Abstract
Background and Objective:
Chronic low-level bacteremia and a systemic inflammatory response have been suggested as a pathogenetic link between periodontal disease and atherosclerosis. The objective of this study is to evaluate the effect of nonsurgical periodontal therapy on various hematological parameters in patients with periodontitis.
Materials and Methods:
A total of 30 periodontitis patients were selected for the study. Clinical parameters such as plaque index, gingival index, and probing pocket depth were assessed. For each patient, venous blood sample were collected, and the estimation of total leukocyte count (TLC), differential leukocyte count, platelet count, and erythrocyte sedimentation rate (ESR) was carried out. All the clinical and hematological parameters were measured at baseline, 1 week and 2 weeks after nonsurgical periodontal therapy.
Results:
The study results showed that there was a statistically significant decrease in TLC, ESR, and platelet count at 1 week and 2 weeks following nonsurgical periodontal therapy.
Conclusion:
In this study, it has been concluded that there is a decrease in the hematological parameters after nonsurgical periodontal therapy, which may also reduce the risk of atherosclerosis formation in the blood vessel and possibly prevent cardiovascular diseases.
Keywords: Atherosclerosis, nonsurgical periodontal therapy, periodontitis, platelet count, total leukocytes count
INTRODUCTION
Cardiovascular diseases (CVDs) comprise a variety of heart and vascular conditions including ischemia, atherosclerosis, peripheral artery disease, infective endocarditis, and acute myocardial infarction.[1] Inflammation is recognized as playing a key role in the pathogenesis of atherosclerosis. Inflammatory cells and cytokines are important not only in the initiation of plaque formation in the blood vessel wall but also in the maintenance and rupture of the plaque and subsequent thrombotic complications.[2,3] Periodontitis is a chronic inflammatory disease characterized by the destruction of alveolar bone, and the loss of the connective tissue supporting the teeth.[4] Triggers of inflammation include smoking, diabetes mellitus, and obesity.[3] These factors (potential confounders) have been related to both periodontal disease and CVD in several studies.[5] Changes in cellular and molecular components of peripheral blood in periodontitis may explain at least in part, the epidemiological observations that periodontitis is associated with CVDs, thromboembolic events, and atherosclerosis.[6]
Traditionally, the total number of white blood cells (leukocytes) and erythrocytes sedimentation rates in peripheral blood has been used as a diagnostic measure to investigate whether a given individual suffers from an infection or inflammatory disease.[6] Leukocytes are an integral part of the innate immune system, these cells are recruited at higher levels during episodes of bacteremia in periodontitis or leakage into the systemic circulation.[6] It has been suggested that the higher numbers of leukocytes increase the blood rheology, make the blood more viscous, and also cells may adhere to the endothelial lining of the blood vessels causing decrease the blood flow. Reduced blood flow could play a role in relation to CVDs, especially in narrow or partly blocked arteries due to atherosclerotic plaque formation.[7,8,9]
Periodontitis has also been shown to be associated with an increase in plasma fibrinogen and an increase in platelet activation, which might contribute to a procoagulant state and thus an increased risk for atherosclerosis and CVD.[10]
The aim of the present study was to investigate the effect of nonsurgical periodontal therapy on total leukocyte count (TLC), differential leukocyte count (neutrophils, lymphocytes, eosinophils, basophils, and monocytes), erythrocyte sedimentation rate (ESR), and total platelet count in patients with periodontitis.
MATERIALS AND METHODS
A total of 30 patients with periodontitis were selected from the Department of Periodontology, Rungta College of Dental Sciences and Research, Bhilai, Chhattisgarh. Systemically, healthy patients within the age group of 20–55 years of both the genders having probing pocket depth (PPD) of ≥5 mm at four sites per tooth in different quadrants of the mouth along with radiographic evidence of bone loss were included in the study.
Patients suffering from any systemic diseases, undergoing any periodontal therapy, taking antibiotic, or having taken antibiotics in the past 3 months, smokers, pregnant women, and lactating mothers were excluded from the study.
The study was approved by the Institutional Ethical Committee. Written consent was obtained from all the patients who were recruited for the study.
