Shenoy, head of the department, Department of Pharmacology, Kasturba Medical College, Mangalore, Manipal University, Karnataka, for aiding in on the Tulsi leaves extract preparation. Dr. Kishore G Bhat, head of the department, Department of Molecular Biology and Immunology, Maratha Mandal's Nathajirao G Halgekar Institute of Dental Sciences and Research Centre, Belgaum, Karnataka, for the support provided in conducting microbiological assays.
REFERENCES
- 1.Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721–32. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dzink JL, Socransky SS, Haffajee AD. The predominant cultivable microbiota of active and inactive lesions of destructive periodontal diseases. J Clin Periodontol. 1988;15:316–23. doi: 10.1111/j.1600-051x.1988.tb01590.x. [DOI] [PubMed] [Google Scholar]
- 3.Carranza FA, Newman MG, Takei HH, Klokkevold PR. Carranza's Clinical Periodontology. 10th ed. St. Louis, Mo: Saunders Elsevier; 2006. [Google Scholar]
- 4.Gmür R, Wyss C, Xue Y, Thurnheer T, Guggenheim B. Gingival crevice microbiota from Chinese patients with gingivitis or necrotizing ulcerative gingivitis. Eur J Oral Sci. 2004;112:33–41. doi: 10.1111/j.0909-8836.2004.00103.x. [DOI] [PubMed] [Google Scholar]
- 5.Miura M, Hamachi T, Fujise O, Maeda K. The prevalence and pathogenic differences of Porphyromonas gingivalis fimA genotypes in patients with aggressive periodontitis. J Periodontal Res. 2005;40:147–52. doi: 10.1111/j.1600-0765.2005.00779.x. [DOI] [PubMed] [Google Scholar]
- 6.Ishikawa I, Kawashima Y, Oda S, Iwata T, Arakawa S. Three case reports of aggressive periodontitis associated with Porphyromonas gingivalis in younger patients. J Periodontal Res. 2002;37:324–32. doi: 10.1034/j.1600-0765.2002.01613.x. [DOI] [PubMed] [Google Scholar]
- 7.Chahbouni H, Maltouf AF, Ennibi O. Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis in aggressive periodontitis in Morocco – Preliminary study. Odontostomatol Trop. 2013;36:5–10. [PubMed] [Google Scholar]
- 8.Siddiqui HH. Safety of herbal drugs – An overview. Drugs News Views. 1993;1:7–10. [Google Scholar]
- 9.Abdollahzadeh SH, Mashouf R, Mortazavi H, Moghaddam M, Roozbahani N, Vahedi M. Antibacterial and antifungal activities of punica granatum peel extracts against oral pathogens. J Dent (Tehran) 2011;8:1–6. [PMC free article] [PubMed] [Google Scholar]
- 10.Li M, Xu Z. Quercetin in a lotus leaves extract may be responsible for antibacterial activity. Arch Pharm Res. 2008;31:640–4. doi: 10.1007/s12272-001-1206-5. [DOI] [PubMed] [Google Scholar]
- 11.Sethi J, Sood S, Seth S, Talwar A. Evaluation of hypoglycemic and antioxidant effect of Ocimum sanctum. Indian J Clin Biochem. 2004;19:152–5. doi: 10.1007/BF02894276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prakash P, Gupta N. Therapeutic uses of Ocimum sanctum Linn (Tulsi) with a note on eugenol and its pharmacological actions: A short review. Indian J Physiol Pharmacol. 2005;49:125–31. [PubMed] [Google Scholar]
- 13.Devi PU, Ganasoundari A. Modulation of glutathione and antioxidant enzymes by Ocimum sanctum and its role in protection against radiation injury. Indian J Exp Biol. 1999;37:262–8. [PubMed] [Google Scholar]
- 14.Sharma P, Kulshreshta S, Sharma AL. Anti-cataract activity of Ocimum sanctum on experimental cataract. Indian J Pharmacol. 1998;30:16. [Google Scholar]
