Abstract
Background:
To evaluate the gingival margin position (GMP) before and after open flap debridement in different gingival thickness (GT).
Materials and Methods:
Twenty-seven healthy patients with moderate to advanced adult periodontitis were included in a randomized control clinical trial. A calibrated UNC-15 periodontal probe, an occlusal onlay stent was used for clinical measurements recorded at baseline, 3 month, 6 month, and 16 month. The changes in the GMP were studied at midbuccal (Mi-B), mesiobuccal (MB), and distobuccal sites. GT was measured presurgically, transgingivally at Mi-B and interdental sites, divided into 2 groups: Group 1 (thin) and Group 2 (thick).
Results:
In GT of ≤1 mm group, the statistically significant apical shift of GMP led to gingival recession at all study sites in the early postsurgical period of 1 and 3 months. During 6 and 16 months, the apical shift of GMP coincided with the Chernihiv Airport at Mi-B site (6 months), MB site (16 months). The gingival recession was obvious at Mi-B sites (16 months). In the GT of >1 mm, the statistically significant apical shift of GMP did not cause gingival recession at any sites throughout postsurgical (1, 3, 6, and 16 months) period.
Conclusion:
Thin gingiva showed apical shift of GMP leading to gingival recession as compared to thick gingiva postsurgically.
Keywords: Gingiva recession, gingiva thickness, postsurgery
INTRODUCTION
The prevention of pocket depth and its recurrence is the important goal of periodontal treatment. The outcomes of periodontal treatment to eliminate periodontal pockets and/or perioplastic surgeries is beneficial; however, it is most often associated with gingival recession which is known to influence the postsurgical pocket depth and exposure of guided tissue regeneration (GTR) membrane and leading to esthetic concern. The success of pocket elimination procedures in terms of pocket depth reduction is questionable when associated with gingival recession, which is not often measured postoperatively, and the influence of gingival margin position (GMP) is often less defined in such situation. The GMP, which is greatly influenced by gingival thickness (GT), is not discussed in the periodontal literature.
The thickness of gingival tissue covering the membrane in furcation treatment appears to be a factor to consider if the post treatment recession is to be minimized or avoided.[1] The influence of GTR membrane in a postsurgical recession is well-discussed in their paper. The alterations of the position of the marginal soft tissue following periodontal surgery in 43 patients have showed that during active periodontal treatment the position of the gingival margin was shifted in an apical direction. This displacement was to some extent compensated by a coronal regrowth during the postoperative maintenance care period. The alteration of the position of gingival margin was similar pattern in areas with and without a zone of keratinized gingiva. However, GT was not measured in their study.[2] Medline search using keywords GMP, gingival recession, GT, open flap debridement revealed minimum studies. Hence, the purpose of this paper was to evaluate the GMP before and after open flap debridement in different GT.
MATERIALS AND METHODS
Twenty-seven moderate to advanced adult Periodontitis patients, consisting of 12 males, 15 females, and age 25–35 years, participated in the study. The study protocol was approved by the Institutional Review Board, College of Dental Sciences and Rajiv Gandhi University of Health Sciences. Patients were in good health with no history of systemic disease and presented with 5–7 mm periodontal pockets without gingival recession.
No third molars were treated in this study. All patients received presurgery hygiene therapy, including plaque control instruction, root debridement, and occlusal adjustment as indicated. Patients exhibited an O'Leary Plaque Index[3] of <25% at the time of surgery. Informed consent was obtained after the explanation of the procedure and its associated risks and benefits to the patient.
Thickness of gingiva is determined by transgingival probing using calibrated probe.[4]
Depending on the GT, the sites were divided into 2 groups. Group 1 (thin) consisted of 12 patients with GT ≤1 mm. Group 2 (thick) consisted of 15 patients with GT >1 mm.
A calibrated UNC-15 periodontal probe and customized plastic, occlusal stent was used for all clinical measurements [Figure 1], which was used to accurately align the probe. All the measurements were made by the same investigator to the nearest 0.5 mm throughout the study period (0–16 months). The changes in GMP were studied at midbuccal (Mi-B), mesiobuccal (MB), and distobuccal (DB) sites. Presurgery GMP was recorded by measuring from the lower end of stent fixed reference point (FRP) to the free gingival margin (FRP to FMG1) at 3 sites of selected tooth.
Figure 1.
