Abstract
Purpose
Pigs expressing neither galactose-α1,3-galactose (Gal) nor N-glycolylneuraminic acid (NeuGc) take xenotransplantation one step closer to the clinic. Our aims were (i) to document the lack of NeuGc expression on corneas and aortas, and cultured endothelial cells (aortic [AECs]; corneal [CECs]) of GTKO/NeuGcKO pigs, and (ii) to investigate whether the absence of NeuGc reduced human antibody binding to the tissues and cells.
Methods
Wild-type (WT), GTKO, and GTKO/NeuGcKO pig were used for the study. Human tissues and cultured cells were negative controls. Immunofluorescence staining was performed using anti-Gal and anti-NeuGc antibodies, and to determine human IgM and IgG binding to tissues. Flow cytometric analysis was used to determine Gal and NeuGc expression on cultured CECs and AECs and to measure human IgM/IgG binding to these cells.
Results
Both Gal and NeuGc were detected on WT pig corneas and aortas. Although GTKO pigs expressed NeuGc, neither human nor GTKO/NeuGcKO pigs expressed Gal or NeuGc. Human IgM/IgG binding to corneas and aortas from GTKO and GTKO/NeuGcKO pigs was reduced compared to binding to WT pigs. Human antibody binding to GTKO/NeuGcKO AECs was significantly less than to GTKO AECs, but there was no significant difference in binding between GTKO and GTKO/NeuGcKO CECs.
Conclusions
The absence of NeuGc on GTKO aortic tissue and AECs is associated with reduced human antibody binding, and possibly will provide better outcome in clinical xenotransplantation using vascularized organs. For clinical corneal xenotransplantation, the absence of NeuGc expression on GTKO/NeuGcKO pig corneas may not prove an advantage over GTKO corneas.
Keywords: Antibody, anti-pig, Cornea, porcine, Immune response, N-glycolylneuraminic acid, Pig, Xenotransplantation
INTRODUCTION
The production of α1,3-galactosyltransferase gene-knockout (GTKO) pigs in 2003 was a significant advance in the development of xenotransplantation1. In 2013, pigs that lacked two major carbohydrate xenoantigens, galactose-α1,3-galactose (Gal) and N-glycolylneuraminic acid (NeuGc), were introduced (GTKO/NeuGcKO pigs)2. The absence of expression of NeuGc (NeuGcKO pigs) further reduced the xenoantigenicity of pig peripheral blood mononuclear cells (PBMCs) when exposed to human serum, since a significant fraction of human anti-nonGal antibodies is known to be specific for carbohydrate structures with terminal NeuGc3. Most pig organs, except for neural tissue, express NeuGc4, and the extent of expression is similar to, or greater than, the Gal antigen5. Furthermore, almost all healthy humans develop anti-NeuGc antibody3,6, in part due to exposure to dietary NeuGc7. For the purposes of clinical xenotransplantation, the need to delete expression of NeuGc was first suggested by Bouhours et al in 19968.
In 2013, the first GTKO/NeuGcKO pigs were successfully produced by zinc-finger nuclease technology2. Binding of human serum IgM and IgG to GTKO/NeuGcKO pig PBMCs was significantly reduced when compared to binding to GTKO pig PBMCs2,9. However, there is no definitive report using other primary cultured cells so far. We have now investigated NeuGc expression on wild-type (WT) and GTKO pig corneas and aortas and have compared IgM and IgG antibody binding to these tissues.
The cornea is an unusual tissue in terms of its immunologic features, e.g., avascularity, weak expression of major histocompatibility complex antigens, and presence of immunomodulating molecules in the aqueous humor10. Despite these advantageous features, the antigenicity of the pig cornea remains a major barrier to successful xenotransplantation. Nevertheless, the immunologic characteristics of corneas may be different from those of other organs.
Both anti-Gal11 and anti-nonGal12 antibody production have been reported in the pig-to-monkey corneal transplantation model, especially when the graft is rejected. Human patients grafted with pig skin13 or ligaments14 develop high titers of anti-nonGal antibodies. The expression of the sialic acids, N-acetylneuraminic (NeuAc) acid and NeuGc, varies between different pig tissues and cells. These oligosaccharides can be present as glycoprotein or glycolipid5.
Previously, we reported the presence and distribution of Gal and NeuGc on WT and GTKO porcine corneas by immunofluorescence staining15. Gal is mainly expressed on the stromal keratocytes (and weakly on the stroma), with no expression on the corneal epithelium or endothelium in naïve status (i.e., when the cornea is not activated). However, WT pig corneal endothelial cells (CECs) develop Gal epitopes in certain situations (e.g., during in vitro culture16, or when exposed to inflammatory cytokines after xenotransplantation)17. In contrast to Gal, NeuGc is expressed on corneal epithelium and endothelium in addition to stromal keratocytes18. Since healthy CECs are essential to corneal transparency, and thus good vision, after corneal transplantation they represent the most important structures. Antibody binding to the CECs may result in significant injury.