Clinical measurements
For all the patients, the periodontal status was recorded using the following clinical parameters: Plaque index (PI), gingival index (GI), and PPD were measured using University of North Carolina-15 probe.
Blood sampling and treatment
Under aseptic condition, peripheral venous blood was drawn from antecubital fossa as per the WHO guidelines[11] in the morning hours, and the samples were then immediately transported to the laboratory for analysis of all the hematological parameters.
All the clinical and hematological parameters were assessed preoperatively at baseline and at 1 week and 2 weeks after nonsurgical periodontal therapy.
For all the patients, complete supragingival scaling was performed using the ultrasonic scalers, and subgingival scaling and root planing were performed using Gracey curettes in two visits within 24 h. All the patients were given proper oral hygiene instructions along with 0.2% of chlorhexidine digluconate mouth wash twice a day for 10 days as an adjunctive oral hygiene measures.
Statistical analysis
The collected data were subjected to statistical analysis through SPSS Software (SPSS v.16.0 IBM, Armonk, NY). ANOVA test was the statistical method applied. The results were represented in text and tables.
RESULTS
Table 1 shows statistically significant changes in all the clinical parameters from baseline to 1 week and 2 weeks postoperatively.
Table 1.
Clinical parameters at baseline, 1 and 2 weeks
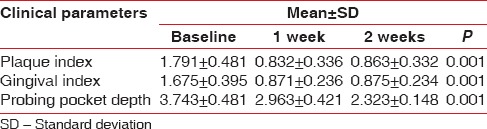
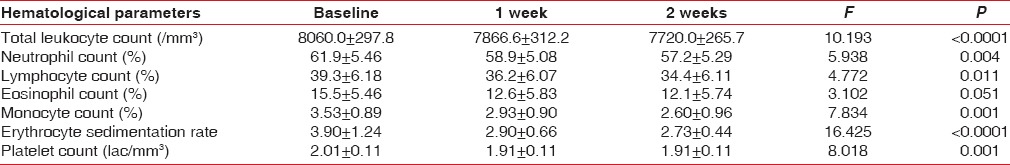
Table 2 shows that there was a statistically significant decrease in TLC following 1 week and 2 weeks after scaling and root planing (at baseline TLC was 7800/mm3, and at 1 weeks 7200/mm3, and at 2 weeks follow-up TLC was 6900/mm3, P < 0.0001). There was a statistically significant decrease in platelet count from 2.1 lac/mm3 preoperatively to 2.0 lac/mm3 at 1 week, and 1.9 lac/mm3 at 2 weeks postoperatively (P < 0.0001). The ESR value also showed a significant decrease after the nonsurgical periodontal therapy. There was no statistically significant difference in the differential leukocyte counts after nonsurgical periodontal therapy.
Table 2.
Hematological values at baseline, 1 and 2 weeks
DISCUSSION
This study shows the beneficial effects of periodontal treatment on possible risk factors for atherosclerotic events due to the improvement of hematological parameters in patients with chronic periodontitis.
Various research studies suggested that periodontitis is associated with increased risk of systemic diseases such as cardiovascular, cerebrovascular diseases, low birth weight of infants, and preterm births. These associations indicate that periodontitis has systemic inflammation thereby increasing the risk of systemic diseases.[12]
The reinstitution of oral hygiene techniques led to the reduction of gingival inflammation within approximately 1 week of plaque removal.[13] In our study, statistically highly significant change was observed in both PI and GI as well as PPD from baseline to 2 weeks. Hence, the 2-week time period may be a justifiable time frame for achieving a reduction in gingival inflammation and thereby reducing systemic inflammation (reduction in TLC and platelet counts). The PPD was significantly reduced in the study groups after periodontal treatment suggesting that periodontal therapy is critical in the context of the design and implementation of a definitive trial.
Leukocytes are the major component of blood cells of the phagocytosis and first cells of the host defense mechanism against infective agents.[14] Periodontitis is a bacterial infection, neutrophils are the initially predominant cells of host defense and have a significant role in inflammation and pathogenesis.[15] Many authors reported that there was a statistical significant decrease in TLC from baseline to 2 weeks following nonsurgical periodontal therapy.[16,17] These findings were similar to our study where TLC was reduced which was statistically significant.