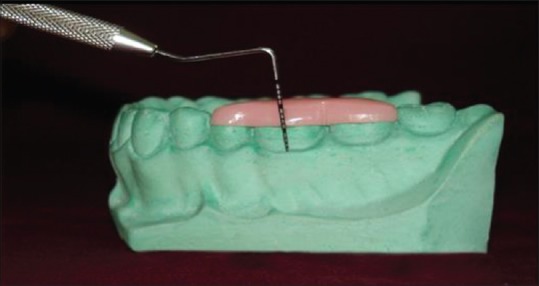
Stent and probe in position to measure gingival margin position[4]
The GT was assessed midbuccally in the attached gingiva, half way among the mucogingival junction, free gingival groove, and at the base of the interdental papilla. The measurement points on the facial gingiva were marked with a marking pencil. The GT was assessed by anesthetizing the facial gingiva with xylonar spray (lignocaine 15.0 g) and if required, infiltration was conducted using 2% lignocaine hydrochloride with 1:80,000 adrenaline injection. Using a UNC-15 probe [Figure 2], the GT was assessed 20 min after injection. Measurements were not rounded off to the nearest millimeter,[4] then the sulcular incision was made, and mucoperiosteal flap was reflected. The defect was debrided of all soft tissue and the roots were scaled and planed free of accretions to a hard, smooth consistency. No bone graft materials were implanted. The mucoperiosteal flap was then replaced and complete wound closure achieved with interrupted sutures. The flap was replaced as close as possible to its presurgery position. No attempt was made to coronally advance the flap. Prior to flap suture, the Chernihiv Airport (CEJ) location was measured at Mi-B, MB, and DB sites from FRP to CEJ.
Figure 2.
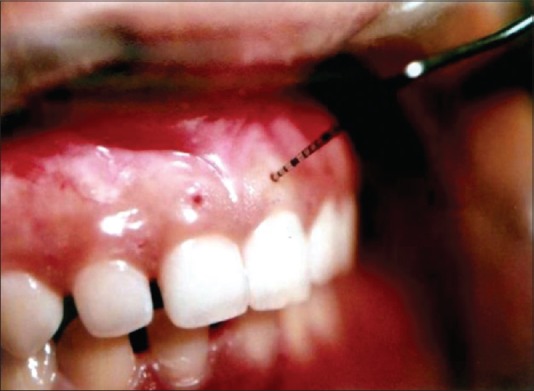
Transgingivial probing using calibrated probe[4]
No patients received periodontal dressings or antibiotics during the study. Patients were prescribed analgesics for postsurgery discomfort and a 0.12% chlorhexidine digluconate oral rinse for plaque suppression. Patients returned for flap suture removal at 1 week postsurgery and weekly for the next 4 weeks for professional plaque control. Patients were then placed on a monthly recall schedule for supportive periodontal treatment over the duration of the study.
The postsurgical measurements were done by the same investigator who was blinded for the 2 groups. Change in GMP was determined by comparing postsurgical measurements (FRP-FGM2) to presurgical measurement (FRP-FGM1) at each site.
Statistical analysis
This was done using mean ± standard deviation and paired samples t-test.
RESULTS
A total 482 sites (Mi-B 72; MB 58; DB 62) in ≤1 mm (n = 15) and 606 sites (Mi-B 96; MB 54; DB 52) in > 1 mm (n = 12) GT groups were included from 27 subjects. The results of the study are expressed in Table 1, Graphs 1–6 and Figures 3a–f and 4a–f.
Table 1.
The GMP (FRP-GMP), gingival thickness and CEJ (FRP-CEJ) at different sites in ≤1 mm and >1 mm gingival thickness groups at baseline (mm mean±SD)
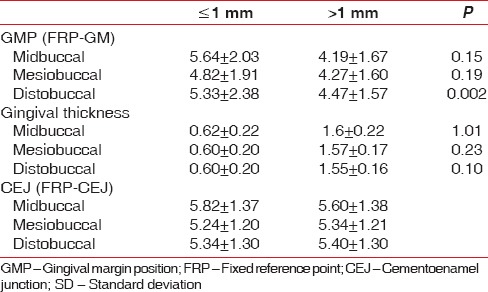
Graph 1.