Our aims in the present study were (i) to document the absence of Gal and NeuGc expression on the cornea as well as aorta, and on cultured cells, from GTKO/NeuGcKO pigs, (ii) to compare human IgM and IgG binding to corneas and aortic tissues from these pigs with binding to WT and GTKO pig and human corneas and aortic tissues, and (iii) to compare this binding to that to cultured cells (aortic endothelial cells [AECs]; corneal endothelial cells [CECs]) from WT, GTKO, and GTKO/NeuGcKO pigs and humans.
MATERIALS AND METHODS
Preparation of corneas
All procedures used in this study conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. In addition, all in vitro human study protocols were approved by the Research Ethics Committee at the University of Pittsburgh. The samples were obtained in accordance with the Declaration of Helsinki. Participants gave informed consent per the guidelines of the Institutional Review Board of the University of Pittsburgh (IRB0608179).
Eyes from 6 month-old WT (Large White) pigs (n=3) were obtained from a local slaughterhouse. Eyes from GTKO pigs (on a Large White background) were obtained from Revivicor (Blacksburg, VA; n=3) and from GTKO pigs (on a mixed background, NSRRC;0009) from the National Swine Resource and Research Center (NSRRC, Columbia, MO; n=2). All GTKO pigs were the result of natural breeding, with the original founder pigs derived from nuclear transfer/embryo transfer.
Eyes from GTKO/NeuGcKO pigs (by zinc finger nuclease technology on a Yorkshire/Chester White background) were provided by the Department of Surgery, Indiana University School of Medicine (Indianapolis, IN; n=2), and eyes from GTKO/NeuGcKO pigs (by nuclear transfer/embryo transfer on a Large White background) were provided by Revivicor (n=6).
Corneas from deceased humans (blood type O) that were not suitable for clinical transplantation were provided by the Pittsburgh Center for Organ Recovery and Education (CORE) with the approval of the University of Pittsburgh Committee for Oversight of Research Involving the Dead (CORID No.231), and in accordance with the guidelines of the Declaration of Helsinki for research involving the use of human tissues16.
Preparation of cultured corneal endothelial cells (CECs)
The eye globes were maintained in sterile wet gauze until the corneas were excised (with at least 1mm of surrounding sclera). From each pair of corneas, one was embedded in optimal cutting temperature compound (Tissue-Tek, Miles Laboratories, Naperville, IL), frozen, and sectioned for immunofluorescence staining. The other provided CECs. Pig and human CECs were isolated, cultured, and passaged as previously described19 and used after passage 2 to 4.
Preparation of aortas and cultured aortic endothelial cells (AECs)
Thoracic aortas from all of the above pigs were obtained, and pig (p) AECs were cultured and passaged as previously described20. All cells were cultured in collagen-I-coated 25- or 75-cm2 tissue culture flasks (BD Biosciences, San Jose, CA).
Immunofluorescence staining for Gal and NeuGc on corneas and aortas
Staining for expression of Gal and NeuGc was carried out as previously described15. Gal staining was with fluorescein isothiocyanate (FITC)-conjugated BSI-B4 lectiin (isolectin B4 from Bandeiraea simplicifolia; 10 mg/mL; Sigma–Aldrich, St. Louis, MO). NeuGc staining was with a chicken-derived anti-NeuGc immunohistochemistry kit (Sialix, Cambridge, MA), following the manufacturer's instructions. DAPI (4,6-diamidino-2-phenylindole, Invitrogen, Waltham, MA) stained nuclei in all cases.
Immunofluorescence staining for human IgM and IgG binding to corneas and aortas
IgM and IgG binding assays using human serum were carried out as previously described15. Heat-inactivated pooled serum from healthy human volunteers (n=5, including all ABO blood types) was diluted to 20% for IgM and to 5% for IgG binding. Corneal or aortic tissues were incubated with pooled human serum for 60min at room temperature. Phosphate buffered serum (PBS; Invitrogen) was used as a negative control. The slides were then washed with PBS and blocked with 10% goat serum for 30min at room temperature. FITC-conjugated goat-derived anti-human IgM (μ chain– specific) or IgG (γ chain–specific) polyclonal antibody (concentration 1:100; Invitrogen) was applied for 30min at room temperature for detection of IgM or IgG binding. DAPI was applied for nuclear staining and the slides were examined by fluorescence microscopy (Nikon, Tokyo, Japan).