In this study, a reduction in counts of individual WBCs, i.e., neutrophils, lymphocytes, eosinophils, and monocytes, were also observed, but this decrease was statistically nonsignificant. No difference was found with respect to basophil count in this study. These findings were similar to the Banthia et al. study.[18]
In our study, there was a statistical decrease in ESR after nonsurgical periodontal therapy due to a decrease in chronic infection which is in accordance with the study by Hutter et al.[12] The determination of ESR is helpful in assessing the progress of patients treated for certain chronic inflammatory disorders.
Platelets have their main function in hemostasis, but they also play a role in inflammatory and immune processes.[19] Their number increases in chronic inflammation. Griesshammer et al.[20] in a study of 732 patients with elevated platelet counts (>500 × 103) reported that infection was the underlying cause of thrombocytosis in 21% of the subjects studied. Wakai et al.[21] have also reported increased platelet counts in patients with periodontitis.
Thaulow et al.[22] assessed the possible association between blood platelet count and the coronary heart diseases over 150 patients where he found that platelet counts were positively related to the risk of cardiovascular death. Hence, an increase in platelets might be another underlying mechanism for the possible link between periodontal inflammation and CVD.
In our study, a statistically significant decrease in platelet counts after nonsurgical periodontal therapy has been reported. Similar results were reported by Christan et al.[16] who showed a decrease in platelet counts after periodontal therapy. Taylor et al.[17] also reported a statistically significant decrease in platelet count after full-mouth tooth extraction.
CONCLUSION
The positive effect of nonsurgical periodontal therapy in reducing leukocyte count and platelet count could be helpful in preventing CVDs. Periodontal microorganisms and their products invade the tissues to enter the blood stream and attaches to the vascular endothelial cells and may help in the formation of atherosclerotic lesions. Nonsurgical periodontal therapy aims to reduce the number of periodontal pathogens thereby reduces the inflammation and which may indirectly decreases the risk of CVDs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
REFERENCES
- 1.Persson GR, Persson RE. Cardiovascular disease and periodontitis: An update on the associations and risk. J Clin Periodontol. 2008;35(8 Suppl):362–79. doi: 10.1111/j.1600-051X.2008.01281.x. [DOI] [PubMed] [Google Scholar]
- 2.Fuster V, Moreno PR, Fayad ZA, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque: Part I: Evolving concepts. J Am Coll Cardiol. 2005;46:937–54. doi: 10.1016/j.jacc.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 3.Tiong AY, Brieger D. Inflammation and coronary artery disease. Am Heart J. 2005;150:11–8. doi: 10.1016/j.ahj.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 4.Alman AC, Johnson LR, Calverley DC, Grunwald GK, Lezotte DC, Harwood JE, et al. Loss of alveolar bone due to periodontal disease exhibits a threshold on the association with coronary heart disease. J Periodontol. 2011;82:1304–13. doi: 10.1902/jop.2011.100647. [DOI] [PubMed] [Google Scholar]
- 5.Joshipura K, Zevallos JC, Ritchie CS. Strength of evidence relating periodontal disease and atherosclerotic disease. Compendium. 2009;30:430–9. [PMC free article] [PubMed] [Google Scholar]
- 6.Loos BG. Systemic markers of inflammation in periodontitis. J Periodontol. 2005;76(11 Suppl):2106–15. doi: 10.1902/jop.2005.76.11-S.2106. [DOI] [PubMed] [Google Scholar]