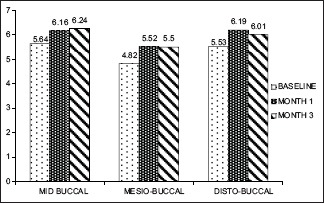
Gingival thickness ≤1 mm (baseline, 1 month, 3 month)
Graph 6.
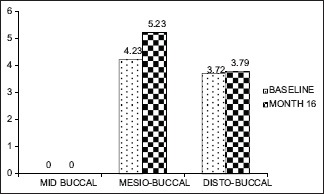
Gingival thickness >1 mm (baseline, 16 month)
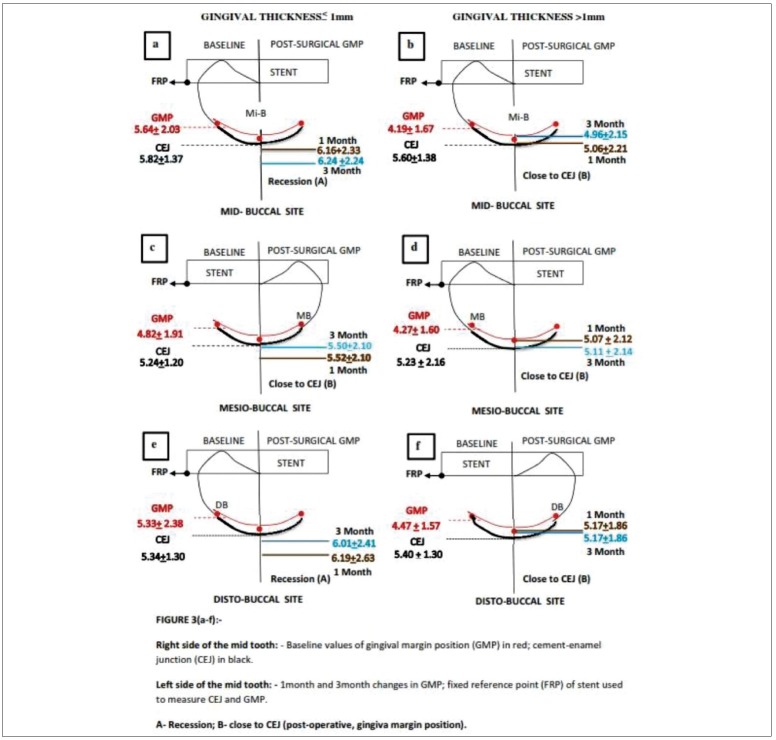
Figure 3.
(a-f) Diagrammatic representation of gingival margin position in gingival thickness ≤1 mm or >1 mm (1 month and 3 month)
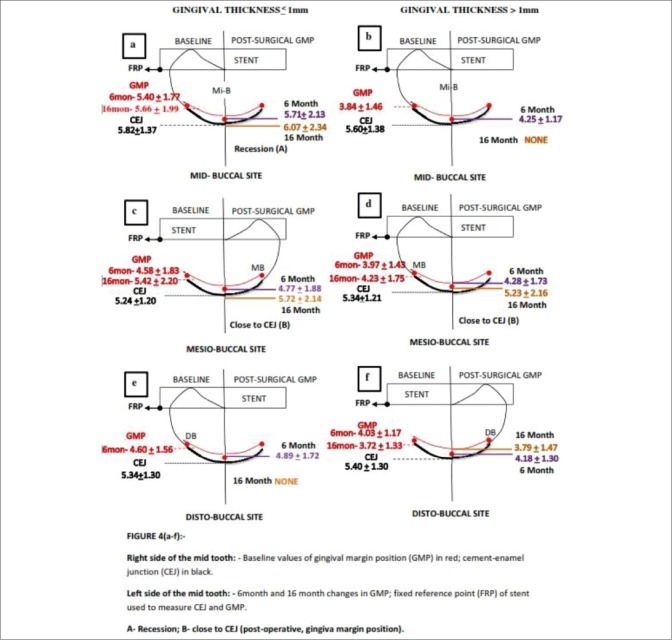
Figure 4.
(a-f) Diagrammatic representation of gingival margin position in gingival thickness ≤1 mm or >1 mm (6 month and 16 month)
Graph 4.
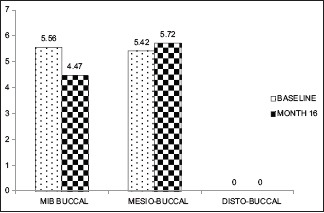
Gingival thickness ≤1 mm (baseline, 16 month)
Graph 5.