Flow cytometric analysis for Gal and NeuGc expression on, and human IgM/IgG binding to, cultured CECs and AECs
Surface expression of Gal and NeuGc, and human IgM/IgG binding to CECs and AECs were detected by flow cytometry (BD LSR II; BD Biosciences), as previously described16. Serum samples from healthy human volunteers (n=7, including all ABO blood types) were pooled. CECs or AECs were diluted to 105 cells per tube in FACS buffer (PBS containing 1% BSA and 0.1% NaN3). The antibodies used for immunofluorescence staining were also used for the detection of antibody binding.
Statistical methods
The statistical significance of differences was determined by Student's t or nonparametric tests, as appropriate, using GraphPad Prism version 4 (GraphPad Software, San Diego, CA). Values are presented with mean value. Differences were considered to be significant at p<0.05.
RESULTS
Expression of Gal and NeuGc by immunofluorescence or flow cytometry
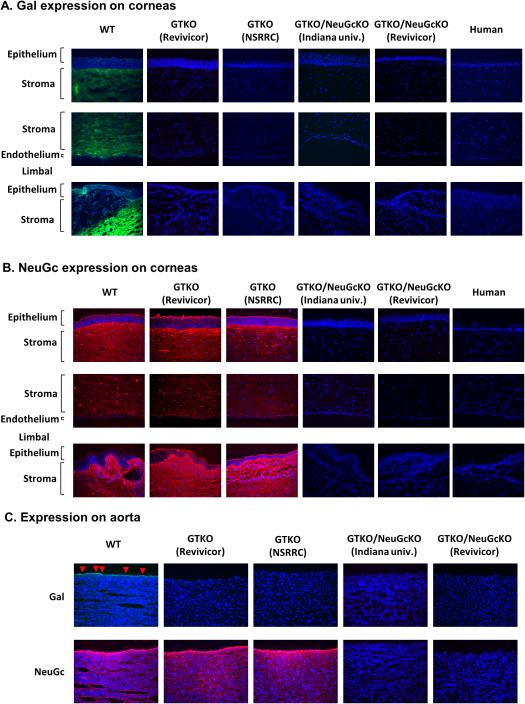
The tissue structure and cell morphology of corneas from genetically-engineered pigs, including GTKO/NeuGcKO pigs, were not different from those of WT pigs20 (data not shown). WT pig corneas and aortas expressed Gal (Fig. 1A,C) and NeuGc (Fig.1B,C) as did CECs (Fig. 2A,B). GTKO pig corneas and aortas (from pigs of two different genetic backgrounds) were negative for Gal expression, but positive for NeuGc (Fig. 1A,B,C), as were GTKO CECs (Fig. 2A,B) and AECs (Fig. 2C,D).
Figure 1.
(A) Gal (green) expression on corneas by immunofluorescence WT pig corneas expressed Gal (largely on anterior stroma), whereas GTKO, GTKO/NeuGcKO, and human corneas were negative for Gal expression.
(B) NeuGc (red) expression on corneas by immunofluorescence Corneas from WT pigs and GTKO pigs of two different genetic backgrounds expressed NeuGc on the epithelial, stromal, and endothelial cells (and stroma), but NeuGc was not detectable on either GTKO/NeuGcKO pig or human corneas.
(C) Expression of Gal and NeuGc on aortas Aortas from WT pigs expressed Gal on the endothelial cells (red arrows) and NeuGc. GTKO pigs of two different genetic backgrounds expressed NeuGc, but not Gal. Neither Gal nor NeuGc was detectable on GTKO/NeuGcKO pig aortas from two different genetic backgrounds. Figures are representative of at least 3 different experiments. (Magnification 200x; nuclei – blue; Gal – green; NeuGc – red).
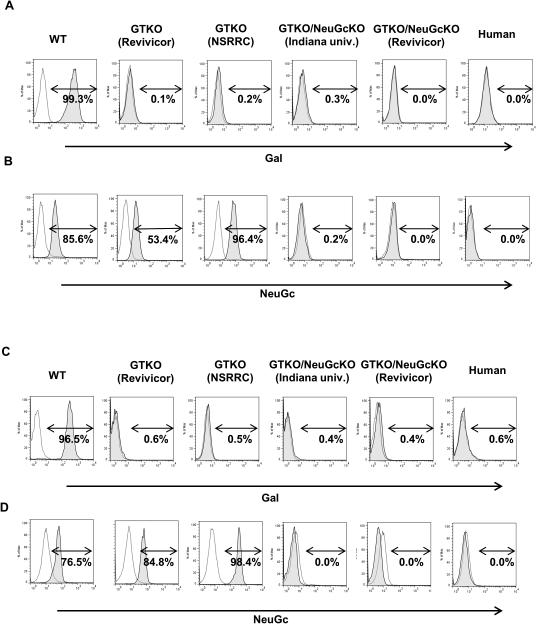
Figure 2. Gal and NeuGc expression on CECs (A, B) and AECs (C, D) by flow cytometry.