- 7.Kannel WB, Anderson K, Wilson PW. White blood cell count and cardiovascular disease. Insights from the Framingham Study. JAMA. 1992;267:1253–6. [PubMed] [Google Scholar]
- 8.Phillips AN, Neaton JD, Cook DG, Grimm RH, Shaper AG. Leukocyte count and risk of major coronary heart disease events. Am J Epidemiol. 1992;136:59–70. doi: 10.1093/oxfordjournals.aje.a116421. [DOI] [PubMed] [Google Scholar]
- 9.Sweetnam PM, Thomas HF, Yarnell JW, Baker IA, Elwood PC. Total and differential leukocyte counts as predictors of ischemic heart disease: The Caerphilly and Speedwell studies. Am J Epidemiol. 1997;145:416–21. doi: 10.1093/oxfordjournals.aje.a009123. [DOI] [PubMed] [Google Scholar]
- 10.Al-Rasheed A. Elevation of white blood cells and platelet counts in patients having chronic periodontitis. Saudi Dent J. 2012;24:17–21. doi: 10.1016/j.sdentj.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhingra N, Diepart M, Dziekan G, Khamassi S, Otaiza F, Wilburn S, et al. WHO Guidelines on drawing Blood: Best Practices in Phlebotomy. Geneva, Switzerland: WHO Press; 2010. [Google Scholar]
- 12.Hutter JW, van der Velden U, Varoufaki A, Huffels RA, Hoek FJ, Loos BG. Lower numbers of erythrocytes and lower levels of hemoglobin in periodontitis patients compared to control subjects. J Clin Periodontol. 2001;28:930–6. doi: 10.1034/j.1600-051x.2001.028010930.x. [DOI] [PubMed] [Google Scholar]
- 13.Loe H, Theilade E, Jensen SB. Experimental gingivitis in man. J Periodontol. 1965;36:177–87. doi: 10.1902/jop.1965.36.3.177. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal S, Huang PJ, Piesco NP, Suyuki JB, Riccelli AE, Johns LP. Altered neutrophil function in localized juvenile periodontitis: Intrinsic or induced? J Periodontol. 1996;67:337–44. doi: 10.1902/jop.1996.67.3s.337. [DOI] [PubMed] [Google Scholar]
- 15.Miyasaki KT. The neutrophil: Mechanisms of controlling periodontal bacteria. J Periodontol. 1991;62:761–74. doi: 10.1902/jop.1991.62.12.761. [DOI] [PubMed] [Google Scholar]
- 16.Christan C, Dietrich T, Hägewald S, Kage A, Bernimoulin JP. White blood cell count in generalized aggressive periodontitis after non-surgical therapy. J Clin Periodontol. 2002;29:201–6. doi: 10.1034/j.1600-051x.2002.290303.x. [DOI] [PubMed] [Google Scholar]
- 17.Taylor BA, Tofler GH, Carey HM, Morel-Kopp MC, Philcox S, Carter TR, et al. Full-mouth tooth extraction lowers systemic inflammatory and thrombotic markers of cardiovascular risk. J Dent Res. 2006;85:74–8. doi: 10.1177/154405910608500113. [DOI] [PubMed] [Google Scholar]
- 18.Banthia R, Jain P, Banthia P, Belludi S, Parwani S, Jain A. Effect of phase I periodontal therapy on pro-coagulant state in chronic periodontitis patients – A clinical and haematological study. J Ir Dent Assoc. 2013;59:183–8. [PubMed] [Google Scholar]
- 19.Klinger MH, Jelkmann W. Role of blood platelets in infection and inflammation. J Interferon Cytokine Res. 2002;22:913–22. doi: 10.1089/10799900260286623. [DOI] [PubMed] [Google Scholar]
- 20.Griesshammer M, Bangerter M, Sauer T, Wennauer R, Bergmann L, Heimpel H. Aetiology and clinical significance of thrombocytosis: Analysis of 732 patients with an elevated platelet count. J Intern Med. 1999;245:295–300. doi: 10.1046/j.1365-2796.1999.00452.x. [DOI] [PubMed] [Google Scholar]
- 21.Wakai K, Kawamura T, Umemura O, Hara Y, Machida J, Anno T, et al. Associations of medical status and physical fitness with periodontal disease. J Clin Periodontol. 1999;26:664–72. doi: 10.1034/j.1600-051x.1999.261006.x. [DOI] [PubMed] [Google Scholar]
- 22.Thaulow E, Erikssen J, Sandvik L, Stormorken H, Cohn PF. Blood platelet count and function are related to total and cardiovascular death in apparently healthy men. Circulation. 1991;84:613–7. doi: 10.1161/01.cir.84.2.613. [DOI] [PubMed] [Google Scholar]