- 15.Suanarunsawat T, Boonnak T, Na Ayutthaya WD, Thirawarapan S. Anti-hyperlipidemic and cardioprotective effects of Ocimum sanctum L. fixed oil in rats fed a high fat diet. J Basic Clin Physiol Pharmacol. 2010;21:387–400. doi: 10.1515/jbcpp.2010.21.4.387. [DOI] [PubMed] [Google Scholar]
- 16.Mondal S, Varma S, Bamola VD, Naik SN, Mirdha BR, Padhi MM, et al. Double-blinded randomized controlled trial for immunomodulatory effects of Tulsi (Ocimum sanctum Linn.) leaf extract on healthy volunteers. J Ethnopharmacol. 2011;136:452–6. doi: 10.1016/j.jep.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Gupta SK, Prakash J, Srivastava S. Validation of traditional claim of Tulsi, Ocimum sanctum Linn. as a medicinal plant. Indian J Exp Biol. 2002;40:765–73. [PubMed] [Google Scholar]
- 18.Khosla MK. Sacred tulsi (Ocimum sanctum L.) in traditional medicine and pharmacology. Anc Sci Life. 1995;15:53–61. [PMC free article] [PubMed] [Google Scholar]
- 19.Gürkan A, Cinarcik S, Hüseyinov A. Adjunctive subantimicrobial dose doxycycline: Effect on clinical parameters and gingival crevicular fluid transforming growth factor-beta levels in severe, generalized chronic periodontitis. J Clin Periodontol. 2005;32:244–53. doi: 10.1111/j.1600-051X.2005.00663.x. [DOI] [PubMed] [Google Scholar]
- 20.Agarwal P, Nagesh L, Murlikrishnan Evaluation of the antimicrobial activity of various concentrations of Tulsi (Ocimum sanctum) extract against Streptococcus mutans: An in vitro study. Indian J Dent Res. 2010;21:357–9. doi: 10.4103/0970-9290.70800. [DOI] [PubMed] [Google Scholar]
- 21.Westphal JF, Vetter D, Brogard JM. Hepatic side-effects of antibiotics. J Antimicrob Chemother. 1994;33:387–401. doi: 10.1093/jac/33.3.387. [DOI] [PubMed] [Google Scholar]
- 22.Dancer SJ. How antibiotics can make us sick: The less obvious adverse effects of antimicrobial chemotherapy. Lancet Infect Dis. 2004;4:611–9. doi: 10.1016/S1473-3099(04)01145-4. [DOI] [PubMed] [Google Scholar]
- 23.Greenstein G. Clinical significance of bacterial resistance to tetracyclines in the treatment of periodontal diseases. J Periodontol. 1995;66:925–32. doi: 10.1902/jop.1995.66.11.925. [DOI] [PubMed] [Google Scholar]
- 24.Sloan B, Scheinfeld N. The use and safety of doxycycline hyclate and other second-generation tetracyclines. Expert Opin Drug Saf. 2008;7:571–7. doi: 10.1517/14740338.7.5.571. [DOI] [PubMed] [Google Scholar]
- 25.Smith K, Leyden JJ. Safety of doxycycline and minocycline: A systematic review. Clin Ther. 2005;27:1329–42. doi: 10.1016/j.clinthera.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Agarwal P, Nagesh L. Comparative evaluation of efficacy of 0.2% chlorhexidine, listerine and tulsi extract mouth rinses on salivary Streptococcus mutans count of high school children – RCT. Contemp Clin Trials. 2011;32:802–8. doi: 10.1016/j.cct.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Wolinsky LE, Mania S, Nachnani S, Ling S. The inhibiting effect of aqueous Azadirachta indica (Neem) extract upon bacterial properties influencing in vitro plaque formation. J Dent Res. 1996;75:816–22. doi: 10.1177/00220345960750021301. [DOI] [PubMed] [Google Scholar]
- 28.Polaquini SR, Svidzinski TI, Kemmelmeier C, Gasparetto A. Effect of aqueous extract from Neem (Azadirachta indica A. Juss) on hydrophobicity, biofilm formation and adhesion in composite resin by Candida albicans. Arch Oral Biol. 2006;51:482–90. doi: 10.1016/j.archoralbio.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Dhanavade M, Jalkute C, Ghosh J, Sonawane K. Study antimicrobial activity of lemon (Citrus lemon L.) peel extract. Br J Pharmacol Toxicol. 2011;2:119–22. [Google Scholar]