(A, C) WT pCECs and pAECs expressed Gal whereas CECs and AECs from GTKO, GTKO/NeuGcKO, and human corneas and aortas did not. (B, D) WT and GTKO pCECs and pAECs expressed NeuGc to varying extents. NeuGc was not expressed on either GTKO/NeuGcKO pig or human CECs and AECs. Figures are representative of experiments with WT cells (n=3), Revivicor GTKO cells (n=3), NSRRC GTKO cells (n=2), Indiana university GTKO/NeuGcKO cells (n=2), Revivicor GTKO/NeuGcKO cells (CECs; n=6, AECs; n=2 respectively), and human cells (n=2). rMFI= relative mean fluorescence intensity
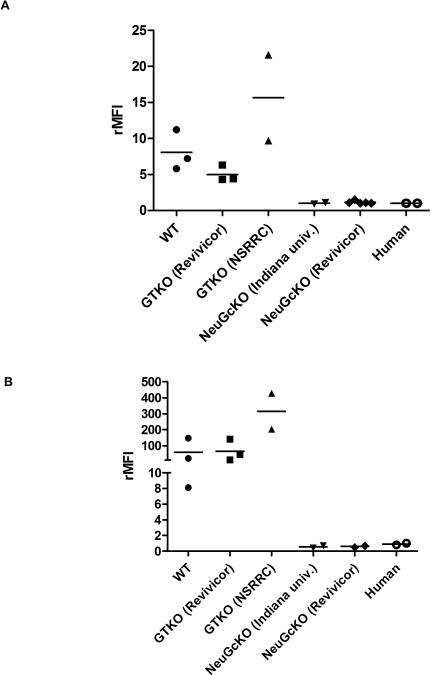
By flow cytometry, the expression level of NeuGc on CECs from GTKO pigs from the NSRRC was higher than on CECs from GTKO pigs from Revivicor and WT corneas (the rMFI of two NSRRC pigs was 9.7 and 21.6, respectively, and of three Revivicor pigs, 4.3, 6.3 and 4.4, respectively [Fig. 3A]), though statistical analysis could not be carried out due to the small number of corneas tested. Similar to CECs, the expression level of NeuGc on AECs from the NSRRC was higher than on AECs of GTKO pigs from Revivicor and WT aortas (Fig. 3B). GTKO/NeuGcKO pig corneas, aortas (Fig. 1), cultured pCECs (Fig. 2A,B) and pAECs (from both sources, Fig. 2C,D) did not express either Gal or NeuGc, as was the case for human corneas and CECs (Fig. 1,2).
Figure 3. Levels of NeuGc expression on CECs (A) and AECs (B) by flow cytometry.
(A, B) The level of NeuGc expression of pCECs and pAECs from different sources differed. Expression of NeuGc on CECs of NSRRC GTKO pigs appeared to be higher than on CECs of Revivicor GTKO pigs. Statistical analysis was not possible due to the small number of samples tested. Figures are from different samples from WT pigs (n=3), Revivicor GTKO pigs (n=3), NSRRC GTKO pigs (n=2), Indiana university GTKO/NeuGcKO pigs (n=2), Revivicor GTKO/NeuGcKO pigs (CECs; n=6, AECs; n=2 respectively), and humans (n=2). rMFI= relative mean fluorescence intensity
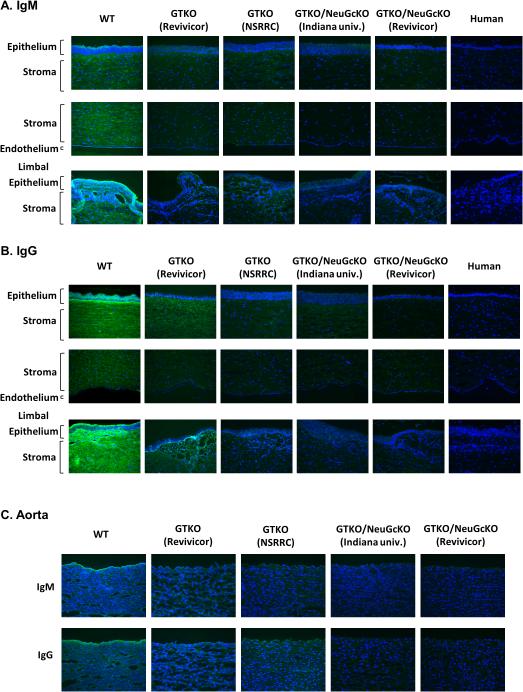
Human IgM and IgG antibody binding to corneas and aortas by immunofluorescence
Compared to binding to WT pig corneas, human IgM and IgG binding to GTKO pig corneas was decreased (Fig. 4A,B). Fluorescence intensity appeared even less to GTKO/NeuGcKO pig corneas, particularly to the endothelium, but there was still some binding when compared to human corneas (Fig. 4A,B). Human IgM and IgG bound primarily to endothelium of pig aortic tissue (Fig. 4C), and appeared to be related to expression of NeuGc epitopes. Compared to binding to WT pig aortas, human IgM and IgG binding to GTKO pig aortas was greatly decreased, especially to endothelium, and it was further decreased to GTKO/NeuGcKO pig aortas (Fig. 4C).