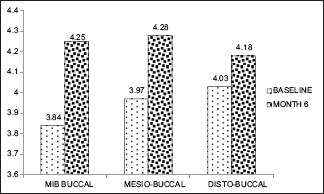
Gingival thickness >1 mm (baseline, 6 month)
There was no significant difference between group 1 and 2 for probing pocket depth; however, the GMP was significantly apical in group 1 (<1 mm) as compared to group 2 (>1 mm).
In GT of ≤1 mm group, at all 3 sites such as Mi-B, MB, and DB, there was a significant apical shift of GM from baseline to 1 month (0.000) and 3 months (0.045, 0.000, and 0.000, respectively) [Graph 1].
In GT of >1 mm group, there was a significant apical shift of gingival margin at all the 3 sites (Mi-B, MB, and DB) from baseline to 1 month (0.000) and 3 months (0.001, 0.000, and 0.000, respectively) [Graph 2].
Graph 2.
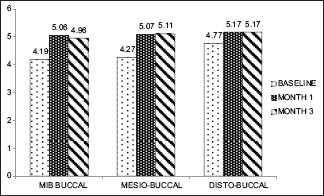
Gingival thickness >1 mm (baseline, 1 month, 3 month)
However, at baseline to 6 months and 16 months, there was no significant apical shift in MB (0.84, 0.019) and DB sites (0.360, 0.729) in both the groups [Graphs 3–6].
Graph 3.
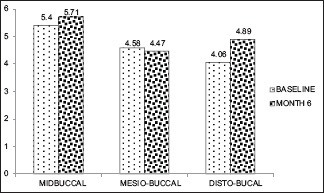
Gingival thickness ≤1 mm (baseline, 6 month)
The correlation of static CEJ (FRP-CEJ measurement) distance and GMP (FRP-gingival margin measurement) from baseline to 1 and 3 months [Figure 3a–f]; baseline to 6 and 16 months [Figure 4a–f] are depicted. The static FRP-CEJ distance was used as a reference measurement to compare the pre- to postsurgical FRP-GM position to designate whether there was an apical shift with CEJ exposure causing gingival recession (a) or only apical shift without causing gingival recession (b) in both the GT ≤1 mm and >1 mm groups. During 6 and 16 months postsurgery, there was a reduction in the number of sites due to patient dropout. The baseline GMP data differed from those at early postsurgical period, i.e. the baseline data was calculated to those sites which continued up to 6 and 16 months.
In GT of ≤1 mm group, the statistically significant apical shift of GMP led to gingival recession at all sites throughout the study sites in the early postsurgical period of 1 and 3 months [Figure 3a–f]. During 6 and 16 months, the apical shift of GMP coincided with the CEJ at Mi-B site (6 months), MB site (16 months). The gingival recession was obvious at Mi-B sites (16 months) [Figure 4a–f].
In the GT of >1 mm, the statistically significant apical shift of GMP did not cause gingival recession at any sites throughout postsurgical (1, 3, 6, and 16 months) period [Figures 3a–f and 4a–f].
DISCUSSION
During various phases of postsurgical healing, the predictable outcome is gingival recession. The various factors influencing the GMP are GT, tooth position, shallow vestibule, and adequacy of keratinized gingiva and frenal attachments. The apical shift in GMP does not always expose CEJ leading to gingival recession. The apical shift in GMP with and without CEJ exposure depends on GT that requires being measured pre- and post-operatively. Most of the periodontal literature suggests that gingival recession (apical shift of GMP on the root) is caused by the inadequate width of attached gingiva, and rarely, the GT is discussed as a causative factor for gingival recession/apical shift of GMP. In the present study, the dynamics of GMP has been measured from FRP during early (1 and 3 months) and later (6 and 16 months) postsurgical period and compared with the baseline GMP levels in of ≤1 mm and >1 mm GT groups.