- 30.Miyake Y, Hiramitsu M. Isolation and extraction of antimicrobial substances against oral bacteria from lemon peel. J Food Sci Technol. 2011;48:635–9. doi: 10.1007/s13197-011-0330-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahmad I, Aqil F, Owais M. Medicinal Plants into Drugs. Weinheim, Germany: WILEY-VCH Verlag GmbH & Co. KGaA; 2006. Modern Phytomedicine: Turning Medicinal Plants into Drugs. [Google Scholar]
- 32.Stingu CS, Jentsch H, Eick S, Schaumann R, Knöfler G, Rodloff A. Microbial profile of patients with periodontitis compared with healthy subjects. Quintessence Int. 2012;43:e23–31. [PubMed] [Google Scholar]
- 33.Zambon JJ. Periodontal diseases: Microbial factors. Ann Periodontol. 1996;1:879–925. doi: 10.1902/annals.1996.1.1.879. [DOI] [PubMed] [Google Scholar]
- 34.Offenbacher S. Periodontal diseases: Pathogenesis. Ann Periodontol. 1996;1:821–78. doi: 10.1902/annals.1996.1.1.821. [DOI] [PubMed] [Google Scholar]
- 35.Genco RJ. Current view of risk factors for periodontal diseases. J Periodontol. 1996;67(10 Suppl):1041–9. doi: 10.1902/jop.1996.67.10.1041. [DOI] [PubMed] [Google Scholar]
- 36.Socransky SS, Haffajee AD. The bacterial etiology of destructive periodontal disease: Current concepts. J Periodontol. 1992;63(4 Suppl):322–31. doi: 10.1902/jop.1992.63.4s.322. [DOI] [PubMed] [Google Scholar]
- 37.Tatakis DN, Kumar PS. Etiology and pathogenesis of periodontal diseases. Dent Clin North Am. 2005;49:491–516, v. doi: 10.1016/j.cden.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 38.van Winkelhoff AJ, Loos BG, van der Reijden WA, van der Velden U. Porphyromonas gingivalis, Bacteroides forsythus and other putative periodontal pathogens in subjects with and without periodontal destruction. J Clin Periodontol. 2002;29:1023–8. doi: 10.1034/j.1600-051x.2002.291107.x. [DOI] [PubMed] [Google Scholar]
- 39.Vishwabhan S, Birendra VK, Vishal S. A review on ethnomedical uses of Ocimum sanctum (Tulsi) Int Res J Pharm. 2011;2:1–3. [Google Scholar]
- 40.Okigbo RN, Mmeka EC. An appraisal of phytomedicine in Africa. KMITL Sci Technol J. 2006;6:83–94. [Google Scholar]
- 41.Singhal G, Bhavesh R, Kasariya K, Sharma AR, Singh RP. Biosynthesis of silver nanoparticles using Ocimum sanctum (Tulsi) leaf extract and screening its antimicrobial activity. J Nanopart Res. 2011;13:2981–8. [Google Scholar]
- 42.Nahak G, Mishra RC, Sahu RK. Taxonomic distribution, medicinal properties and drug development potentiality of Ocimum (Tulsi) Drug Invent Today. 2011;3:95–113. [Google Scholar]
- 43.Rathod G. In vitro antibacterial study of two commonly used medicinal plants in ayurveda neem Azadirachta indica L and Tulsi Ocimum sanctum L. Int J Pharm Biol. 2012;3:582–6. [Google Scholar]
- 44.Shah S, Trivedi B, Patel J. Evaluation and comparison of antimicrobial activity of Tulsi (Ocimum Sanctum), Neem (Azadirachta indica) and triphala extract against Streptococcus. Natl J Int Res Med. 2014;5:17–21. [Google Scholar]
- 45.Prasannabalaji N. Antibacterial activities of some Indian traditional plant extracts. Asian Pac J Trop Dis. 2012;2:S291–5. [Google Scholar]
- 46.Prasad D, Kunnaiah R. Punica granatum: A review on its potential role in treating periodontal disease. J Indian Soc Periodontol. 2014;18:428–32. doi: 10.4103/0972-124X.138678. [DOI] [PMC free article] [PubMed] [Google Scholar]