Figure 4. Human antibody binding to pig and human corneas by immunofluorescence.
(A) Human IgM binding to corneas after co-culture with 20% pooled human serum. Compared to binding to WT corneas, human IgM binding to GTKO corneas was decreased and further decreased to GTKO/NeuGcKO corneas. However, there was still some binding, especially in the limbal area. There was minimal IgM binding to a human cornea.
(B) Human IgG binding to corneas after co-culture with 5% pooled human serum. Compared to binding to WT corneas, human IgG binding to GTKO corneas was decreased and further decreased to GTKO/NeuGcKO corneas. There was minimal IgG binding to a human cornea.
(C) Human IgM and IgG binding to aortas after co-culture with pooled human serum (20% for IgM and 5% for IgG respectively)
Compared to binding to WT aortas, human IgM/IgG binding to GTKO aorta was decreased, and further decreased to GTKO/NeuGcKO aortas, particularly to the aortic endothelium.
Figures are representative of at least 3 different experiments. (Magnification 200x; nuclei – blue; IgM - green, IgG – green)
Human IgM and IgG antibody binding to CECs and AECs by flow cytometry
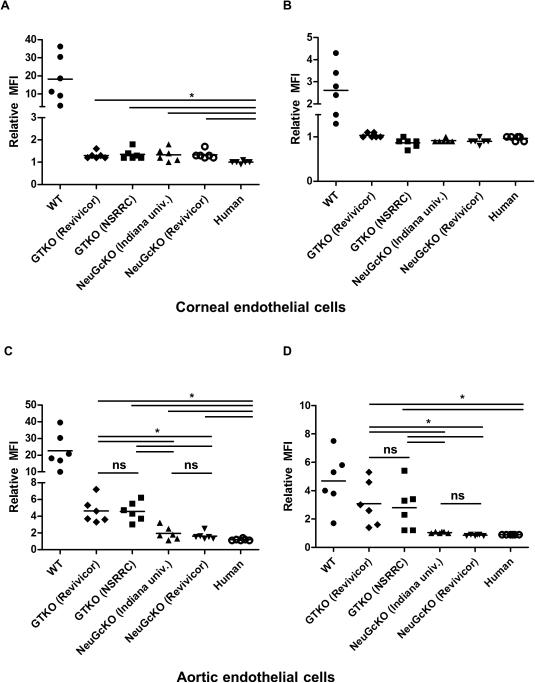
Binding of human IgM and IgG to GTKO pCECs was greatly reduced compared to that to WT pCECs (Fig. 5A,B). There was no obvious difference in human IgM/IgG binding to the CECs from GTKO pigs (from 2 different genetic backgrounds) and GTKO/NeuGcKO pigs (from 2 different genetic backgrounds). In contrast to pCECs, there was significant further reduction of human IgM/IgG binding to GTKO/NeuGcKO pAECs in comparison to GTKO pAECs (Fig. 5C,D). There was no significant difference in binding to pAECs from the two different types of GTKO pig or GTKO/NeuGcKO pig.
Figure 5. Human IgM and IgG antibody binding to CECs (A and B) and AECs (C and D) by flow cytometry using different individual human sera.
(A) Human IgM binding to WT pCECs was variable, but binding to GTKO and GTKO/NeuGcKO pig CECs and to human CECs was significantly decreased (n=6, *p<0.05). Binding of human IgM antibody to human CECs was significantly lower than to all other pCECs (*p<0.05). There was no significant difference in binding to GTKO and GTKO/NeuGcKO pCECs.
(B) Human IgG binding to WT CECs was variable, but binding to GTKO, GTKO/NeuGcKO, or human CECs was significantly decreased (n=6, *p<0.05). There was no significant difference in binding to GTKO, GTKO/NeuGcKO, and human CECs. (C) Human IgM binding to WT pAECs was variable, but binding to GTKO and GTKO/NeuGcKO pAECs was significantly decreased (n=6, *p<0.05). There was no significant difference in IgM binding to pAECs from GTKO pigs of the two different genetic backgrounds, but binding to GTKO pAECs was significantly greater than to GTKO/NeuGcKO pAECs (* p<0.05; ns=not significant). There was no significant difference in IgM binding to pAECs from GTKO/NeuGcKO pigs of the two different genetic backgrounds. Binding of human IgM antibody to human CECs was significantly lower than to all other pCECs (* p<0.05).