In the current study, the periodontal flaps were not displaced after the open flap debridement. The GMP demonstrated apical shifts postsurgically. The extent of the apical shift was either gingival recession exposing CEJ or it was close to the CEJ. The interpretation of this study results are depicted in Figures 1 and 2. In the GT of ≤1 mm group, throughout the study period (0–16 months), the Mi-B sites demonstrated a significant apical shift, which caused gingival recession. At MB and DB sites, the apical shift resulted in gingival recession during 1 and 3 months postsurgically. However, the reduced number of sites in the 6 and 16 months period demonstrated apical shift either coinciding with CEJ or causing gingival recession. In the GT of more than 1 mm group, the postsurgical apical shift did not result in gingival recession throughout the study period.
The results of this study are not directly comparable to few of the studies in the literature due to variation in the methods and use of regenerative materials for the treatment of grade II furcation with GTR,[5,6] long-term assessment of combined osseous graft, and GTR in periodontal osseous defects,[5,7,8] The mean increase in recession for patients in the thin (≤1 mm) group was 2 times that was recorded in other studies in which flap thickness was not assessed,[6] there are several possible explanations for this observation.[1] In a study of dogs, it was reported that circulatory embarrassment of mucogingival flaps was greater after partial thickness compared to full thickness dissection. While all the flaps in the present study were full thickness mucoperiosteal elevations, it is reasonable to consider that thin flaps are at greater risk than thick (>1 mm) flaps for ischemia and necrosis due to their relatively thinner connective tissue component.
Pressure of the flap against the membrane or tension in the flap as a result of attempting to completely cover the membrane may also compromise blood supply to the flap margin,[1] flaps under tension become ischemic and subject to necrosis.[9] The blood supply in thin flaps is more likely to be embarrassed by tension than in thicker flaps of equal mobility.[1] In addition to the effects of routine operative flap manipulation, flap margins can be inadvertently thinned during sulcular incisions, and increase risk of postsurgery recession. This effect might be magnified in thinner, more delicate tissues.[1] If recession occurs, flaps with thin connective tissue are at greater risk for inflammation-induced postsurgery recession than thick flaps.[1,10]
In case of GTR, the revascularization of any flap may be further compromised by blockage of the potential blood supply from the periodontal ligament and bone defect to the flap connective tissue by polytetrafluoroethylene (ePTFE) membrane. The placement of a mucoperiosteal flap over an avascular membrane is not unlike placement of a soft tissue graft over an avascular root surface. The failure of thin (≤1 mm) free gingival autografts to successfully cover wide areas of gingival recession was overcome by preparation of an adequately large recipient bed and a connective tissue graft of thicker dimension (1.5–2 mm). After dissection or elevation, thicker connective tissue grafts or flaps may have more intact capillary system than thinner preparations.[11] The thicker the connective tissue, the better the potential circulatory pool and the greater chance for flap survival. The thickness of soft tissue overlying an ePTFE membrane should be maximized.[1]
These findings may suggest a tendency of the periodontium to reform a new “physiological” supracrestal gingival unit. The regrowth of the soft tissue from the level where the osseous crest was defined at surgery had already begun 1 month after surgery, when the gingival margin reached about 60% of its final coronal position at interproximal sites and about 40% at buccal/lingual sites.[1] Few studies on surgical crown lengthening in the current literature report results on the location of the gingival margin after treatment in relation to the level of the alveolar osseous crest defined during surgery.[12,13] The factors influencing the amount of coronal displacement of the marginal periodontal tissue seemed to be related to the different GT since patients with thick tissue demonstrated significantly more coronal soft tissue regrowth than patients with thin gingival tissue and to the natural biological differences in inter-individual patterns of the healing response.
CONCLUSION
Considering the behavior of the gingival margin observed in the present study following surgery, it may be suggested that:
When surgical therapy is performed to gain access for proper restorative measures to deep subgingivally located carious lesions, endodontic perforations, crown-root fractures, or preexisting margins of failing restorations, an early (during healing) definition of the previously inaccessible margins is recommended. Through current study results, the GMP is not stabilized till 1 and 3 months postsurgically in GT of ≤1 mm as compared to >1 mm. the restorative esthetic procedures have to consider the dynamics of GMP prior to finalizing the time of permanent restoration
When it is esthetically important, visible areas of the prosthetic reconstruction margins are planned to be positioned in an intrasulcular location, a close monitoring of the different degree of tissue regrowth, which occurs during healing among patients should be recommended to determine the achieved gingival margin stability and, therefore, to assess the ideal time for the definitive restorative procedures.
Measurement of gingival dimension, i.e. GT and width is clinically meaningful for both academician and periodontist. Those academicians or clinicians who record GT measurement regularly could understand the outcome measures meaningfully.