(D) Human IgG binding to WT and GTKO pAECs and was variable, but almost no binding to GTKO/NeuGcKO pAECs was detected. Compared to WT pAECs, human IgG binding to GTKO and GTKO/NeuGcKO pAECs was significantly decreased (n=6, * p<0.05). There was no significant difference in IgG binding to GTKO pAECs of the two different genetic backgrounds, but binding to GTKO pAECs was significantly greater than to GTKO/NeuGcKO pAECs (* p<0.05; ns=not significant). There was no significant difference in IgG binding to pAECs from GTKO/NeuGcKO pigs of the two different genetic backgrounds.
DISCUSSION
Neither Gal nor NeuGc could be detected in corneas/CECs (or aortas/AECs) from GTKO/NeuGcKO pigs, as is the case with human corneas and aortas. Nevertheless, some human antibodies, noticeably IgG, bound to the corneas and aortas, suggesting that there are remaining xenoantigens on GTKO/NeuGcKO pig corneas and aortas, as suggested by others22. An antibody directed to nonGal/nonNeuGc antigens has been proposed23, but its specificity remains unknown. Byrne et al have identified β1,4 N-acetylgalactosaminyl transferase as an antigen of significance in xenotransplantation24, but its relevance to corneal transplantation is unknown. Whether antibody binding to non-Gal, non-NeuGc epitopes on a corneal graft (or other organ) would be detrimental to its long-term outcome needs to be investigated.
A higher expression of NeuGc has been demonstrated on GTKO pig tissue (i.e., heart, liver, kidney) and cells (fibroblast cells) compared to NeuGc expression on WT cells, suggesting that deletion of the α1,3-galactosyltransferase gene leads to a ‘compensatory’ increased expression of sialylated glycans, including NeuGc 25,26. In contrast, in our previous experience we did not document any difference in expression of NeuGc between WT and GTKO pig corneas by immunofluorescence staining15. However, in the present study, by flow cytometry we detected varying levels of expression of NeuGc depending on the genetic background of the pig. Cells (both pAECs and pCECs) from the GTKO pigs provided by the NSRRC appeared to have greater expression of NeuGc than WT pigs, but cells from GTKO pigs provided by Revivicor showed similar or even lower expression than WT pigs, but whether this difference is significant remains uncertain. Expression of NeuGc would need to be measured in various strains of pig using a quantifiable method.
These perceived different levels of NeuGc expression did not correlate with the extent of human antibody binding. Pigs from the NSRRC had higher NeuGc expression, but human antibody binding to the tissues or cells was not more than to those from Revivicor pigs. The variable level of NeuGc expression on pCECs and pAECs may therefore not be sufficiently different to show disparity in antibody binding. The differing backgrounds (strains) of the pigs or different techniques of genetic modification may have modified surface carbohydrate composition. The extent of antibody binding may also be influenced by the presence of anti-nonGal, anti-nonNeuGc antibodies in the serum.
When using CECs as the target cells, there were no significant differences in human IgM or IgG binding to GTKO and GTKO/NeuGcKO CECs. There was minimal antibody binding to CECs from either GTKO or GTKO/NeuGcKO pigs; nevertheless, the binding of IgM was significantly higher than to human cells. There was no binding of IgG to all GTKO, GTKO/NeuGcKO and human cells (rMFI =< 1). This result differs from that reported using PBMCs2,9 and AECs as target cells, which showed greater reduction in antibody binding to GTKO/NeuGcKO pig cells than to GTKO pig cells. This may possibly be explained by low immunogenicity of pig CECs compared to pig PBMCs and AECs16. Both Gal16 and NeuGc expression are significantly lower on CECs than on comparable AECs (mean rMFI of NeuGc level of pAECs =142.3 ±145.1 [n=7] vs. mean rMFI of NeuGc level of pCECs =9.24 ±6.0 [n=7]; p=0.02). Removal of Gal epitopes alone appears to be sufficient to significantly reduce the humoral barrier to CECs, but not to AECs.
Whether human anti-NeuGc antibodies are less or more detrimental to a pig graft than anti-Gal antibodies is uncertain. Although human anti-NeuGc antibodies have a lower IgM/IgG ratio and lower titers of pre-existing natural antibody, the presence of NeuGc epitopes on the pig corneal endothelium (where Gal is absent) may be important and may be associated with greater injury4. The in vivo human elicited antibody response to NeuGc has not been measured, but may be considerable, thus initiating rejection. Furthermore, the interactions of NeuGc with circulating anti-NeuGc antibodies may potentially incite inflammation27, and therefore the absence of NeuGc may reduce both the humoral and inflammatory barriers to corneal xenotransplantation. Long-term assessment of the relationship between NeuGc and inflammation will be necessary.
In summary, by reducing human xenoreactive antibody binding, the development of pigs deficient in both Gal and NeuGc may reduce immunologic and/or inflammatory injury to a pig corneal xenograft in humans (but not in Old World nonhuman primates, which express NeuGc), but will not prevent all antibody binding. Identification and deletion of other xenoantigens may be necessary to provide complete protection of a pig corneal xenograft.
ACKNOWLEDGEMENTS
Research on xenotransplantation at the University of Pittsburgh is funded in part by NIH grant # IU19A1090959-01, NIH grant #U01A1066331, and by Sponsored Research Agreements with Revivicor Inc., Blacksburg, VA. Burcin Ekser, MD, was a recipient of a NIH NIAID T32 AI 074490 Training Grant. Hidetaka Hara MD, PhD was supported in part by NIH grant #1RO3A1096296-01. NSRRC is funded by NIH grant U42ODO11140.
ABBREVIATIONS
- AECs
aortic endothelial cells
- CECs
corneal endothelial cells
- Gal
galactose-α1,3-galactose
- GTKO
α1,3-galactosyltransferase gene-knockout
- NeuGc
N-glycolylneuraminic acid
- NeuGcKO
NeuGc gene-knockout
- p
pig
Footnotes
CONFLICT OF INTEREST
David Ayares and Jagdeece Ramsoondar are employees of Revivicor Inc. No other author has a conflict of interest.
REFERENCES
- 1.Phelps CJ, Koike C, Vaught TD, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299:411–414. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lutz AJ, Li P, Estrada JL, et al. Double knockout pigs deficient in N-glycolylneuraminic acid and galactose alpha-1,3-galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation. 2013;20:27–35. doi: 10.1111/xen.12019. [DOI] [PubMed] [Google Scholar]
- 3.Zhu A, Hurst R. Anti-N-glycolylneuraminic acid antibodies identified in healthy human serum. Xenotransplantation. 2002;9:376–381. doi: 10.1034/j.1399-3089.2002.02138.x. [DOI] [PubMed] [Google Scholar]
- 4.Salama A, Evanno G, Harb J, et al. Potential deleterious role of anti-Neu5Gc antibodies in xenotransplantation. Xenotransplantation. 2015;22:85–94. doi: 10.1111/xen.12142. [DOI] [PubMed] [Google Scholar]
- 5.Miwa Y, Kobayashi T, Nagasaka T, et al. Are N-glycolylneuraminic acid (Hanganutziu-Deicher) antigens important in pig-to-human xenotransplantation? Xenotransplantation. 2004;11:247–253. doi: 10.1111/j.1399-3089.2004.00126.x. [DOI] [PubMed] [Google Scholar]
- 6.Padler-Karavani V, Varki A. Potential impact of the non-human sialic acid N-glycolylneuraminic acid on transplant rejection risk. Xenotransplantation. 2011;18:1–5. doi: 10.1111/j.1399-3089.2011.00622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bardor M, Nguyen DH, Diaz S, et al. Mechanism of uptake and incorporation of the non-human sialic acid N-glycolylneuraminic acid into human cells. J Biol Chem. 2005;80:4228–4237. doi: 10.1074/jbc.M412040200. [DOI] [PubMed] [Google Scholar]
- 8.Bouhours D, Pourcel C, Bouhours JE. Simultaneous expression by porcine aorta endothelial cells of glycosphingolipids bearing the major epitope for human xenoreactive antibodies (Galα1–3Gal), blood group H determinant andN-glycolylneuraminic acid. Glycoconjugate Journal. 1996;13:947–953. doi: 10.1007/BF01053190. [DOI] [PubMed] [Google Scholar]
- 9.Burlak C, Paris LL, Lutz AJ, et al. Reduced binding of human antibodies to cells from GGTA1/CMAH KO pigs. Am J Transplant. 2014;14:1895–1900. doi: 10.1111/ajt.12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hara H, Cooper DK. The immunology of corneal xenotransplantation: a review of the literature. Xenotransplantation. 2010;17:338–349. doi: 10.1111/j.1399-3089.2010.00608.x. [DOI] [PubMed] [Google Scholar]
- 11.Choi HJ, Kim MK, Lee HJ, et al. Efficacy of pig-to-rhesus lamellar corneal xenotransplantation. Invest Ophthalmol Vis Sci. 2011;52:6643–6650. doi: 10.1167/iovs.11-7273. [DOI] [PubMed] [Google Scholar]
- 12.Vabres B, Le Bas-Bernardet S, Riochet D, et al. hCTLA4-Ig transgene expression in keratocytes modulates rejection of corneal xenografts in a pig to non-human primate anterior lamellar keratoplasty model. Xenotransplantation. 2014;21:431–443. doi: 10.1111/xen.12107. [DOI] [PubMed] [Google Scholar]