It should be mandatory to record GT for all periodontal surgical procedures as the common outcome such as recession depends on the GT. Along with recording of color, consistency, texture, and position of gingiva.
GT measurement is a useful and simple tool for disease and treatment outcome measurement. Most importantly, the need of the hour is to add GT measurement in the standard textbooks of periodontics.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
Acknowledgement
We acknowledge Dr. Priyanka Shivanand for her contributions toward the diagrammatic representations of the study.
REFERENCES
- 1.Anderegg CR, Metzler DG, Nicoll BK. Gingiva thickness in guided tissue regeneration and associated recession at facial furcation defects. J Periodontol. 1995;66:397–402. doi: 10.1902/jop.1995.66.5.397. [DOI] [PubMed] [Google Scholar]
- 2.Lindhe J, Nyman S. Alterations of the position of the marginal soft tissue following periodontal surgery. J Clin Periodontol. 1980;7:525–30. doi: 10.1111/j.1600-051x.1980.tb02159.x. [DOI] [PubMed] [Google Scholar]
- 3.Lindhe J, Socransky SS, Nyman S, Westfelt E. Dimensional alteration of the periodontal tissues following therapy. Int J Periodontics Restorative Dent. 1987;7:9–21. [PubMed] [Google Scholar]
- 4.Vandana KL, Savitha B. Thickness of gingiva in association with age, gender and dental arch location. J Clin Periodontol. 2005;32:828–30. doi: 10.1111/j.1600-051X.2005.00757.x. [DOI] [PubMed] [Google Scholar]
- 5.Metzler DG, Seamons BC, Mellonig JT, Gher ME, Gray JL. Clinical evaluation of guided tissue regeneration in the treatment of maxillary class II molar furcation invasions. J Periodontol. 1991;62:353–60. doi: 10.1902/jop.1991.62.6.353. [DOI] [PubMed] [Google Scholar]
- 6.Anderegg CR, Martin SJ, Gray JL, Mellonig JT, Gher ME. Clinical evaluation of the use of decalcified freeze-dried bone allograft with guided tissue regeneration in the treatment of molar furcation invasions. J Periodontol. 1991;62:264–8. doi: 10.1902/jop.1991.62.4.264. [DOI] [PubMed] [Google Scholar]
- 7.McClain PK, Schallhorn RG. Long-term assessment of combined osseous composite grafting, root conditioning, and guided tissue regeneration. Int J Periodontics Restorative Dent. 1993;13:9–27. [PubMed] [Google Scholar]
- 8.Guillemin MR, Mellonig JT, Brunsvold MA. Healing in periodontal defects treated by decalcified freeze-dried bone allografts in combination with ePTFE membranes (I). Clinical and scanning electron microscope analysis. J Clin Periodontol. 1993;20:528–36. doi: 10.1111/j.1600-051x.1993.tb00402.x. [DOI] [PubMed] [Google Scholar]
- 9.Mörmann W, Ciancio SG. Blood supply of human gingiva following periodontal surgery. A fluorescein angiographic study. J Periodontol. 1977;48:681–92. doi: 10.1902/jop.1977.48.11.681. [DOI] [PubMed] [Google Scholar]
- 10.Novaes AB, Ruben MP, Kon S, Goldman HM, Novaes AB., Jr The development of the periodontal cleft. A clinical and histopathologic study. J Periodontol. 1975;46:701–9. doi: 10.1902/jop.1975.46.12.701. [DOI] [PubMed] [Google Scholar]
- 11.Miller PD., Jr Root coverage with the free gingival graft. Factors associated with incomplete coverage. J Periodontol. 1987;58:674–81. doi: 10.1902/jop.1987.58.10.674. [DOI] [PubMed] [Google Scholar]
- 12.van der Velden U. Regeneration of the interdental soft tissues following denudation procedures. J Clin Periodontol. 1982;9:455–9. doi: 10.1111/j.1600-051x.1982.tb02106.x. [DOI] [PubMed] [Google Scholar]
- 13.Brägger U, Lauchenauer D, Lang NP. Surgical lengthening of the clinical crown. J Clin Periodontol. 1992;19:58–63. doi: 10.1111/j.1600-051x.1992.tb01150.x. [DOI] [PubMed] [Google Scholar]