- 13.Scobie L, Padler-Karavani V, Le Bas-Bernardet S, et al. Long-term IgG response to porcine Neu5Gc antigens without transmission of PERV in burn patients treated with porcine skin xenografts. J Immunol. 2013;191:2907–2915. doi: 10.4049/jimmunol.1301195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stone KR, Abdel-Motal UM, Walgenbach AW, et al. Replacement of human anterior cruciate ligaments with pig ligaments: a model for anti-non-gal antibody response in long-term xenotransplantation. Transplantation. 2007;83:211–219. doi: 10.1097/01.tp.0000250598.29377.13. [DOI] [PubMed] [Google Scholar]
- 15.Cohen D, Miyagawa Y, Mehra R, et al. Distribution of non-gal antigens in pig cornea: relevance to corneal xenotransplantation. Cornea. 2014;33:390–397. doi: 10.1097/ICO.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 16.Hara H, Koike N, Long C, et al. Initial in vitro investigation of the human immune response to corneal cells from genetically engineered pigs. Invest Ophthalmol Vis Sci. 2011;52:5278–5286. doi: 10.1167/iovs.10-6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi HJ, Lee JJ, Kim DH, et al. Blockade of CD40-CD154 Costimulatory Pathway Promotes Long-Term Survival of Full-Thickness Porcine Corneal Grafts in Nonhuman Primates: Clinically Applicable Xenocorneal Transplantation. Am J Transplant. 2015;15:628–41. doi: 10.1111/ajt.13057. [DOI] [PubMed] [Google Scholar]
- 18.Kim YG, Oh JY, Gil GC, et al. Identification of alpha-Gal and non-Gal epitopes in pig corneal endothelial cells and keratocytes by using mass spectrometry. Curr Eye Res. 2009;34:877–895. doi: 10.3109/02713680903184243. [DOI] [PubMed] [Google Scholar]
- 19.Fujita M, Mehra R, Lee SE, et al. Comparison of proliferative capacity of genetically-engineered pig and human corneal endothelial cells. Ophthalmic Res. 2013;49:127–138. doi: 10.1159/000342978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hara H, Long C, Lin YJ, et al. In vitro investigation of pig cells for resistance to human antibody-mediated rejection. Transpl Int. 2008;21:1163–1174. doi: 10.1111/j.1432-2277.2008.00736.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee SE, Mehra R, Fujita M, et al. Characterization of porcine corneal endothelium for xenotransplantation. Semin Ophthalmol. 2014;29:127–35. doi: 10.3109/08820538.2013.787104. [DOI] [PubMed] [Google Scholar]
- 22.Burlak C, Bern M, Brito AE, et al. N-linked glycan profiling of GGTA1/CMAH knockout pigs identifies new potential carbohydrate xenoantigens. Xenotransplantation. 2013;20:277–291. doi: 10.1111/xen.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barone A, Benktander J, Teneberg S, et al. Characterization of acid and non-acid glycosphingolipids of porcine heart valve cusps as potential immune targets in biological heart valve grafts. Xenotransplantation. 2014;21:510–522. doi: 10.1111/xen.12123. [DOI] [PubMed] [Google Scholar]
- 24.Byrne GW, Du Z, Stalboerger P, et al. Cloning and expression of porcine beta1,4 N-acetylgalactosaminyl transferase encoding a new xenoreactive antigen. Xenotransplantation. 2014;21:543–554. doi: 10.1111/xen.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park HM, Kim YW, Kim KJ, et al. Comparative N-Linked Glycan Analysis of Wild-Type and alpha1,3-Galactosyltransferase Gene Knock-Out Pig Fibroblasts Using Mass Spectrometry Approaches. Mol Cells. 2015;38:65–74. doi: 10.14348/molcells.2015.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park JY, Park MR, Kwon DN, et al. Alpha 1,3-galactosyltransferase deficiency in pigs increases sialyltransferase activities that potentially raise non-gal xenoantigenicity. J Biomed Biotechnol. 2011;2011:560850. doi: 10.1155/2011/560850. doi: 10.1155/2011/560850. Epub 2011 Oct 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samraj AN, Pearce OM, Laubli H, et al. A red meat-derived glycan promotes inflammation and cancer progression. Proc Natl Acad Sci U S A. 2015;112:542–547. doi: 10.1073/pnas.1417508112. [DOI] [PMC free article] [PubMed] [Google Scholar]